Focal Salvage Treatment of Radiorecurrent Prostate Cancer: A Narrative Review of Current Strategies and Future Perspectives
Abstract
1. Introduction
2. Whole-Gland Primary Radiotherapy
3. Recurrence Risk and Location
4. Traditional Approach to Radiorecurrent Prostate Cancer
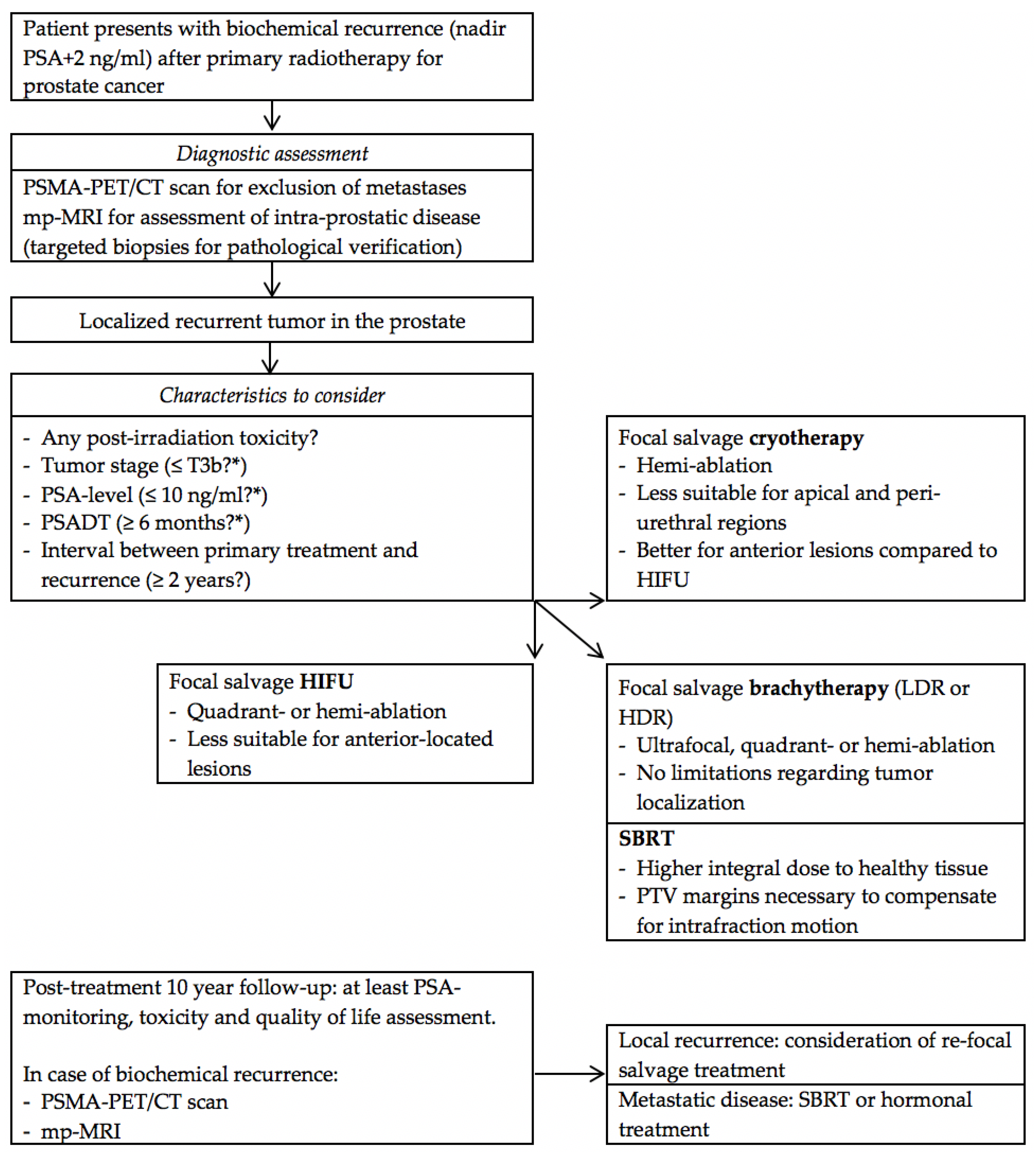
5. Focal Treatment of Radiorecurrent Prostate Cancer
5.1. Diagnostic Assessment
5.1.1. Excluding Metastatic Disease
5.1.2. Assessing and Targeting Intra-Prostatic Disease
5.1.3. Biopsies
5.2. Current Focal Salvage Series
5.3. Future Prospects Regarding MRI-Guided Radiotherapy
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cornford, P.; Bellmunt, J.; Bolla, M.; Briers, E.; De Santis, M.; Gross, T.; Henry, A.M.; Joniau, S.; Lam, T.B.; Mason, M.D.; et al. Eau-estro-siog guidelines on prostate cancer. Part II: Treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur. Urol. 2017, 71, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, F.C.; Donovan, J.L.; Lane, J.A.; Mason, M.; Metcalfe, C.; Holding, P.; Davis, M.; Peters, T.J.; Turner, E.L.; Martin, R.M.; et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N. Engl. J. Med. 2016, 375, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Sheets, N.C.; Goldin, G.H.; Meyer, A.M.; Wu, Y.; Chang, Y.; Sturmer, T.; Holmes, J.A.; Reeve, B.B.; Godley, P.A.; Carpenter, W.R.; et al. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA 2012, 307, 1611–1620. [Google Scholar] [CrossRef] [PubMed]
- Biegala, M.; Hydzik, A. Analysis of dose distribution in organs at risk in patients with prostate cancer treated with the intensity-modulated radiation therapy and arc technique. J. Med. Phys. 2016, 41, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Aluwini, S.; Pos, F.; Schimmel, E.; van Lin, E.; Krol, S.; van der Toorn, P.P.; de Jager, H.; Dirkx, M.; Alemayehu, W.G.; Heijmen, B.; et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): Acute toxicity results from a randomised non-inferiority phase 3 trial. Lancet. Oncol. 2015, 16, 274–283. [Google Scholar] [CrossRef]
- Aluwini, S.; Pos, F.; Schimmel, E.; Krol, S.; van der Toorn, P.P.; de Jager, H.; Alemayehu, W.G.; Heemsbergen, W.; Heijmen, B.; Incrocci, L. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): Late toxicity results from a randomised, non-inferiority, phase 3 trial. Lancet. Oncol. 2016, 17, 464–474. [Google Scholar] [CrossRef]
- Dearnaley, D.; Syndikus, I.; Mossop, H.; Khoo, V.; Birtle, A.; Bloomfield, D.; Graham, J.; Kirkbride, P.; Logue, J.; Malik, Z.; et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet. Oncol. 2016, 17, 1047–1060. [Google Scholar] [CrossRef]
- Musunuru, H.B.; Quon, H.; Davidson, M.; Cheung, P.; Zhang, L.; D’Alimonte, L.; Deabreu, A.; Mamedov, A.; Loblaw, A. Dose-escalation of five-fraction SABR in prostate cancer: Toxicity comparison of two prospective trials. Radiother. Oncol. 2016, 118, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Catton, C.N.; Lukka, H.; Gu, C.S.; Martin, J.M.; Supiot, S.; Chung, P.W.M.; Bauman, G.S.; Bahary, J.P.; Ahmed, S.; Cheung, P.; et al. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J. Clin. Oncol. 2017, 35, 1884–1890. [Google Scholar] [CrossRef] [PubMed]
- Dearnaley, D.; Hall, E. How will the CHHiP trial affect the future of prostate radiotherapy? Expert Rev. Anticancer Ther. 2018, 18, 607–609. [Google Scholar] [CrossRef]
- Grimm, P.; Billiet, I.; Bostwick, D.; Dicker, A.P.; Frank, S.; Immerzeel, J.; Keyes, M.; Kupelian, P.; Lee, W.R.; Machtens, S.; et al. Comparative analysis of prostate-specific antigen free survival outcomes for patients with low, intermediate and high risk prostate cancer treatment by radical therapy. Results from the prostate cancer results study group. BJU Int. 2012, 109, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Morton, G.C.; Hoskin, P.J. Brachytherapy: Current status and future strategies-can high dose rate replace low dose rate and external beam radiotherapy? Clin. Oncol. (R. Coll. Radiol.) 2013, 25, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Prada, P.J.; Ferri, M.; Cardenal, J.; Blanco, A.G.; Anchuelo, J.; Diaz de Cerio, I.; Vazquez, A.; Pacheco, M.; Raba, I.; Ruiz, S. High-dose-rate interstitial brachytherapy as monotherapy in one fraction of 20.5 gy for the treatment of localized prostate cancer: Toxicity and 6-year biochemical results. Brachytherapy 2018. [Google Scholar] [CrossRef]
- Morris, W.J.; Tyldesley, S.; Rodda, S.; Halperin, R.; Pai, H.; McKenzie, M.; Duncan, G.; Morton, G.; Hamm, J.; Murray, N. Androgen suppression combined with elective nodal and dose escalated radiation therapy (the ASCENDE-RT trial): An analysis of survival endpoints for a randomized trial comparing a low-dose-rate brachytherapy boost to a dose-escalated external beam boost for high- and intermediate-risk prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Zumsteg, Z.S.; Spratt, D.E.; Romesser, P.B.; Pei, X.; Zhang, Z.; Polkinghorn, W.; McBride, S.; Kollmeier, M.; Yamada, Y.; Zelefsky, M.J. The natural history and predictors of outcome following biochemical relapse in the dose escalation era for prostate cancer patients undergoing definitive external beam radiotherapy. Eur. Urol. 2015, 67, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Wise, A.M.; Stamey, T.A.; McNeal, J.E.; Clayton, J.L. Morphologic and clinical significance of multifocal prostate cancers in radical prostatectomy specimens. Urology 2002, 60, 264–269. [Google Scholar] [CrossRef]
- Andreoiu, M.; Cheng, L. Multifocal prostate cancer: Biologic, prognostic, and therapeutic implications. Hum. Pathol. 2010, 41, 781–793. [Google Scholar] [CrossRef]
- Liu, W.; Laitinen, S.; Khan, S.; Vihinen, M.; Kowalski, J.; Yu, G.; Chen, L.; Ewing, C.M.; Eisenberger, M.A.; Carducci, M.A.; et al. Copy number analysis indicates monoclonal origin of lethal metastatic prostate cancer. Nat. Med. 2009, 15, 559–565. [Google Scholar] [CrossRef]
- Ahmed, H.U. The index lesion and the origin of prostate cancer. N. Engl. J. Med. 2009, 361, 1704–1706. [Google Scholar] [CrossRef]
- Ahmed, H.U.; Pendse, D.; Illing, R.; Allen, C.; van der Meulen, J.H.; Emberton, M. Will focal therapy become a standard of care for men with localized prostate cancer? Nature clinical practice. Oncology 2007, 4, 632–642. [Google Scholar] [CrossRef]
- Arrayeh, E.; Westphalen, A.C.; Kurhanewicz, J.; Roach, M., 3rd; Jung, A.J.; Carroll, P.R.; Coakley, F.V. Does local recurrence of prostate cancer after radiation therapy occur at the site of primary tumor? Results of a longitudinal MRI and MRSI study. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, e787–e793. [Google Scholar] [CrossRef]
- Cellini, N.; Morganti, A.G.; Mattiucci, G.C.; Valentini, V.; Leone, M.; Luzi, S.; Manfredi, R.; Dinapoli, N.; Digesu, C.; Smaniotto, D. Analysis of intraprostatic failures in patients treated with hormonal therapy and radiotherapy: Implications for conformal therapy planning. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 595–599. [Google Scholar] [CrossRef]
- Chopra, S.; Toi, A.; Taback, N.; Evans, A.; Haider, M.A.; Milosevic, M.; Bristow, R.G.; Chung, P.; Bayley, A.; Morton, G.; et al. Pathological predictors for site of local recurrence after radiotherapy for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, e441–e448. [Google Scholar] [CrossRef]
- Pucar, D.; Hricak, H.; Shukla-Dave, A.; Kuroiwa, K.; Drobnjak, M.; Eastham, J.; Scardino, P.T.; Zelefsky, M.J. Clinically significant prostate cancer local recurrence after radiation therapy occurs at the site of primary tumor: Magnetic resonance imaging and step-section pathology evidence. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Jalloh, M.; Leapman, M.S.; Cowan, J.E.; Shinohara, K.; Greene, K.L.; Roach, M., 3rd; Chang, A.J.; Chan, J.M.; Simko, J.P.; Carroll, P.R. Patterns of local failure following radiation therapy for prostate cancer. J. Urol. 2015, 194, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Chade, D.C.; Eastham, J.; Graefen, M.; Hu, J.C.; Karnes, R.J.; Klotz, L.; Montorsi, F.; van Poppel, H.; Scardino, P.T.; Shariat, S.F. Cancer control and functional outcomes of salvage radical prostatectomy for radiation-recurrent prostate cancer: A systematic review of the literature. Eur. Urol. 2012, 61, 961–971. [Google Scholar] [CrossRef]
- Wenske, S.; Quarrier, S.; Katz, A.E. Salvage cryosurgery of the prostate for failure after primary radiotherapy or cryosurgery: Long-term clinical, functional, and oncologic outcomes in a large cohort at a tertiary referral centre. Eur. Urol. 2013, 64, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.K.; Martinez, C.H.; Lu, C.; Ng, C.K.; Pautler, S.E.; Chin, J.L. Disease-free survival following salvage cryotherapy for biopsy-proven radio-recurrent prostate cancer. Eur. Urol. 2011, 60, 405–410. [Google Scholar] [CrossRef]
- Murat, F.J.; Poissonnier, L.; Rabilloud, M.; Belot, A.; Bouvier, R.; Rouviere, O.; Chapelon, J.Y.; Gelet, A. Mid-term results demonstrate salvage high-intensity focused ultrasound (HIFU) as an effective and acceptably morbid salvage treatment option for locally radiorecurrent prostate cancer. Eur. Urol. 2009, 55, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Crouzet, S.; Murat, F.J.; Pommier, P.; Poissonnier, L.; Pasticier, G.; Rouviere, O.; Chapelon, J.Y.; Rabilloud, M.; Belot, A.; Mege-Lechevallier, F.; et al. Locally recurrent prostate cancer after initial radiation therapy: Early salvage high-intensity focused ultrasound improves oncologic outcomes. Radiother. Oncol. 2012, 105, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.P.; Weinberg, V.; Shinohara, K.; Roach, M., 3rd; Nash, M.; Gottschalk, A.; Chang, A.J.; Hsu, I.C. Salvage HDR brachytherapy for recurrent prostate cancer after previous definitive radiation therapy: 5-year outcomes. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Kollmeier, M.A.; McBride, S.; Taggar, A.; Anderson, E.; Lin, M.; Pei, X.; Weiji, S.; Voros, L.; Cohen, G.; Yamada, Y.; et al. Salvage brachytherapy for recurrent prostate cancer after definitive radiation therapy: A comparison of low-dose-rate and high-dose-rate brachytherapy and the importance of prostate-specific antigen doubling time. Brachytherapy 2017, 16, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Wojcieszek, P.; Szlag, M.; Glowacki, G.; Cholewka, A.; Gawkowska-Suwinska, M.; Kellas-Sleczka, S.; Bialas, B.; Fijalkowski, M. Salvage high-dose-rate brachytherapy for locally recurrent prostate cancer after primary radiotherapy failure. Radiother. Oncol. 2016, 119, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.L.; Alibhai, S.M.; Basaria, S.; D’Amico, A.V.; Kantoff, P.W.; Keating, N.L.; Penson, D.F.; Rosario, D.J.; Tombal, B.; Smith, M.R. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur. Urol. 2015, 67, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.; Moman, M.R.; van der Poel, H.G.; Vergunst, H.; de Jong, I.J.; Vijverberg, P.L.; Battermann, J.J.; Horenblas, S.; van Vulpen, M. Patterns of outcome and toxicity after salvage prostatectomy, salvage cryosurgery and salvage brachytherapy for prostate cancer recurrences after radiation therapy: A multi-center experience and literature review. World J. Urol. 2013, 31, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.; Kwok, J.; Pickles, T.; Tyldesley, S.; Black, P.C. Underutilization of local salvage therapy after radiation therapy for prostate cancer. Urol. Oncol. 2014, 32, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.K.; Sadetsky, N.; Konety, B.R.; Resnick, M.I.; Carroll, P.R. Cancer of the Prostate Strategic Urological Research, E. Treatment failure after primary and salvage therapy for prostate cancer: Likelihood, patterns of care, and outcomes. Cancer 2008, 112, 307–314. [Google Scholar] [CrossRef]
- Abuzallouf, S.; Dayes, I.; Lukka, H. Baseline staging of newly diagnosed prostate cancer: A summary of the literature. J. Urol. 2004, 171, 2122–2127. [Google Scholar] [CrossRef]
- Hovels, A.M.; Heesakkers, R.A.; Adang, E.M.; Jager, G.J.; Strum, S.; Hoogeveen, Y.L.; Severens, J.L.; Barentsz, J.O. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: A meta-analysis. Clin. Radiol. 2008, 63, 387–395. [Google Scholar] [CrossRef]
- Evangelista, L.; Guttilla, A.; Zattoni, F.; Muzzio, P.C.; Zattoni, F. Utility of choline positron emission tomography/computed tomography for lymph node involvement identification in intermediate- to high-risk prostate cancer: A systematic literature review and meta-analysis. Eur. Urol. 2013, 63, 1040–1048. [Google Scholar] [CrossRef]
- Evangelista, L.; Zattoni, F.; Guttilla, A.; Saladini, G.; Zattoni, F.; Colletti, P.M.; Rubello, D. Choline pet or PET/CT and biochemical relapse of prostate cancer: A systematic review and meta-analysis. Clin. Nucl. Med. 2013, 38, 305–314. [Google Scholar] [CrossRef]
- Wallitt, K.L.; Khan, S.R.; Dubash, S.; Tam, H.H.; Khan, S.; Barwick, T.D. Clinical pet imaging in prostate cancer. Radiographics 2017, 37, 1512–1536. [Google Scholar] [CrossRef]
- Perera, M.; Papa, N.; Christidis, D.; Wetherell, D.; Hofman, M.S.; Murphy, D.G.; Bolton, D.; Lawrentschuk, N. Sensitivity, specificity, and predictors of positive (68)Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: A systematic review and meta-analysis. Eur. Urol. 2016, 70, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Afshar-Oromieh, A.; Zechmann, C.M.; Malcher, A.; Eder, M.; Eisenhut, M.; Linhart, H.G.; Holland-Letz, T.; Hadaschik, B.A.; Giesel, F.L.; Debus, J.; et al. Comparison of pet imaging with a (68)Ga-labelled PSMA ligand and (18)F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Afshar-Oromieh, A.; Malcher, A.; Eder, M.; Eisenhut, M.; Linhart, H.G.; Hadaschik, B.A.; Holland-Letz, T.; Giesel, F.L.; Kratochwil, C.; Haufe, S.; et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: Biodistribution in humans and first evaluation of tumor lesions. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Perera, M.; Murphy, D.; Lawrentschuk, N. Prostate-specific membrane antigen positron emission tomography/computed tomography in locally advanced, recurrent, and metastatic prostate cancer. JAMA Oncol. 2018, 4, 748–749. [Google Scholar] [CrossRef]
- Zacho, H.D.; Nielsen, J.B.; Afshar-Oromieh, A.; Haberkorn, U.; deSouza, N.; De Paepe, K.; Dettmann, K.; Langkilde, N.C.; Haarmark, C.; Fisker, R.V.; et al. Prospective comparison of (68)Ga-PSMA PET/CT, (18)F-sodium fluoride PET/CT and diffusion weighted-MRI at for the detection of bone metastases in biochemically recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1884–1897. [Google Scholar] [CrossRef]
- Kanthabalan, A.; Shah, T.; Arya, M.; Punwani, S.; Bomanji, J.; Haroon, A.; Illing, R.O.; Latifoltojar, A.; Freeman, A.; Jameson, C.; et al. The forecast study-focal recurrent assessment and salvage treatment for radiorecurrent prostate cancer. Contemp. Clin. Trials 2015, 44, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Futterer, J.J.; Briganti, A.; De Visschere, P.; Emberton, M.; Giannarini, G.; Kirkham, A.; Taneja, S.S.; Thoeny, H.; Villeirs, G.; Villers, A. Can clinically significant prostate cancer be detected with multiparametric magnetic resonance imaging? A systematic review of the literature. Eur. Urol. 2015, 68, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.U.; El-Shater Bosaily, A.; Brown, L.C.; Gabe, R.; Kaplan, R.; Parmar, M.K.; Collaco-Moraes, Y.; Ward, K.; Hindley, R.G.; Freeman, A.; et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): A paired validating confirmatory study. Lancet 2017, 389, 815–822. [Google Scholar] [CrossRef]
- Le, J.D.; Tan, N.; Shkolyar, E.; Lu, D.Y.; Kwan, L.; Marks, L.S.; Huang, J.; Margolis, D.J.; Raman, S.S.; Reiter, R.E. Multifocality and prostate cancer detection by multiparametric magnetic resonance imaging: Correlation with whole-mount histopathology. Eur. Urol. 2015, 67, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.A.; Chung, P.; Sweet, J.; Toi, A.; Jhaveri, K.; Menard, C.; Warde, P.; Trachtenberg, J.; Lockwood, G.; Milosevic, M. Dynamic contrast-enhanced magnetic resonance imaging for localization of recurrent prostate cancer after external beam radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 425–430. [Google Scholar] [CrossRef]
- Roy, C.; Foudi, F.; Charton, J.; Jung, M.; Lang, H.; Saussine, C.; Jacqmin, D. Comparative sensitivities of functional MRI sequences in detection of local recurrence of prostate carcinoma after radical prostatectomy or external-beam radiotherapy. AJR Am. J. Roentgenol. 2013, 200, W361–W368. [Google Scholar] [CrossRef]
- Arumainayagam, N.; Kumaar, S.; Ahmed, H.U.; Moore, C.M.; Payne, H.; Freeman, A.; Allen, C.; Kirkham, A.; Emberton, M. Accuracy of multiparametric magnetic resonance imaging in detecting recurrent prostate cancer after radiotherapy. BJU Int. 2010, 106, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Gaur, S.; Turkbey, B. Prostate MR imaging for posttreatment evaluation and recurrence. Radiol. Clin. North. Am. 2018, 56, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Oppenheimer, D.C.; Weinberg, E.P.; Hollenberg, G.M.; Meyers, S.P. Multiparametric magnetic resonance imaging of recurrent prostate cancer. J. Clin. Imaging Sci. 2016, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Panebianco, V.; Barchetti, F.; Grompone, M.D.; Colarieti, A.; Salvo, V.; Cardone, G.; Catalano, C. Magnetic resonance imaging for localization of prostate cancer in the setting of biochemical recurrence. Urol. Oncol. 2016, 34, 303–310. [Google Scholar] [CrossRef]
- Patel, P.; Mathew, M.S.; Trilisky, I.; Oto, A. Multiparametric MR imaging of the prostate after treatment of prostate cancer. Radiographics 2018, 38, 437–449. [Google Scholar] [CrossRef]
- Hotker, A.M.; Meier, A.; Mazaheri, Y.; Zheng, J.; Capanu, M.; Chaim, J.; Sosa, R.; Coleman, J.; Hricak, H.; Akin, O. Temporal changes in MRI appearance of the prostate after focal ablation. Abdom. Radiol. 2018. [Google Scholar] [CrossRef]
- Taneja, S.; Jena, A.; Taneja, R.; Singh, A.; Ahuja, A. Effect of combined (68)Ga-PSMAHBED-CC uptake pattern and multiparametric MRI derived with simultaneous PET/MRI in the diagnosis of primary prostate cancer: Initial experience. AJR Am. J. Roentgenol. 2018, 210, 1338–1345. [Google Scholar] [CrossRef]
- Afshar-Oromieh, A.; Avtzi, E.; Giesel, F.L.; Holland-Letz, T.; Linhart, H.G.; Eder, M.; Eisenhut, M.; Boxler, S.; Hadaschik, B.A.; Kratochwil, C.; et al. The diagnostic value of PET/CT imaging with the (68)Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Woo, S.; Kim, Y.J.; Suh, C.H. Impact of (68)Ga-PSMA PET on the management of patients with prostate cancer: A systematic review and meta-analysis. Eur. Urol. 2018, 74, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Kasivisvanathan, V.; Rannikko, A.S.; Borghi, M.; Panebianco, V.; Mynderse, L.A.; Vaarala, M.H.; Briganti, A.; Budaus, L.; Hellawell, G.; Hindley, R.G.; et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N. Engl. J. Med. 2018, 378, 1767–1777. [Google Scholar] [CrossRef] [PubMed]
- Tran, M.; Thompson, J.; Bohm, M.; Pulbrook, M.; Moses, D.; Shnier, R.; Brenner, P.; Delprado, W.; Haynes, A.M.; Savdie, R.; et al. Combination of multiparametric MRI and transperineal template-guided mapping biopsy of the prostate to identify candidates for hemi-ablative focal therapy. BJU Int. 2016, 117, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Wegelin, O.; Exterkate, L.; Somford, D.; Barentsz, J.; Van Der Leest, M.; Kummer, A.; Vreuls, W.; De Bruin, P.; Bosch, R.; Van Melick, H. The future trial; a multicenter RCT on three techniques of MRI targeted prostate biopsy. EAU 2018, 17, e699–e700. [Google Scholar] [CrossRef]
- Crook, J.; Malone, S.; Perry, G.; Bahadur, Y.; Robertson, S.; Abdolell, M. Postradiotherapy prostate biopsies: What do they really mean? Results for 498 patients. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 355–367. [Google Scholar] [CrossRef]
- Kass-Iliyya, A.; Jovic, G.; Murphy, C.; Fisher, C.; Syndikus, I.; Jose, C.; Scrase, C.D.; Graham, J.D.; Nicol, D.; Sydes, M.R.; et al. Two-years postradiotherapy biopsies: Lessons from mrc rt01 trial. Eur. Urol. 2018, 73, 968–976. [Google Scholar] [CrossRef]
- Kanthabalan, A.; Abd-Alazeez, M.; Arya, M.; Allen, C.; Freeman, A.; Jameson, C.; Kirkham, A.; Mitra, A.V.; Payne, H.; Punwani, S.; et al. Transperineal magnetic resonance imaging-targeted biopsy versus transperineal template prostate mapping biopsy in the detection of localized radio-recurrent prostate cancer. Clin. Oncol. (R. Coll. Radiol.) 2016, 28, 568–576. [Google Scholar] [CrossRef]
- Crook, J.M.; Bahadur, Y.A.; Robertson, S.J.; Perry, G.A.; Esche, B.A. Evaluation of radiation effect, tumor differentiation, and prostate specific antigen staining in sequential prostate biopsies after external beam radiotherapy for patients with prostate carcinoma. Cancer 1997, 79, 81–89. [Google Scholar] [CrossRef]
- Goldstein, N.S.; Martinez, A.; Vicini, F.; Stromberg, J. The histology of radiation therapy effect on prostate adenocarcinoma as assessed by needle biopsy after brachytherapy boost. Correlation with biochemical failure. Am. J. Clin. Pathol. 1998, 110, 765–775. [Google Scholar] [CrossRef]
- Grignon, D.J.; Sakr, W.A. Histologic effects of radiation therapy and total androgen blockade on prostate cancer. Cancer 1995, 75, 1837–1841. [Google Scholar] [CrossRef]
- De Castro Abreu, A.L.; Bahn, D.; Leslie, S.; Shoji, S.; Silverman, P.; Desai, M.M.; Gill, I.S.; Ukimura, O. Salvage focal and salvage total cryoablation for locally recurrent prostate cancer after primary radiation therapy. BJU Int. 2013, 112, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Kongnyuy, M.; Berg, C.J.; Kosinski, K.E.; Habibian, D.J.; Schiff, J.T.; Corcoran, A.T.; Katz, A.E. Salvage focal cryosurgery may delay use of androgen deprivation therapy in cryotherapy and radiation recurrent prostate cancer patients. Int. J. Hyperthermia. 2017, 33, 810–813. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Elshafei, A.; Agarwal, G.; Ruckle, H.; Powsang, J.; Jones, J.S. Salvage focal prostate cryoablation for locally recurrent prostate cancer after radiotherapy: Initial results from the cryo on-line data registry. Prostate 2015, 75, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kanthabalan, A.; Peters, M.; Van Vulpen, M.; McCartan, N.; Hindley, R.G.; Emara, A.; Moore, C.M.; Arya, M.; Emberton, M.; Ahmed, H.U. Focal salvage high-intensity focused ultrasound in radiorecurrent prostate cancer. BJU Int. 2017, 120, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Kunogi, H.; Wakumoto, Y.; Yamaguchi, N.; Horie, S.; Sasai, K. Focal partial salvage low-dose-rate brachytherapy for local recurrent prostate cancer after permanent prostate brachytherapy with a review of the literature. J. Contemp. Brachytherapy 2016, 8, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.; Maenhout, M.; van der Voort van Zyp, J.R.; Moerland, M.A.; Moman, M.R.; Steuten, L.M.; van Deursen, M.J.; van Vulpen, M. Focal salvage iodine-125 brachytherapy for prostate cancer recurrences after primary radiotherapy: A retrospective study regarding toxicity, biochemical outcome and quality of life. Radiother. Oncol. 2014, 112, 77–82. [Google Scholar] [CrossRef]
- Zamboglou, C.; Rischke, H.C.; Meyer, P.T.; Knobe, S.; Volgeova-Neher, N.; Kollefrath, M.; Jilg, C.A.; Grosu, A.L.; Baltas, D.; Kroenig, M. Single fraction multimodal image guided focal salvage high-dose-rate brachytherapy for recurrent prostate cancer. J. Contemp. Brachytherapy 2016, 8, 241–248. [Google Scholar] [CrossRef]
- Maenhout, M.; Peters, M.; van Vulpen, M.; Moerland, M.A.; Meijer, R.P.; van den Bosch, M.; Nguyen, P.L.; Frank, S.J.; van der Voort van Zyp, J.R.N. Focal MRI-guided salvage high-dose-rate brachytherapy in patients with radiorecurrent prostate cancer. Technol. Cancer Res. Treat. 2017, 16, 1194–1201. [Google Scholar] [CrossRef]
- Murgic, J.; Morton, G.; Loblaw, A.; D’Alimonte, L.; Ravi, A.; Wronski, M.; Davidson, M.; Haider, M.; Commisso, K.; Zhang, L.; et al. Focal salvage high dose-rate brachytherapy for locally recurrent prostate cancer after primary radiation therapy failure: Results from a prospective clinical trial. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 561–567. [Google Scholar] [CrossRef]
- Jereczek-Fossa, B.A.; Beltramo, G.; Fariselli, L.; Fodor, C.; Santoro, L.; Vavassori, A.; Zerini, D.; Gherardi, F.; Ascione, C.; Bossi-Zanetti, I.; et al. Robotic image-guided stereotactic radiotherapy, for isolated recurrent primary, lymph node or metastatic prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Mbeutcha, A.; Chauveinc, L.; Bondiau, P.Y.; Chand, M.E.; Durand, M.; Chevallier, D.; Amiel, J.; Kee, D.L.; Hannoun-Levi, J.M. Salvage prostate re-irradiation using high-dose-rate brachytherapy or focal stereotactic body radiotherapy for local recurrence after definitive radiation therapy. Radiat. Oncol. 2017, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Golbari, N.M.; Katz, A.E. Salvage therapy options for local prostate cancer recurrence after primary radiotherapy: A literature review. Curr. Urol. Rep. 2017, 18, 63. [Google Scholar] [CrossRef] [PubMed]
- Zdrojowy, R.; Dembowski, J.; Malkiewicz, B.; Tupikowski, K.; Krajewski, W. Salvage local therapy for radiation-recurrent prostate cancer-where are we? Cent. European J. Urol. 2016, 69, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Duijzentkunst, D.A.; Peters, M.; van der Voort van Zyp, J.R.; Moerland, M.A.; van Vulpen, M. Focal salvage therapy for local prostate cancer recurrences after primary radiotherapy: A comprehensive review. World J. Urol. 2016, 34, 1521–1531. [Google Scholar] [CrossRef] [PubMed]
- Eiber, M.; Weirich, G.; Holzapfel, K.; Souvatzoglou, M.; Haller, B.; Rauscher, I.; Beer, A.J.; Wester, H.J.; Gschwend, J.; Schwaiger, M.; et al. Simultaneous (68)Ga-PSMA HBED-CC PET/MRI improves the localization of primary prostate cancer. Eur. Urol. 2016, 70, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Kaljouw, E.; Pieters, B.R.; Kovacs, G.; Hoskin, P.J. A delphi consensus study on salvage brachytherapy for prostate cancer relapse after radiotherapy, a Uro-GEC study. Radiother. Oncol. 2016, 118, 122–130. [Google Scholar] [CrossRef]
- Peters, M.; Kanthabalan, A.; Shah, T.T.; McCartan, N.; Moore, C.M.; Arya, M.; van der Voort van Zyp, J.R.; Moerland, M.A.; Hindley, R.G.; Emberton, M.; et al. Development and internal validation of prediction models for biochemical failure and composite failure after focal salvage high intensity focused ultrasound for local radiorecurrent prostate cancer: Presentation of risk scores for individual patient prognoses. Urol. Oncol. 2018, 36, 13.e1–13.e10. [Google Scholar] [CrossRef]
- Barret, E.; Turkbey, B.; Puech, P.; Durand, M.; Panebianco, V.; Futterer, J.J.; Renard-Penna, R.; Rouviere, O. Update on the ICUD-SIU consultation on multi-parametric magnetic resonance imaging in localized prostate cancer. World J. Urol. 2018. [Google Scholar] [CrossRef]
- Ashrafi, A.N.; Tafuri, A.; Cacciamani, G.E.; Park, D.; de Castro Abreu, A.L.; Gill, I.S. Focal therapy for prostate cancer: Concepts and future directions. Curr. Opin. Urol. 2018, 28, 536–543. [Google Scholar] [CrossRef]
- Lagemaat, M.W.; Philips, B.W.; Vos, E.K.; van Uden, M.J.; Futterer, J.J.; Jenniskens, S.F.; Scheenen, T.W.; Maas, M.C. Feasibility of multiparametric magnetic resonance imaging of the prostate at 7 T. Invest. Radiol. 2017, 52, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Onofrey, J.A.; Staib, L.H.; Sarkar, S.; Venkataraman, R.; Nawaf, C.B.; Sprenkle, P.C.; Papademetris, X. Learning non-rigid deformations for robust, constrained point-based registration in image-guided mr-trus prostate intervention. Med. Image Anal. 2017, 39, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Tharmalingam, H.; Alonzi, R.; Hoskin, P.J. The role of magnetic resonance imaging in brachytherapy. Clin. Oncol. (R. Coll. Radiol.) 2018. [Google Scholar] [CrossRef] [PubMed]
- Van den Bosch, M.R.; Moman, M.R.; van Vulpen, M.; Battermann, J.J.; Duiveman, E.; van Schelven, L.J.; de Leeuw, H.; Lagendijk, J.J.; Moerland, M.A. MRI-guided robotic system for transperineal prostate interventions: Proof of principle. Phys. Med. Biol. 2010, 55, N133–N140. [Google Scholar] [CrossRef] [PubMed]
- Borot de Battisti, M.; Maenhout, M.; Denis de Senneville, B.; Hautvast, G.; Binnekamp, D.; Lagendijk, J.J.; van Vulpen, M.; Moerland, M.A. An automated optimization tool for high-dose-rate (HDR) prostate brachytherapy with divergent needle pattern. Phys. Med. Biol. 2015, 60, 7567–7583. [Google Scholar] [CrossRef] [PubMed]
- Kontaxis, C.; Bol, G.H.; Kerkmeijer, L.G.W.; Lagendijk, J.J.W.; Raaymakers, B.W. Fast online replanning for interfraction rotation correction in prostate radiotherapy. Med. Phys. 2017, 44, 5034–5042. [Google Scholar] [CrossRef]

| Focal Salvage Treatment | Study | Ablation Extent | Patients | Median Follow-up | bFFS | GU/GI Toxicity | QoL |
|---|---|---|---|---|---|---|---|
| Brachytherapy | |||||||
| LDR | Kunogi et al. [76] | Ultrafocal (145 Gy) | 12 | 56 months | 78% at 4 years | No grade 3 | NA |
| Peters et al. [77] | Ultrafocal (144 Gy) | 20 | 36 months | 60% at 3 years | 5% grade 3 GU | Increase in urinary symptoms | |
| HDR | Zamboglou et al. [78] | Ultrafocal (18 Gy) | 2 | 6 months | 100% at 6 months | No grade 3 | NA |
| Maenhout et al. [79] | Ultrafocal (19 Gy) | 17 | 10 months | 92% at 1 year | 6% grade 3 GU | NA | |
| Murgic et al. [80] | Quadrant (27 Gy in 2 fractions) | 15 | 36 months | 61% at 3 years | 7% grade 3 GU | No significant change | |
| Cryotherapy | de Castro Abreu et al. [72] | Hemi | 25 | 31 months | 54% at 5 years | No incontinence, no fistula | NA |
| Kongnyuy et al. [73] | Hemi | 65 | 27 months | 48% at 3 years | 6% incontinence | NA | |
| Li et al. [74] | NA | 91 | 15 months | 47% at 5 years | 6% incontinence, 7% retention, 3% fistula | NA | |
| HIFU | Kanthabalan et al. [75] | Ultrafocal (11%), quadrant (55%), hemi (34%) | 150 | 35 months | 48% at 3 years | 8% bladder neck stricture, 2% fistula | NA |
| SBRT | Jereczek-Fossa et al. [81] | Ultrafocal (30 Gy in 5 fractions) | 15 | 10 months | 22% at 2.5 years | 7% grade 3 GU | NA |
| Mbeutcha et al. [82] | Ultrafocal (35 Gy in 5 fractions) | 18 | 15 months | 56% at 1 year | No grade 3 | NA |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Son, M.; Peters, M.; Moerland, M.; Kerkmeijer, L.; Lagendijk, J.; Van der Voort van Zyp, J. Focal Salvage Treatment of Radiorecurrent Prostate Cancer: A Narrative Review of Current Strategies and Future Perspectives. Cancers 2018, 10, 480. https://doi.org/10.3390/cancers10120480
Van Son M, Peters M, Moerland M, Kerkmeijer L, Lagendijk J, Van der Voort van Zyp J. Focal Salvage Treatment of Radiorecurrent Prostate Cancer: A Narrative Review of Current Strategies and Future Perspectives. Cancers. 2018; 10(12):480. https://doi.org/10.3390/cancers10120480
Chicago/Turabian StyleVan Son, Marieke, Max Peters, Marinus Moerland, Linda Kerkmeijer, Jan Lagendijk, and Jochem Van der Voort van Zyp. 2018. "Focal Salvage Treatment of Radiorecurrent Prostate Cancer: A Narrative Review of Current Strategies and Future Perspectives" Cancers 10, no. 12: 480. https://doi.org/10.3390/cancers10120480
APA StyleVan Son, M., Peters, M., Moerland, M., Kerkmeijer, L., Lagendijk, J., & Van der Voort van Zyp, J. (2018). Focal Salvage Treatment of Radiorecurrent Prostate Cancer: A Narrative Review of Current Strategies and Future Perspectives. Cancers, 10(12), 480. https://doi.org/10.3390/cancers10120480





