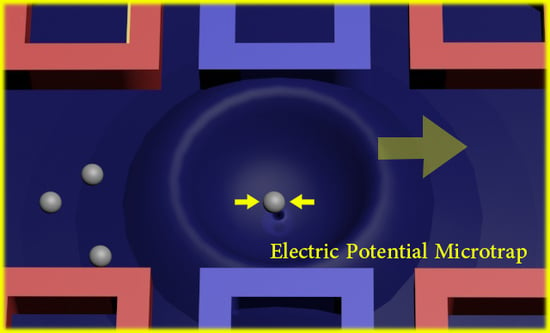
A Handy Liquid Metal Based Non-Invasive Electrophoretic Particle Microtrap
Abstract
:1. Introduction
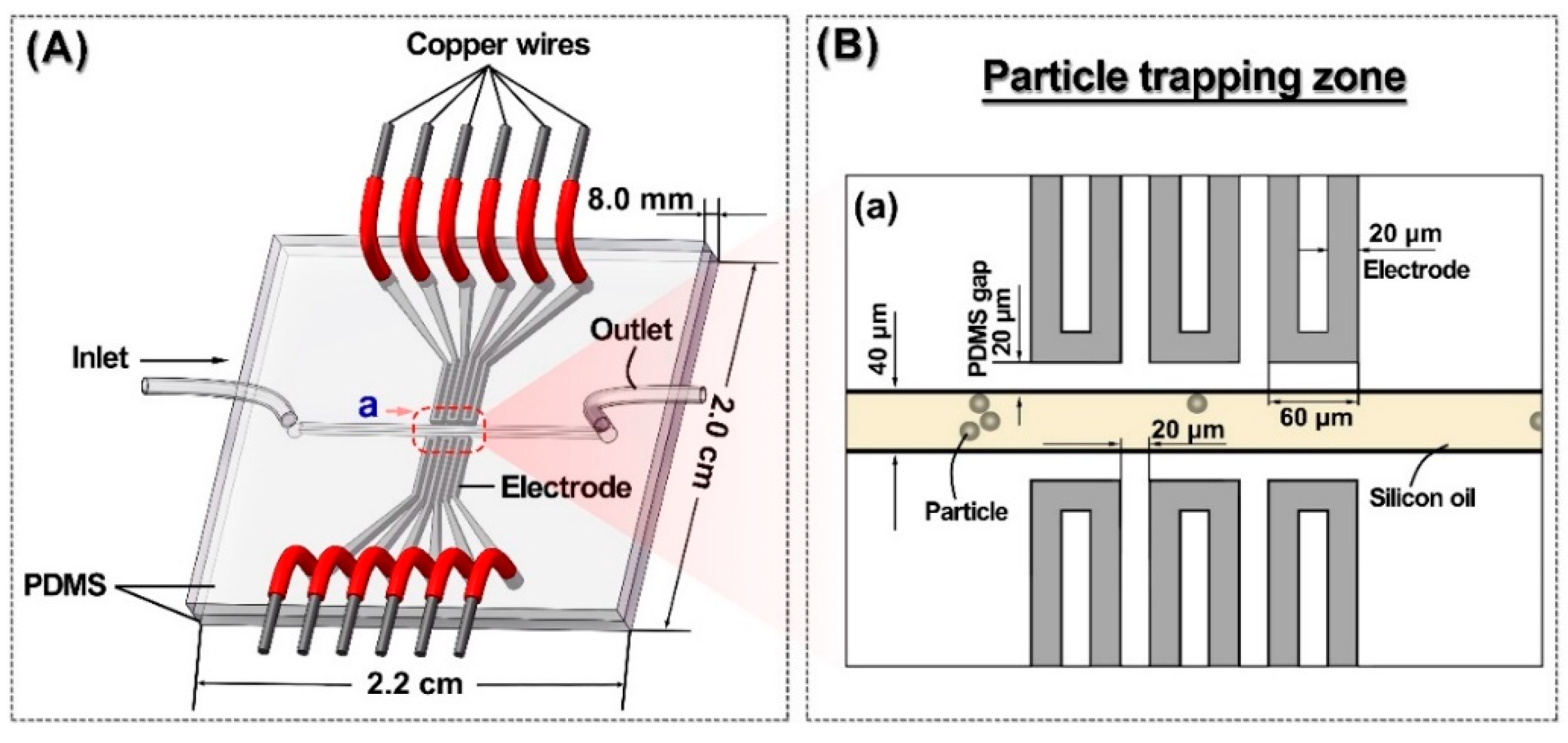
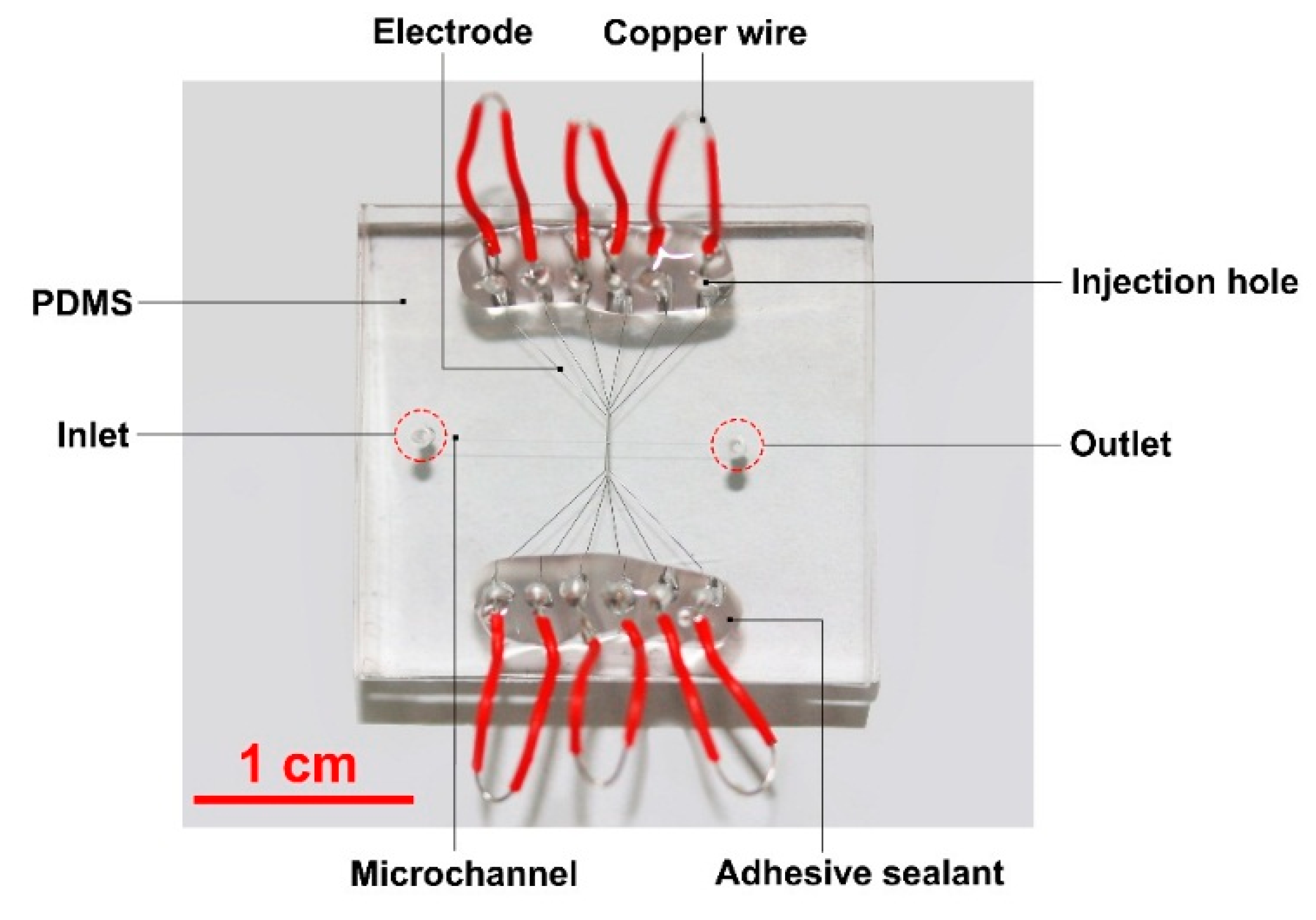
2. Liquid Metal Based Particle Microtrap
3. Experimental Details
4. Results
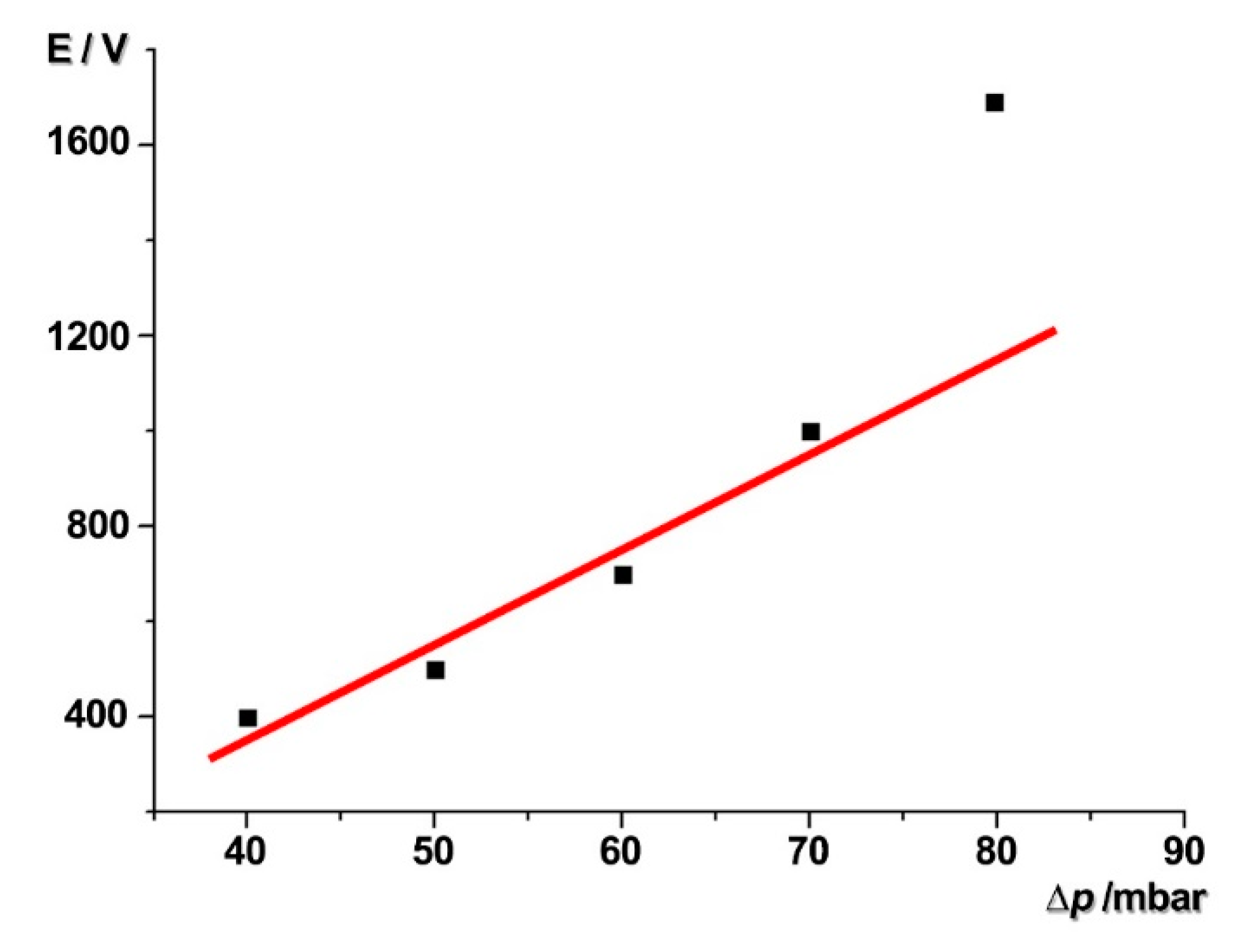
4.1. Electric Potential Particle Trap Design
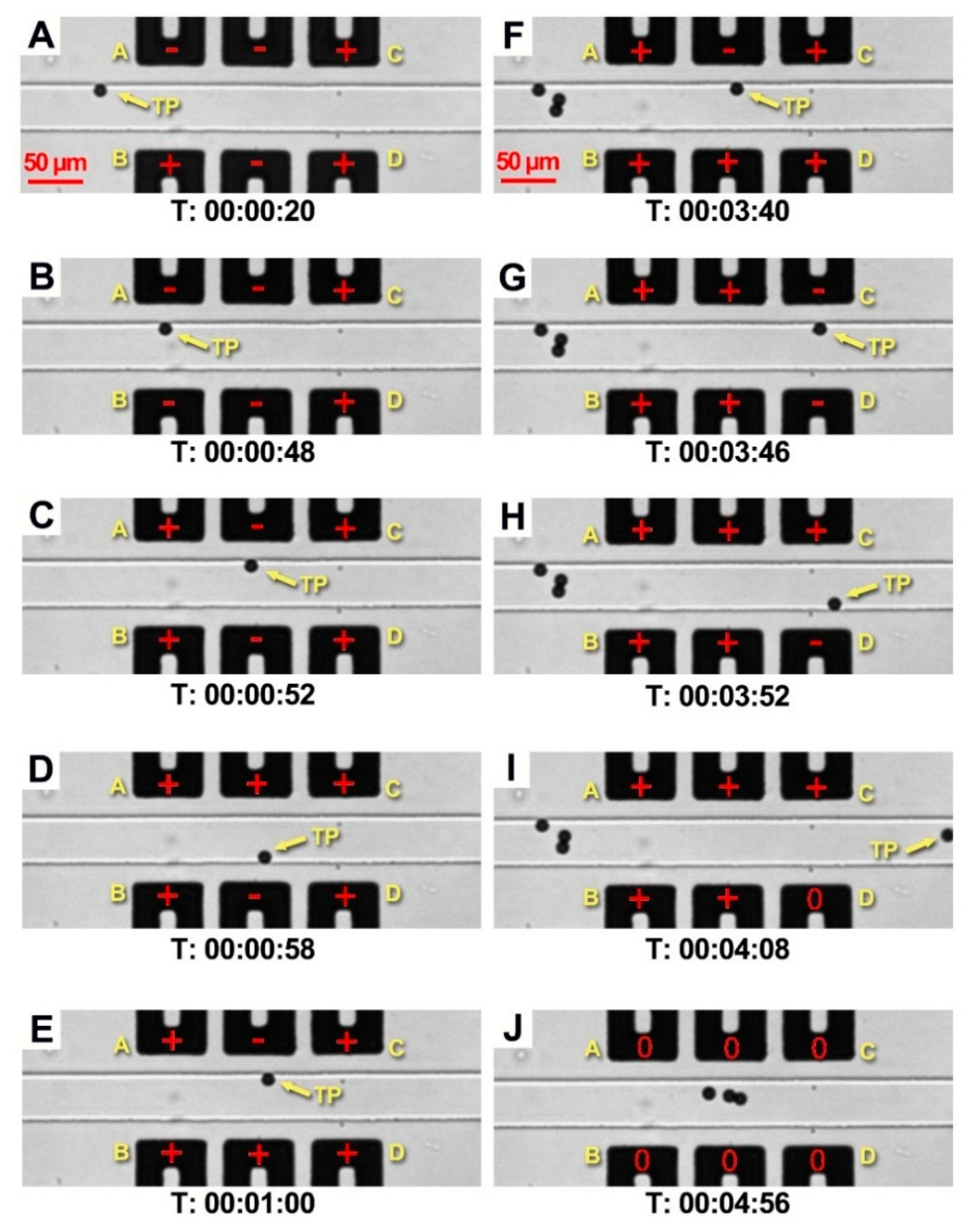
4.2. Particle Capture and Release
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Albrecht, T.; Macpherson, J.; Magnussen, O.; Fermin, D.; Crooks, R.; Gooding, J.; Hersbach, T.; Kanoufi, F.; Schuhmann, W.; Bentley, C.; et al. Electrochemistry of single nanoparticles: General discussion. Faraday Discuss. 2016, 193, 387–413. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.T.; Rotem, A.; Heyman, J.A.; Weitz, D.A. Droplet microfluidics for high-throughput biological assays. Lab Chip 2012, 12, 2146–2155. [Google Scholar] [CrossRef] [PubMed]
- Teh, S.Y.; Lin, R.; Hung, L.H.; Lee, A.P. Droplet microfluidics. Lab Chip 2008, 8, 198–220. [Google Scholar] [CrossRef] [PubMed]
- Sciambi, A.; Abate, A.R. Generating electric fields in PDMS microfluidic devices with salt water electrodes. Lab Chip 2014, 14, 2605–2609. [Google Scholar] [CrossRef] [PubMed]
- Faraghat, S.A.; Hoettges, K.F.; Steinbach, M.K.; van der Veen, D.R.; Brackenbury, W.J.; Henslee, E.A.; Labeed, F.H.; Hughes, M.P. High-throughput, low-loss, low-cost, and label-free cell separation using electrophysiology-activated cell enrichment. Proc. Natl. Acad. Sci. USA 2017, 114, 4591–4596. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.V.; Salmon, G.L.; Ros, A. Continuous separation of DNA molecules by size using insulator-based dielectrophoresis. Anal. Chem. 2017, 89, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Rambach, R.W.; Linder, K.; Heymann, M.; Franke, T. Droplet trapping and fast acoustic release in a multi-height device with steady-state flow. Lab Chip 2017, 17, 3422–3430. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.Y.; Zhang, W.; Baratchi, S.; Nasabi, M.; Kalantarzadeh, K.; Khoshmanesh, K. Modifying Dielectrophoretic Response of Nonviable Yeast Cells by Ionic Surfactant Treatment. Anal. Chem. 2013, 85, 6364–6371. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.Y.; Yi, P.; Soffe, R.; Nahavandi, S.; Shukla, R.; Khoshmanesh, K. Using dielectrophoresis to study the dynamic response of single budding yeast cells to Lyticase. Anal. Bioanal. Chem. 2015, 407, 3437–3448. [Google Scholar] [CrossRef] [PubMed]
- Voldman, J.; Braff, R.A.; Toner, M.; Gray, M.L.; Schmidt, M.A. Holding forces of single-particle dielectrophoretic traps. Biophys. J. 2001, 80, 531–542. [Google Scholar] [CrossRef]
- Zhang, C.; Khoshmanesh, K.; Mitchell, A.; Kalantar-Zadeh, K. Dielectrophoresis for manipulation of micro/nano particles in microfluidic systems. Anal. Bioanal. Chem. 2010, 396, 401–420. [Google Scholar] [CrossRef] [PubMed]
- Cole, R.H.; Tang, S.Y.; Siltanen, C.A.; Shahi, P.; Zhang, J.Q.; Poust, S.; Gartner, Z.J.; Abate, A.R. Printed droplet microfluidics for on demand dispensing of picoliter droplets and cells. Proc. Natl. Acad. Sci. USA 2017, 114, 8728–8733. [Google Scholar] [CrossRef] [PubMed]
- Collins, D.J.; Alan, T.; Neild, A. The particle valve: On-demand particle trapping, filtering, and release from a microfabricated polydimethylsiloxane membrane using surface acoustic waves. Appl. Phys. Lett. 2014, 105. [Google Scholar] [CrossRef]
- Ahmed, H.; Destgeer, G.; Park, J.; Jin, H.J.; Ahmad, R.; Park, K.; Sung, H.J. A pumpless acoustofluidic platform for size-selective concentration and separation of microparticles. Anal. Chem. 2017, 89, 13575–13581. [Google Scholar] [CrossRef] [PubMed]
- Sesen, M.; Alan, T.; Neild, A. Microfluidic on-demand droplet merging using surface acoustic waves. Lab Chip 2014, 14, 3325–3333. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.J.; Destgeer, G.; Park, J.; Ahmed, H.; Park, K.; Sung, H.J. On-demand droplet capture and release using microwell-assisted surface acoustic waves. Anal. Chem. 2017, 89, 2211–2215. [Google Scholar]
- Jin, H.J.; Lee, K.H.; Destgeer, G.; Kang, S.L.; Cho, H.; Ha, B.H.; Sung, H.J. In situ seriate droplet coalescence under an optical force. Microfluid. Nanofluid. 2015, 18, 1247–1254. [Google Scholar]
- Ashkin, A. Acceleration and trapping of particles by radiation pressure. Phys. Rev. Lett 1970, 24, 156–159. [Google Scholar] [CrossRef]
- Mirsaidov, U.; Scrimgeour, J.; Timp, W.; Beck, K.; Mir, M.; Matsudaira, P.; Timp, G. Live cell lithography: Using optical tweezers to create synthetic tissue. Lab Chip 2008, 8, 2174–2181. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.C.; Ohta, A.T.; Wu, M.C. Massively parallel manipulation of single cells and microparticles using optical images. Nature 2005, 436, 370–372. [Google Scholar]
- Dienerowitz, M.; Heitkamp, T.; Gottschall, T.; Limpert, J.; Borsch, M. Confining Brownian motion of single nanoparticles in an ABELtrap. Proc. SPIE 2017, 10120, 1012017. [Google Scholar] [CrossRef]
- Fields, A.P.; Cohen, A.E. Electrokinetic trapping at the one nanometer limit. Proc. Natl. Acad. Sci. USA 2011, 108, 8937–8942. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.A.; Lew, M.D.; Moerner, W.E. Extending microscopic resolution with single-molecule imaging and active control. Annu. Rev. Biophys. 2012, 41, 321–342. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Stott, M.A.; Hawkins, A.R.; Schmidt, H. Design and characterization of integrated 2D ABEL trap. In Proceedings of the IEEE Photonics Conference, Waikoloa, HI, USA, 2–6 October 2016; pp. 374–375. [Google Scholar]
- Han, J.H.; Kim, H.J.; Sudheendra, L.; Hass, E.A.; Gee, S.J.; Hammock, B.D.; Kennedy, I.M. Electrophoretic build-up of multi nanoparticle array for a highly sensitive immunoassay. Biosens. Bioelectron. 2013, 41, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Ramónazcón, J.; Yasukawa, T.; Mizutani, F. Sensitive and spatially multiplexed detection system based on dielectrophoretic manipulation of DNA-encoded particles used as immunoreactions platform. Anal. Chem. 2011, 83, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Rosales, C.; Lim, K.M. Numerical comparison between Maxwell stress method and equivalent multipole approach for calculation of the dielectrophoretic force in single-cell traps. Electrophoresis 2005, 26, 2057–2065. [Google Scholar] [CrossRef] [PubMed]
- Hart, M.B.; Sivaprakasam, V.; Czege, J.; Eversole, J.D. Using a Linear Electrodynamic Quadrupole as a Particle Trap. In Proceedings of the Optical Trapping Applications, San Diego, CA, USA, 2–5 April 2017. [Google Scholar]
- Gao, M.; Gui, L. A handy liquid metal based electroosmotic flow pump. Lab Chip 2014, 14, 1866–1872. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Gui, L. Development of a fast thermal response microfluidic system using liquid metal. J. Micromech. Microeng. 2016, 26, 075005. [Google Scholar] [CrossRef]
- Hallfors, N.; Khan, A.; Dickey, M.D.; Taylor, A.M. Integration of pre-aligned liquid metal electrodes for neural stimulation within a user-friendly microfluidic platform. Lab Chip 2013, 13, 522–526. [Google Scholar] [CrossRef] [PubMed]
- So, J.H.; Dickey, M.D. Inherently aligned microfluidic electrodes composed of liquid metal. Lab Chip 2011, 11, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.Y.; Zhu, J.; Sivan, V.; Gol, B.; Soffe, R.; Zhang, W.; Mitchell, A.; Khoshmanesh, K. Creation of liquid metal 3D microstructures using dielectrophoresis. Adv. Funct. Mater. 2015, 25, 4445–4452. [Google Scholar] [CrossRef]
- Huckel, E. Die kataphorese der kugel. Physikalische Zeitschrift 1924, 25, 204–210. [Google Scholar]
- Kuo, A.C. Poly (dimethylsiloxane); Oxford University Press: Oxford, UK, 1999; pp. 411–435. [Google Scholar]
- Product Data Sheets. Available online: https://www.xiameter.com/en/Products/Pages/ProductDetail.aspx pid=01013084&R=X68EN&C=US#characteristicsAnchor (accessed on 23 January 2017).
- Erhard, P.; Etling, D.; Muller, U.; Riedel, U.; Sreenivasan, K.R.; Warnatz, J. Prandtl-Essentials of Fluid Mechanics; Springer Science & Business Media: New York, NY, USA, 2010; Volume 158. [Google Scholar]
- Yang, X.-H.; Tan, S.; Ding, Y.; Liu, J. Flow and thermal modeling and optimization of micro/mini-channel heat sink. Appl. Therm. Eng. 2017, 117, 289–296. [Google Scholar] [CrossRef]
- Harms, T.M.; Kazmierczak, M.J.; Gerner, F.M. Developing convective heat transfer in deep rectangular microchannels. Int. J. Heat Fluid Flow 1999, 20, 149–157. [Google Scholar] [CrossRef]
- Tian, L.; Gao, M.; Gui, L. A Microfluidic chip for liquid metal droplet generation and sorting. Micromachines 2017, 8, 39. [Google Scholar] [CrossRef]
- Murali, S.; Jagtiani, A.V.; Xia, X.; Carletta, J.; Jiang, Z. A microfluidic Coulter counting device for metal wear detection in lubrication oil. Rev. Sci. Instrum. 2009, 80, 016105. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, L.; Zhang, L.; Gao, M.; Deng, Z.; Gui, L. A Handy Liquid Metal Based Non-Invasive Electrophoretic Particle Microtrap. Micromachines 2018, 9, 221. https://doi.org/10.3390/mi9050221
Tian L, Zhang L, Gao M, Deng Z, Gui L. A Handy Liquid Metal Based Non-Invasive Electrophoretic Particle Microtrap. Micromachines. 2018; 9(5):221. https://doi.org/10.3390/mi9050221
Chicago/Turabian StyleTian, Lu, Lunjia Zhang, Meng Gao, Zhongshan Deng, and Lin Gui. 2018. "A Handy Liquid Metal Based Non-Invasive Electrophoretic Particle Microtrap" Micromachines 9, no. 5: 221. https://doi.org/10.3390/mi9050221
APA StyleTian, L., Zhang, L., Gao, M., Deng, Z., & Gui, L. (2018). A Handy Liquid Metal Based Non-Invasive Electrophoretic Particle Microtrap. Micromachines, 9(5), 221. https://doi.org/10.3390/mi9050221