Abstract
The efficacy of proton beam therapy (PBT) for hepatocellular carcinoma (HCC) has been reported, but insertion of fiducial markers in the liver is usually required. We evaluated the efficacy and toxicity of respiratory-gated PBT without fiducial markers for HCC located within 2 cm of the gastrointestinal tract. From March 2011 to December 2015 at our institution, 40 patients were evaluated (median age, 72 years; range, 38–87 years). All patients underwent PBT at a dose of 60 to 80 cobalt gray equivalents (CGE) in 20 to 38 fractions. The median follow-up period was 19.9 months (range, 1.2–72.3 months). The median tumor size was 36.5 mm (range, 11–124 mm). Kaplan–Meier estimates of the 2-year overall survival, progression-free survival, and local tumor control rates were 76%, 60%, and 94%, respectively. One patient (2.5%) developed a grade 3 gastric ulcer and one (2.5%) developed grade 3 ascites retention; none of the remaining patients developed grade >3 toxicities (National Cancer Institute Common Terminology Criteria for Adverse Events ver. 4.0.). This study indicates that PBT without fiducial markers achieves good local control without severe treatment-related toxicity of the gastrointestinal tract for HCC located within 2 cm of the gastrointestinal tract.
1. Introduction
Hepatocellular carcinoma (HCC) is one of the most important cancers worldwide because of its generally poor prognosis [1,2]. In addition to radiotherapy, the treatment strategies for HCC include surgery, transcatheter artery chemoembolization, radiofrequency ablation (RFA), and liver transplantation [3,4]. Radiation therapy has historically been considered ineffective for treatment of HCC. Because the liver is a radiosensitive organ, it is difficult to achieve a sufficient irradiation dose to treat HCC [5,6]. Proton beam therapy (PBT) for HCC has recently been reported to achieve good local control and less toxicity [7,8,9].
In contrast, the gastrointestinal (GI) tract is a serial organ that is sensitive to radiation [6]. A high dose of radiation therapy may cause severe damage [10]. Therefore, HCC adjacent to the GI tract should be carefully treated using the high-precision irradiation technique. For high-accuracy irradiation, Tsukuba University developed a respiratory gating system with fiducial markers and reported good treatment outcomes [8,11]. Furthermore, a four-dimensional computed tomography (4D-CT) technique was recently used to analyze the respiratory motion of the target and was shown to be useful for high-accuracy radiotherapy [12,13].
In this study, we used 4D-CT planning to measure tumor motion and retrospectively assessed the outcome of treatment for HCC adjacent to the GI tract without fiducial markers using breathing-synchronized irradiation.
2. Results
Of the 40 patients included in this study, 38 completed PBT according to the treatment protocol (Table 1). One of the two who did not complete the protocol had gastric hemorrhage with an ulcer treated by transfusion (grade 3), and PBT was finished earlier than the planned protocol (70 cobalt gray equivalents (CGE) in 35 Fr). The other patient developed uncontrollable ascites (grade 3) and also finished PBT earlier than the planned protocol (52.8 CGE in 24 Fr). Of the 40 cases, 22 were replanned and three were replanned twice. In total, we performed 25 replannings. Furthermore, three patients underwent unexpected replanning because of an unpredictable change in the GI tract position or shape at the time of 5-, 6-, or 10-fraction treatment (Table 2).

Table 1.
Patient and tumor characteristics.

Table 2.
Treatment protocols.
All patients were followed up until death or December 2017. The median follow-up time was 19.9 months (range, 1.2–72.3 months). The median survival time was 20.8 months. The overall survival (OS) rates after 1 and 2 years were 86% (95% CI, 75%–98%) and 76% (62%–91%), respectively. Of all potential prognostic factors in the univariate analysis, age, sex (female), number of tumors, and PS were associated with OS. In the multivariate analyses using Cox regression analysis, sex (female) and PS were significantly associated with good OS (Table 3).

Table 3.
Variables affecting overall survival.
The progression-free survival (PFS) rates after 1 and 2 years were 70% (95% confidence interval (CI), 55–86%) and 60% (42–79%), respectively. The local tumor control (LTC) rate after 2 years was 94% (95% CI, 83–100%) (Figure 1).
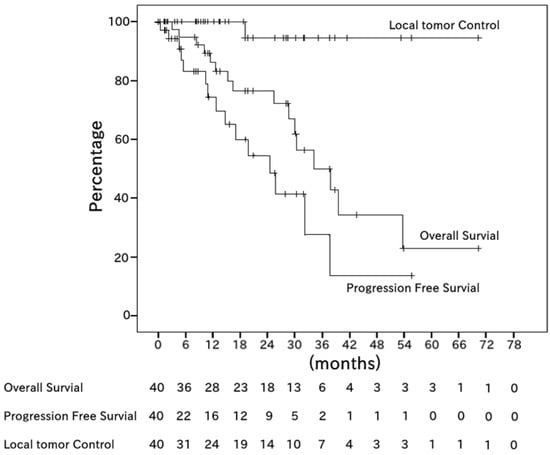
Figure 1.
Kaplan–Meier estimates of overall survival (OS), progression-free survival (PFS), and local tumor control (LTC) among all 40 patients. The median OS period was 20.8 months (range, 1.3–72.3 months). The median PFS period was 9.8 months (range, 0.0–57.2 months). The 2-year OS, PFS, and LTC rates were 76%, 60%, and 94%, respectively.
With respect to acute treatment-related toxicity, one patient with a bleeding gastric ulcer (grade 3) and another patient with uncontrollable ascites (grade 3) were unable to complete treatment. No patients had any other grade >3 severe acute toxicities; only skin reactions were confirmed (grade 1 or 2). With respect to late toxicity, one patient had GI bleeding (grade 2), and one had a rib fracture with pain (grade 2). No patients had grade >3 severe late treatment-related toxicity, including GI bleeding or perforation.
Figure 2 shows the MRI and PBT planning results of a successfully treated 64-year-old man with a large HCC in the right hepatic lobe (Figure 2a). He was treated with PBT at 80 GCE in 25 fractions (Figure 2b,c). The tumor shrank and was controlled 2 years after PBT (Figure 2d). He showed no severe complications during a 2-year follow-up.
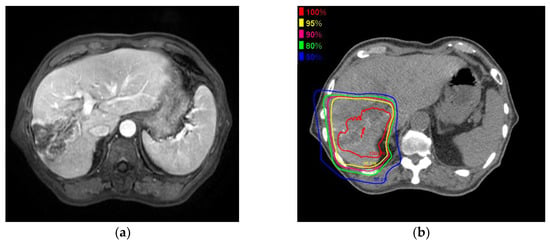
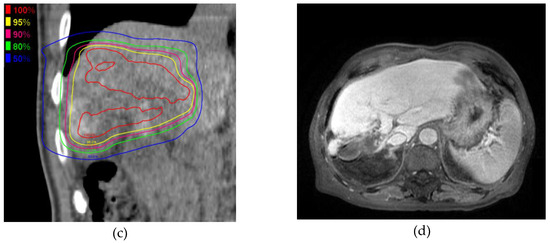
Figure 2.
A 6.3 cm hepatocellular carcinoma (HCC) was treated by proton beam therapy (PBT) in a patient with Child–Pugh class A liver disease and right portal vein tumor thrombosis. (a) Early enhancement of the HCC was shown in the arterial phase of gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid-enhanced dynamic magnetic resonance imaging (Gd-EOB-MRI); (b) The isodose lines were shown on planning CT (axial view); (c) This tumor was close to the colon (coronal view); (d) HCC was shown in the arterial phase of EOB-MRI 2 years after PBT.
3. Discussion
3.1. Treatment Effect
A particle beam has different physical characteristics from a photon beam and can be useful for high-dose irradiation to HCC with a decreased background liver dose compared with photon therapy [14,15,16]. Many cases of good local control by particle beam therapy for HCC have recently been reported [17,18,19]. In PBT for HCC, Fukumitsu et al. [18] reported a 2-year local control rate of 87.8%. In addition, Kawashima et al. [19] reported a 2-year local control rate of 96%. Considering that the GI tract is susceptible to irradiation damage, irradiation doses to the GI tract should be reduced. In particle beam therapy, Abe et al. [20] reported that a carbon ion beam can reduce the GI tract dose more than can stereotactic body radiotherapy. Additionally, Nakayama et al. [21] performed PBT for HCC adjacent to the GI tract and reported good control and less toxicity with fiducial markers. In the present study, the 2-year local control rate was 94%, which is almost the same as in the above study.
Age, sex (female), number of tumors, and PS were associated with OS in the univariate analysis, while sex (female) and PS were associated with OS in the multivariate analysis (Table 3). Komatsu et al. [22] analyzed the factors associated with OS in 150 patients with HCC and reported that age, PS, and Child–Pugh classification significantly influenced OS in the univariate analysis. Tangkijvanich et al. [23] reported that among patients with HCC, female patients tended to have higher survival rates than male patients, but sex was not a predictor of patient survival. The number of tumors (solitary or not) is clinically important and an effective prognostic factor for OS. These clinical prognostic factors for OS in the present study are comparable with those in previous reports [9,24,25,26].
3.2. Adverse Effects
If the organs at risk are close to the irradiation field, more precise treatment is necessary, especially for organs affected by respiratory motion. In previously reported studies of PBT for HCC, fiducial markers were used for treatment [7,8,9,21,22,24]. Nakayama et al. [21] reported that 47 patients with HCC close to the GI tract were treated with PBT using metallic fiducial markers and 4 patients had grade 2 or 3 GI bleeding. In two of our patients, treatment was discontinued due to acute toxicity. The first patient had grade 3 gastric hemorrhage at the end of treatment (70 CGE in 35 Fr); this patient was irradiated at 59.3 CGE in 35 Fr (the 1-cc dose), which is the stomach tolerance dose (Figure 3) [6]. However, patients with liver cirrhosis are at risk of gastric bleeding, and this patient had a history of a gastric ulcer. In such cases, the irradiation dose should be as low as possible. After our experience with this patient, we attempted to reduce the 1-cc dose of the stomach and duodenum to <50 CGE, equivalent to 2 gray. Late gastric toxicity did not appear in this patient’s clinical course, and the HCC was controlled for 3 years.
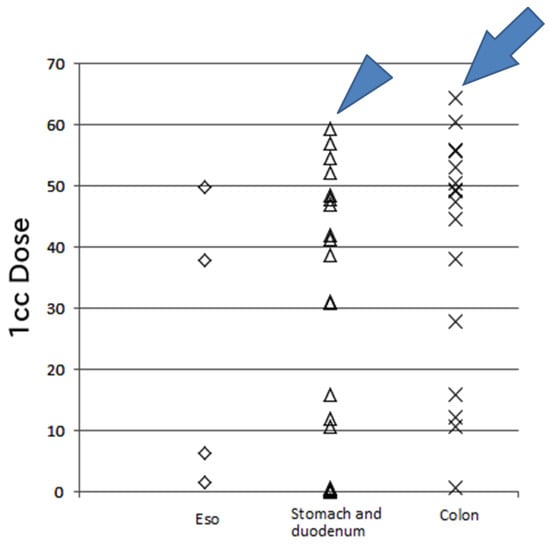
Figure 3.
The 1-cc dose of the gastrointestinal tract. One case (arrowhead) involved a high dose (59.3 CGE) per 35 fractions to the stomach and duodenum, resulting in gastric hemorrhage with an ulcer treated by transfusion (grade 3). Another case (arrow) involved a high dose (64.4 CGE) per 20 fractions to the transverse colon without acute or late toxicity during the 3-year follow-up. Eso: esophagus, CGE: cobalt gray equivalent.
The second patient who discontinued treatment was a 73-year-old woman with hepatitis C-related liver cirrhosis with bile duct invasion (Child–Pugh B; gross tumor volume, 252 mL; liver volume, 1320 mL). She stopped treatment due to ascites at the time of 52.8 CGE/24 Fr irradiation. Patients approaching a state of decompensated liver cirrhosis with a large target volume should be carefully treated and are at risk of developing ascites due to acute liver damage.
One patient with a high dose at the colon was a 38-year-old woman with hepatitis C-related liver cirrhosis (Child–Pugh class A) who developed HCC after transcatheter artery chemoembolization and RFA, and she was treated by PBT (76 CGE in 20 Fr) (Figure 3). Fortunately, she did not develop severe complications throughout a 3-year follow-up. However, the smallest dose possible should be administered to the colon considering the tolerance dose of this organ [6].
In this study, we attempted to reduce the GI tract dose by frequent performance of diagnostic imaging (CT or MRI) and application of adaptive PBT. No patients had severe late treatment-related toxicity, including GI bleeding and perforation.
3.3. Fiducial Markers
In this study, we did not use fiducial markers to achieve less invasive PBT. Fiducial marker insertion to the liver is safe, but in rare cases there is a risk of complications [27,28]. In the present study, most patients had inoperable disease, requiring us to avoid or reduce the risk as much as possible. Without insertion of fiducial markers into the liver, the diaphragm was used as the marker in this technique, and the daily inter-fractional error of the diaphragm in the craniocaudal direction was adjusted by the onboard imaging system. Balter et al. [29] and Yang et al. [30] reported that the diaphragm is an acceptable anatomic landmark for radiographic estimation of liver movement. Furthermore, in our respiratory gating method, the gate width with a duty cycle of about 20% (17–25%) is narrower than that obtained with the general method with a duty cycle of 20% to 40% [31]. The merit of a narrow gate is minimization of respiratory motion; however, the treatment time increases. For some patients, the treatment time was about 30 to 40 minutes because of the irregular respiratory rhythm.
We performed 4D-CT analysis the motion of the target, respiratory gating irradiation with a narrow gate width, frequent checks of changes in the GI tract, and adaptive PBT. Our results are consistent with those in other reports of PBT for HCC with fiducial markers in terms of LTC and adverse effects. Our treatment method is considered useful and safe.
The limitations of the present study include its retrospective design, small number of patients, and short follow-up period. Further prospective studies are necessary to assess a greater number of patients for a longer follow-up period.
4. Patients and Methods
4.1. Patients and Clinical Examination
This retrospective study was approved by the research ethics committee of our institution, and written informed consent for this study was waived because of its retrospective nature. From March 2011 to December 2015, 144 consecutive patients were treated at our institution. The inclusion criteria were (1) a medically inoperable condition considered difficult to control with RFA or patient refusal to undergo surgery or RFA, (2) an Eastern Cooperative Oncology Group performance status (PS) of 0 to 2, (3) hepatic function characterized by a Child–Pugh score of ≤10, (4) no uncontrolled ascites, (5) no extrahepatic metastasis, and (6) HCC adjacent to the digestive tract (≤2 cm distant of the digestive tract). Forty patients were considered eligible for analysis (Table 1). HCC was pathologically or clinically diagnosed by means of early nodular staining regarding the arterial enhancement and venous washout on dynamic CT and/or magnetic resonance imaging (MRI) and using serum levels of tumor markers (α-fetoprotein and des-γ-carboxyprothrombin) [4].
4.2. PBT Planning
Patient setup, imaging planning, and use of the respiratory gating system were previously described [8,32,33]. A respiratory-synchronized 4D-CT system (Aquilion LB TSX-201A; Toshiba Medical Systems Co., Tochigi, Japan) with a respiratory gating system (Anzai Medical Co., Tokyo, Japan) was used under the following conditions: tube voltage, 120 kV; tube current, 200 to 300 mA; rotation time, 500 ms; and breathing synchronization using the 4D helical scan method. The patient was asked to perform stable breathing at a rate of 10 to 15 breaths/min using a metronome to maintain the appropriate rhythm. Patients with HCC close to the upper GI tract were examined in a fasting state. The reconstruction conditions of the CT images were as follows: slice thickness, 2 mm; slice interval (gap), 0.4 mm; and field of view set to match the physique of the patient.
The target delineation method was previously described [8]. Targets were contoured on the end of the exhalation phase of the 4D-CT image. The internal target volume was determined as the clinical target volume plus a 5- or 10-mm margin from the 4D-CT analyses. The internal margin was customized based on the amount of respiratory-induced motion visualized in the gate width for 1 s; a duty cycle of about 20% was centered at end-expiration [31]. The planning target volume was determined as the clinical target volume plus a 5-mm margin in all directions using a radiation treatment planning system with a proton pencil beam algorithm (XiO-N; Elekta, Mitsubishi Electric Corporation, Kobe, Japan). The beam angles were selected according to the following protocol: The treatment plan mainly involved more than two ports containing the perpendicular directions, and the beam directions were mainly selected to avoid the GI tract while cutting the radiation field using patient collimators [21]. Furthermore, patients with HCC in contact with the GI tract underwent replanning after 30 to 40 CGE irradiation with a cone-down boost (Table 2).
Additionally, the treatment plan was determined according to the following protocol: as large as the percentage volume of the non-irradiated normal liver [34], as low as the maximum exposure dose of the adjacent GI tract, and as low as the maximum 1-cc exposure dose in the adjacent GI tract [35] (Table 4). The total dose at the isocenter was prescribed to cover 95% of the planning target volume. The total dose for the target and organs were evaluated by rigid fusion techniques using commercially available software (MIM Maestro; MIM Vista Corp., Cleveland, OH, USA).

Table 4.
Gastrointestinal tract dose.
4.3. PBT
The PBT system used proton beams ranging from 150 to 230 mega-electron volts (MeV) generated through a linear accelerator and synchrotron. The 3D conformal proton dose distributions were formed based on a broad-beam method involving a couple of wobbling electromagnets, a scatter plate, a range modulator, and a multi-leaf collimator and in which the patient’s collimator and bolus were practically used. The patient’s collimators and boluses were custom-made to conform the beams to the treatment planning data (Mitsubishi Electric Corporation).
Nine protocols for respiratory-gated proton therapy (52.8–80.0 CGE in 20–38 fractions using 150-, 190-, or 230-MeV proton beams) were implemented during the study period using an irradiation schedule of 5 fractions per week (Table 2). The radiation dose was prescribed in CGE using a relative biological effectiveness value of 1.1 based on our preclinical experiments.
These protocols were based on previously reported studies [7,8,21,24]. To reduce the GI tract dose, patients with HCC close to the upper GI tract were treated in a fasting state. Daily irradiation was performed using more than two ports (with the exception of the plan using a one-port beam). The respiratory gating was configured for a beam-on cycle centered at end-exhalation for 1 s (duty cycle of about 20%). In this system, we can adjust the respiratory window; the beam irradiation is automated. If the patient’s respiratory rhythm becomes irregular or the patient begins coughing, we can push the button and easily avoid irradiation during the respiratory window.
Our image-guided radiation therapy system contains a biplane X-ray system with a full six-axis adjustability couch for the patient. We can correct setup errors matching to the vertebral body using the X-ray system in the anterior–posterior and right–left directions. The kV X-ray fluoroscopy can directly show real-time movement of the diaphragm and gas in the GI tract. Differences can be compared and the distance measured in the craniocaudal direction between the diaphragm contour at the time of planning and the diaphragm position in the deep aspiration phase onboard. We adjust the daily error of the diaphragm position at end-exhalation to plan the position of the diaphragm onboard by moving the treatment couch in the craniocaudal direction.
In the present study, we evaluated the target and GI tract by autoactivation imaging using positron emission tomography–CT. The data acquisition started 5 to 10 minutes after proton beam irradiation to assess the irradiated area on the first day of PBT [36]. Differences in the position or shape and total dose of the target or GI tract were evaluated by rigid fusion techniques using MIM Maestro. If the GI tract received high-dose proton beam irradiation by a change in the position or shape, we re-established the treatment plan. Furthermore, we evaluated the target and GI tract by CT and MRI at 15-fraction irradiation (3 weeks after beginning treatment) for adaptive treatment. If the high-dose irradiation was on the GI tract, the treatment plan was adaptively changed.
4.4. Follow-Up and Toxicity Evaluation
Abdominal CT and MRI examinations were performed at each follow-up and analyzed to determine the treatment effect (every 3 months after completion of PBT for the first 3 years, once every 6 months for the following 3–5 years, and annually thereafter). Tumor local control was assessed by simply determining if tumor regrowth was present using CT and MRI. Toxicity was graded according to the Common Terminology Criteria for Adverse Events ver. 4.
4.5. Statistical Methods
The OS, PFS, LTC rates were analyzed using the Kaplan–Meier method, and a log-rank test was performed to evaluate statistical differences between two categories. OS, PFS, and LTC periods were calculated from the date of PBT start to the date of death or latest follow-up. Multivariate analyses were performed using a Cox regression analysis to identify the most significant independent prognostic factors. The following clinical variables were assessed using multivariate analysis: sex (female), age, chronic liver disease with viral infection, alcoholic liver, Child–Pugh class, operability, PS (0, 1/2), T classification, prior treatment, number of tumors (solitary or not), whole liver volume, tumor size, and gross tumor volume. The hazard ratio and 95% CI were calculated for each independent factor. All statistical analyses were performed using IBM SPSS version 20.0 software (IBM Corp., Armonk, NY, USA). A p-value of <0.05 was considered statistically significant.
5. Conclusions
PBT for HCC located within 2 cm of the GI tract without fiducial markers at our institution is a safe and effective treatment without severe complications. Further investigation and long-term follow-up is necessary.
Acknowledgments
We gratefully acknowledge the work of the members of the Proton Therapy Center and the Radiology Department of Fukui Prefectural Hospital. We also thank all of the patients who participated in the study for their understanding and support. Finally, we thank Angela Morben, DVM, ELS, from Edanz Group (www.edanzediting.com/ac), for editing a draft of this manuscript. This work was supported by JSPS KAKENHI (Grants-in-Aid for Scientific Research) Grant Number 16K10273.
Author Contributions
Miu Mizuhata, Shigeyuki Takamatsu, Tomoyasu Kumano, Satoshi Kobayashi, and Toshifumi Gabata contributed to the study concept and clinical study design. Miu Mizuhata also wrote the initial draft of the manuscript. Shigeyuki Takamatsu, Kazutaka Yamamoto, Mariko Kawamura, and Satoshi Kobayashi assisted in the preparation of the manuscript. Miu Mizuhata and Shigeyuki Takamatsu also contributed to the data collection and analysis. Shigeyuki Takamatsu, Kazutaka Yamamoto, Satoshi Shibata, Sayuri Bou, Yoshitaka Sato, Mariko Kawamura, Satoko Asahi, and Hiroyasu Tamamura contributed to patient care. Yuji Tameshige, Yoshikazu Maeda, and Makoto Sasaki contributed to the proton beam therapy treatment planning. Miu Mizuhata contributed to the critical revision of the article for important intellectual content. Toshifumi Gabata contributed to the final approval of the article. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liver Cancer. Estimated Incidence, Mortality and Prevalence Worldwide in 2012. Available online: http://globocan.iarc.fr/old/FactSheets/cancers/liver-new.asp (accessed on 15 January 2018).
- Forner, A.; Llovet, J.M.; Bruix, J. Hepatocellular carcinoma. Lancet 2012, 379, 1245–1255. [Google Scholar] [CrossRef]
- Forner, A.; Gilabert, M.; Bruix, J.; Raoul, J.L. Treatment of intermediate-stage hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2014, 11, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Sherman, M.; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: An update. Hepatology 2011, 53, 1020–1022. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.; Murikami, K. Preliminary neoplasms of the liver. Results of radiation therapy. Cancer 1960, 13, 714–720. [Google Scholar] [CrossRef]
- Emami, B.; Lyman., J.; Brown, A.; Coia., L.; Goitein, M.; Munzenrider, J.E.; Shank, B.; Solin, L.J.; Wesson, M. Tolerance of normal tissue to therapeutic irradiation. Int. J. Radiat. Oncol. Biol. Phys. 1991, 21, 109–122. [Google Scholar] [CrossRef]
- Komatsu, S.; Fukumoto, T.; Demizu, Y.; Miyawaki, D.; Terashima, K.; Sasaki, R.; Hori, Y.; Hishikawa, Y.; Ku, Y.; Murakami, M. Clinical results and risk factors of proton and carbon ion therapy for hepatocellular carcinoma. Cancer 2011, 117, 4890–4904. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, H.; Sugahara, S.; Tokita, M.; Fukuda, K.; Mizumoto, M.; Abei, M.; Shoda, J.; Sakurai, H.; Tsuboi, K.; Tokuuye, K. Proton beam therapy for hepatocellular carcinoma: The University of Tsukuba experience. Cancer 2009, 115, 5499–5506. [Google Scholar] [CrossRef] [PubMed]
- Chiba, T.; Tokuuye, K.; Matsuzaki, Y.; Sugahara, S.; Chuganji, Y.; Kagei, K.; Shoda, J.; Hata, M.; Abei, M.; Igaki, H.; et al. Proton beam therapy for hepatocellular carcinoma: A retrospective review of 162 patients. Clin. Cancer Res. 2005, 11, 3799–3805. [Google Scholar] [CrossRef] [PubMed]
- Onishi, H.; Ozaki, M.; Kuriyama, K.; Komiyama, T.; Marino, K.; Araya, M.; Saito, R.; Aoki, S.; Maehata, Y.; Tomiaga, L.; et al. Serious gastric ulcer event after stereotactic body radiotherapy (SBRT) delivered with concomitant vinorelbine in a patient with left adrenal metastasis of lung cancer. Acta Oncol. 2010, 51, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Ohara, K.; Okumura, T.; Akisada, M.; Inada, T.; Mori, T.; Yokota, H.; Calaguas, M.J. Irradiation synchronized with respiration gate. Int. J. Radiat. Oncol. Biol. Phys. 1989, 17, 853–857. [Google Scholar] [CrossRef]
- Gabryś, D.; Kulik, R.; Trela, K.; Ślosarek, K. Dosimetric comparison of liver tumour radiotherapy in all respiratory phases and in one phase using 4DCT. Radiother. Oncol. 2011, 100, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.T.; Apisarnthanarax, S.; Yin, L.; Zou, W.; Rosen, M.; Plastaras, J.P.; Ben-Josef, E.; Metz, J.M.; Teo, B.K. Comparative assessment of liver tumor motion using cine-magnetic resonance imaging versus 4-dimensional computed tomography. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Toramatsu, C.; Katoh, N.; Shimizu, S.; Nihongi, H.; Matsuura, T.; Takao, S.; Miyamoto, N.; Suzuki, R.; Sutherland, K.; Kinoshita, R.; et al. What is the appropriate size criterion for proton radiotherapy for hepatocellular carcinoma? A dosimetric comparison of spot-scanning proton therapy versus intensity-modulated radiation therapy. Radiat. Oncol. 2013, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.X.; Fu, S.; Zhang, Q.; Guo, X.M. Charged particle therapy versus photon therapy for patients with hepatocellular carcinoma: A systematic review and meta-analysis. Radiother. Oncol. 2015, 114, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Lin, S.H.; Simone, C.B.; Mehta, M.P. Clinical outcomes and toxicities of proton radiotherapy for gastrointestinal neoplasms: A systematic review. J. Gastrointest. Oncol. 2016, 7, 644–664. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Okumura, T.; Abei, M.; Fukumitsu, N.; Ishige, K.; Mizumoto, M.; Hasegawa, N.; Numajiri, H.; Ohnishi, K.; Ishikawa, H.; et al. Long-term outcomes of proton beam therapy in patients with previously untreated hepatocellular carcinoma. Cancer Sci. 2017, 108, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Fukumitsu, N.; Sugahara, S.; Nakayama, H.; Fukuda, K.; Mizumoto, M.; Abei, M.; Shoda, J.; Thono, E.; Tsuboi, K.; Tokuuye, K. A prospective study of hypofractionated proton beam therapy for patients with hepatocellular carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, M.; Furuse, J.; Nishio, T.; Konishi, M.; Ishii, H.; Kinoshita, T.; Nagase, M.; Nihei, K.; Ogino, T. Phase II study of radiotherapy employing proton beam for hepatocellular carcinoma. J. Clin. Oncol. 2005, 23, 1839–1846. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Saitoh, J.; Kobayashi, D.; Shibuya, K.; Koyama, Y.; Shimada, H.; Shirai, K.; Ohno, T.; Nakano, T. Dosimetric comparison of carbon ion radiotherapy and stereotactic body radiotherapy with photon beams for the treatment of hepatocellular carcinoma. Radiat. Oncol. 2015, 10, 187. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, H.; Sugahara, S.; Fukuda, K.; Abei, M.; Shoda, J.; Sakurai, H.; Tsuboi, K.; Matsuzaki, Y.; Tokuuye, K. Proton beam therapy for hepatocellular carcinoma located adjacent to the alimentary tract. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 992–995. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, S.; Murakami, M.; Fukumoto, T.; Hori, Y.; Hishikawa, Y.; Ku, Y. Risk factors for survival and local recurrence after particle radiotherapy for single small hepatocellular carcinoma. Br. J. Surg. 2011, 98, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Tangkijvanich, P.; Mahachai, V.; Suwangool, P.; Poovorawan, Y. Gender difference in clinicopathologic features and survival of patients with hepatocellular carcinoma. World J. Gastroenterol. 2004, 10, 1547–1550. [Google Scholar] [CrossRef] [PubMed]
- Mizumoto, M.; Okumura, T.; Hashimoto, T.; Fukuda, K.; Oshiro, Y.; Fukumitsu, N.; Abei, M.; Kawaguchi, A.; Hayashi, Y.; Ookawa, A.; et al. Proton beam therapy for hepatocellular carcinoma: a comparison of three treatment protocols. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Hata, M.; Tokuuye, K.; Sugahara, S.; Fukumitsu, N.; Hashimoto, T.; Ohnishi, K.; Nemoto, K.; Ohara, K.; Matsuzaki, Y.; Akine, Y. Proton beam therapy for hepatocellular carcinoma with limited treatment options. Cancer 2006, 107, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Mizumoto, M.; Tokuuye, K.; Sugahara, S.; Nakayama, H.; Fukumitsu, N.; Ohara, K.; Abei, M.; Shoda, J.; Tohno, E.; Minami, M. Proton beam therapy for hepatocellular carcinoma adjacent to the porta hepatis. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Khalsa, B.; Lord, B.; Sandrasegaran, K.; Lall, C. Planting the seeds of success: CT-guided gold seed fiducial marker placement to guide robotic radiosurgery. J. Med. Imaging Radiat. Oncol. 2013, 57, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Brook, O.R.; Gourtsoyianni, S.; Mendiratta-Lala., M.; Mahadevan, A.; Siewert, B.; Sheiman, R.R. Safety profile and technical success of imaging-guided percutaneous fiducial seed placement with and without core biopsy in the abdomen and pelvis. Am. J. Roentgenol. 2012, 198, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Balter, J.M.; Dawson, L.A.; Kazanjian, S.; McGinn, C.; Brock, K.K.; Lawrence, T.; Ten, H.R. Determination of ventilatory liver movement via radiographic evaluation of diaphragm position. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 267–270. [Google Scholar] [CrossRef]
- Yang, J.; Cai, J.; Wang, H.; Chang, Z.; Czito, B.G.; Bashir, M.R.; Palta, M.; Yin, F.F. Is diaphragm motion a good surrogate for liver tumor motion? Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Mageras, G.S.; Yorke, E. Deep Inspiration Breath Hold and Respiratory Gating Strategies for Reducing Organ Motion in Radiation Treatment. Semin. Radiat. Oncol. 2004, 14, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Tsujii, H.; Tsuji, H.; Inada, T.; Maruhashi, A.; Hayakawa, Y.; Takada, Y.; Tada, J.; Fukumoto, S.; Tatuzaki, H.; Ohara, K.; et al. Clinical results of fractionated proton therapy. Int. J. Radiat. Oncol. Biol. Phys. 1993, 25, 49–60. [Google Scholar] [CrossRef]
- Takamatsu, S.; Yamamoto, K.; Maeda, Y.; Kawamura, M.; Shibata, S.; Sato, Y.; Terashima, K.; Shimizu, Y.; Tameshige, Y.; Sasaki, M.; et al. Evaluation of Focal Liver Reaction after Proton Beam Therapy for Hepatocellular Carcinoma Examined Using Gd-EOB-DTPA Enhanced Hepatic Magnetic Resonance Imaging. PLoS ONE 2016, 11, 0167155. [Google Scholar] [CrossRef] [PubMed]
- Mizumoto, M.; Okumura, T.; Hashimoto, T.; Fukuda, K.; Oshiro, Y.; Fukumitsu, N.; Abei, M.; Kawaguchi, A.; Hayashi, Y.; Ohkawa, A.; et al. Evaluation of liver function after proton beam therapy for hepatocellular carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Terashima, K.; Demizu, Y.; Hashimoto, N.; Jin, D.; Mima, M.; Fujii, O.; Niwa, Y.; Takatori, K.; Kitajima, N.; Sirakawa, S.; et al. A phase I/II study of gemcitabine-concurrent proton radiotherapy for locally advanced pancreatic cancer without distant metastasis. Radiother. Oncol. 2012, 103, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Sasaki, R.; Miyawaki, D.; Nishimura, H.; Demizu, Y.; Akagi, T.; Suga, D.; Sakamoto, H.; Murakami, M.; Sugimura, K.; et al. Physiologic reactions after proton beam therapy in patients with prostate cancer: significance of urinary autoactivation. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 580–586. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).