Tackling Cancer Resistance by Immunotherapy: Updated Clinical Impact and Safety of PD-1/PD-L1 Inhibitors
Abstract
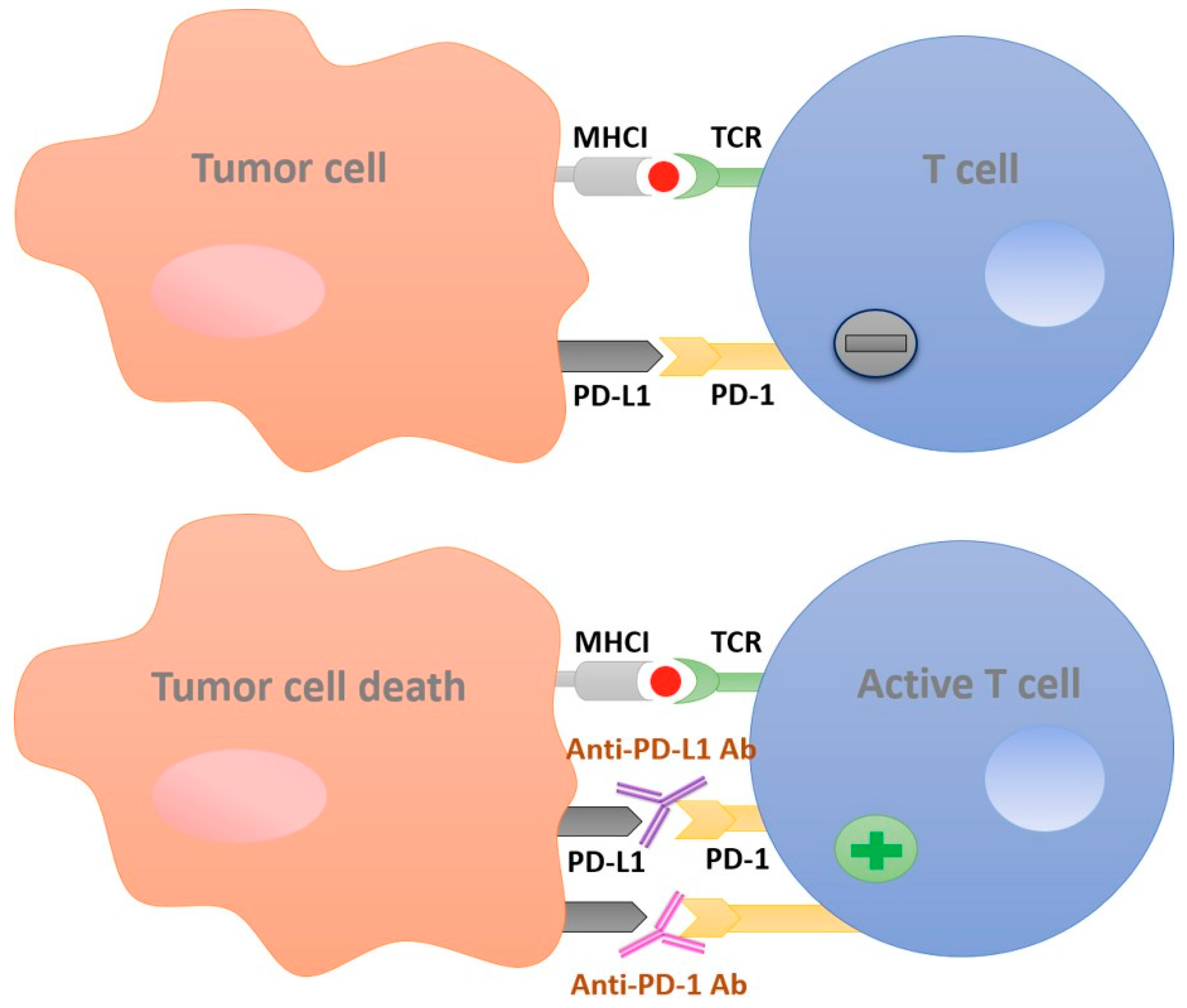
:1. Introduction
2. PD-1 Inhibitors
2.1. Nivolumab
2.2. Pembrolizumab
2.3. Pidilizumab
2.4. AMP-224 and AMP-514
3. PD-L1 Inhibitors
3.1. Atezolizumab
3.2. Durvalumab
3.3. Avelumab
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Burnet, F.M. The concept of immunological surveillance. Prog. Exp. Tumor Res. 1970, 13, 1–27. [Google Scholar] [PubMed]
- De Visser, K.E.; Eichten, A.; Coussens, L.M. Paradoxical roles of the immune system during cancer development. Nat. Rev. Cancer 2006, 6, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Caligiuri, M.A. Human natural killer cells. Blood 2008, 112, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Atkins, M.B.; Sznol, M. Cancer immunotherapy: Past progress and future directions. Semin. Oncol. 2015, 42, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Gubin, M.M.; Schreiber, R.D. Cancer. The odds of immunotherapy success. Science 2015, 350, 158–159. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Flies, D.B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013, 13, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R. Rituximab (monoclonal anti-CD20 antibody): Mechanisms of action and resistance. Oncogene 2003, 22, 7359–7368. [Google Scholar] [CrossRef] [PubMed]
- Couzin-Frankel, J. Breakthrough of the year 2013. Cancer immunotherapy. Science 2013, 342, 1432–1433. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Thomas, L.; Bondarenko, I.; O’Day, S.; Weber, J.; Garbe, C.; Lebbe, C.; Baurain, J.F.; Testori, A.; Grob, J.J.; et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 2011, 364, 2517–2526. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Chinai, J.M.; Janakiram, M.; Chen, F.; Chen, W.; Kaplan, M.; Zang, X. New immunotherapies targeting the PD-1 pathway. Trends Pharmacol. Sci. 2015, 36, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Marincola, F.M.; Jaffee, E.M.; Hicklin, D.J.; Ferrone, S. Escape of human solid tumors from T–cell recognition: Molecular mechanisms and functional significance. In Advances in immunology; Dixon, F.J., Ed.; Academic Press: Cambridge, MA, USA, 1999; Volume 74, pp. 181–273. [Google Scholar]
- Alsaab, H.O.; Sau, S.; Alzhrani, R.; Tatiparti, K.; Bhise, K.; Kashaw, S.K.; Lyer, A.K. PD-1 and PD-L1 Checkpoint signaling Inhibition for cancer immunotherapy: Mechanism, combinations, and clinical outcome. Front. Pharmacol. 2017, 8, 561. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Allison, J.P. The future of immune checkpoint therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Jadus, M.R.; Natividad, J.; Mai, A.; Ouyang, Y.; Lambrecht, N.; Szabo, S.; Ge, L.; Hoa, N.; Dacosta-Iyer, M.G. Lung cancer: A classic example of tumor escape and progression while providing opportunities for immunological intervention. Clin. Dev. Immunol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Holt, G.E.; Podack, E.R.; Raez, L.E. Immunotherapy as a strategy for the treatment of non-small-cell lung cancer. Therapy 2011, 8, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, S.; Gong, D.; Qin, Y.; Shen, Q. Programmed death-1 upregulation is correlated with dysfunction of tumor-infiltrating CD8(+) T lymphocytes in human non-small cell lung cancer. Cell. Mol. Immunol. 2010, 7, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Seetharamu, N.; Preeshagul, I.R.; Sullivan, K.M. New PD-L1 inhibitors in non-small cell lung cancer—Impact of atezolizumab. Lung Cancer 2017, 8, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Jazirehi, A.R.; Lim, A.; Dinh, T. PD-1 inhibition and treatment of advanced melanoma-role of pembrolizumab. Am. J. Cancer Res. 2016, 6, 2117–2128. [Google Scholar] [PubMed]
- Finn, L.; Markovic, S.N.; Joseph, R.W. Therapy for metastatic melanoma: The past, present, and future. BMC Med. 2012, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Sznol, M.; McDermott, D.F.; Kluger, H.M.; Carvajal, R.D.; Sharfman, W.H.; Brahmer, J.R.; Lawrence, D.P.; Atkins, M.B.; Powderly, J.D.; et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J. Clin. Oncol. 2014, 32, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Inman, B.A.; Sebo, T.J.; Frigola, X.; Dong, H.; Bergstralh, E.J.; Frank, I.; Fradet, Y.; Lacombe, L.; Kwon, E.D. PD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: Associations with localized stage progression. Cancer 2007, 109, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Harrington, K.J.; Ferris, R.L.; Blumenschein, G., Jr.; Colevas, A.D.; Fayette, J.; Licitra, L.; Kasper, S.; Even, C.; Vokes, E.E.; Worden, F.; et al. Nivolumab versus standard, single-agent therapy of investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck (CheckMate 141): Health-related quality-of-life results from a randomised, phase 3 trial. Lancet Oncol. 2017, 18, 1104–1115. [Google Scholar] [CrossRef]
- Wilkinson, E. Nivolumab success in untreated metastatic melanoma. Lancet Oncol. 2015, 16, e9. [Google Scholar] [CrossRef]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crino, L.; Eberhardt, W.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Gettinger, S.; Rizvi, N.A.; Chow, L.Q.; Borghaei, H.; Brahmer, J.; Ready, N.; Gerber, D.E.; Shepherd, F.A.; Antonia, S.; Goldman, J.W.; et al. Nivolumab monotherapy for first-line treatment of advanced non-small-cell lung cancer. J. Clin. Oncol. 2016, 34, 2980–2987. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-K.; Satoh, T.; Ryu, M.-H.; Chao, Y.; Kato, K.; Chung, H.C.; Chen, J.-S.; Muro, K.; Kang, W.K.; Yoshikawa, T.; et al. Nivolumab (ONO-4538/BMS-936558) as salvage treatment after second or later-line chemotherapy for advanced gastric or gastro-esophageal junction cancer (AGC): A double-blinded, randomized, phase III trial. J. Clin. Oncol. 2017, 35. [Google Scholar] [CrossRef]
- Le, D.T.; Bendell, J.C.; Calvo, E.; Kim, J.W.; Ascierto, P.A.; Sharma, P.; Ott, P.A.; Bono, P.; Jaeger, D.; Evans, T.R.J.; et al. Safety and activity of nivolumab monotherapy in advanced and metastatic (A/M) gastric or gastroesophageal junction cancer (GC/GEC): Results from the CheckMate-032 study. J. Clin. Oncol. 2016, 34. [Google Scholar] [CrossRef]
- Hamanishi, J.; Mandai, M.; Ikeda, T.; Minami, M.; Kawaguchi, A.; Murayama, T.; Kanai, M.; Mori, Y.; Matsumoto, S.; Chikuma, S.; et al. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J. Clin. Oncol. 2015, 33, 4015–4022. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Baas, P.; Kim, D.W.; Felip, E.; Perez-Gracia, J.L.; Han, J.Y.; Molina, J.; Kim, J.H.; Arvis, C.D.; Ahn, M.J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Plimack, E.R.; Bellmunt, J.; Gupta, S.; Berger, R.; Chow, L.Q.; Juco, J.; Lunceford, J.; Saraf, S.; Perini, R.F.; O’Donnell, P.H. Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE-012): A non-randomised, open-label, phase 1b study. Lancet Oncol. 2017, 18, 212–220. [Google Scholar] [CrossRef]
- Kefford, R.; Ribas, A.; Hamid, O.; Robert, C.; Daud, A.; Wolchok, J.D.; Joshua, A.M.; Hodi, F.S.; Gangadhar, T.C.; Hersey, P.; et al. Clinical efficacy and correlation with tumor PD-L1 expression in patients (pts) with melanoma (MEL) treated with the anti-PD-1 monoclonal antibody MK-3475. J. Clin. Oncol. 2014, 32. [Google Scholar] [CrossRef]
- Hsu, C.; Lee, S.-H.; Ejadi, S.; Even, C.; Cohen, R.B.; Le Tourneau, C.; Mehnert, J.M.; Algazi, A.; van Brummelen, E.M.J.; Saraf, S.; et al. Safety and antitumor activity of pembrolizumab in patients with programmed death-ligand 1–positive nasopharyngeal carcinoma: Results of the KEYNOTE-028 study. J. Clin. Oncol. 2017, 35, 4050–4056. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Puzanov, I.; Dummer, R.; Schadendorf, D.; Hamid, O.; Robert, C.; Hodi, F.S.; Schachter, J.; Pavlick, A.C.; Lewis, K.D.; et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): A randomised, controlled, phase 2 trial. Lancet Oncol. 2015, 16, 908–918. [Google Scholar] [CrossRef]
- Nanda, R.; Chow, L.Q.; Dees, E.C.; Berger, R.; Gupta, S.; Geva, R.; Pusztai, L.; Pathiraja, K.; Aktan, G.; Cheng, J.D.; et al. Pembrolizumab in patients with advanced triple-negative breast cancer: Phase ib keynote-012 study. J. Clin. Oncol. 2016, 34, 2460–2467. [Google Scholar] [CrossRef] [PubMed]
- Varga, A.; Piha-Paul, S.A.; Ott, P.A.; Mehnert, J.M.; Berton-Rigaud, D.; Johnson, E.A.; Cheng, J.D.; Yuan, S.; Rubin, E.H.; Matei, D.E. Antitumor activity and safety of pembrolizumab in patients (pts) with PD-L1 positive advanced ovarian cancer: Interim results from a phase IB study. J. Clin. Oncol. 2015, 33. [Google Scholar] [CrossRef]
- Zinzani, P.L.; Ribrag, V.; Moskowitz, C.H.; Michot, J.M.; Kuruvilla, J.; Balakumaran, A.; Zhang, Y.; Chlosta, S.; Shipp, M.A.; Armand, P. Safety and tolerability of pembrolizumab in patients with relapsed/refractory primary mediastinal large B-cell lymphoma. Blood 2017, 130, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Armand, P.; Nagler, A.; Weller, E.A.; Devine, S.M.; Avigan, D.E.; Chen, Y.-B.; Kaminski, M.S.; Holland, H.K.; Winter, J.N.; Mason, J.R.; et al. Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: Results of an international phase II trial. J. Clin. Oncol. 2013, 31, 4199–4206. [Google Scholar] [CrossRef] [PubMed]
- Berger, R.; Rotem-Yehudar, R.; Slama, G.; Landes, S.; Kneller, A.; Leiba, M.; Koren-Michowitz, M.; Shimoni, A.; Nagler, A. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin. Cancer Res. 2008, 14, 3044–3051. [Google Scholar] [CrossRef] [PubMed]
- Atkins, M.B.; Kudchadkar, R.R.; Sznol, M.; McDermott, D.F.; Lotem, M.; Schachter, J.; Wolchok, J.D.; Urba, W.J.; Kuzel, T.; Schuchter, L.M.; et al. Phase 2, multicenter, safety and efficacy study of pidilizumab in patients with metastatic melanoma. J. Clin. Oncol. 2014, 32. [Google Scholar] [CrossRef]
- LoRusso, P.M.; Powderly, J.; Burris, H.A.; Kittaneh, M.; Grice, J.; Smothers, J.F.; Brett, S.; Fleming, M.; May, R.J.; Marshall, S.; et al. Abstract LB-193: Phase I study of safety, tolerability, pharmacokinetics, and pharmacodynamics of AMP-224 (B7-DC Fc fusion protein) in a regimen containing cyclophosphamide (CTX) in patients with advanced solid tumors. Cancer Res. 2013, 73. [Google Scholar] [CrossRef]
- Infante, J.R.; Goel, S.; Tavakkoli, F.; Marshall, S.; Robbins, P.B.; D’Angelo, G.; Gribbin, M.J.; Karakunnel, J.J.; Naing, A. A phase I, multicenter, open-label, first-in-human study to evaluate MEDI0680, an anti-programmed cell death-1 antibody, in patients with advanced malignancies. J. Clin. Oncol. 2015, 33. [Google Scholar] [CrossRef]
- Hamid, O.; Chow, L.Q.M.; Tavakkoli, F.; Marshall, S.; Gribbin, M.J.; Karakunnel, J.J.; Gray, J.E. Phase I, open-label study of MEDI0680, an anti-programmed cell death-1 (PD-1) antibody, in combination with MEDI4736, an anti-programmed cell death ligand-1 (PD-L1) antibody, in patients with advanced malignancies. J. Clin. Oncol. 2015, 33. [Google Scholar] [CrossRef]
- Socinski, M.; Creelan, B.; Horn, L.; Reck, M.; Paz-Ares, L.; Steins, M.; Felip, E.; van den Heuvel, M.; Ciuleanu, T.E.; Badin, F.; et al. NSCLC, metastatic CheckMate 026: A phase 3 trial of nivolumab vs investigator’s choice (IC) of platinum-based doublet chemotherapy (PT-DC) as first-line therapy for stage iv/recurrent programmed death ligand 1 (PD-L1)-positive NSCLC. Ann. Oncol. 2016, 27. [Google Scholar] [CrossRef]
- Shin, T.; Kennedy, G.; Gorski, K.; Tsuchiya, H.; Koseki, H.; Azuma, M.; Yagita, H.; Chen, L.; Powell, J.; Pardoll, D.; et al. Cooperative B7-1/2 (CD80/CD86) and B7-DC costimulation of CD4+ T cells independent of the PD-1 receptor. J. Exp. Med. 2003, 198, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Nakahama, K.; Tamiya, A.; Taniguchi, Y.; Sasaki, Y.; Akira, M.; Atagi, S. Severe acute interstitial lung disease after nivolumab in three non-small cell lung cancer patients with imaging findings of airway obstruction adjacent to lung tumors. J. Infect. Chemother. 2017, 23, 826–829. [Google Scholar] [CrossRef] [PubMed]
- Khoja, L.; Butler, M.O.; Kang, S.P.; Ebbinghaus, S.; Joshua, A.M. Pembrolizumab. J. Immunother. Cancer 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Sul, J.; Blumenthal, G.M.; Jiang, X.; He, K.; Keegan, P.; Pazdur, R. Fda approval summary: Pembrolizumab for the treatment of patients with metastatic non-small cell lung cancer whose tumors express programmed death-ligand 1. Oncologist 2016, 21, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Bagcchi, S. Pembrolizumab for treatment of refractory melanoma. Lancet Oncol. 2014, 15, e419. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Bellmunt, J.; de Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.-L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Bajorin, D.F.; Plimack, E.R.; Siefker-Radtke, A.O.; Choueiri, T.K.; De Wit, R.; Sonpavde, G.; Gipson, A.; Brown, H.; Mai, Y.; Pang, L.; et al. KEYNOTE-052: Phase 2 study of pembrolizumab (MK-3475) as first-line therapy for patients (pts) with unresectable or metastatic urothelial cancer ineligible for cisplatin-based therapy. J. Clin. Oncol. 2015, 33. [Google Scholar] [CrossRef]
- Balar, A.V.; Castellano, D.E.; O’Donnell, P.H.; Grivas, P.; Vuky, J.; Powles, T.; Plimack, E.R.; Hahn, N.M.; Wit, R.D.; Pang, L.; et al. Pembrolizumab as first-line therapy in cisplatin-ineligible advanced urothelial cancer: Results from the total KEYNOTE-052 study population. J. Clin. Oncol. 2017, 35. [Google Scholar] [CrossRef]
- Arenbergerova, M.; Fialova, A.; Arenberger, P.; Gkalpakiotis, S.; Jirasek, T.; Srp, A.; Novotna, A.; Frankova, H. Killing two birds with one stone: Response to pembrolizumab in a patient with metastatic melanoma and B-cell chronic lymphocytic leukemia. J. Eur. Acad. Dermatol. Venereol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.-R.; Tsai, F.-P.; Wu, T.-W. Indirect comparison between pembrolizumab and nivolumab for the treatment of non-small cell lung cancer: A meta-analysis of randomized clinical trials. Int. Immunopharmacol. 2017, 49, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Fessas, P.; Lee, H.; Ikemizu, S.; Janowitz, T. A molecular and preclinical comparison of the PD-1–Targeted T-cell checkpoint inhibitors nivolumab and pembrolizumab. Semin. Oncol. 2017, 44, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Carotta, S. Targeting NK cells for anticancer immunotherapy: Clinical and preclinical approaches. Front. Immunol. 2016, 7, 152. [Google Scholar] [CrossRef] [PubMed]
- Guillaume, T.; Rubinstein, D.B.; Symann, M. Immune reconstitution and immunotherapy after autologous hematopoietic stem cell transplantation. Blood 1998, 92, 1471–1490. [Google Scholar] [PubMed]
- Infante, J.R.; Powderly, J.D.; Burris, H.A.; Kittaneh, M.; Grice, J.H.; Smothers, J.F.; Brett, S.; Fleming, M.E.; May, R.; Marshall, S.; et al. Clinical and pharmacodynamic (PD) results of a phase i trial with AMP-224 (B7-DC Fc) that binds to the PD-1 receptor. J. Clin. Oncol. 2013, 31. [Google Scholar] [CrossRef]
- Duffy, A.G.; Makarova-Rusher, O.V.; Fioravanti, S.; Walker, M.; Venkatesan, A.; Abi-Jaoudeh, N.; Wood, B.J.; Citrin, D.E.; Greten, T.F. A pilot study of AMP-224—a PD-1 inhibitor—in combination with stereotactic body radiation therapy (SBRT) in patients with metastatic colorectal cancer. J. Clin. Oncol. 2015, 33. [Google Scholar] [CrossRef]
- Chen, D.S.; Irving, B.A.; Hodi, F.S. Molecular pathways: Next-generation immunotherapy—inhibiting programmed death-ligand 1 and programmed death-1. Clin. Cancer Res. 2012, 18, 6580–6587. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.M.; Suzman, D.; Maher, V.E.; Zhang, L.; Tang, S.; Ricks, T.; Palmby, T.; Fu, W.; Liu, Q.; Goldberg, K.B.; et al. FDA approval summary: Atezolizumab for the treatment of patients with progressive advanced urothelial carcinoma after platinum-containing chemotherapy. Oncologist 2017, 22, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Balar, A.V.; Galsky, M.D.; Rosenberg, J.E.; Powles, T.; Petrylak, D.P.; Bellmunt, J.; Loriot, Y.; Necchi, A.; Hoffman-Censits, J.; Perez-Gracia, J.L.; et al. Atezolizumab as first-line therapy in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial. Lancet 2017, 389, 67–76. [Google Scholar] [CrossRef]
- Fehrenbacher, L.; Spira, A.; Ballinger, M.; Kowanetz, M.; Vansteenkiste, J.; Mazieres, J.; Park, K.; Smith, D.; Artal-Cortes, A.; Lewanski, C.; et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): A multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016, 387, 1837–1846. [Google Scholar] [CrossRef]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C.; et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 2017, 389, 255–265. [Google Scholar] [CrossRef]
- Hamid, O.; Sosman, J.A.; Lawrence, D.P.; Sullivan, R.J.; Ibrahim, N.; Kluger, H.M.; Boasberg, P.D.; Flaherty, K.; Hwu, P.; Ballinger, M.; et al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic melanoma (mM). J. Clin. Oncol. 2013, 31. [Google Scholar] [CrossRef]
- Emens, L.A.; Braiteh, F.S.; Cassier, P.; DeLord, J.-P.; Eder, J.P.; Shen, X.; Xiao, Y.; Wang, Y.; Hegde, P.S.; Chen, D.S.; et al. Abstract: PD1-6: Inhibition of PD-L1 by MPDL3280A leads to clinical activity in patients with metastatic triple-negative breast cancer. Cancer Res. 2015, 75. [Google Scholar] [CrossRef]
- McDermott, D.F.; Atkins, M.B.; Motzer, R.J.; Rini, B.I.; Escudier, B.J.; Fong, L.; Joseph, R.W.; Pal, S.K.; Sznol, M.; Hainsworth, J.D.; et al. A phase II study of atezolizumab (atezo) with or without bevacizumab (bev) versus sunitinib (sun) in untreated metastatic renal cell carcinoma (mRCC) patients (pts). J. Clin. Oncol. 2017, 35. [Google Scholar] [CrossRef]
- Rizvi, N.A.; Brahmer, J.R.; Ou, S.-H.I.; Segal, N.H.; Khleif, S.; Hwu, W.-J.; Gutierrez, M.; Schoffski, P.; Hamid, O.; Weiss, J.; et al. Safety and clinical activity of MEDI4736, an anti-programmed cell death-ligand 1 (PD-L1) antibody, in patients with non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2015, 33. [Google Scholar] [CrossRef]
- Segal, N.H.; Antonia, S.J.; Brahmer, J.R.; Maio, M.; Blake-Haskins, A.; Li, X.; Vasselli, J.; Ibrahim, R.A.; Lutzky, J.; Khleif, S. Preliminary data from a multi-arm expansion study of MEDI4736, an anti-PD-L1 antibody. J. Clin. Oncol. 2014, 32. [Google Scholar] [CrossRef]
- Massard, C.; Gordon, M.S.; Sharma, S.; Rafii, S.; Wainberg, Z.A.; Luke, J.; Curiel, T.J.; Colon-Otero, G.; Hamid, O.; Sanborn, R.E.; et al. Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J. Clin. Oncol. 2016, 34, 3119–3125. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.; Heery, C.R.; Patel, M.R.; Infante, J.R.; Iannotti, N.; Leach, J.W.; Wang, D.; Chandler, J.C.; Arkenau, H.-T.; Taylor, M.H.; et al. Avelumab (MSB0010718C; anti-PD-L1) in patients with advanced cancer: Safety data from 1300 patients enrolled in the phase 1b JAVELIN solid tumor trial. J. Clin. Oncol. 2016, 34. [Google Scholar] [CrossRef]
- Apolo, A.B.; Infante, J.R.; Hamid, O.; Patel, M.R.; Wang, D.; Kelly, K.; Mega, A.E.; Britten, C.D.; Mita, A.C.; Ravaud, A.; et al. Safety, clinical activity, and PD-L1 expression of avelumab (MSB0010718C), an anti-PD-L1 antibody, in patients with metastatic urothelial carcinoma from the JAVELIN solid tumor phase Ib trial. J. Clin. Oncol. 2016, 34. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Russell, J.; Hamid, O.; Bhatia, S.; Terheyden, P.; D’Angelo, S.P.; Shih, K.C.; Lebbe, C.; Linette, G.P.; Milella, M.; et al. Avelumab in patients with chemotherapy-refractory metastatic merkel cell carcinoma: A multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 1374–1385. [Google Scholar] [CrossRef]
- Herzberg, B.; Campo, M.J.; Gainor, J.F. Immune checkpoint inhibitors in non-small cell lung cancer. Oncologist 2017, 22, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Besse, B.; Johnson, M.; Janne, P.A.; Garassino, M.; Eberhardt, W.E.E.; Besse, B.; Johnson, M.; Janne, P.A.; Garassino, M.; Eberhardt, W.E.E.; et al. 16LBA phase II, single-arm trial (BIRCH) of atezolizumab as first-line or subsequent therapy for locally advanced or metastatic PD-L1-selected non-small cell lung cancer (NSCLC). Eur. J. Cancer 2015, 51, S717–S718. [Google Scholar] [CrossRef]
- Basappa, N.; Pouliot, F. New research in kidney cancer, ASCO-GU 2017. Can. Urol. Assoc. J. 2017, 11, S163–S165. [Google Scholar] [CrossRef] [PubMed]
- Johansson, H.; Andersson, R.; Bauden, M.; Hammes, S.; Holdenrieder, S.; Ansari, D. Immune checkpoint therapy for pancreatic cancer. World J. Gastroenterol. 2016, 22, 9457–9476. [Google Scholar] [CrossRef] [PubMed]
- Borazanci, E.H.; Hong, D.S.; Gutierrez, M.; Rasco, D.W.; Reid, T.R.; Veeder, M.H.; Tawashi, A.; Lin, J.; Dimery, I.W. Ibrutinib + durvalumab (MEDI4736) in patients (pts) with relapsed or refractory (R/R) pancreatic adenocarcinoma (PAC): A phase Ib/II multicenter study. J. Clin. Oncol. 2016, 34. [Google Scholar] [CrossRef]
- A Study to Assess the Safety, Tolerability, Pharmacokinetics and Anti-tumour Activity of Ascending doses of Selumetinib in Combination with MEDI4736 in Patients with Advanced Solid Tumours (Clinicaltrials.Gov Identifier: Nct02586987). 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT02586987 (accessed on 10 December 2017).
- Syed, Y.Y. Durvalumab: First global approval. Drugs 2017, 77, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Carrera, W.; Baartman, B.J.; Kosmorsky, G. A case report of drug-induced myopathy involving extraocular muscles after combination therapy with tremelimumab and durvalumab for non-small cell lung cancer. Neuroophthalmology 2017, 41, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Boyerinas, B.; Jochems, C.; Fantini, M.; Heery, C.R.; Gulley, J.L.; Tsang, K.Y.; Schlom, J. Antibody-dependent cellular cytotoxicity activity of a novel anti-PD-L1 antibody avelumab (MSB0010718C) on human tumor cells. Cancer Immunol. Res. 2015, 3, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S. Avelumab: First global approval. Drugs 2017, 77, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Sunshine, J.; Taube, J.M. PD-1/PD-L1 inhibitors. Curr. Opin. Pharmacol. 2015, 23, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Tolba, M.F.; Omar, H.A. Immunotherapy, an evolving approach for the management of triple negative breast cancer: Converting non-responders to responders. Crit. Rev. Oncol. Hematol. 2018. [Google Scholar] [CrossRef]
- Michot, J.M.; Bigenwald, C.; Champiat, S.; Collins, M.; Carbonnel, F.; Postel-Vinay, S.; Berdelou, A.; Varga, A.; Bahleda, R.; Hollebecque, A.; et al. Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur. J. Cancer 2016, 54, 139–148. [Google Scholar] [CrossRef] [PubMed]
| Drug | Phase | Indication (Case) | Number of Patients | Median OS | ORR (%) | PFS | Control Drug | NCT Number | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Nivolumab | III | B-RAF negative MM | 418 | - | 40 | 5.1 m | Dacarbazine | NCT01721772 | [27] |
| I/II | Melanoma pretreated | 107 | 17 m | 31 | 3.7 m | - | NCT00730639 | [23] | |
| III | Advanced NSCLC | 272 | 9.2 m | 20 | 3.5 m | Docetaxel | NCT01642004 | [28] | |
| III | Advanced NSCLC | 582 | 12.2 m | 19 | 2.3 m | Docetaxel | NCT01673867 | [28,29] | |
| I | NSCLC | 52 | 19.4 m | 28 | 3.6 m | Docetaxel | NCT01454102 | [30] | |
| III | GEJC | 493 | 5.32 m | 11.2 | 1.61 m | Placebo | NCT02267343 | [31] | |
| I/II | Advanced and metastatic GEJC | 59 | 6.8 m | 18 | - | - | NCT01928394 | [32] | |
| III | Head and neck cancer | 361 | 7.5 m | - | 2 m | Methotrexate/Docetaxel/Cetuximab | NCT02105636 | [25] | |
| II | Ovarian cancer | 20 | 20 m | - | 3.5 m | - | - | [33] | |
| Pembrolizumab | II/III | NSCLC | 1034 | 12.7 m | 44.8 | 4 m | Docetaxel | NCT01905657 | [34] |
| IB | Urothelial cancer | 33 | - | 38 | - | - | NCT02335424 | [35] | |
| II | MM | 150 | - | - | 31 wk | - | - | [36] | |
| IB | Metastatic nasopharyngeal carcinoma | 27 | - | 25.9 | - | - | NCT02054806 | [37] | |
| I | MM | 135 | - | 41 | 38% (10 mg/kg) | - | NCT1295827 | [38] | |
| IB | TNBC | 27 | - | 18.5 | - | - | NCT02447003 | [39] | |
| IB | Ovarian cancer | 26 | - | - | - | - | NCT02054806 | [40] | |
| IB | rrPMBCL | 18 | - | 41 | - | - | NCT01953692. | [41] | |
| Pizilizumab | II | DLBCL | 66 | - | 72 | 72% | NCT00532259 | [42] | |
| I | Hematological malignancies | 17 | - | - | - | - | - | [43] | |
| II | Melanoma | 103 | - | 5.9 | 2.8 m | - | NCT01435369 | [44] | |
| AMP-224 | I | Solid tumors | 6 | - | - | - | - | - | [45] |
| AMP-514 | I | Advanced malignancies | 150 | - | - | - | - | NCT02118337 | [46,47] |
| Drug | Clinical Phase | Indication (Case) | Number of Patients | OS | ORR (%) | PFS | Control Drug | NCT Number | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Atezolizumab | - | Urothelial cancer | 310 | - | 14.8 | - | - | NCT02108652 | [66] |
| II | Urothelial cancer | 119 | 15.9 m | 23 | 2.7 m | - | NCT02108652 | [67] | |
| II | NSCLC | 287 | 12.6 m | - | - | Docetaxel | NCT01903993 | [68] | |
| III | NSCLC | 1225 | 13.8 m | 13 | 2.8 m | Docetaxel | NCT02008227 | [69] | |
| I | MM | 45 | - | 26 | 35% | - | NCT01375842 | [70] | |
| I | TNBC | 21 | - | 24 | - | - | NCT01375842 | [71] | |
| II | mRCC | 305 | - | - | 6.1 m | Sunitinib | NCT01984242 | [72] | |
| Durvalumab | I/II | NSCLC | 198 | - | 14 | - | - | NCT01693562 | [73] |
| I/II | Advanced solid tumors | 151 | - | - | - | - | NCT01693562 | [74] | |
| IB | Urothelial bladder cancer | 61 | - | 31 | - | - | NCT01693562 | [75] | |
| Avelumab | I | Solid tumors | >1700 | - | - | - | - | NCT01772004 | [76] |
| IB | MUC | 44 | - | 40 | 70% | - | NCT01772004 | [77] | |
| II | mMCC | 88 | 11.3 m | 31.8 | 2.7 m | - | NCT02155647 | [78] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdin, S.M.; Zaher, D.M.; Arafa, E.-S.A.; Omar, H.A. Tackling Cancer Resistance by Immunotherapy: Updated Clinical Impact and Safety of PD-1/PD-L1 Inhibitors. Cancers 2018, 10, 32. https://doi.org/10.3390/cancers10020032
Abdin SM, Zaher DM, Arafa E-SA, Omar HA. Tackling Cancer Resistance by Immunotherapy: Updated Clinical Impact and Safety of PD-1/PD-L1 Inhibitors. Cancers. 2018; 10(2):32. https://doi.org/10.3390/cancers10020032
Chicago/Turabian StyleAbdin, Shifaa M., Dana M. Zaher, El-Shaimaa A. Arafa, and Hany A. Omar. 2018. "Tackling Cancer Resistance by Immunotherapy: Updated Clinical Impact and Safety of PD-1/PD-L1 Inhibitors" Cancers 10, no. 2: 32. https://doi.org/10.3390/cancers10020032
APA StyleAbdin, S. M., Zaher, D. M., Arafa, E.-S. A., & Omar, H. A. (2018). Tackling Cancer Resistance by Immunotherapy: Updated Clinical Impact and Safety of PD-1/PD-L1 Inhibitors. Cancers, 10(2), 32. https://doi.org/10.3390/cancers10020032





