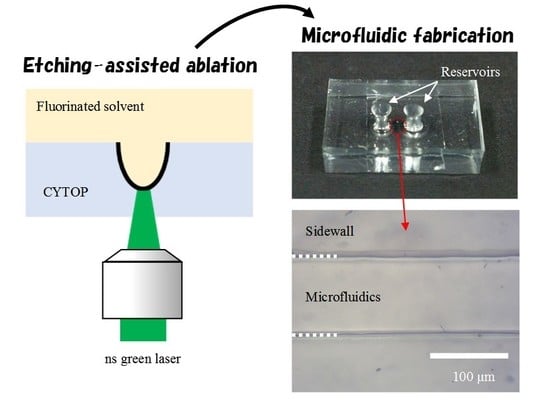
Etching-Assisted Ablation of the UV-Transparent Fluoropolymer CYTOP Using Various Laser Pulse Widths and Subsequent Microfluidic Applications
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
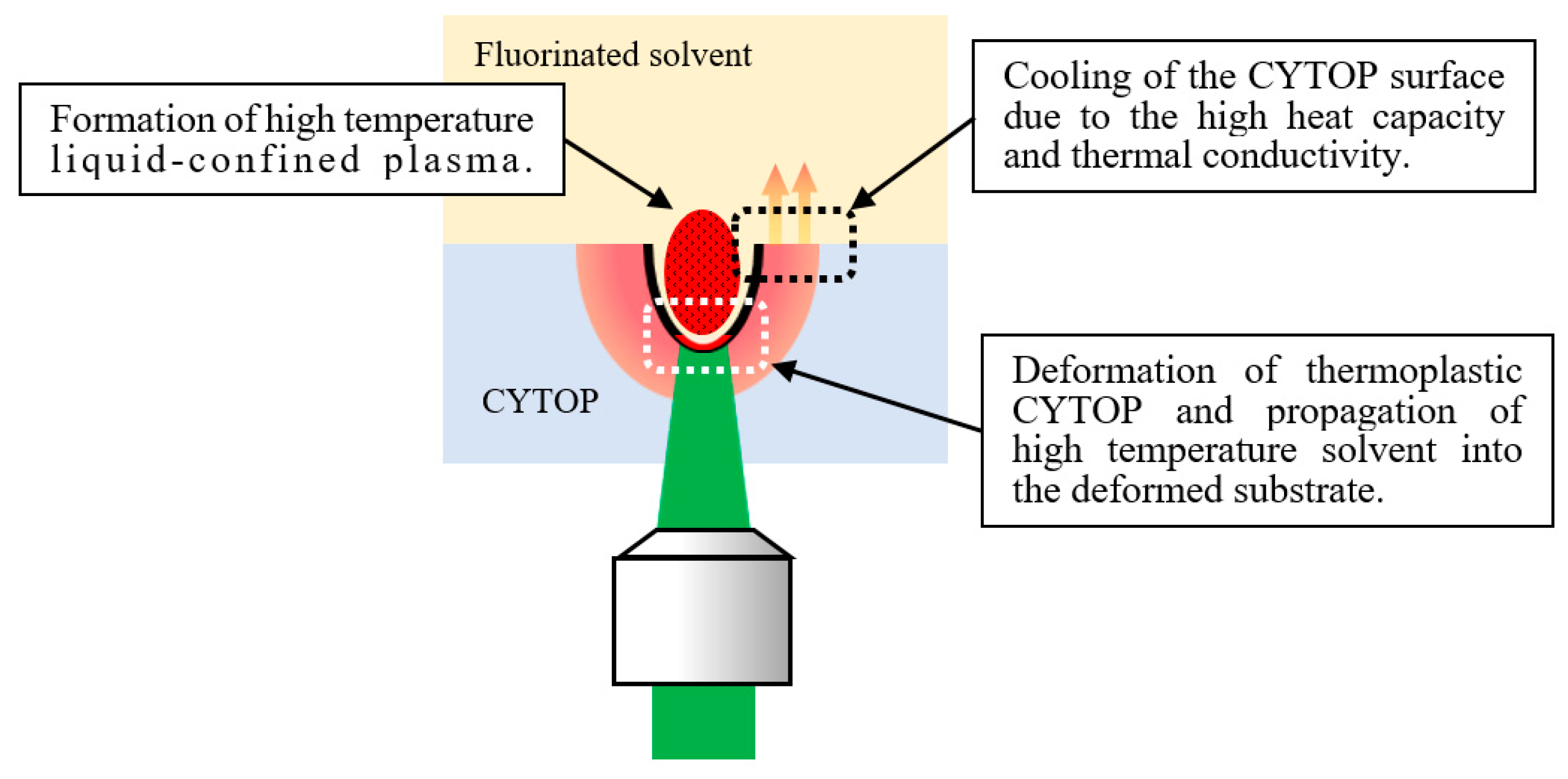
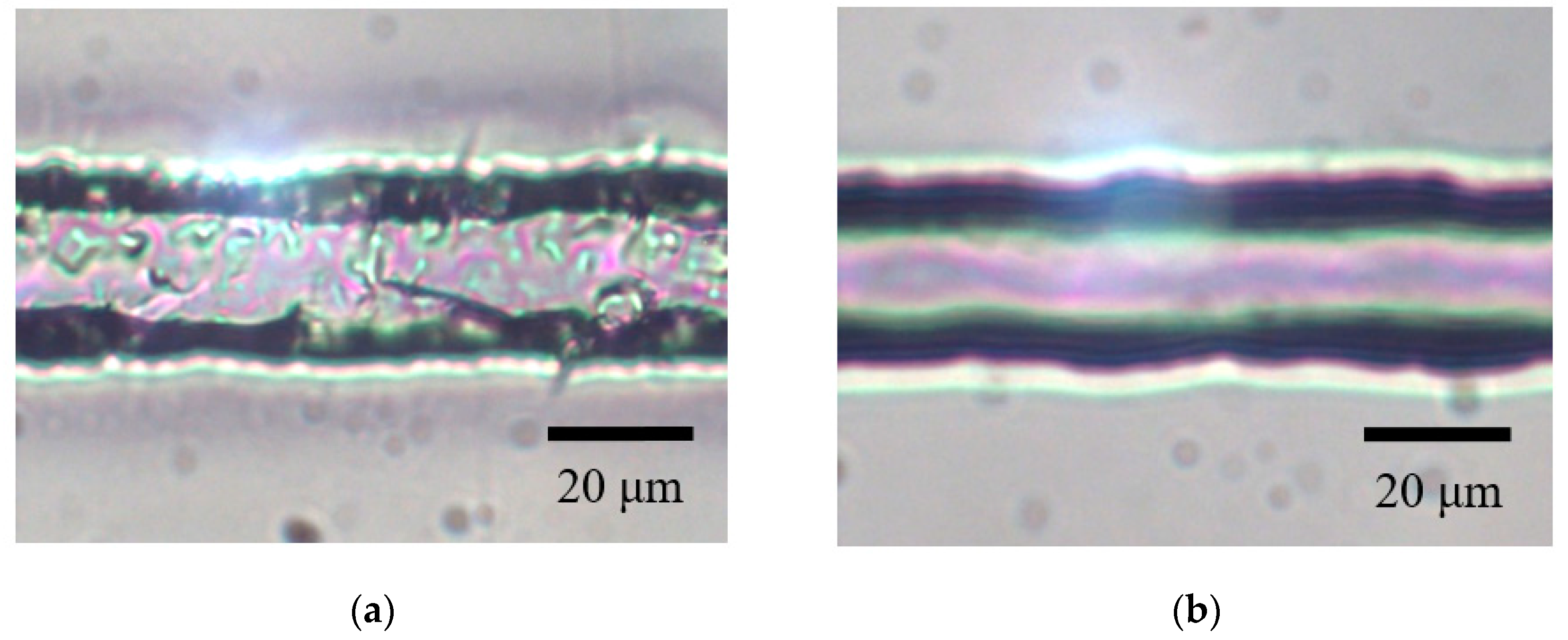
3.1. Etching-Assisted Ablation vs. Ablation Followed by Etching
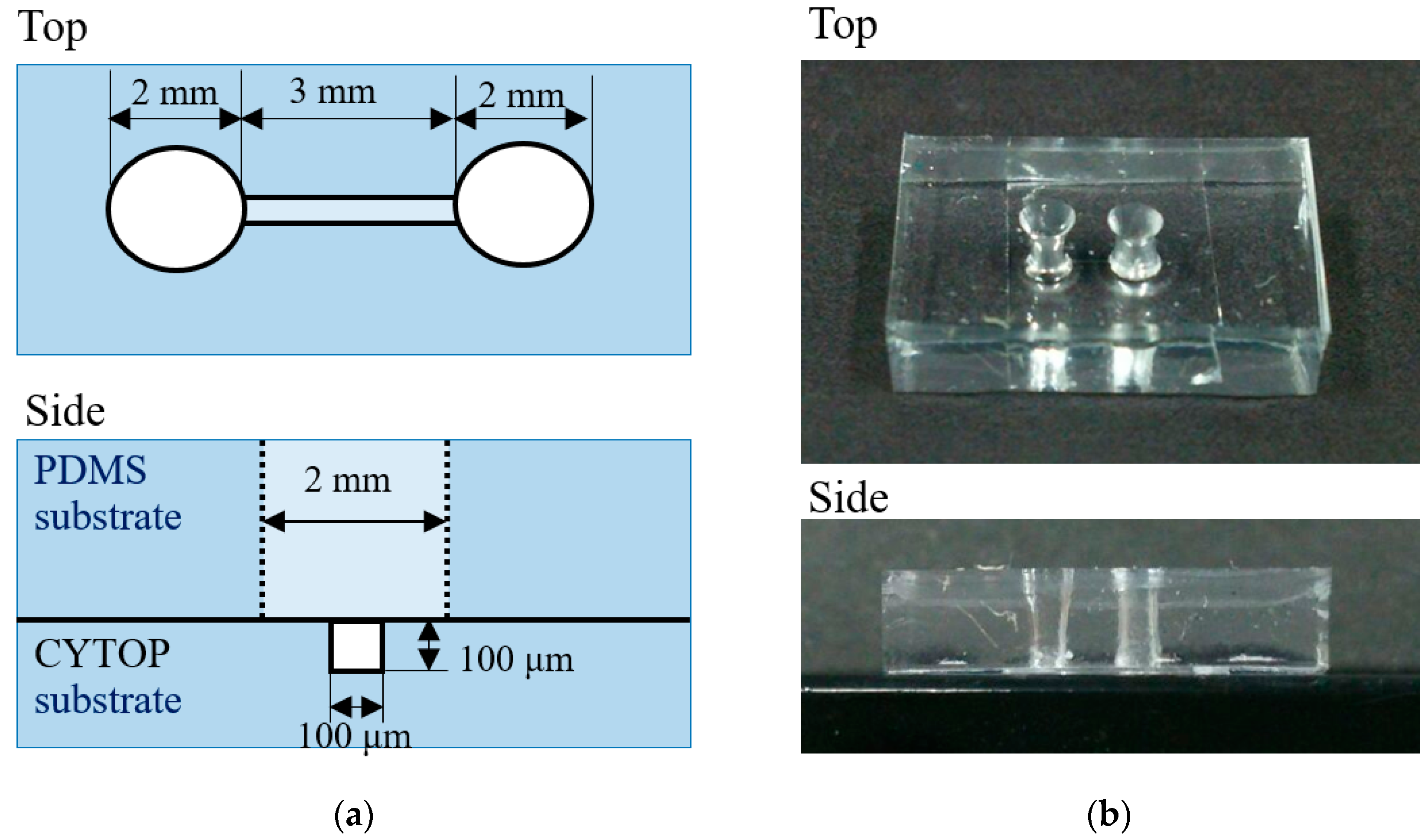
3.2. Microfluidic Fabrication Using Etching-Assisted ns Laser Ablation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xia, Z.; Wedel, A.; Danz, R. Charge storage and its dynamics in porous polytetrafluoroethylene (PTFE) film electrets. IEEE Trans. Dielectr. Electr. Insul. 2003, 10, 102–108. [Google Scholar]
- Lopez, J.R.; Aviles, M.A.A.; Bard, A.J. Selective insulation with poly(tetrafluoroethylene) of substrate electrodes for electrochemical background reduction in scanning electrochemical microscopy. Anal. Chem. 2008, 80, 1813–1818. [Google Scholar] [CrossRef] [PubMed]
- Su, F.H.; Zhang, Z.Z.; Liu, W.M. Study on the friction and wear properties of glass fabric composites filled with nano- and micro-particles under different conditions. Mater. Sci. Eng. A 2005, 392, 359–365. [Google Scholar] [CrossRef]
- Turrentine, M.W.; McCarthy, R.P.; Vijay, P.; McConnell, K.W.; Brown, J.W. PTFE monocusp valve reconstruction of the right ventricular outflow tract. Ann. Thorac. Surg. 2002, 73, 871–880. [Google Scholar] [CrossRef]
- Catanese, J.; Cooke, D.; Maas, C.; Pruitt, L. Mechanical properties of medical grade expanded polytetrafluoroethylene: The effects of internodal distance, density, and displacement rate. J. Biomed. Mater. Res. Part A 1999, 48, 187–192. [Google Scholar] [CrossRef]
- Liang, F.; Lehr, J.; Danielczak, L.; Leask, R.; Kietzig, A.M. Robust non-wetting PTFE surfaces by femtosecond laser machining. Int. J. Mol. Sci. 2014, 15, 13681–13696. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, V.F.; Correia, D.M.; Ribeiro, C.; Fernandes, M.M.; Méndez, S.L. Fluorinated polymers as smart materials for advanced biomedical applications. Polymers 2018, 10, 161. [Google Scholar] [CrossRef]
- Teng, H. Overview of the development of the fluoropolymer industry. Appl. Sci. 2012, 2, 496–512. [Google Scholar] [CrossRef]
- CYTOP®-Functions and Features. Available online: https://www.agc-chemicals.com/jp/en/fluorine/products/detail/feature/index.html?pCode=JP-EN-F019 (accessed on 28 November 2018).
- Hanada, Y.; Ogawa, T.; Koike, K.; Sugioka, K. Making the invisible visible: A microfluidic chip using a low refractive index polymer. Lab Chip 2016, 16, 2481–2486. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Mayne-Branton, V. Self-developing photoetching of poly(ethylene terephtalate) films by far-ultraviolet excimer laser radiation. Appl. Phys. Lett. 1982, 41, 567–578. [Google Scholar] [CrossRef]
- Pfleging, W.; Bernauer, W.; Hanemann, T.; Torge, M. Rapid fabrication of microcomponents—UV-laser assisted prototyping, laser micro-machining of mold inserts and replication via photomolding. Microsyst. Technol. 2002, 9, 67–74. [Google Scholar] [CrossRef]
- Pan, C.T.; Yang, H.; Wei, M.K. 248 nm excimer laser drilling PI film for nozzle plate application. Int. J. Adv. Manuf. Technol. 2007, 34, 889–897. [Google Scholar] [CrossRef]
- Li, H.; Fan, Y.; Kodzius, R.; Foulds, I.G. Fabrication of polystyrene microfluidic devices using a pulsed CO2 laser system. Microsyst. Technol. 2012, 18, 373–379. [Google Scholar] [CrossRef]
- Yan, Z.; Huang, X.; Yang, C. Rapid prototyping of single-layer microfluidic PDMS devices with abrupt depth variations under non-clean-room conditions by using laser ablation and UV-curable polymer. Microfluid Nanofluid 2017, 21, 108–117. [Google Scholar] [CrossRef]
- Kusaka, Y.; Hirata, A.; Ushijima, H. Direct adhesion contrast patterning on PDMS substrate by ArF excimer laser scanning for on-demand printing of functional layers. Int. Adv. Manuf. Technol. 2018, 99, 859–865. [Google Scholar] [CrossRef]
- Urech, L.; Lippert, T. Photochemistry and Photophysics of Polymer Materials; Wiley: New York, NY, USA, 2010; pp. 541–568. [Google Scholar]
- Juha, L.; Bittner, M.; Chvostova, D.; Krasa, J.; Otcenasek, Z.; Präg, A.R.; Ullschmied, J. Ablation of organic polymers by 46.9-nm-laser radiation. Appl. Phys. Lett. 2005, 86, 034109. [Google Scholar] [CrossRef]
- Obata, K.; Sugioka, K.; Midorikawa, K. F2 laser ablation of UV transparent polymer material. JLMN 2006, 1, 28–32. [Google Scholar] [CrossRef]
- Ogawa, T.; Hanada, Y. Microfabrication of the UV transparent polymer CYTOP using a conventional pulsed green laser. Appl. Phys. A 2016, 122, 156–162. [Google Scholar] [CrossRef]

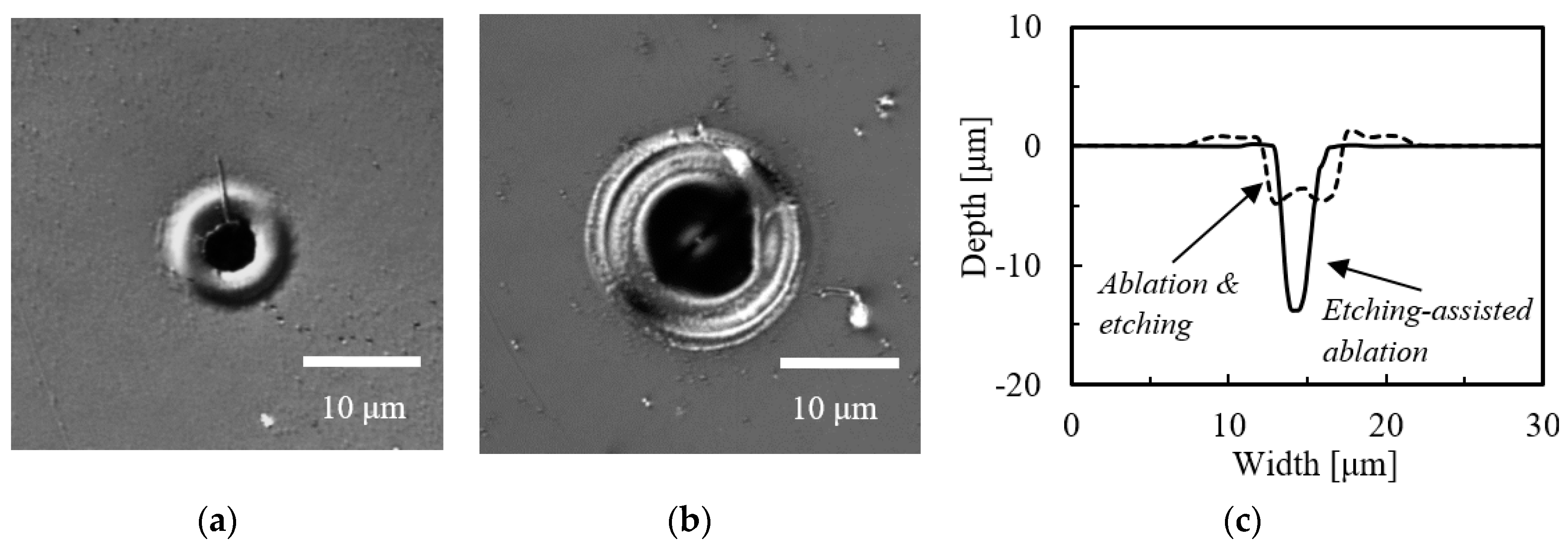
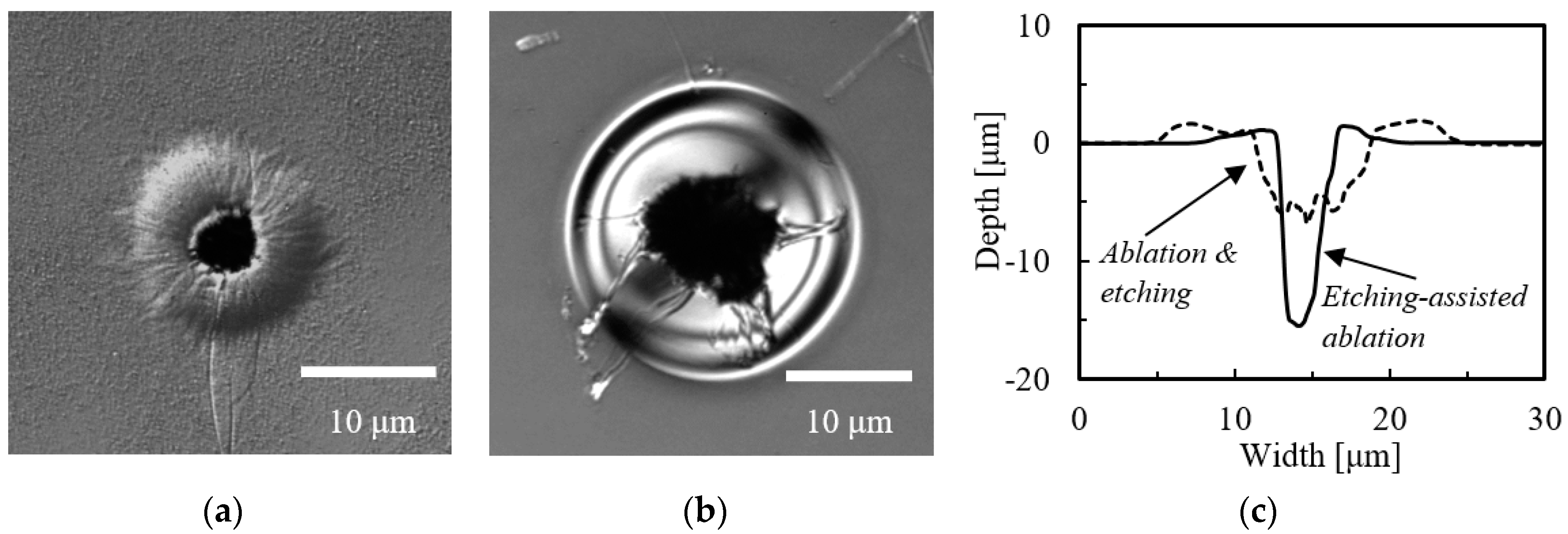
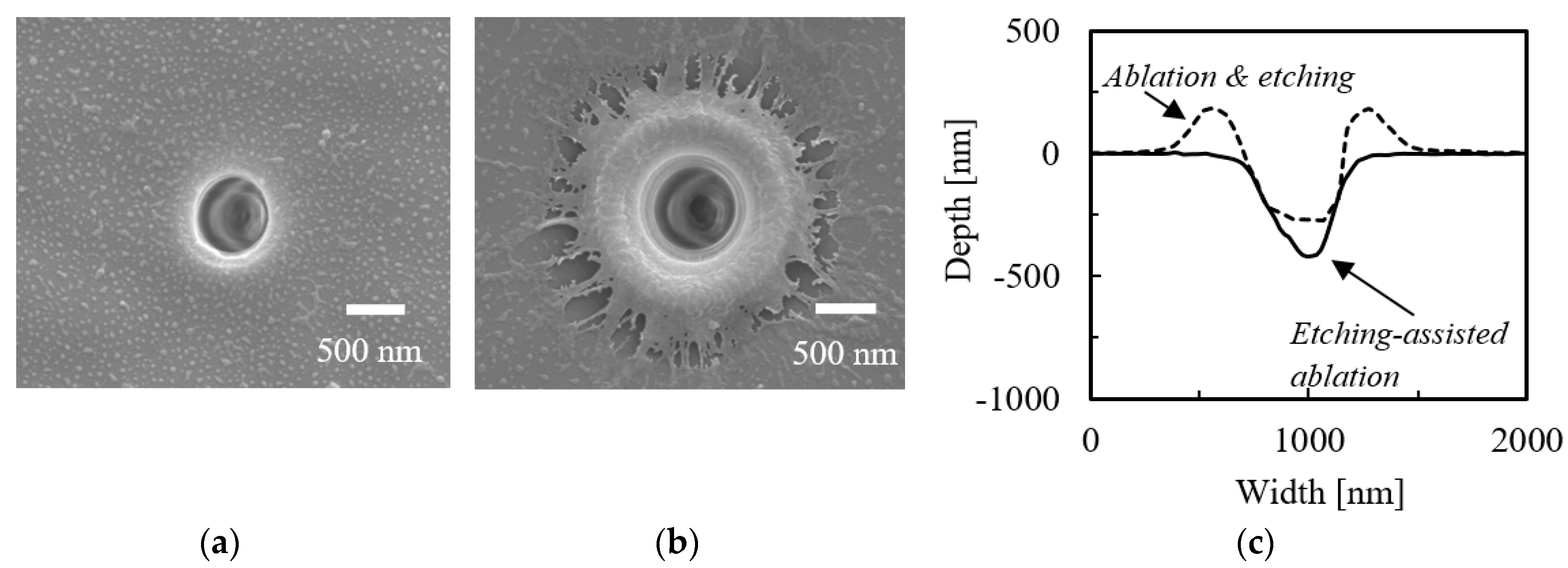

| Laser & Method | Diameter [μm] | Depth [μm] | Aspect Ratio | |
|---|---|---|---|---|
| ns laser | Ablation & etching | 7.5 ± 0.03 | 5.4 ± 0.02 | 0.72 |
| Etching assisted ablation | 4.4 ± 0.05 | 15.5 ± 0.02 | 3.52 | |
| ps laser | Ablation & etching | 5.7 ± 0.05 | 5.2 ± 0.01 | 0.91 |
| Etching assisted ablation | 4.1 ± 0.02 | 13.7 ± 0.05 | 3.34 | |
| fs laser | Ablation & etching | 0.70 ± 0.02 | 0.27 ± 0.03 | 0.39 |
| Etching assisted ablation | 0.71 ± 0.03 | 0.42 ± 0.01 | 0.59 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemoto, K.; Hanada, Y. Etching-Assisted Ablation of the UV-Transparent Fluoropolymer CYTOP Using Various Laser Pulse Widths and Subsequent Microfluidic Applications. Micromachines 2018, 9, 662. https://doi.org/10.3390/mi9120662
Nemoto K, Hanada Y. Etching-Assisted Ablation of the UV-Transparent Fluoropolymer CYTOP Using Various Laser Pulse Widths and Subsequent Microfluidic Applications. Micromachines. 2018; 9(12):662. https://doi.org/10.3390/mi9120662
Chicago/Turabian StyleNemoto, Keisuke, and Yasutaka Hanada. 2018. "Etching-Assisted Ablation of the UV-Transparent Fluoropolymer CYTOP Using Various Laser Pulse Widths and Subsequent Microfluidic Applications" Micromachines 9, no. 12: 662. https://doi.org/10.3390/mi9120662
APA StyleNemoto, K., & Hanada, Y. (2018). Etching-Assisted Ablation of the UV-Transparent Fluoropolymer CYTOP Using Various Laser Pulse Widths and Subsequent Microfluidic Applications. Micromachines, 9(12), 662. https://doi.org/10.3390/mi9120662