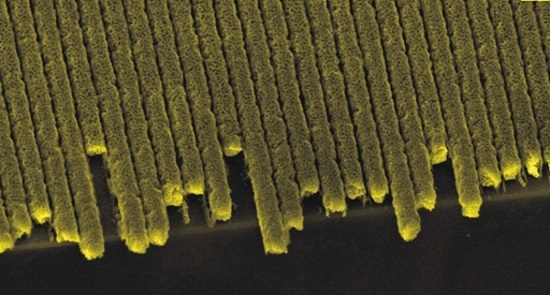
Large-Scale Fabrication of Porous Gold Nanowires via Laser Interference Lithography and Dealloying of Gold–Silver Nano-Alloys
Abstract
:1. Introduction
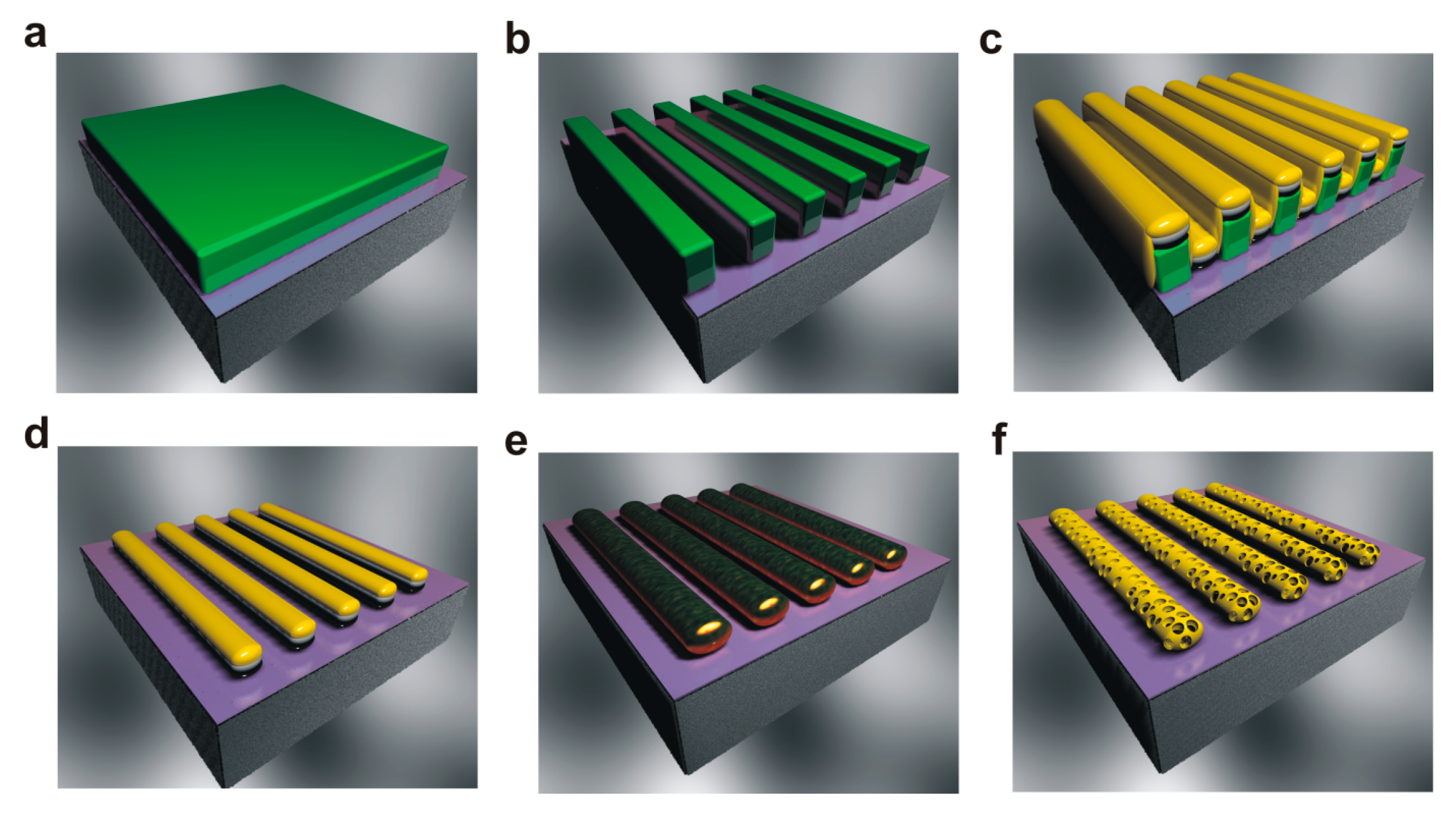
2. Fabrication Scheme
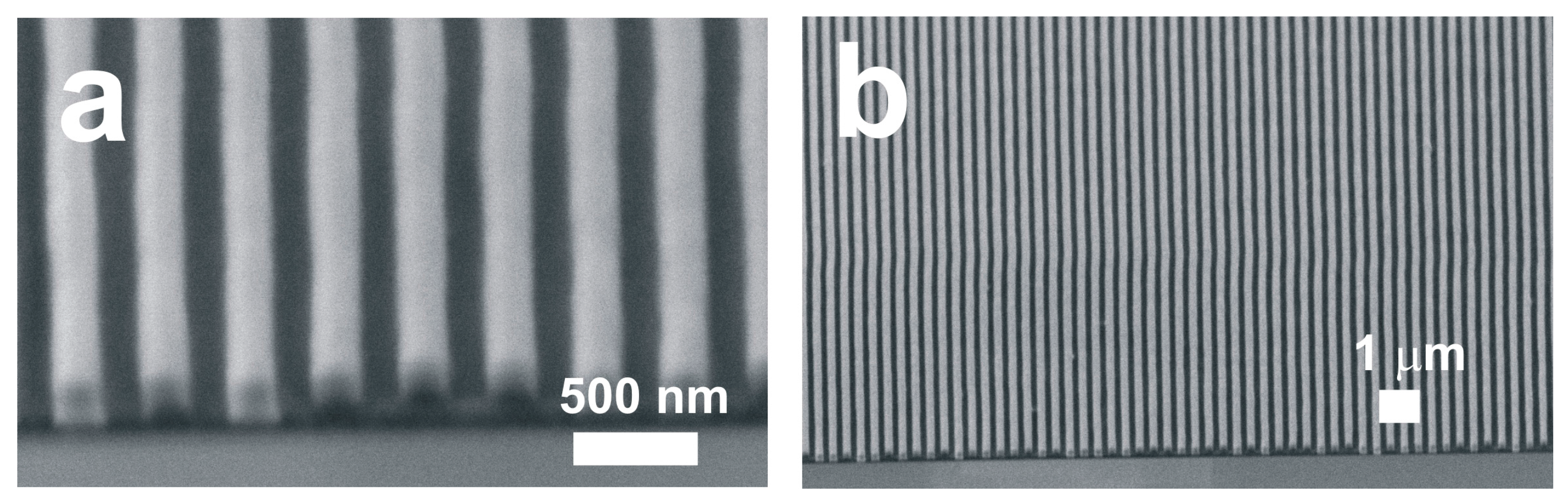
3. Materials and Methods
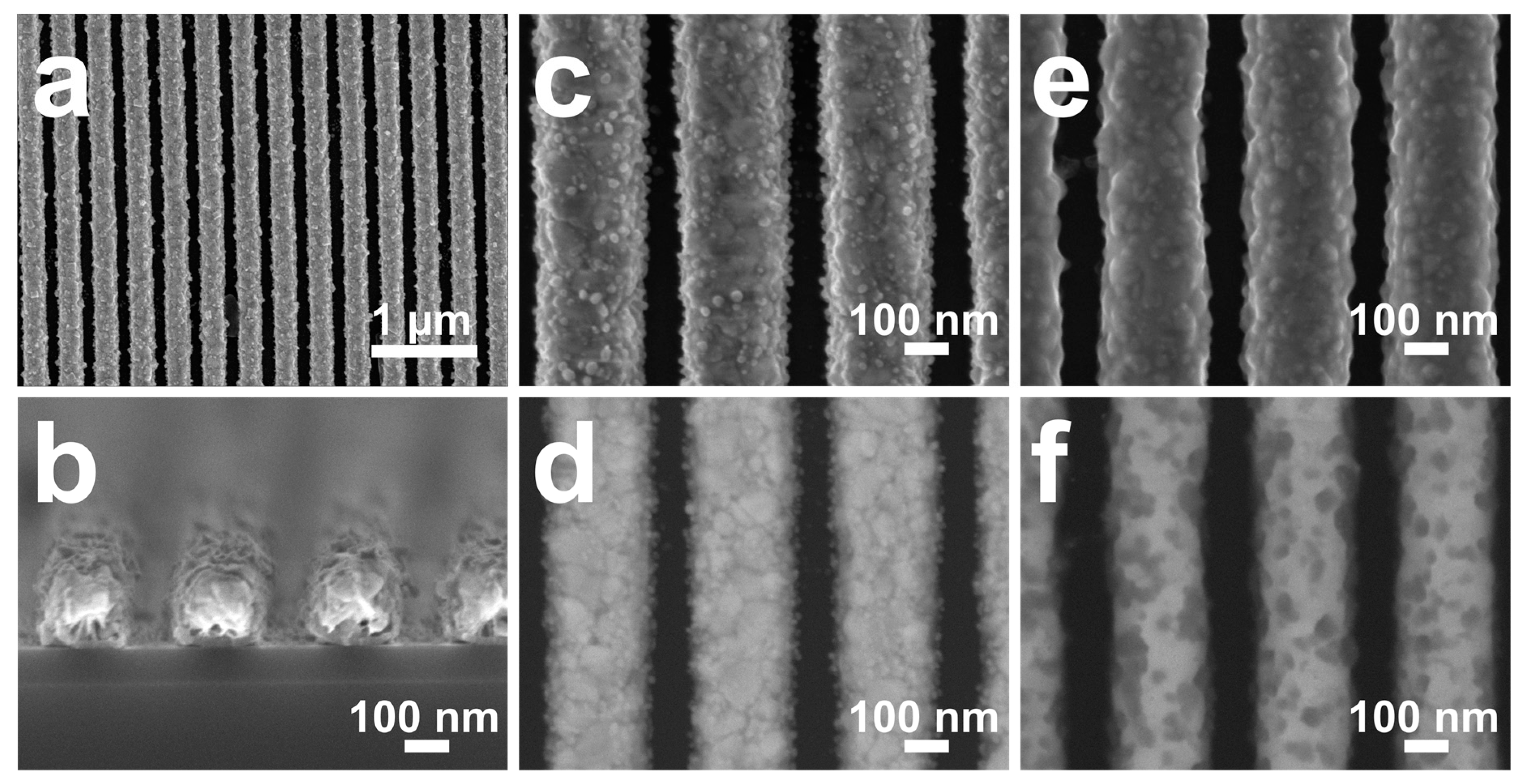
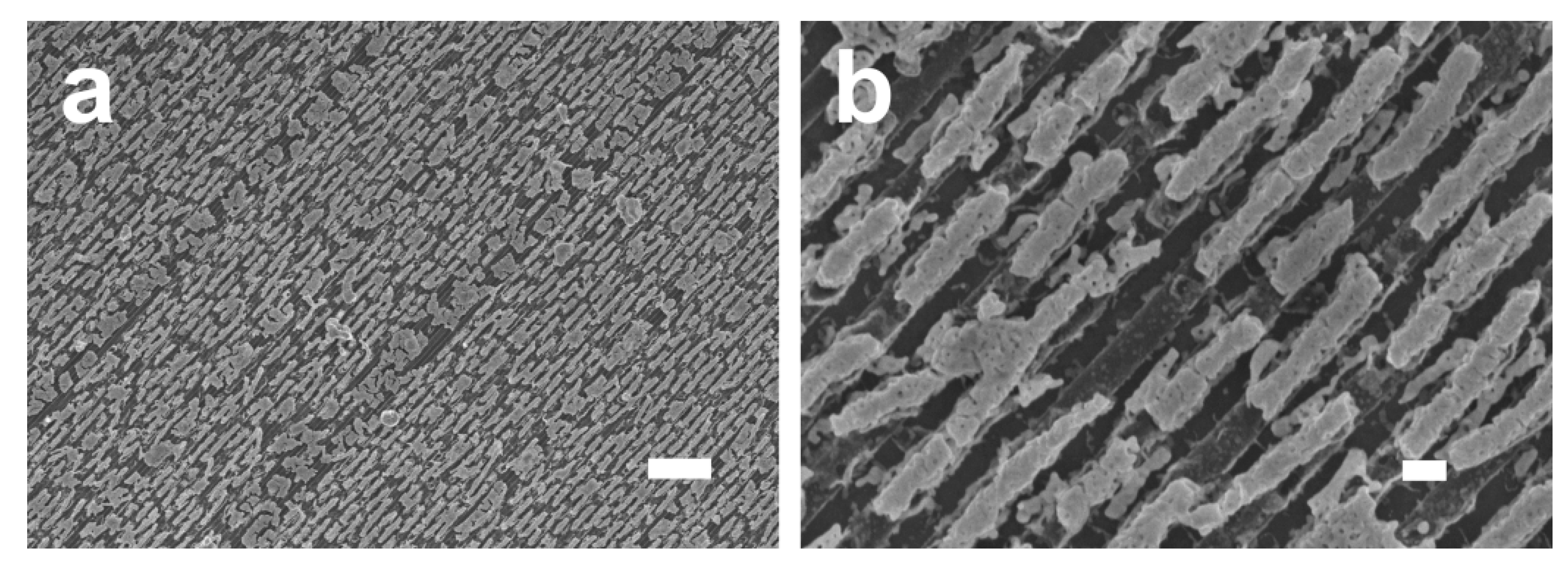
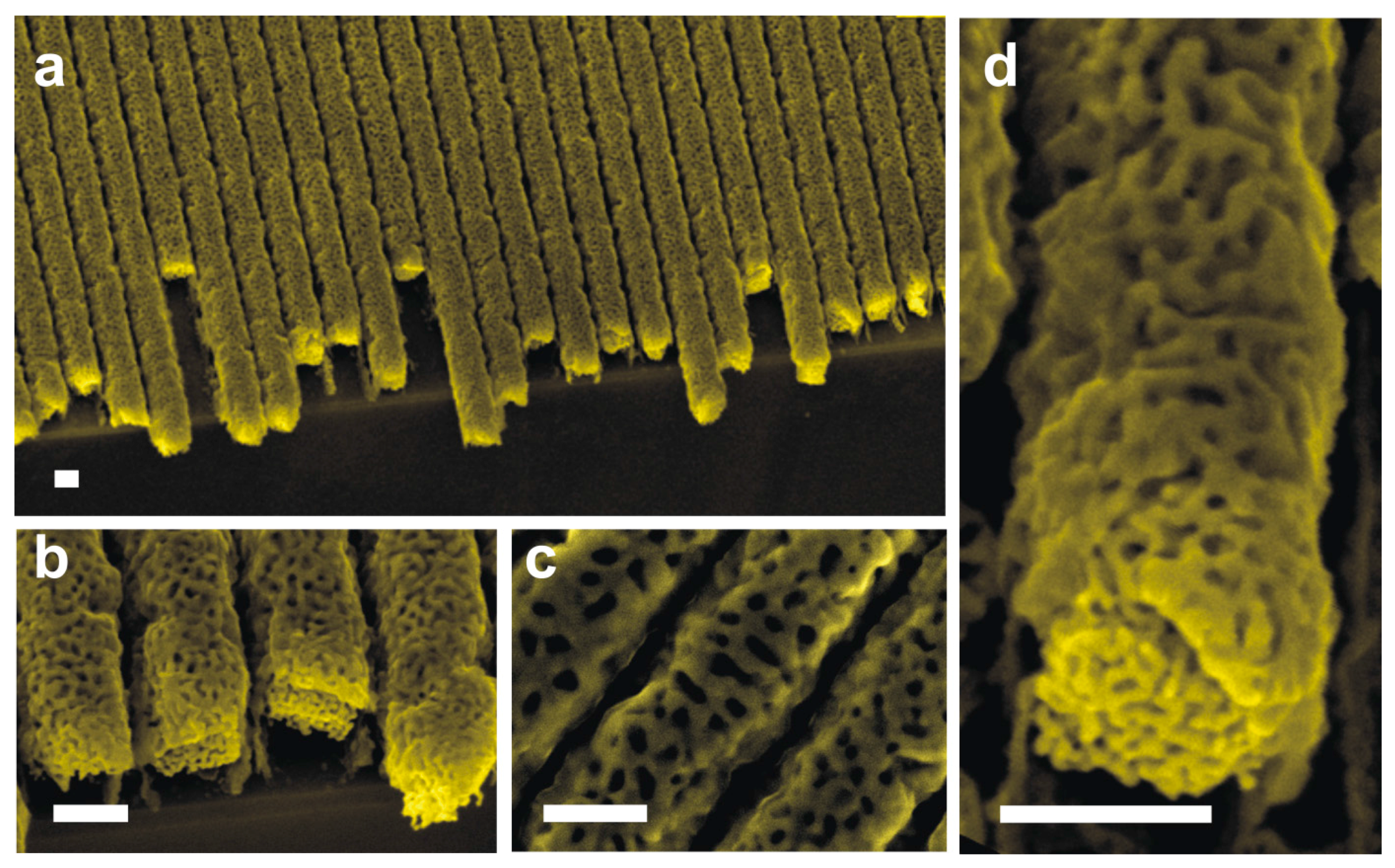
4. Results
5. Comparison to Template Technique
6. Conclusions
Author Contributions
Conflicts of Interest
References
- Xu, C.; Wang, R.; Chen, M.; Zhang, Y.; Ding, Y. Dealloying to nanoporous Au/Pt alloys and their structure sensitive electrocatalytic properties. Phys. Chem. Chem. Phys. 2010, 12, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Carmo, M.; Sekol, R.C.; Ding, S.; Kumar, G.; Schroers, J.; Taylor, A.D. Bulk Metallic Glass Nanowire Architecture for Electrochemical Applications. ACS Nano 2011, 5, 2979–2983. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.A.R.; Wang, L.; Biener, J.; Seker, E.; Biener, M.M.; Matthews, M.J. Engineering on-chip nanoporous gold material libraries via precision photothermal treatment. Nanoscale 2016, 8, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Xu, H.; Xiao, Y.; Liu, Y.; Yuan, A.; Shen, X. Facile Fabrication and Enhanced Sensing Properties of Hierarchically Porous CuO Architectures. ACS Appl. Mater. Interfaces 2012, 4, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Kramer, D.; Viswanath, R.N.; Weissmüller, J. Surface-Stress Induced Macroscopic Bending of Nanoporous Gold Cantilevers. Nano Lett. 2004, 4, 793–796. [Google Scholar] [CrossRef]
- Weissmüller, J.; Viswanath, R.N.; Kramer, D.; Zimmer, P.; Würschum, R.; Gleiter, H. Charge-induced reversible strain in a metal. Science 2003, 300, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Sattayasamitsathit, S.; Gu, Y.; Kaufmann, K.; Minteer, S.; Polsky, R.; Wang, J. Tunable hierarchical macro/mesoporous gold microwires fabricated by dual-templating and dealloying processes. Nanoscale 2013, 5, 7849–7854. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; He, J.; Zhou, W.L.; Wiley, J.B. Synthesis of porous wires from directed assemblies of nanospheres. J. Am. Chem. Soc. 2003, 125, 16166–16167. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xia, Y. Mechanistic Study on the Replacement Reaction between Silver Nanostructures and Chloroauric Acid in Aqueous Medium. J. Am. Chem. Soc. 2004, 126, 3892–3901. [Google Scholar] [CrossRef] [PubMed]
- El Mel, A.-A.; Chettab, M.; Gautron, E.; Chauvin, A.; Humbert, B.; Mevellec, J.-Y.; Delacote, C.; Thiry, D.; Stephant, N.; Ding, J.; et al. Galvanic Replacement Reaction: A Route to Highly Ordered Bimetallic Nanotubes. J. Phys. Chem. C 2016, 120, 17652–17659. [Google Scholar] [CrossRef]
- El Mel, A.-A.; Stephant, N.; Hamon, J.; Thiry, D.; Chauvin, A.; Chettab, M.; Gautron, E.; Konstantinidis, S.; Granier, A.; Tessier, P.-Y. Creating nanoporosity in silver nanocolumns by direct exposure to radio-frequency air plasma. Nanoscale 2016, 8, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Erlebacher, J.; Aziz, M.J.; Karma, A.; Dimitrov, N.; Sieradzki, K. Evolution of nanoporosity in dealloying. Nature 2001, 410, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Erlebacher, J. Nanoporous Metals with Controlled Multimodal Pore Size Distribution. J. Am. Chem. Soc. 2003, 125, 7772–7773. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, A.; Txia Cha Heu, W.; Tessier, P.-Y.; El Mel, A.-A. Impact of the morphology and composition on the dealloying process of co-sputtered silver-aluminum alloy thin films: Impact of morphology and composition on dealloying of Ag-Al films. Phys. Status Solidi B 2016, 253, 2167–2174. [Google Scholar] [CrossRef]
- Erlebacher, J. An Atomistic Description of Dealloying. J. Electrochem. Soc. 2004, 151, C614. [Google Scholar] [CrossRef]
- Biener, J.; Nyce, G.W.; Hodge, A.M.; Biener, M.M.; Hamza, A.V.; Maier, S.A. Nanoporous Plasmonic Metamaterials. Adv. Mater. 2008, 20, 1211–1217. [Google Scholar] [CrossRef]
- McCue, I.; Benn, E.; Gaskey, B.; Erlebacher, J. Dealloying and Dealloyed Materials. Annu. Rev. Mater. Res. 2016, 46, 263–286. [Google Scholar] [CrossRef]
- Erlebacher, J.; Sieradzki, K. Pattern formation during dealloying. Scr. Mater. 2003, 49, 991–996. [Google Scholar] [CrossRef]
- Pickering, H.W.; Wagner, C. Electrolytic dissolution of binary alloys containing a noble metal. J. Electrochem. Soc. 1967, 114, 698–706. [Google Scholar] [CrossRef]
- Qian, L.H.; Chen, M.W. Ultrafine nanoporous gold by low-temperature dealloying and kinetics of nanopore formation. Appl. Phys. Lett. 2007, 91, 083105. [Google Scholar] [CrossRef]
- Morrish, R.; Dorame, K.; Muscat, A.J. Formation of nanoporous Au by dealloying AuCu thin films in HNO3. Scr. Mater. 2011, 64, 856–859. [Google Scholar] [CrossRef]
- El Mel, A.-A.; Boukli-Hacene, F.; Molina-Luna, L.; Bouts, N.; Chauvin, A.; Thiry, D.; Gautron, E.; Gautier, N.; Tessier, P.-Y. Unusual Dealloying Effect in Gold/Copper Alloy Thin Films: The Role of Defects and Column Boundaries in the Formation of Nanoporous Gold. ACS Appl. Mater. Interfaces 2015, 7, 2310–2321. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Xu, L.; Tian, F.; Ding, Y. Au–Ag alloy nanoporous nanotubes. Nano Res. 2009, 2, 386–393. [Google Scholar] [CrossRef]
- Chen, B.; Meng, G.; Huang, Q.; Huang, Z.; Xu, Q.; Zhu, C.; Qian, Y.; Ding, Y. Green Synthesis of Large-Scale Highly Ordered Core@Shell Nanoporous Au@Ag Nanorod Arrays as Sensitive and Reproducible 3D SERS Substrates. ACS Appl. Mater. Interfaces 2014, 6, 15667–15675. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.-H.; Park, S. Platinum-Coated, Nanoporous Gold Nanorod Arrays: Synthesis and Characterization. Adv. Mater. 2007, 19, 1612–1615. [Google Scholar] [CrossRef]
- Liu, Z.; Searson, P.C. Single Nanoporous Gold Nanowire Sensors. J. Phys. Chem. B 2006, 110, 4318–4322. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Lee, W.; Huang, Z.; Scholz, R.; Gösele, U. Fabrication and characterization of a flow-through nanoporous gold nanowire/AAO composite membrane. Nanotechnology 2008, 19, 335604. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Searson, P.C. Fabrication of nanoporous gold nanowires. Appl. Phys. Lett. 2002, 81, 4437. [Google Scholar] [CrossRef]
- Chauvin, A.; Delacôte, C.; Molina-Luna, L.; Duerrschnabel, M.; Boujtita, M.; Thiry, D.; Du, K.; Ding, J.; Choi, C.-H.; Tessier, P.-Y.; et al. Planar Arrays of Nanoporous Gold Nanowires: When Electrochemical Dealloying Meets Nanopatterning. ACS Appl. Mater. Interfaces 2016, 8, 6611–6620. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, A.; Delacôte, C.; Boujtita, M.; Angleraud, B.; Ding, J.; Choi, C.-H.; Tessier, P.-Y.; El Mel, A.-A. Dealloying of gold–copper alloy nanowires: From hillocks to ring-shaped nanopores. Beilstein J. Nanotechnol. 2016, 7, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- El Mel, A.A.; Gautron, E.; Choi, C.H.; Angleraud, B.; Granier, A.; Tessier, P.Y. Titanium carbide/carbon composite nanofibers prepared by a plasma process. Nanotechnology 2010, 21, 435603. [Google Scholar] [CrossRef] [PubMed]
- Wathuthanthri, I.; Liu, Y.; Du, K.; Xu, W.; Choi, C.-H. Simple Holographic Patterning for High-Aspect-Ratio Three-Dimensional Nanostructures with Large Coverage Area. Adv. Funct. Mater. 2013, 23, 608–618. [Google Scholar] [CrossRef]
- Wathuthanthri, I.; Mao, W.; Choi, C.-H. Two degrees-of-freedom Lloyd–mirror interferometer for superior pattern coverage area. Opt. Lett. 2011, 36, 1593–1595. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Wathuthanthri, I.; Liu, Y.; Xu, W.; Choi, C.-H. Wafer-Scale Pattern Transfer of Metal Nanostructures on Polydimethylsiloxane (PDMS) Substrates via Holographic Nanopatterns. ACS Appl. Mater. Interfaces 2012, 4, 5505–5514. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Wathuthanthri, I.; Choi, C.-H. Tunable two-mirror interference lithography system for wafer-scale nanopatterning. Opt. Lett. 2011, 36, 3176–3178. [Google Scholar] [CrossRef] [PubMed]
- Okman, O.; Kysar, J.W. Fabrication of crack-free blanket nanoporous gold thin films by galvanostatic dealloying. J. Alloy. Compd. 2011, 509, 6374–6381. [Google Scholar] [CrossRef]
- Parida, S.; Kramer, D.; Volkert, C.A.; Rösner, H.; Erlebacher, J.; Weissmüller, J. Volume Change during the Formation of Nanoporous Gold by Dealloying. Phys. Rev. Lett. 2006, 97, 035504. [Google Scholar] [CrossRef] [PubMed]
- Dotzler, C.J.; Ingham, B.; Illy, B.N.; Wallwork, K.; Ryan, M.P.; Toney, M.F. In Situ Observation of Strain Development and Porosity Evolution in Nanoporous Gold Foils. Adv. Funct. Mater. 2011, 21, 3938–3946. [Google Scholar] [CrossRef]
- Zhang, L.; Lang, X.; Hirata, A.; Chen, M. Wrinkled Nanoporous Gold Films with Ultrahigh Surface-Enhanced Raman Scattering Enhancement. ACS Nano 2011, 5, 4407–4413. [Google Scholar] [CrossRef] [PubMed]
- Schubert, I.; Sigle, W.; Burr, L.; van Aken, P.A.; Trautmann, C.; Toimil-Molares, M.E. Fabrication and plasmonic characterization of Au nanowires with controlled surface morphology. Adv. Mater. Lett. 2015, 6, 377–382. [Google Scholar] [CrossRef]
- Chien, F.-C.; Lin, C.-Y.; Yih, J.-N.; Lee, K.-L.; Chang, C.-W.; Wei, P.-K.; Sun, C.-C.; Chen, S.-J. Coupled waveguide–surface plasmon resonance biosensor with subwavelength grating. Biosens. Bioelectron. 2007, 22, 2737–2742. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Choy, W.C.H.; Huo, L.; Xie, F.; Sha, W.E.I.; Ding, B.; Guo, X.; Li, Y.; Hou, J.; You, J.; et al. Dual Plasmonic Nanostructures for High Performance Inverted Organic Solar Cells. Adv. Mater. 2012, 24, 3046–3052. [Google Scholar] [CrossRef] [PubMed]
- Erlebacher, J.; Ding, Y. Method of Forming Nanoporous Membranes. U.S. Patent 6,805,972, 19 October 2004. [Google Scholar]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chauvin, A.; Stephant, N.; Du, K.; Ding, J.; Wathuthanthri, I.; Choi, C.-H.; Tessier, P.-Y.; El Mel, A.-A. Large-Scale Fabrication of Porous Gold Nanowires via Laser Interference Lithography and Dealloying of Gold–Silver Nano-Alloys. Micromachines 2017, 8, 168. https://doi.org/10.3390/mi8060168
Chauvin A, Stephant N, Du K, Ding J, Wathuthanthri I, Choi C-H, Tessier P-Y, El Mel A-A. Large-Scale Fabrication of Porous Gold Nanowires via Laser Interference Lithography and Dealloying of Gold–Silver Nano-Alloys. Micromachines. 2017; 8(6):168. https://doi.org/10.3390/mi8060168
Chicago/Turabian StyleChauvin, Adrien, Nicolas Stephant, Ke Du, Junjun Ding, Ishan Wathuthanthri, Chang-Hwan Choi, Pierre-Yves Tessier, and Abdel-Aziz El Mel. 2017. "Large-Scale Fabrication of Porous Gold Nanowires via Laser Interference Lithography and Dealloying of Gold–Silver Nano-Alloys" Micromachines 8, no. 6: 168. https://doi.org/10.3390/mi8060168
APA StyleChauvin, A., Stephant, N., Du, K., Ding, J., Wathuthanthri, I., Choi, C.-H., Tessier, P.-Y., & El Mel, A.-A. (2017). Large-Scale Fabrication of Porous Gold Nanowires via Laser Interference Lithography and Dealloying of Gold–Silver Nano-Alloys. Micromachines, 8(6), 168. https://doi.org/10.3390/mi8060168