Loss of Myeloid-Specific TGF-β Signaling Decreases CTHRC1 to Downregulate bFGF and the Development of H1993-Induced Osteolytic Bone Lesions
Abstract
1. Introduction
2. Results
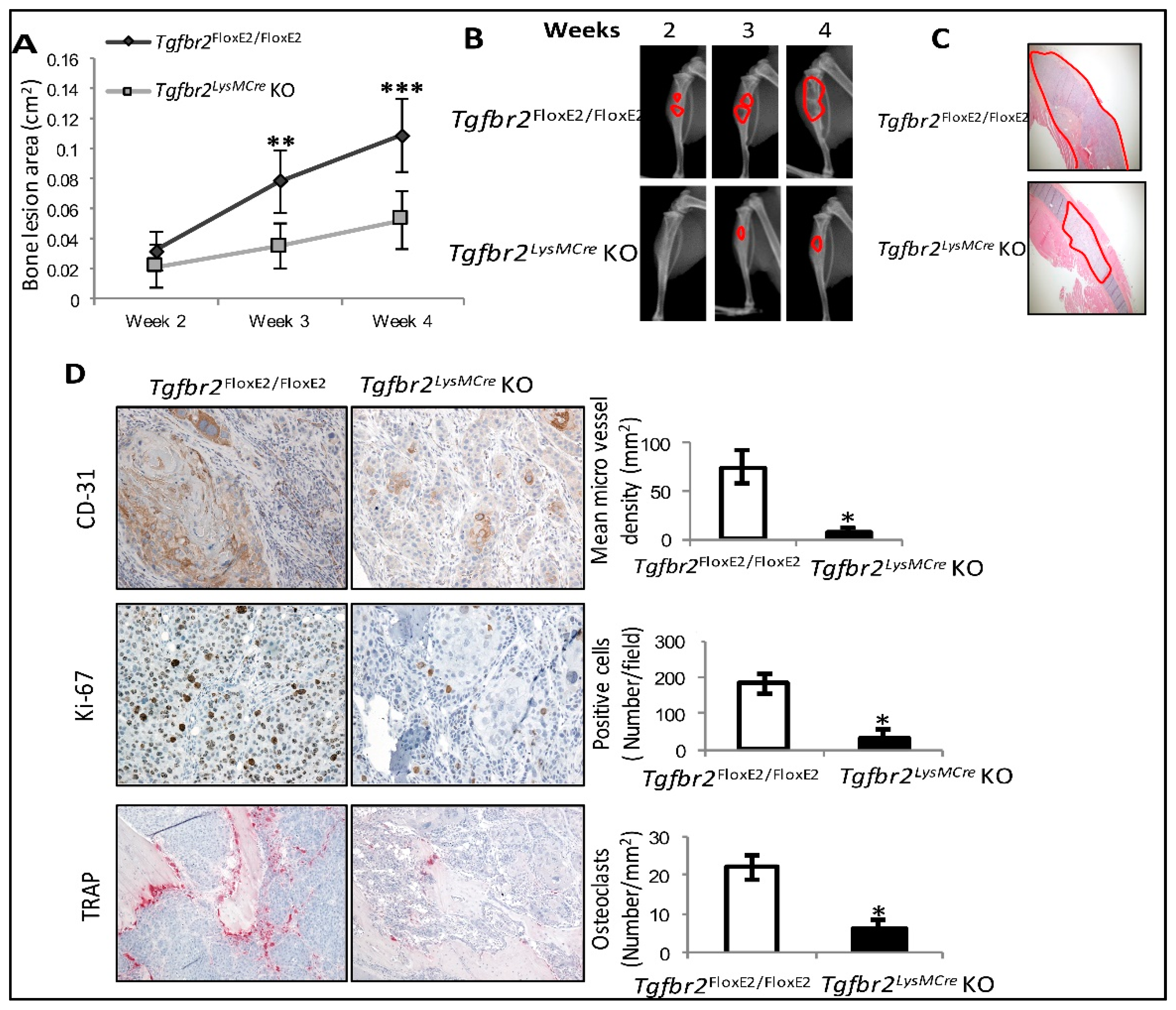
2.1. Osteolytic Bone Lesions Were Inhibited in Tgfbr2LysMCre KO Mice
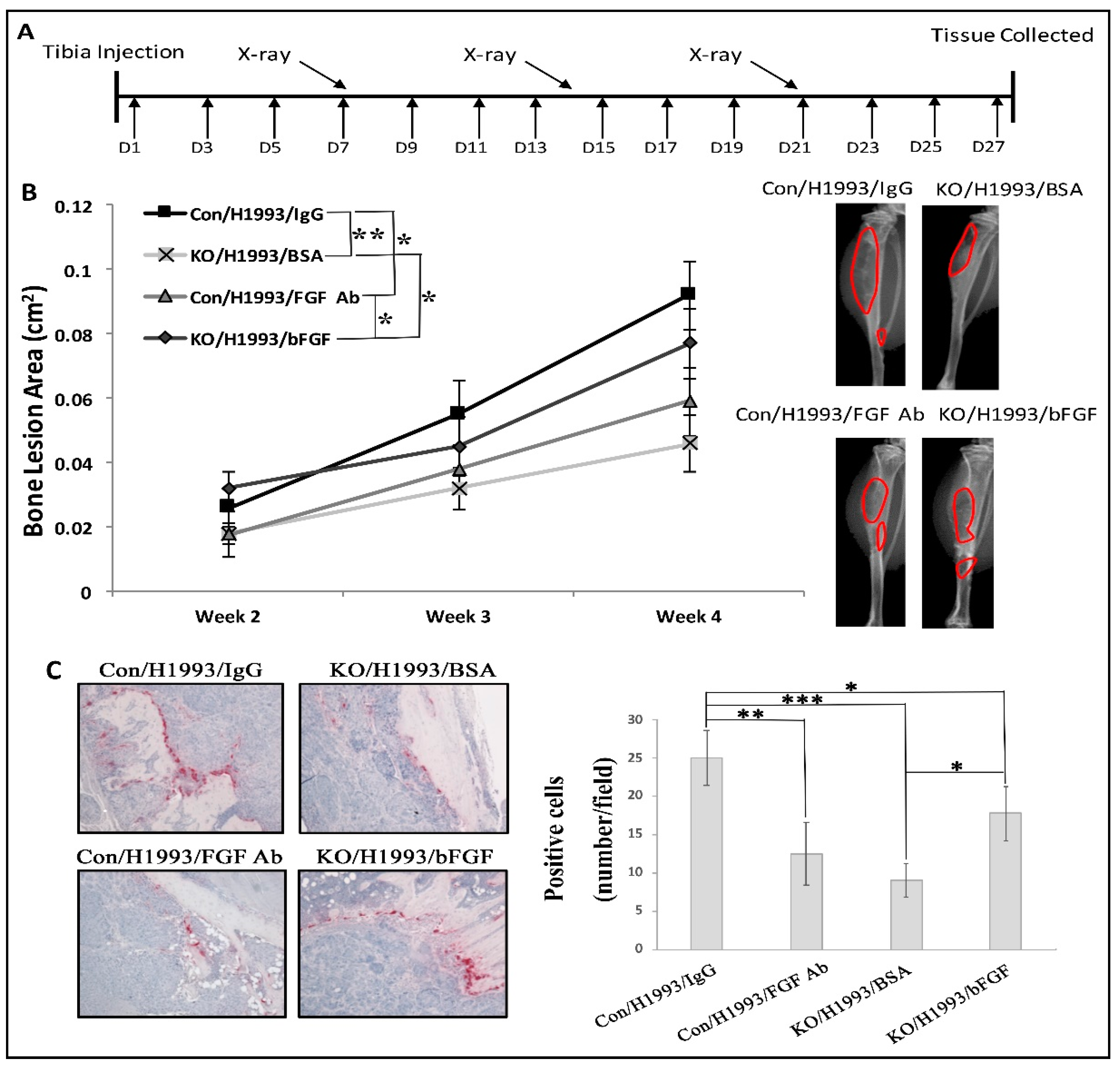
2.2. Myeloid-Specific TGF-β Signaling Promoted H1993-Induced Bone Lesions and bFGF Expression
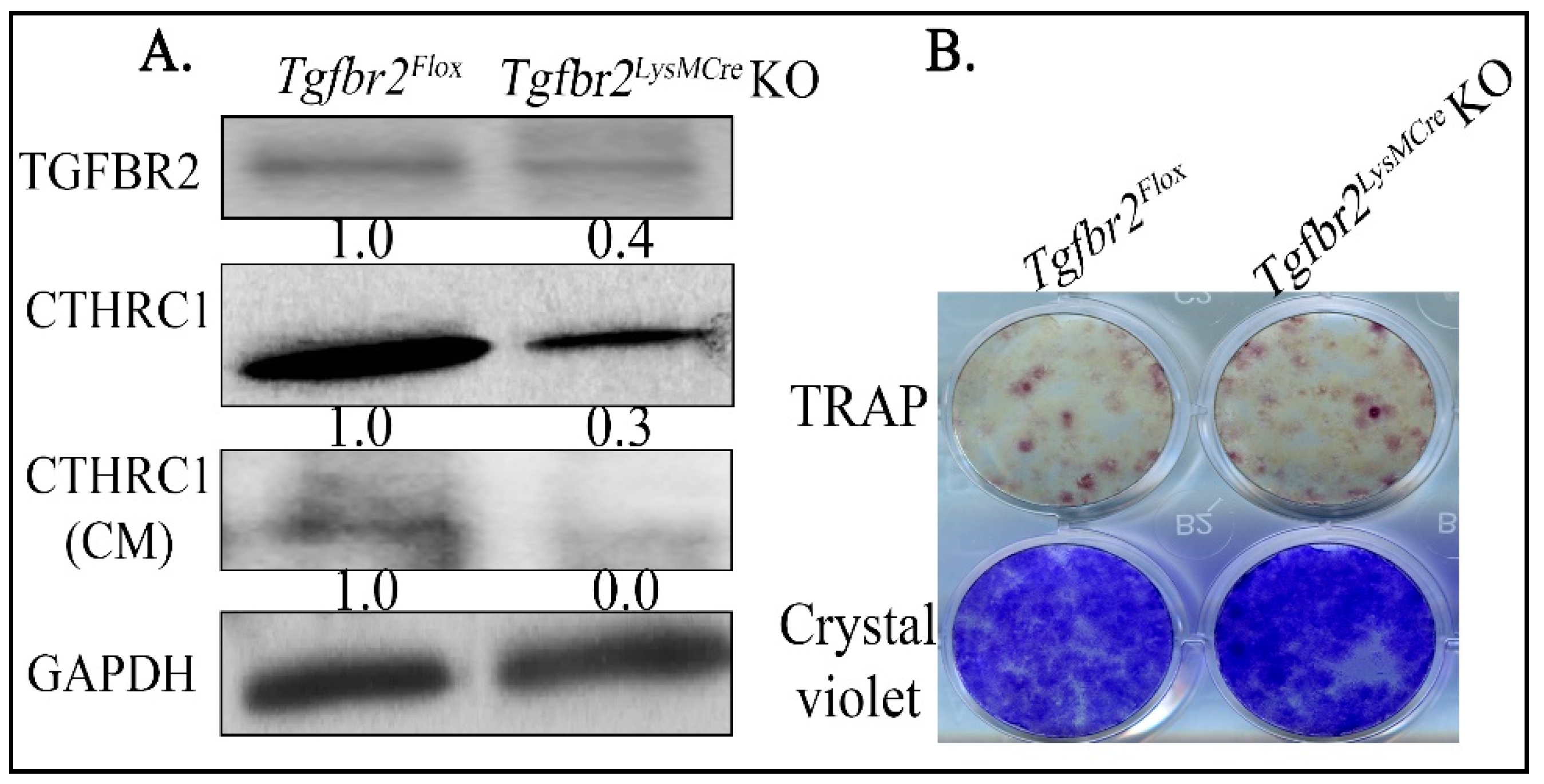
2.3. Loss of TGFBR2 in Osteoclasts Decreased the Expression and Secretion of CTHRC1
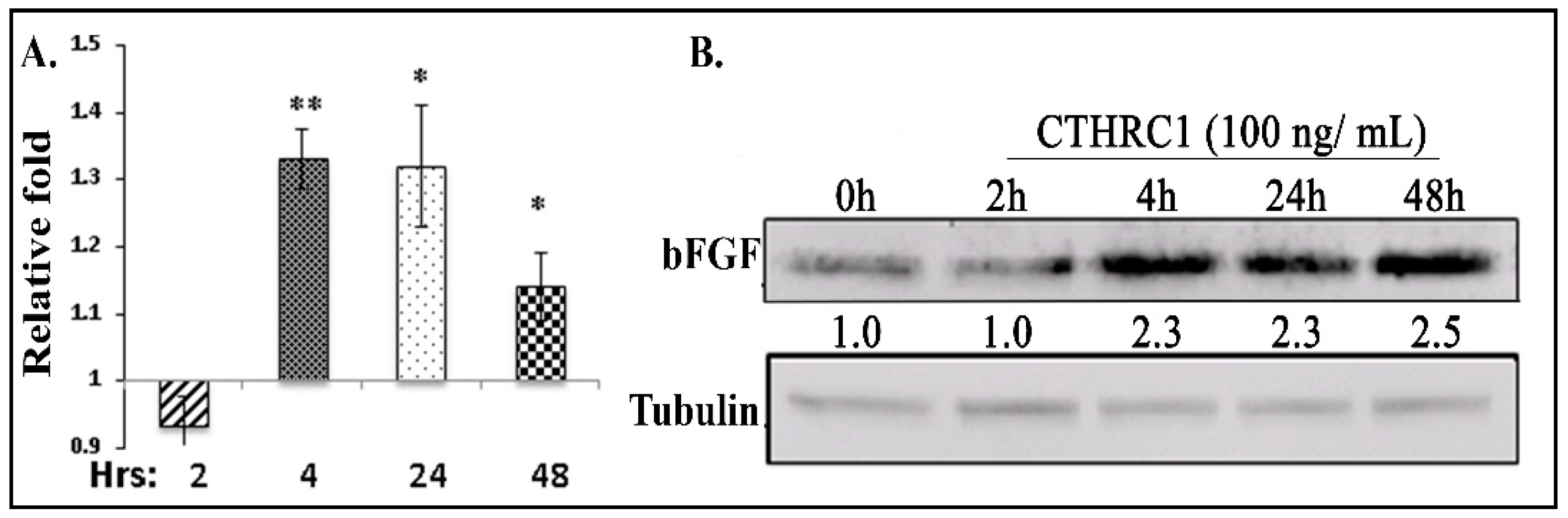
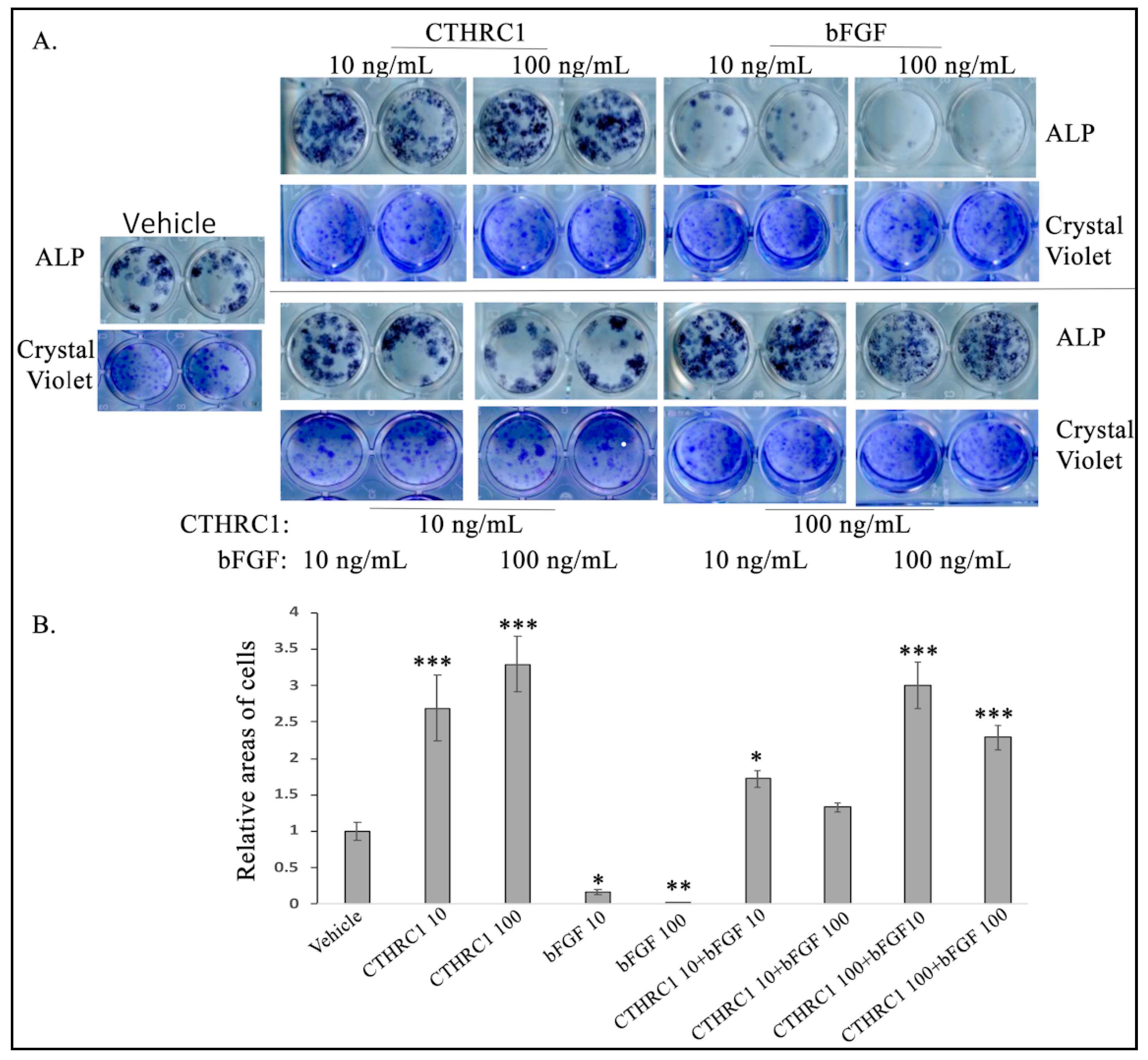
2.4. CTHRC1 Induced the Expression of bFGF in Osteoblasts and Osteoblastogenesis
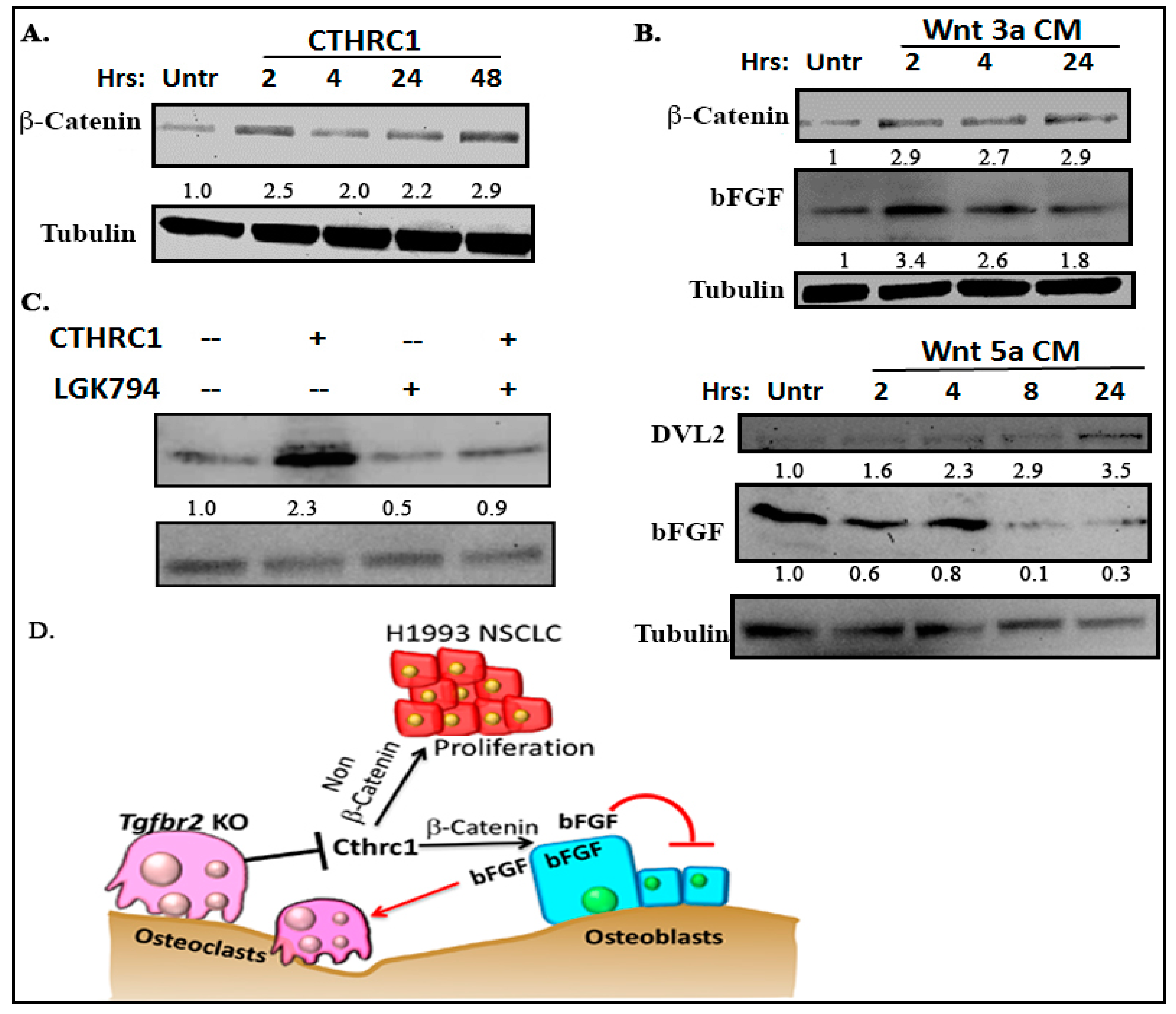
2.5. CTHRC1 Promoted bFGF Expression in a Wnt/β-Catenin-Dependent Manner
3. Discussion
4. Materials and Methods
4.1. Cells Cultures and Animals
4.2. Injections and Radiographic Imaging
4.3. Histology, Histomorphometry, and Immunohistochemistry
4.4. Protein Extraction and Western Blots
4.5. RNA Extraction and Quantitative Real-Time RT-PCR (qRT-PCR)
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wilkinson, A.N.; Viola, R.; Brundage, M.D. Managing skeletal related events resulting from bone metastases. BMJ 2008, 337, e2041. [Google Scholar] [CrossRef] [PubMed]
- Talreja, D.B. Importance of antiresorptive therapies for patients with bone metastases from solid tumors. Cancer Manag. Res. 2012, 4, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yu, Q.F.; Peng, A.F.; Tong, W.L.; Liu, J.M.; Liu, Z.L. The risk factors of bone metastases in patients with lung cancer. Sci. Rep. 2017, 7, e8970. [Google Scholar] [CrossRef] [PubMed]
- Kong, P.; Yan, J.; Liu, D.; Ji, Y.; Wang, Y.; Zhuang, J.; Wang, J.; Hu, X.; Yue, X. Skeletal-related events and overall survival of patients with bone metastasis from nonsmall cell lung cancer—A retrospective analysis. Medicine (Baltimore) 2017, 96, e9327. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, H.; Yamada, K.; Sugiura, T.; Hida, T.; Mitsudomi, T. Predictors of survival in patients with bone metastasis of lung cancer. Clin. Orthop. Relat. Res. 2008, 466, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Kuchuk, M.; Kuchuk, I.; Sabri, E.; Hutton, B.; Clemons, M.; Wheatley-Price, P. The incidence and clinical impact of bone metastases in non-small cell lung cancer. Lung Cancer 2015, 89, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Suva, L.J.; Washam, C.; Nicholas, R.W.; Griffin, R.J. Bone metastasis: Mechanisms and therapeutic opportunities. Nat. Rev. Endocrinol. 2011, 7, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Bussard, K.M.; Gay, C.V.; Mastro, A.M. The bone microenvironment in metastasis: What is special about bone? Cancer Meta. Rev. 2008, 27, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.J. Manipulating the environment of cancer cells in bone: A novel therapeutic approach. J. Clin. Investig. 2002, 110, 1399–1401. [Google Scholar] [CrossRef] [PubMed]
- Roodman, G.D. Mechanisms of bone metastasis. N. Engl. J. Med. 2004, 350, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Mundy, G.R. Metastasis to bone: Causes, consequences and therapeutic opportunities. Nat. Rev. Cancer 2002, 2, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Shi Y, M.J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 2003, 113, 685–700. [Google Scholar] [CrossRef]
- Massague, J. TGFβ signalling in context. Nat. Rev. Mol. Cell Biol. 2012, 13, 616–630. [Google Scholar] [CrossRef] [PubMed]
- Ikushima, H.; Miyazono, K. TGFβ signalling: A complex web in cancer progression. Nat. Rev. Cancer 2010, 10, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Eser, P.O.; Janne, P.A. TGFβ pathway inhibition in the treatment of non-small cell lung cancer. Pharmacol. Ther. 2018, 184, 112–130. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Gara, S.K.; Achyut, B.R.; Li, Z.; Yan, H.H.; Day, C.P.; Weiss, J.M.; Trinchieri, G.; Morris, J.C.; Yang, L. TGF-β signaling in myeloid cells is required for tumor metastasis. Cancer Discov. 2013, 3, 936–951. [Google Scholar] [CrossRef] [PubMed]
- Engblom, C.; Pfirschke, C.; Pittet, M.J. The role of myeloid cells in cancer therapies. Nat. Rev. Cancer 2016, 16, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Bronte, V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 2012, 12, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Montero, A.J.; Diaz-Montero, C.M.; Kyriakopoulos, C.E.; Bronte, V.; Mandruzzato, S. Myeloid-derived suppressor cells in cancer patients: A clinical perspective. J. Immunother. 2012, 35, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Vander Ark, A.; Lee, P.; Hostetter, G.; Bhowmick, N.A.; Matrisian, L.M.; Williams, B.O.; Miranti, C.K.; Li, X. Myeloid-specific TGF-β signaling in bone promotes basic-FGF and breast cancer bone metastasis. Oncogene 2016, 35, 2370–2378. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Vander Ark, A.; Daft, P.; Woodford, E.; Wang, J.; Madaj, Z.; Li, X. Loss of TGF-β signaling in osteoblasts increases basic-FGF and promotes prostate cancer bone metastasis. Cancer Lett. 2018, 418, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Pyagay, P.; Heroult, M.; Wang, Q.; Lehnert, W.; Belden, J.; Liaw, L.; Friesel, R.E.; Lindner, V. Collagen triple helix repeat containing 1, a novel secreted protein in injured and diseased arteries, inhibits collagen expression and promotes cell migration. Circ. Res. 2005, 96, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Wang, T.H.; Hsu, H.C.; Yuan, R.H.; Jeng, Y.M. Overexpression of CTHRC1 in hepatocellular carcinoma promotes tumor invasion and predicts poor prognosis. PLoS ONE 2013, 8, e70324. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, S.; Fumoto, T.; Matsuoka, K.; Park, K.A.; Aburatani, H.; Kato, S.; Ito, M.; Ikeda, K. Osteoclast-secreted CTHRC1 in the coupling of bone resorption to formation. J. Clin. Investig. 2013, 123, 3914–3924. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.H.; Chen, J.; Wang, X.P.; Wu, Y.Q.; Peng, H.T.; Lin, X.H.; Wang, W.J. Collagen triple helix repeat containing-1 negatively regulated by microRNA-30c promotes cell proliferation and metastasis and indicates poor prognosis in breast cancer. J. Exp. Clin. Cancer Res. 2017, 36, e92. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Cui, Y.; Liu, J.; Zhu, X.; Wu, H.; Yang, Z.; Ke, Z. Multidimensional Roles of Collagen Triple Helix Repeat Containing 1 (CTHRC1) in Malignant Cancers. J. Cancer 2016, 7, 2213–2220. [Google Scholar] [CrossRef] [PubMed]
- Ke, Z.; He, W.; Lai, Y.; Guo, X.; Chen, S.; Li, S.; Wang, Y.; Wang, L. Overexpression of collagen triple helix repeat containing 1 (CTHRC1) is associated with tumour aggressiveness and poor prognosis in human non-small cell lung cancer. Oncotarget 2014, 5, 9410–9424. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Zhang, H.; Wang, Y.; Zhou, Y.; Luo, Y.; Cui, Y.; Jiang, N.; Jiang, W.; Wang, H.; Xu, D.; et al. CTHRC1 induces non-small cell lung cancer (NSCLC) invasion through upregulating MMP-7/MMP-9. BMC Cancer 2018, 18, e400. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.Z.; Zhuang, C.; Yang, X.M.; Zhang, Z.Z.; Ma, H.; Zhang, W.M.; You, H.; Qin, W.; Gu, J.; Yang, S.; et al. CTHRC1 acts as a prognostic factor and promotes invasiveness of gastrointestinal stromal tumors by activating Wnt/PCP-Rho signaling. Neoplasia 2014, 16, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Nishimura, O.; Misaki, K.; Nishita, M.; Minami, Y.; Yonemura, S.; Tarui, H.; Sasaki, H. Cthrc1 selectively activates the planar cell polarity pathway of Wnt signaling by stabilizing the Wnt-receptor complex. Dev. Cell 2008, 15, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. Wnt/Planar cell polarity signaling: A new paradigm for cancer therapy. Mol. Cancer Ther. 2009, 8, 2103–2109. [Google Scholar] [CrossRef] [PubMed]
- Goetz, R.; Mohammadi, M. Exploring mechanisms of FGF signalling through the lens of structural biology. Nat. Rev. Mol. Cell Biol. 2013, 14, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Teti, A. Mechanisms of osteoclast-dependent bone formation. Bonekey Rep. 2013, 2, e449. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, Y.C.; Chen, X.Y.; Shen, Z.Y.; Cao, H.; Zhang, Y.J.; Yu, J.; Zhu, J.D.; Lu, Y.Y.; Fang, J.Y. CTHRC1 is upregulated by promoter demethylation and transforming growth factor-beta1 and may be associated with metastasis in human gastric cancer. Cancer Sci. 2012, 103, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Sachie, A.W.; Hiratsuka, H.A.; Maru, Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat. Cell Biol. 2006, 8, 1369–1375. [Google Scholar]
- Liu, J.; Pan, S.; Hsieh, M.H.; Ng, N.; Sun, F.; Wang, T.; Kasibhatla, S.; Schuller, A.G.; Li, A.G.; Cheng, D.; et al. Targeting Wnt-driven cancer through the inhibition of Porcupine by LGK974. Proc. Natl. Acad. Sci. USA 2013, 110, 20224–20229. [Google Scholar] [CrossRef] [PubMed]
- Page, J.M.; Merkel, A.R.; Ruppender, N.S.; Guo, R.; Dadwal, U.C.; Cannonier, S.A.; Basu, S.; Guelcher, S.A.; Sterling, J.A. Matrix rigidity regulates the transition of tumor cells to a bone-destructive phenotype through integrin β3 and TGF-β receptor type II. Biomaterials 2015, 64, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Duinhouwer, L.E.; Tuysuz, N.; Rombouts, E.W.; Ter Borg, M.N.; Mastrobattista, E.; Spanholtz, J.; Cornelissen, J.J.; Ten Berge, D.; Braakman, E. Wnt3a protein reduces growth factor-driven expansion of human hematopoietic stem and progenitor cells in serum-free cultures. PLoS ONE 2015, 10, e0119086. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, A.; Loots, G.G. Transcriptional control of Sost in bone. Bone 2017, 96, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Ten Dijke, P.; Krause, C.; de Gorter, D.J.; Lowik, C.W.; van Bezooijen, R.L. Osteocyte-derived sclerostin inhibits bone formation: Its role in bone morphogenetic protein and Wnt signaling. J. Bone Joint. Surg. Am. 2008, 90, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Moester, M.J.; Papapoulos, S.E.; Lowik, C.W.; van Bezooijen, R.L. Sclerostin: Current knowledge and future perspectives. Calcif. Tissue Int. 2010, 87, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Ostapoff, K.T.; Cenik, B.K.; Wang, M.; Ye, R.; Xu, X.; Nugent, D.; Hagopian, M.M.; Topalovski, M.; Rivera, L.B.; Carroll, K.D.; et al. Neutralizing murine TGFβR2 promotes a differentiated tumor cell phenotype and inhibits pancreatic cancer metastasis. Cancer Res. 2014, 74, 4996–5007. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.; Agyin, J.K.; Wang, L.; Tang, Y.; Lei, X.; Story, B.M.; Cornell, J.E.; Pollock, B.H.; Mundy, G.R.; Sun, L.Z. Inhibition of pulmonary and skeletal metastasis by a transforming growth factor-beta type I receptor kinase inhibitor. Cancer Res. 2006, 66, 6714–6721. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.J.; Selander, K.; Chirgwin, J.M.; Dallas, M.; Grubbs, B.G.; Wieser, R.; Massague, J.; Mundy, G.R.; Guise, T.A. TGF-β signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J. Clin. Investig. 1999, 103, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Colak, S.; Ten Dijke, P. Targeting TGF-β Signaling in Cancer. Trends Cancer 2017, 3, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.W.; Hsu, E.C.; Lee, S.S.; Lang, Y.D.; Lin, Y.C.; Chang, C.Y.; Lee, S.Y.; Gu, D.L.; Shih, J.H.; Ho, C.M.; et al. PSPC1 mediates TGF-beta1 autocrine signalling and Smad2/3 target switching to promote EMT, stemness and metastasis. Nat. Cell Biol. 2018, 20, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, Q.; Hou, J.; Gu, Y.; Zhang, Y.; Chen, Z.; Fan, J.; Zhou, W.; Qiu, S.; Zhang, Y.; et al. Tumor-Induced Generation of Splenic Erythroblast-like Ter-Cells Promotes Tumor Progression. Cell 2018, 173, 634–648. [Google Scholar] [CrossRef] [PubMed]
- Mariathasan, S.; Turley, S.J.; Nickles, D.; Castiglioni, A.; Yuen, K.; Wang, Y.; Kadel, E.E., III; Koeppen, H.; Astarita, .L.; Cubas, R.; et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018, 554, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Zhang, D.; Xu, C.; Hance, K.W.; Marelli, B.; Qi, J.; Yu, H.; Qin, G.; Sircar, A.; Hernandez, V.M.; et al. Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD-L1 and TGF-β. Sci. Transl. Med. 2018, 10, e5488. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, N.A.; Chytil, A.; Plieth, D.; Gorska, A.E.; Dumont, N.; Shappell, S.; Washington, M.K.; Neilson, E.G.; Moses, H.L. TGF-β signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science 2004, 303, 848–851. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, N.A.; Chytil, A.; Plieth, D.; Gorska, A.E.; Dumont, N.; Shappell, S.; Washington, M.K.; Neilson, E.G.; Moses, H.L. TGF-β signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science 2004, 6, 848–851. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Placencio, V.; Iturregui, J.M.; Uwamariya, C.; Sharif-Afshar, A.R.; Koyama, T.; Hayward, S.W.; Bhowmick, N.A. Prostate tumor progression is mediated by a paracrine TGF-β/Wnt3a signaling axis. Oncogene 2008, 27, 7118–7130. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sterling, J.A.; Fan, K.H.; Vessella, R.L.; Shyr, Y.; Hayward, S.W.; Matrisian, L.M.; Bhowmick, N.A. Loss of TGF-β responsiveness in prostate stromal cells alters chemokine levels and facilitates the development of mixed osteoblastic/osteolytic bone lesions. Mol. Cancer Res. 2012, 10, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Neuzillet, C.; Tijeras-Raballand, A.; Cohen, R.; Cros, J.; Faivre, S.; Raymond, E.; de Gramont, A. Targeting the TGFβ pathway for cancer therapy. Pharmacol. Ther. 2015, 147, 22–31. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganguly, S.S.; Daft, P.G.; Cao, J.; Meng, X.; Zhong, Z.A.; Vander Ark, A.; Meadows, A.; Madaj, Z.; Williams, B.; Li, X. Loss of Myeloid-Specific TGF-β Signaling Decreases CTHRC1 to Downregulate bFGF and the Development of H1993-Induced Osteolytic Bone Lesions. Cancers 2018, 10, 463. https://doi.org/10.3390/cancers10120463
Ganguly SS, Daft PG, Cao J, Meng X, Zhong ZA, Vander Ark A, Meadows A, Madaj Z, Williams B, Li X. Loss of Myeloid-Specific TGF-β Signaling Decreases CTHRC1 to Downregulate bFGF and the Development of H1993-Induced Osteolytic Bone Lesions. Cancers. 2018; 10(12):463. https://doi.org/10.3390/cancers10120463
Chicago/Turabian StyleGanguly, Sourik S., Paul G. Daft, Jingchen Cao, Xiangqi Meng, Zhendong A. Zhong, Alexandra Vander Ark, Austin Meadows, Zach Madaj, Bart Williams, and Xiaohong Li. 2018. "Loss of Myeloid-Specific TGF-β Signaling Decreases CTHRC1 to Downregulate bFGF and the Development of H1993-Induced Osteolytic Bone Lesions" Cancers 10, no. 12: 463. https://doi.org/10.3390/cancers10120463
APA StyleGanguly, S. S., Daft, P. G., Cao, J., Meng, X., Zhong, Z. A., Vander Ark, A., Meadows, A., Madaj, Z., Williams, B., & Li, X. (2018). Loss of Myeloid-Specific TGF-β Signaling Decreases CTHRC1 to Downregulate bFGF and the Development of H1993-Induced Osteolytic Bone Lesions. Cancers, 10(12), 463. https://doi.org/10.3390/cancers10120463




