Intensity Modulated Radiotherapy (IMRT) + Carbon Ion Boost for Adenoid Cystic Carcinoma of the Minor Salivary Glands in the Oral Cavity
Abstract
1. Introduction
2. Results
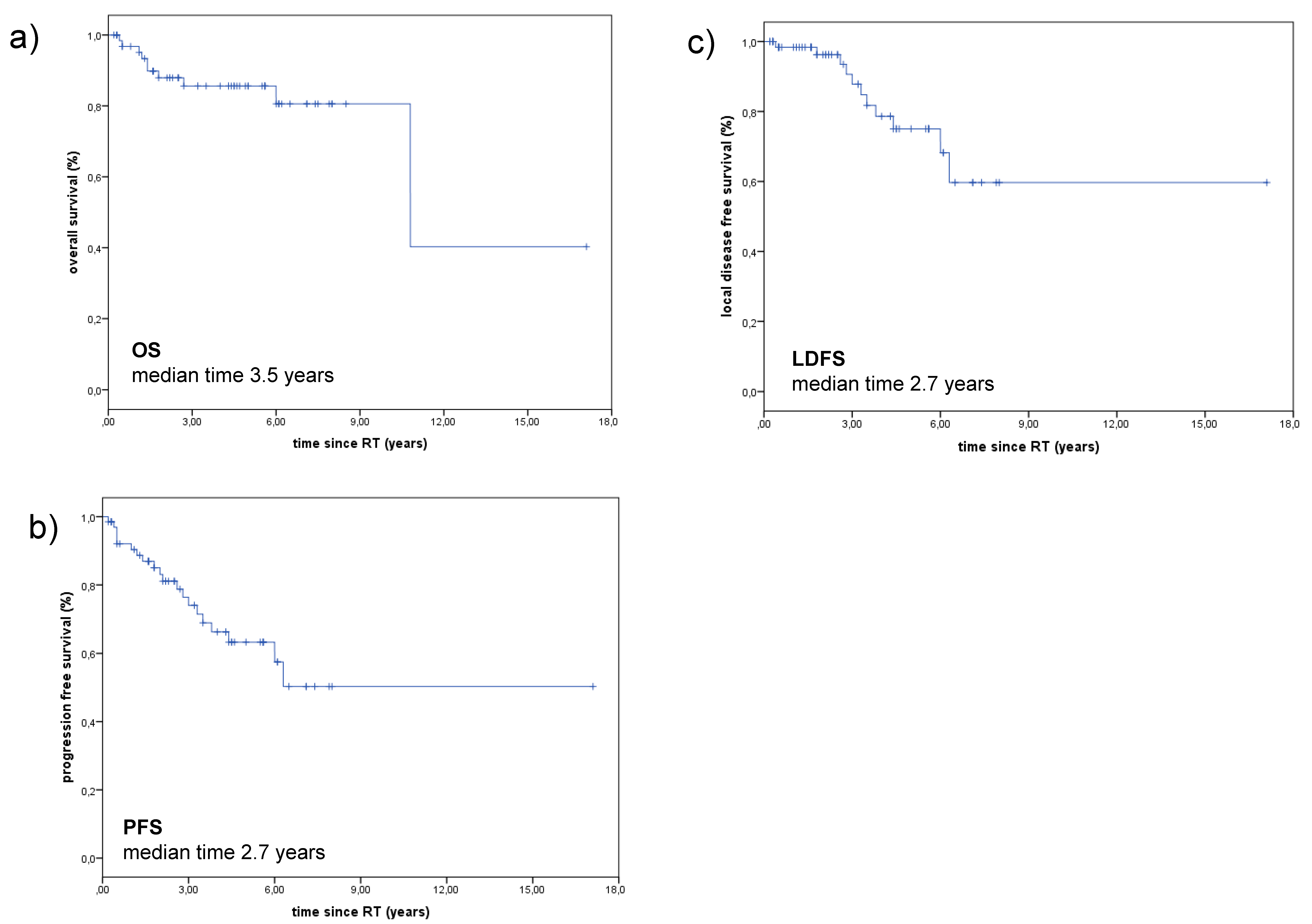
2.1. Treatment Outcome
2.2. Prognostic Factors
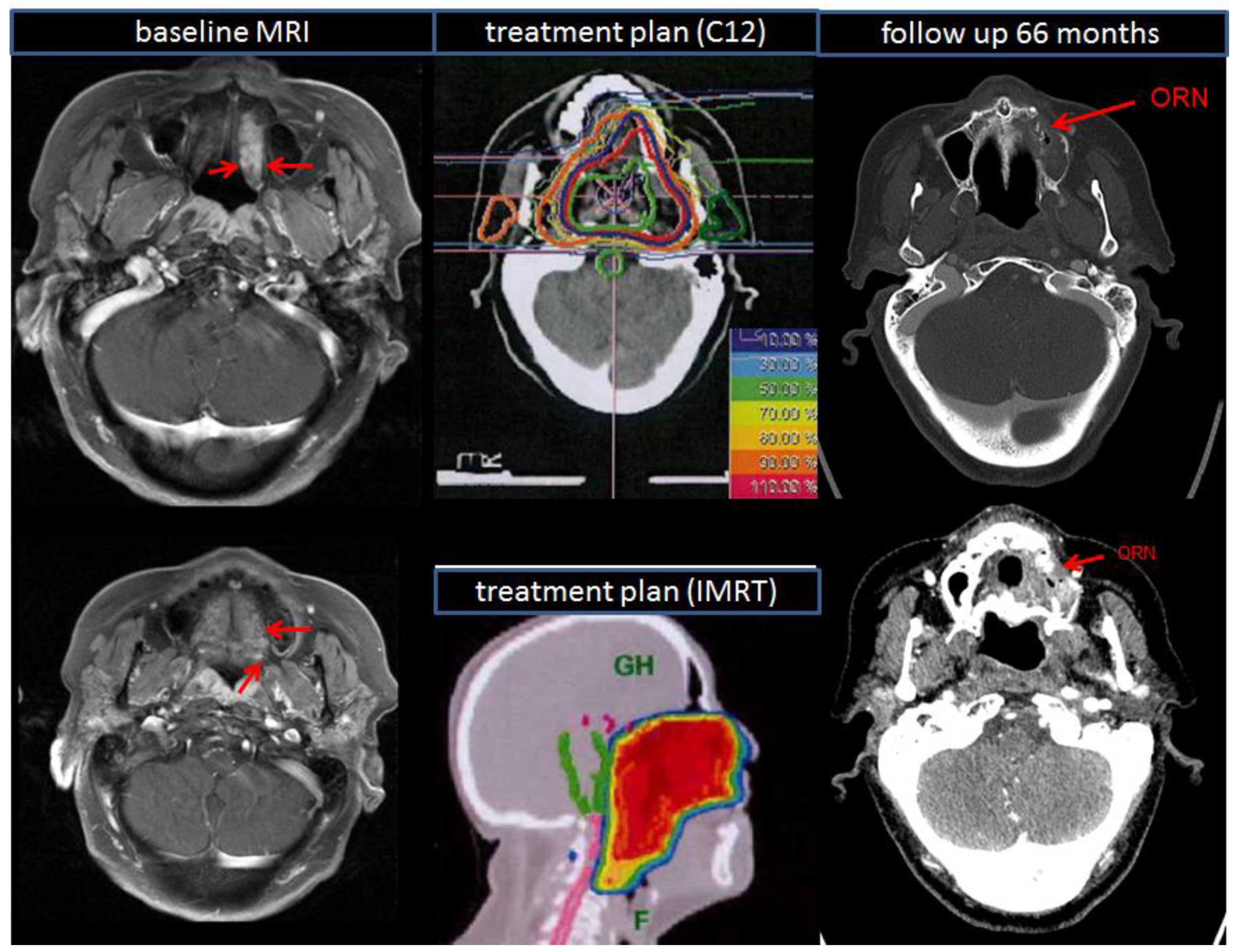
2.3. Treatment Toxicity
3. Discussion
4. Materials and Methods
4.1. Demographic and Patient Characteristics
4.2. Pre-Treatment Imaging
4.3. Treatment Modalities — Combined RT (IMRT+C12)
4.4. Follow-Up
4.5. Overall Survival (OS)
4.6. Progression-Free Survival (PFS)
4.7. Local Disease-Free Survival (LDFS)
4.8. Treatment Toxicity
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bradley, P.J. Adenoid cystic carcinoma of the head and neck: A review. Curr. Opin. Otolaryngol. Head Neck Surg. 2004, 12, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Bjorndal, K.; Krogdahl, A.; Therkildsen, M.H.; Charabi, B.; Kristensen, C.A.; Andersen, E.; Schytte, S.; Primdahl, H.; Johansen, J.; Pedersen, H.B.; et al. Salivary adenoid cystic carcinoma in Denmark 1990–2005: Outcome and independent prognostic factors including the benefit of radiotherapy. Results of the Danish Head and Neck Cancer Group (DAHANCA). Oral Oncol. 2015, 51, 1138–1142. [Google Scholar] [CrossRef] [PubMed]
- DeAngelis, A.F.; Tsui, A.; Wiesenfeld, D.; Chandu, A. Outcomes of patients with adenoid cystic carcinoma of the minor salivary glands. Int. J. Oral Maxillofac. Surg. 2011, 40, 710–714. [Google Scholar] [CrossRef] [PubMed]
- Hyam, D.M.; Veness, M.J.; Morgan, G.J. Minor salivary gland carcinoma involving the oral cavity or oropharynx. Aust. Dent. J. 2004, 49, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, A.G.; Amaral, A.L.; Prado, L.A.; Kligerman, J.; Silveira, T.R. Adenoid cystic carcinoma of salivary glands. A study of 61 cases with clinicopathologic correlation. Cancer 1986, 57, 312–319. [Google Scholar] [CrossRef]
- Kokemueller, H.; Eckardt, A.; Brachvogel, P.; Hausamen, J.E. Adenoid cystic carcinoma of the head and neck--a 20 years experience. Int. J. Oral Maxillofac. Surg. 2004, 33, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.J.; Pambuccian, S.E.; Ondrey, F.G.; Adams, G.L.; Gaffney, P.M. Genes associated with early development, apoptosis and cell cycle regulation define a gene expression profile of adenoid cystic carcinoma. Oral Oncol. 2006, 42, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Speight, P.M.; Barrett, A.W. Salivary gland tumours. Oral Dis. 2002, 8, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Triantafillidou, K.; Dimitrakopoulos, J.; Iordanidis, F.; Koufogiannis, D. Management of adenoid cystic carcinoma of minor salivary glands. J. Oral Maxillofac. Surg. 2006, 64, 1114–1120. [Google Scholar] [CrossRef]
- Coca-Pelaz, A.; Rodrigo, J.P.; Bradley, P.J.; Vander Poorten, V.; Triantafyllou, A.; Hunt, J.L.; Strojan, P.; Rinaldo, A.; Haigentz, M., Jr.; Takes, R.P.; et al. Adenoid cystic carcinoma of the head and neck--An update. Oral Oncol. 2015, 51, 652–661. [Google Scholar] [CrossRef]
- Coca-Pelaz, A.; Rodrigo, J.P.; Triantafyllou, A.; Hunt, J.L.; Rinaldo, A.; Strojan, P.; Haigentz, M., Jr.; Mendenhall, W.M.; Takes, R.P.; Vander Poorten, V.; et al. Salivary mucoepidermoid carcinoma revisited. Eur. Arch. Oto-Rhino-Laryngol. 2015, 272, 799–819. [Google Scholar] [CrossRef]
- Mucke, T.; Tannapfel, A.; Kesting, M.R.; Wagenpfeil, S.; Robitzky, L.K.; Wolff, K.D.; Holzle, F. Adenoid cystic carcinomas of minor salivary glands. Auris Nasus Larynx 2010, 37, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Ertner, D.; Nikoghosyan, A.; Didinger, B.; Munter, M.; Jakel, O.; Karger, C.P.; Debus, J. Therapy strategies for locally advanced adenoid cystic carcinomas using modern radiation therapy techniques. Cancer 2005, 104, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Debus, J.; Engenhart-Cabillic, R.; Kraft, G.; Wannenmacher, M. The role of high-LET radiotherapy compared to conformal photon radiotherapy in adenoid cystic carcinoma. Strahlenther. Onkol. 1999, 175, 63–65. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.D.; Poulakis, M.; Nikoghosyan, A.V.; Welzel, T.; Uhl, M.; Federspil, P.A.; Freier, K.; Krauss, J.; Hoss, A.; Haberer, T.; et al. High-LET radiotherapy for adenoid cystic carcinoma of the head and neck: 15 years’ experience with raster-scanned carbon ion therapy. Radiother. Oncol. 2016, 118, 272–280. [Google Scholar] [CrossRef]
- Chang, C.F.; Hsieh, M.Y.; Chen, M.K.; Chou, M.C. Adenoid cystic carcinoma of head and neck: A retrospective clinical analysis of a single institution. Auris Nasus Larynx 2018, 45, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.M.; Bucci, M.K.; Weinberg, V.; Garcia, J.; Quivey, J.M.; Schechter, N.R.; Phillips, T.L.; Fu, K.K.; Eisele, D.W. Adenoid cystic carcinoma of the head and neck treated by surgery with or without postoperative radiation therapy: Prognostic features of recurrence. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Ellington, C.L.; Goodman, M.; Kono, S.A.; Grist, W.; Wadsworth, T.; Chen, A.Y.; Owonikoko, T.; Ramalingam, S.; Shin, D.M.; Khuri, F.R.; et al. Adenoid cystic carcinoma of the head and neck: Incidence and survival trends based on 1973-2007 Surveillance, Epidemiology, and End Results data. Cancer 2012, 118, 4444–4451. [Google Scholar] [CrossRef]
- He, S.; Li, P.; Zhong, Q.; Hou, L.; Yu, Z.; Huang, Z.; Chen, X.; Fang, J.; Chen, X. Clinicopathologic and prognostic factors in adenoid cystic carcinoma of head and neck minor salivary glands: A clinical analysis of 130 cases. Am. J. Otolaryngol. 2017, 38, 157–162. [Google Scholar] [CrossRef]
- Mendenhall, W.M.; Morris, C.G.; Amdur, R.J.; Werning, J.W.; Hinerman, R.W.; Villaret, D.B. Radiotherapy alone or combined with surgery for adenoid cystic carcinoma of the head and neck. Head Neck 2004, 26, 154–162. [Google Scholar] [CrossRef]
- Mendenhall, W.M.; Morris, C.G.; Amdur, R.J.; Werning, J.W.; Villaret, D.B. Radiotherapy alone or combined with surgery for salivary gland carcinoma. Cancer 2005, 103, 2544–2550. [Google Scholar] [CrossRef] [PubMed]
- Da Cruz Perez, D.E.; de Abreu Alves, F.; Nobuko Nishimoto, I.; de Almeida, O.P.; Kowalski, L.P. Prognostic factors in head and neck adenoid cystic carcinoma. Oral Oncol. 2006, 42, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Mizoe, J.E.; Hasegawa, A.; Jingu, K.; Takagi, R.; Bessyo, H.; Morikawa, T.; Tonoki, M.; Tsuji, H.; Kamada, T.; Tsujii, H.; et al. Results of carbon ion radiotherapy for head and neck cancer. Radiother. Oncol. 2012, 103, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Spiro, R.H. Distant metastasis in adenoid cystic carcinoma of salivary origin. Am. J. Surg. 1997, 174, 495–498. [Google Scholar] [CrossRef]
- Huber, P.E.; Debus, J.; Latz, D.; Zierhut, D.; Bischof, M.; Wannenmacher, M.; Engenhart-Cabillic, R. Radiotherapy for advanced adenoid cystic carcinoma: Neutrons, photons or mixed beam? Radiother. Oncol. 2001, 59, 161–167. [Google Scholar] [CrossRef]
- Shah, J.P.; Gil, Z. Current concepts in management of oral cancer--surgery. Oral Oncol. 2009, 45, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Asikainen, P.J.; Kullaa, A.M.; Koistinen, A.; Schulten, E.; Ten Bruggenkate, C.M. A phd completed. The effect of radiotherapy on oral mucosa cell morphology. Ned. Tijdschr. Tandheelkd. 2018, 125, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Cheriex, K.C.; Nijhuis, T.H.; Mureau, M.A. Osteoradionecrosis of the jaws: A review of conservative and surgical treatment options. J. Reconstr. Microsurg. 2013, 29, 69–75. [Google Scholar] [CrossRef]
- Chronopoulos, A.; Zarra, T.; Ehrenfeld, M.; Otto, S. Osteoradionecrosis of the jaws: Definition, epidemiology, staging and clinical and radiological findings. A concise review. Int. Dent. J. 2018, 68, 22–30. [Google Scholar] [CrossRef]
- Dai, T.; Tian, Z.; Wang, Z.; Qiu, W.; Zhang, Z.; He, Y. Surgical management of osteoradionecrosis of the jaws. J. Craniofac. Surg. 2015, 26, e175–e179. [Google Scholar] [CrossRef]
- Rice, N.; Polyzois, I.; Ekanayake, K.; Omer, O.; Stassen, L.F. The management of osteoradionecrosis of the jaws--a review. Surgeon 2015, 13, 101–109. [Google Scholar] [CrossRef] [PubMed]


| Authors/Year | Number of Patients | Median Follow Up (Months) | RT Modality | Treatment Intention | T4-Stage | LC | OS | Conclusion for Using C12 |
|---|---|---|---|---|---|---|---|---|
| Jensen et al. 2015 [12] | 53 | 42 | Combined (IMRT+C12) | R1, R2, definitive | 57% | 3-years: 81.9% | 3-years: 78.4% | less toxicity in combined group |
| Jensen et al. 2015 [12] | 58 | 74 | Combined (IMRT+C12) | definitive, R2 | 90% | 5-years: 59.6% | 10-years: 44.2% | LC, OS, PFS better in combined group |
| Jensen et al. 2015 [12] | 37 | 63 | photons alone | definitive, R2 | 94% | 5-years: 39.9% | 10-years: 19.6% | LC, OS, PFS better in combined group |
| Jensen et al. 2016 [12] | 309 | 34 | Combined (IMRT+C12) | R1, R2, definitive | 60% | 3-years: 83.7% | 3-years: 88.9% | good LC in combined group |
| Early Treatment Toxicity | No of Patients | Late Treatment Toxicity | No of Patients | |||
|---|---|---|---|---|---|---|
| CTC grade | n | % | CTC grade | n | % | |
| Mukositis | ||||||
| 1 | 8 | 11.9 | 1 | 7 | 10.4 | |
| 2 | 21 | 31.3 | 2 | 2 | 4.5 | |
| 3 | 35 | 52.2 | ||||
| Dermatitis | ||||||
| 1 | 38 | 56.7 | 1 | 11 | 16.4 | |
| 2 | 22 | 32.9 | ||||
| 3 | 5 | 7.5 | ||||
| Dysphagia | ||||||
| 1 | 13 | 19.4 | 1 | 13 | 19.4 | |
| 2 | 39 | 58.2 | ||||
| 3 | 8 | 11.9 | ||||
| Xerostomia | ||||||
| 1 | 43 | 64.2 | 1 | 33 | 49.3 | |
| 2 | 8 | 11.9 | 2 | 7 | 10.4 | |
| Epitheliolysis | ||||||
| 3 | 11 | 16.4 | ||||
| Osteoradionecrosis | ||||||
| 3 | 2 | 3.0 | ||||
| Hearing impairment | ||||||
| 13 | 19.4 | 8 | 11.9 | |||
| Loss of taste | ||||||
| 50 | 74.6 | |||||
| Trismus | ||||||
| 16 | 23.9 | 20 | 29.9 | |||
| Edema | ||||||
| 2 | 3.0 | 1 | 1.5 | |||
| Fatigue | ||||||
| 59 | 88.1 | |||||
| Hair loss | ||||||
| 3 | 4.5 | |||||
| Characteristics | No of Patients (%) |
|---|---|
| gender | |
| male | 27 (40.3%) |
| female | 40 (59.7%) |
| T-stage | |
| 1 | 6 (9.0%) |
| 2 | 5 (7.5%) |
| 3 | 12 (17.9%) |
| 4 | 43 (64.2%) |
| N-stage | |
| 0 | 51 (76.1%) |
| + | 16 (23.9%) |
| Resection margin | |
| 0 | 10 (14.9%) |
| 1 | 52 (77.6%) |
| 2 | 5 (7.5%) |
| Locations in oral cavity | |
| buccal | 8 (11.9%) |
| palate (soft/hard) | 35 (52.2%) |
| tongue | 5 (7.5%) |
| maxilla | 19 (28.4%) |
| Characteristics | No of Patients |
|---|---|
| irradiation | |
| photons + carbon ions | 67 |
| median IMRT dose | Gy (range) |
| 50 (48–56) | |
| median C12 dose | |
| 24 (18–24) | |
| median dose of cervical lymphatic drainage | |
| 50 (48–56) | |
| cumulative dose (IMRT + C12) | |
| 74 (68–74) | |
| median CTV volume (ccm) | |
| IMRT | 346 ccm (range: 21–921 ccm) |
| C12 | 134 ccm (range: 21–411 ccm) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lang, K.; Baur, M.; Akbaba, S.; Held, T.; Kargus, S.; Bougatf, N.; Bernhardt, D.; Freier, K.; Plinkert, P.K.; Rieken, S.; et al. Intensity Modulated Radiotherapy (IMRT) + Carbon Ion Boost for Adenoid Cystic Carcinoma of the Minor Salivary Glands in the Oral Cavity. Cancers 2018, 10, 488. https://doi.org/10.3390/cancers10120488
Lang K, Baur M, Akbaba S, Held T, Kargus S, Bougatf N, Bernhardt D, Freier K, Plinkert PK, Rieken S, et al. Intensity Modulated Radiotherapy (IMRT) + Carbon Ion Boost for Adenoid Cystic Carcinoma of the Minor Salivary Glands in the Oral Cavity. Cancers. 2018; 10(12):488. https://doi.org/10.3390/cancers10120488
Chicago/Turabian StyleLang, Kristin, Melissa Baur, Sati Akbaba, Thomas Held, Steffen Kargus, Nina Bougatf, Denise Bernhardt, Kolja Freier, Peter K. Plinkert, Stefan Rieken, and et al. 2018. "Intensity Modulated Radiotherapy (IMRT) + Carbon Ion Boost for Adenoid Cystic Carcinoma of the Minor Salivary Glands in the Oral Cavity" Cancers 10, no. 12: 488. https://doi.org/10.3390/cancers10120488
APA StyleLang, K., Baur, M., Akbaba, S., Held, T., Kargus, S., Bougatf, N., Bernhardt, D., Freier, K., Plinkert, P. K., Rieken, S., Debus, J., & Adeberg, S. (2018). Intensity Modulated Radiotherapy (IMRT) + Carbon Ion Boost for Adenoid Cystic Carcinoma of the Minor Salivary Glands in the Oral Cavity. Cancers, 10(12), 488. https://doi.org/10.3390/cancers10120488





