Multiple and Periodic Measurement of RBC Aggregation and ESR in Parallel Microfluidic Channels under On-Off Blood Flow Control
Abstract
1. Introduction
2. Materials and Methods
2.1. Blood Sample Preparation
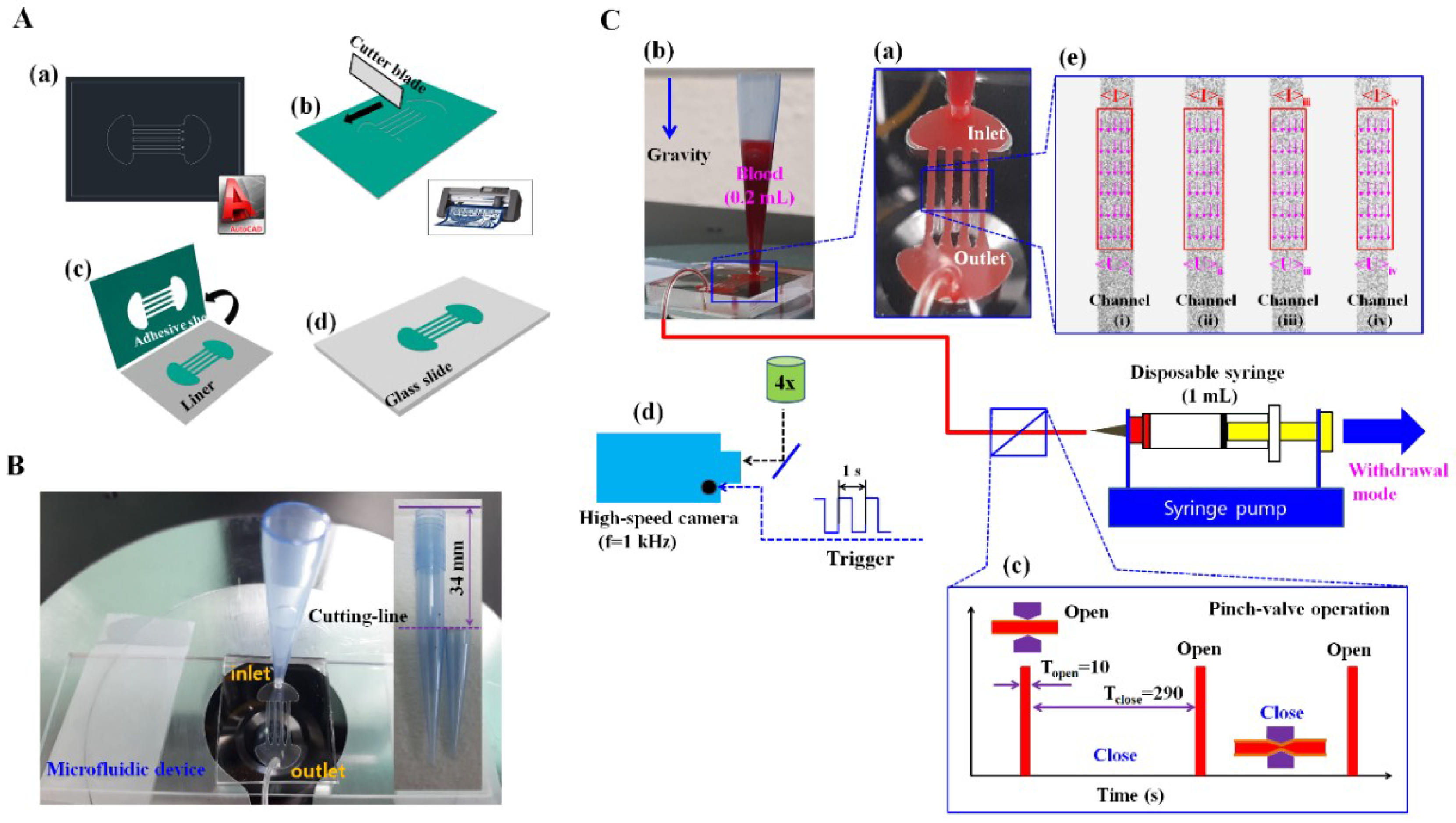
2.2. Fabrication of a Microfluidic Device and Experimental Procedure
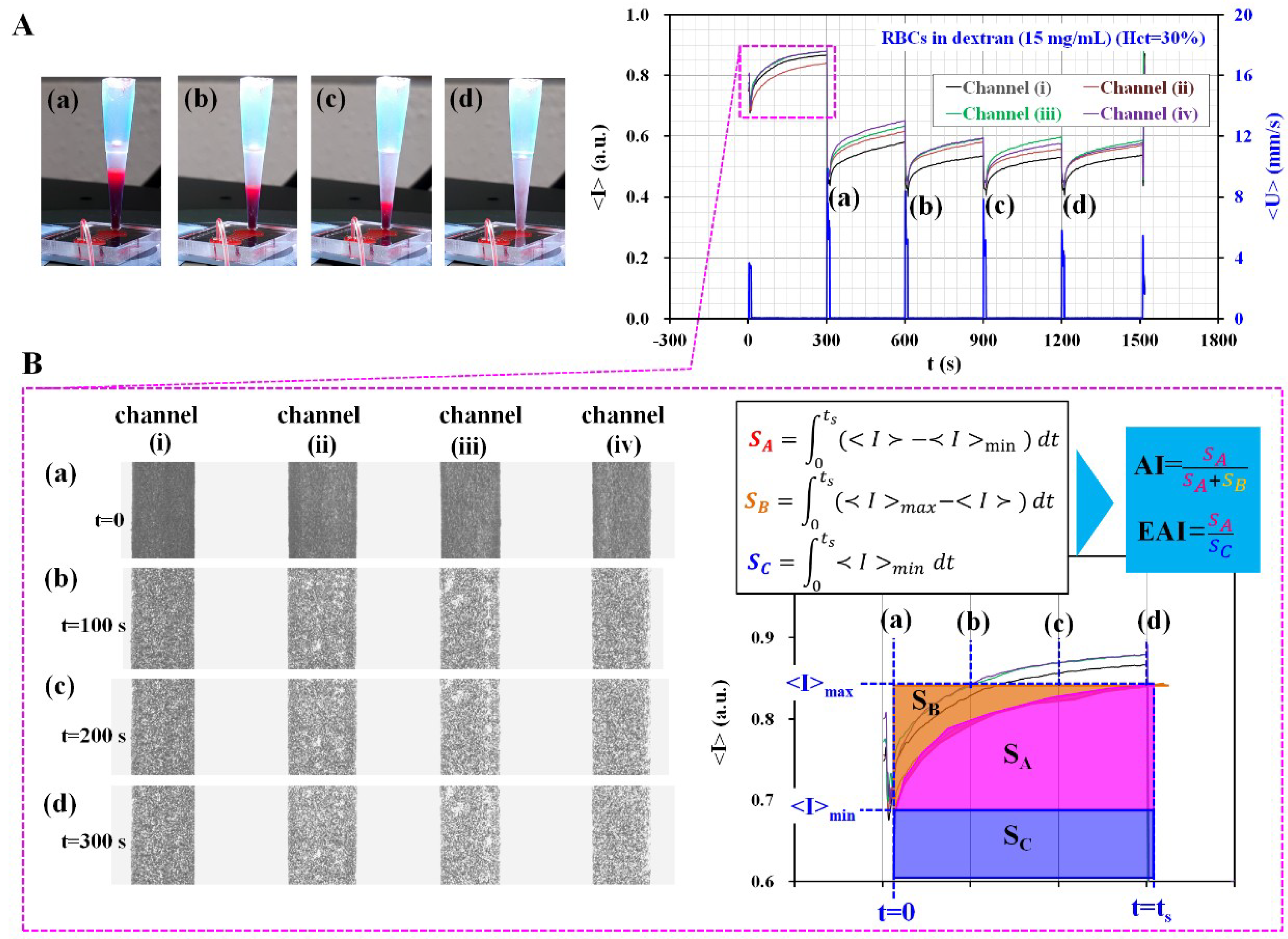
2.3. The Proposed Method for Quantifying RBCs Aggregation and ESR over Time
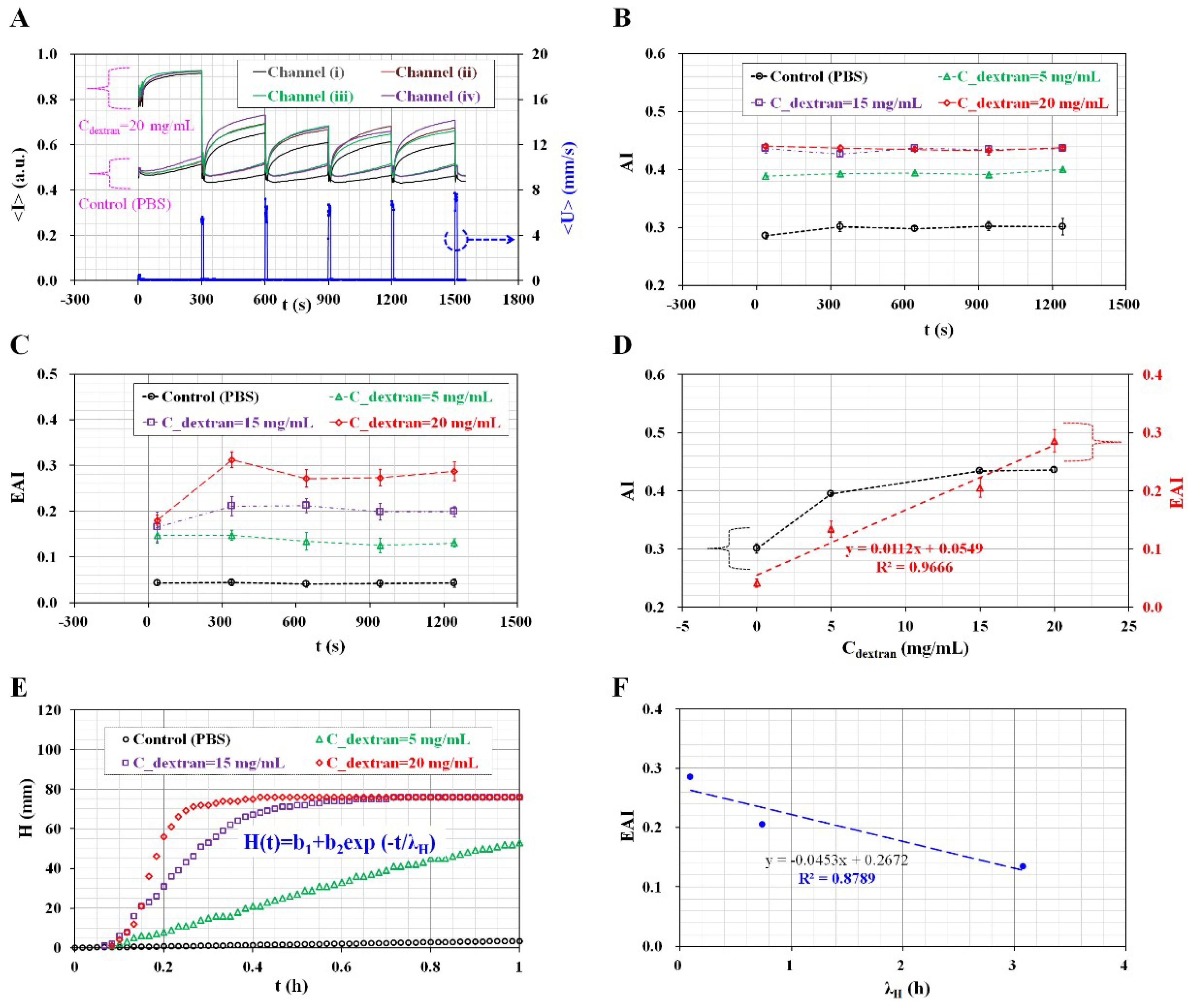
2.4. Quantifications of Image Intensity (<I>) and Blood Velocity (<U>)
3. Results and Discussion
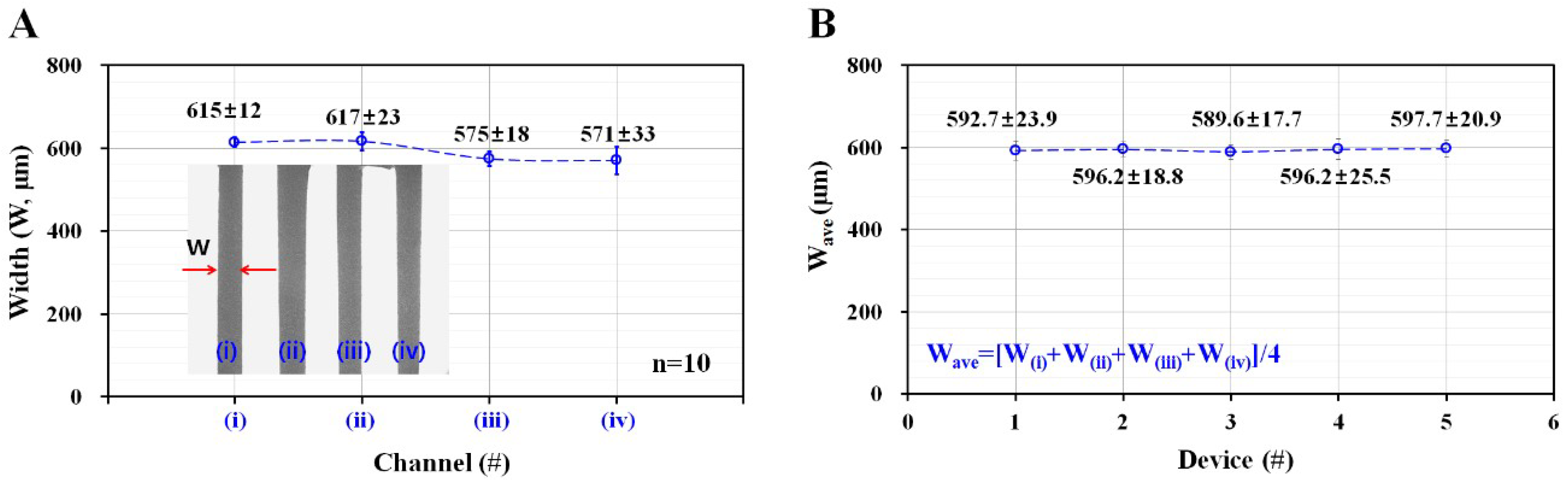
3.1. Variation of Width in Microfluidic Channel Fabricated by Using an Adhesive Sheet for Master Mold
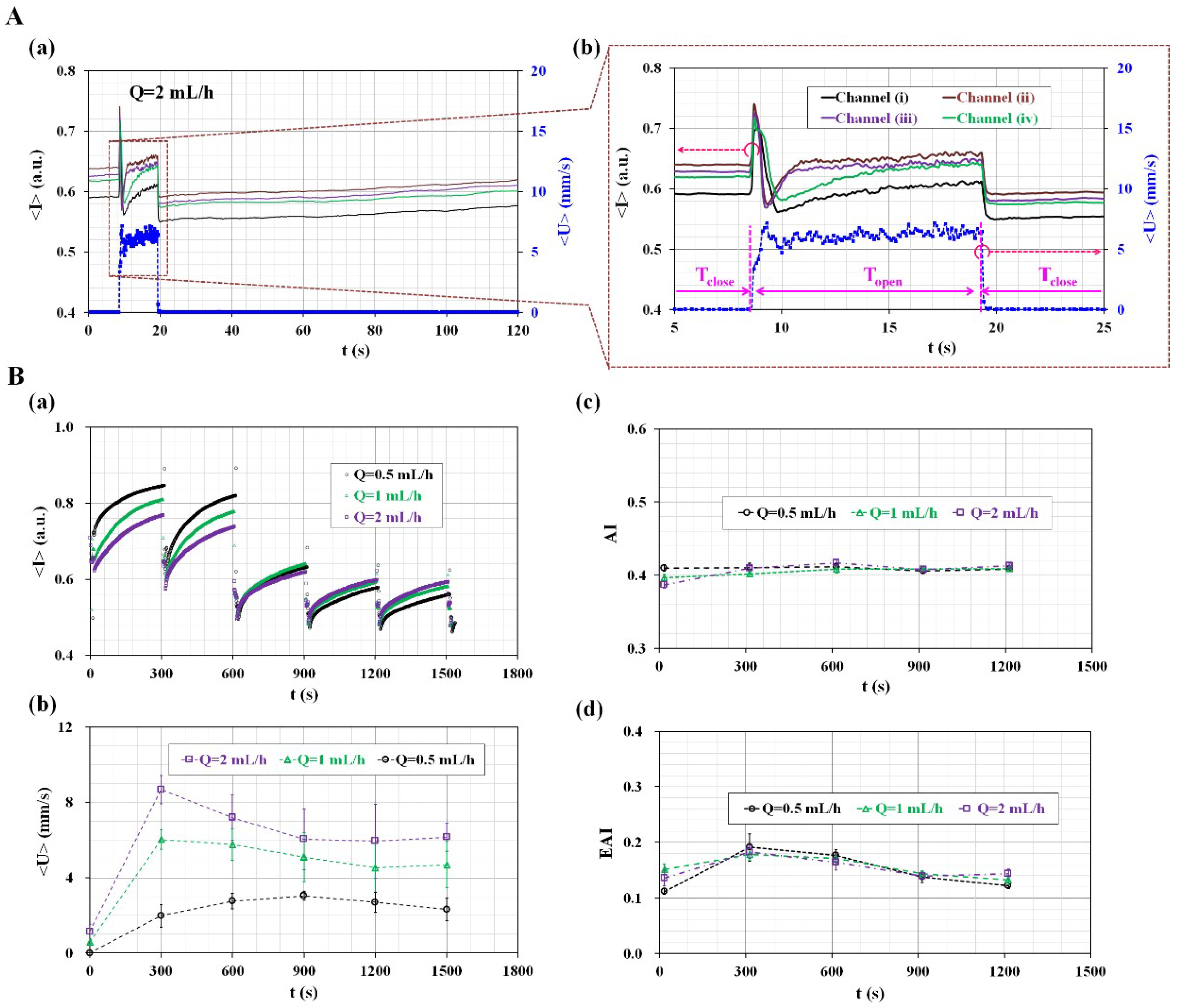
3.2. Quantitative Evaluation of the Effects of Pinch-Valve Operation and Syringe Pump Flow-Rate
3.3. Quantitative Evaluation of the Channel Number for Evaluating Variations of AI and EAI
3.4. Quantitative Evaluation of the Effect of Hematocrit Variations
3.5. Quantitative Evaluation of RBC Aggregation under Continuous for Homogeneous Aggregated RBCs
3.6. Quantitative Comparison between the Proposed Method and the Modified Conventional ESR Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lanotte, L.; Mauer, J.; Mendez, S.; Fedosov, D.A.; Fromental, J.-M.; Claveria, V.; Nicoud, F.; Gompper, G.; Abkarian, M. Red cells’ dynamic morphologies govern blood shear thinning under microcirculatory flow conditions. Proc. Natl. Acad. Sci. USA 2016, 113, 13289–13294. [Google Scholar] [CrossRef] [PubMed]
- Baskurt, O.K.; Meiselman, H.J. Time course of electrical impedance during red blood cell aggregation in a glass tube: Comparison with light transmittance. IEEE Trans. Biomed. Eng. 2010, 57, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Isiksacan, Z.; Erel, O.; Elbuken, C. A portable microfluidic system for rapid measurement of the erythrocyte sedimentation rate. Lab Chip 2016, 16, 4682–4690. [Google Scholar] [CrossRef] [PubMed]
- Popel, A.S.; Johnson, P.C. Microcirculation and hemorheology. Annu. Rev. Fluid Mech. 2005, 37, 43–69. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.J.; Popel, A.S.; Intaglietta, M.; Johnson, P.C. Rheological effects of red blood cell aggregation in the venous network: A review of recent studies. Biorheology 2001, 38, 263–274. [Google Scholar] [PubMed]
- Yayan, J. Erythrocyte sedimentation rate as a marker for coronary heart disease. Vasc. Health Risk Manag. 2012, 8, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Bochen, K.; Krasowska, A.; Milaniuk, S.; Kulczyńska, M.; Prystupa, A.; Dzida, G. Erythrocyte sedimentation rate-an old marker with new applications. JPCCR 2011, 5, 50–55. [Google Scholar]
- Piva, E.; Pajola, R.; Temporin, V.; Plebani, M. A new turbidimetric standard to improve the quality assurance of the erythrocyte sedimentation rate measurement. Clin. Biochem. 2007, 40, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Larsson, A.; Hansson, L.-O. Inflammatory activity: Capillary electrophoresis provides more information than erythrocyte sedimentation rate. Upsala J. Med. Sci. 2005, 110, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Fabry, T.L. Mechanism of erythrocyte aggregation and sedimentation. Blood 1987, 70, 1572–1576. [Google Scholar] [PubMed]
- Cha, C.-H.; Park, C.-J.; Cha, Y.J.; Kim, H.K.; Kim, D.H.; Bae, J.H.; Jung, J.-S.; Jang, S.; Chi, H.-S.; Lee, D.S.; et al. Erythrocyte sedimentation rate measurements by test 1 better reflect inflammation than do those by the Westergren method in patients with malignancy, autoimmune disease, or infection. Am. J. Clin. Pathol. 2009, 131, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Plebani, M.; Toni, S.D.; Sanzari, M.C.; Bernardi, D.; Stockreiter, E. A New method for mMeasuring the erythrocyte sedimentation rate. Am. J. Clin. Pathol. 1998, 110, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Kaliviotis, E.; Sherwood, M.; Balabani, S. Partitioning of red blood cell aggregates in bifurcating microscale flows. Sci. Rep. 2017, 7, 44563. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J. Continuous and simultaneous measurement of the biophysical properties of blood in a microfluidic environment. Analyst 2016, 141, 6583–6597. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Ha, Y.-R.; Lee, S.-J. Microfluidic-based measurement of erythrocyte sedimentation rate for biophysical assessment of blood in an in vivo malaria-infected mouse. Biomicrofluidics 2014, 8, 044114. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Hou, J.X.; Suh, J.-S. Measurement of cell aggregation characteristics by analysis of laser-backscattering in a microfluidic rheometry. Korea-Aust. Rheol. J. 2007, 19, 61–66. [Google Scholar]
- Yeom, E.; Lee, S.J. Microfluidic-based speckle analysis for sensitive measurement of erythrocyte aggregation: A comparison of four methods for detection of elevated erythrocyte aggregation in diabetic rat blood. Biomicrofluidics 2015, 9, 024110. [Google Scholar] [CrossRef] [PubMed]
- Zhbanov, A.; Yang, S. Effects of aggregation on blood sedimentation and conductivity. PLoS ONE 2015, 10, e0129337. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J. Microfluidic-based measurement method of red blood cell aggregation under hematocrit variations. Sensors 2017, 17, 2037. [Google Scholar] [CrossRef] [PubMed]
- Uyuklu, M.; Cengiz, M.; Ulker, P.; Hever, T.; Tripette, J.; Connes, P.; Nemeth, N.; Meiselman, H.J.; Baskurt, O.K. Effects of storage duration and temperature of human blood on red cell deformability and aggregation. Clin. Hemorheol. Microcirc. 2009, 41, 269–278. [Google Scholar] [PubMed]
- Lim, H.-J.; Nam, J.-H.; Lee, B.-K.; Suh, J.-S.; Shin, S. Alteration of red blood cell aggregation during blood storage. Korea-Aust. Rheol. J. 2011, 23, 67–70. [Google Scholar] [CrossRef]
- Berezina, T.L.; Zaets, S.B.; Morgan, C.; Spillert, C.R.; Kamiyama, M.; Spolarics, Z.; Deitch, E.A.; Machiedo, G.W. Influence of storage on red blood cell rheological properties. J. Surg. Res. 2002, 102, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Bartholomeusz, D.A.; Boutté, R.; Andrade, J.D. Xurography: Rapid prototyping of microstructures using a cutting plotter. J. Microelectromech. Syst. 2005, 14, 1364–1374. [Google Scholar] [CrossRef]
- Faustino, V.; Catarino, S.O.; Lima, R.; Minas, G. Biomedical microfluidic devices by using low-cost fabrication techniques: A review. J. Biomech. 2016, 49, 2280–2292. [Google Scholar] [CrossRef] [PubMed]
- Pinto, E.; Faustino, V.; Rodrigues, R.O.; Pinho, D.; Garcia, V.; Miranda, J.M.; Lima, R. A rapid and low-cost nonlithographic method to fabricate biomedical microdevices for blood flow analysis. Micromachines 2015, 6, 121–135. [Google Scholar] [CrossRef]
- Islam, M.; Natu, R.; Martinez-Duarte, R. A study on the limits and advantages of using a desktop cutter plotter to fabricate microfluidic networks. Microfluid. Nanofluid. 2015, 19, 973–985. [Google Scholar] [CrossRef]
- Bento, D.; Sousa, L.; Yaginuma, T.; Garcia, V.; Lima, R.; Miranda, J.M. Microbubble moving in blood flow in microchannels: Effect on the cell-free layer and cell local concentration. Biomed. Microdevices 2017, 19, 6. [Google Scholar] [CrossRef] [PubMed]
- Yeom, E.; Kim, H.M.; Park, J.H.; Choi, W.; Doh, J.; Lee, S.J. Microfluidic system for monitoring temporal variations of hemorheological properties and platelet adhesion in LPS-injected rats. Sci. Rep. 2017, 7, 1801. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, Y.J.; Kim, B.J. Multiple and Periodic Measurement of RBC Aggregation and ESR in Parallel Microfluidic Channels under On-Off Blood Flow Control. Micromachines 2018, 9, 318. https://doi.org/10.3390/mi9070318
Kang YJ, Kim BJ. Multiple and Periodic Measurement of RBC Aggregation and ESR in Parallel Microfluidic Channels under On-Off Blood Flow Control. Micromachines. 2018; 9(7):318. https://doi.org/10.3390/mi9070318
Chicago/Turabian StyleKang, Yang Jun, and Byung Jun Kim. 2018. "Multiple and Periodic Measurement of RBC Aggregation and ESR in Parallel Microfluidic Channels under On-Off Blood Flow Control" Micromachines 9, no. 7: 318. https://doi.org/10.3390/mi9070318
APA StyleKang, Y. J., & Kim, B. J. (2018). Multiple and Periodic Measurement of RBC Aggregation and ESR in Parallel Microfluidic Channels under On-Off Blood Flow Control. Micromachines, 9(7), 318. https://doi.org/10.3390/mi9070318





