Hydrothermally Synthesized PPy/VO2 Nanorod Composites for High-Performance Aqueous Zinc-Ion Battery Cathodes
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
2.2. Preparation of PPy Nanorods
2.3. Synthesis of PPy/VO2
2.4. Electrode Fabrication and Cell Construction
2.5. Characterization Techniques
3. Results and Discussion
3.1. Material Characterization
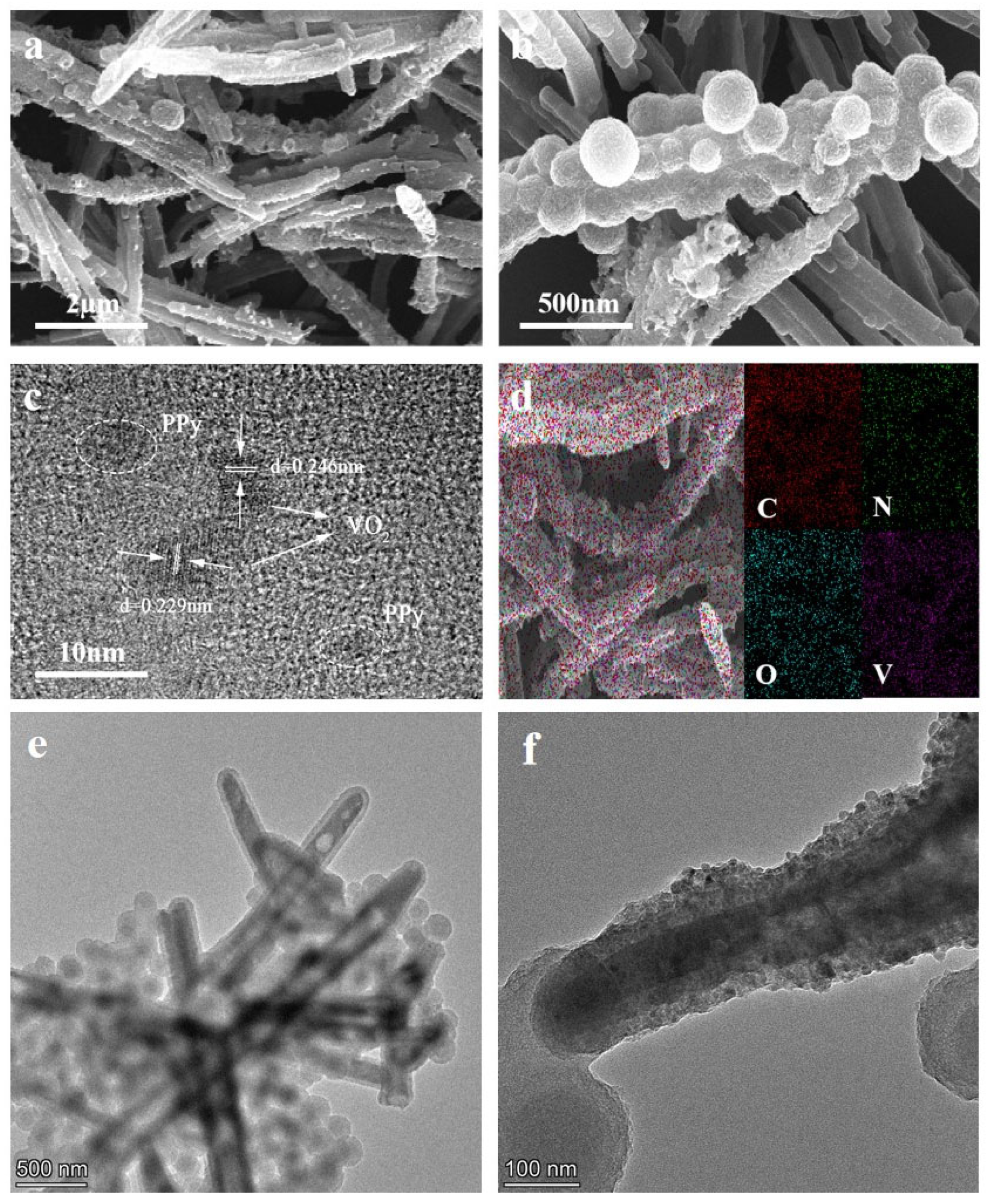
3.1.1. Scanning Electron Microscopy (SEM) and Energy Dispersive Spectroscopy (EDS) Analysis
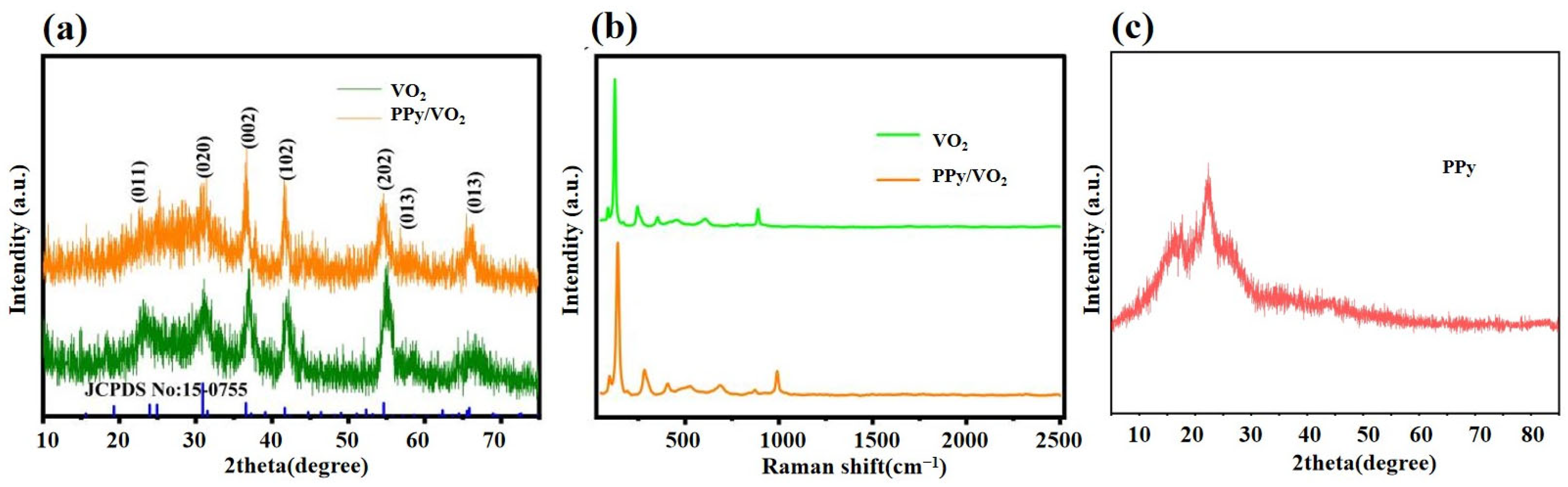
3.1.2. X-Ray Diffraction (XRD) and Raman Spectroscopy Analysis
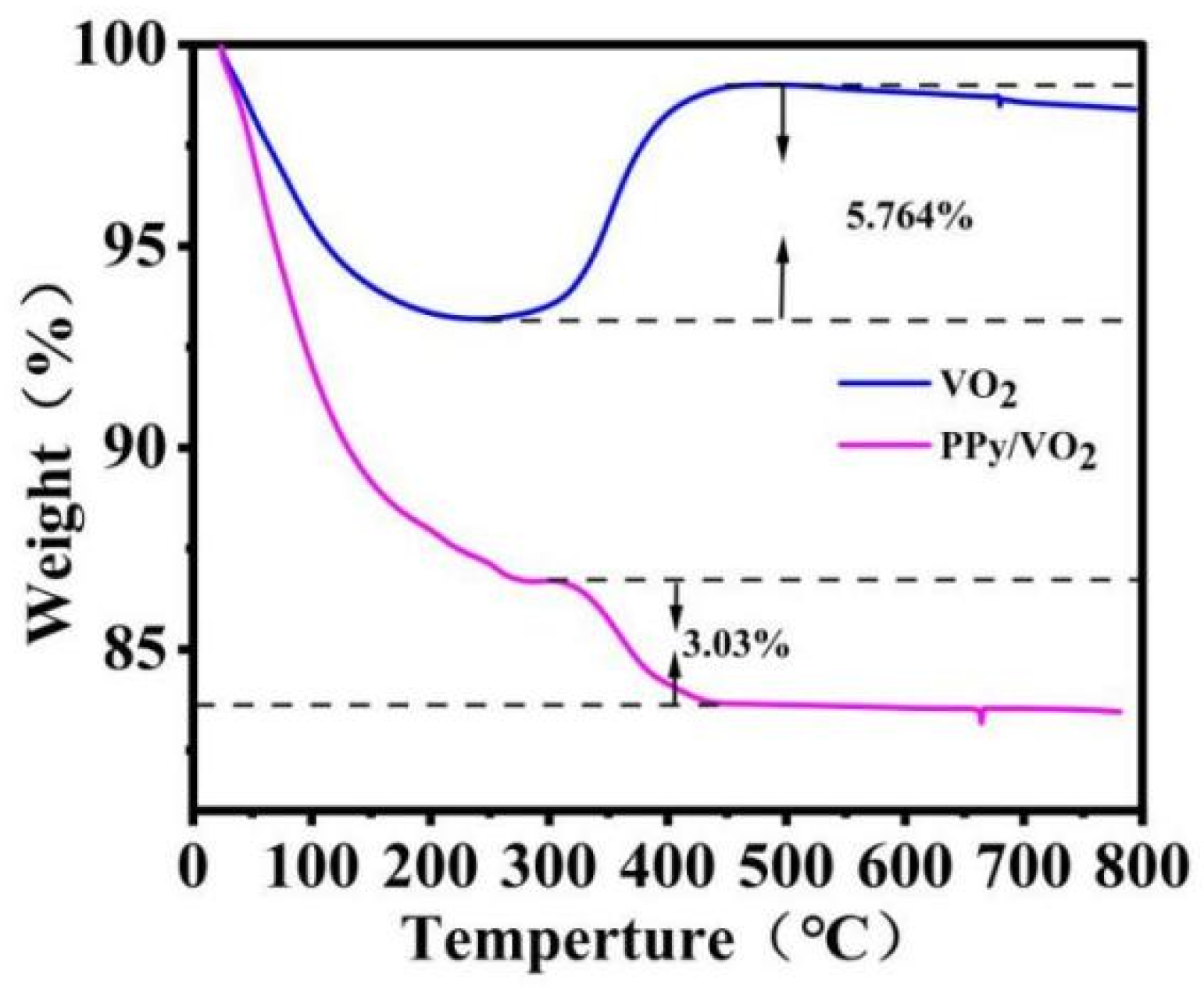
3.1.3. Thermogravimetric (TGA) Analysis
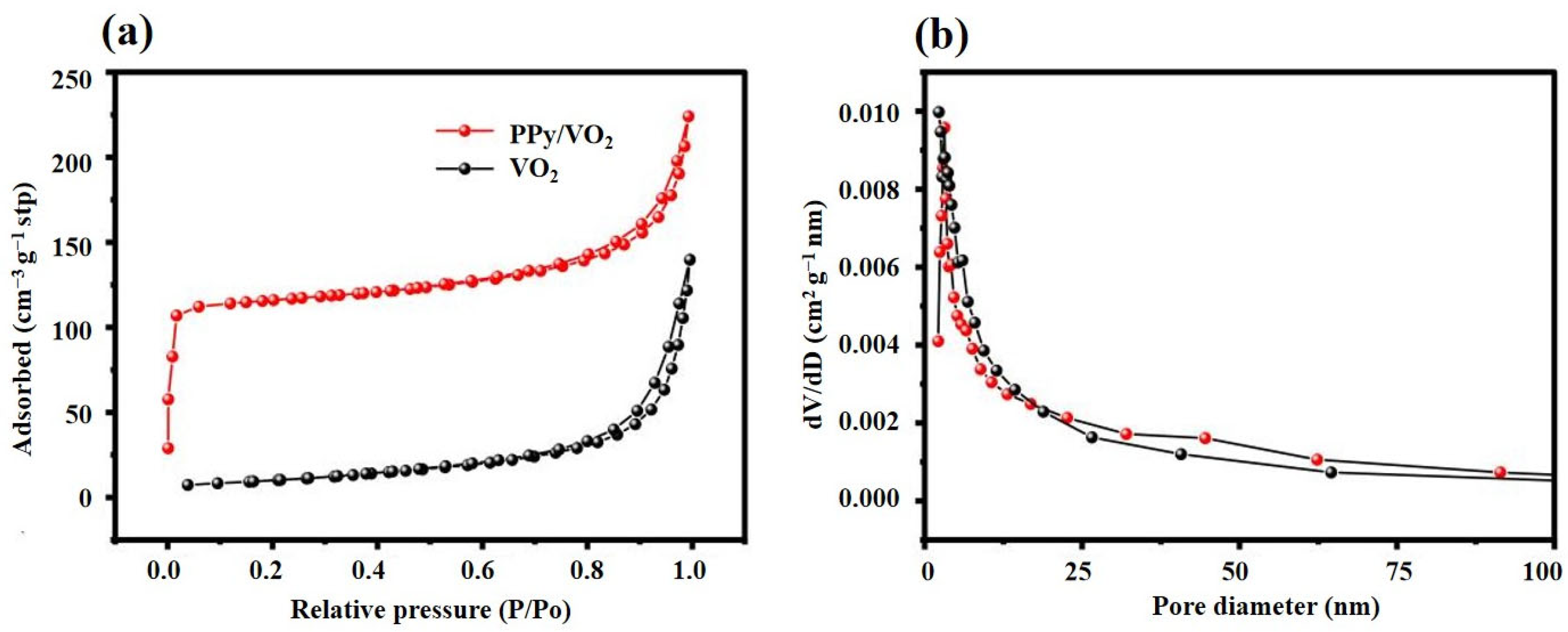
3.1.4. Nitrogen Adsorption/Desorption Test
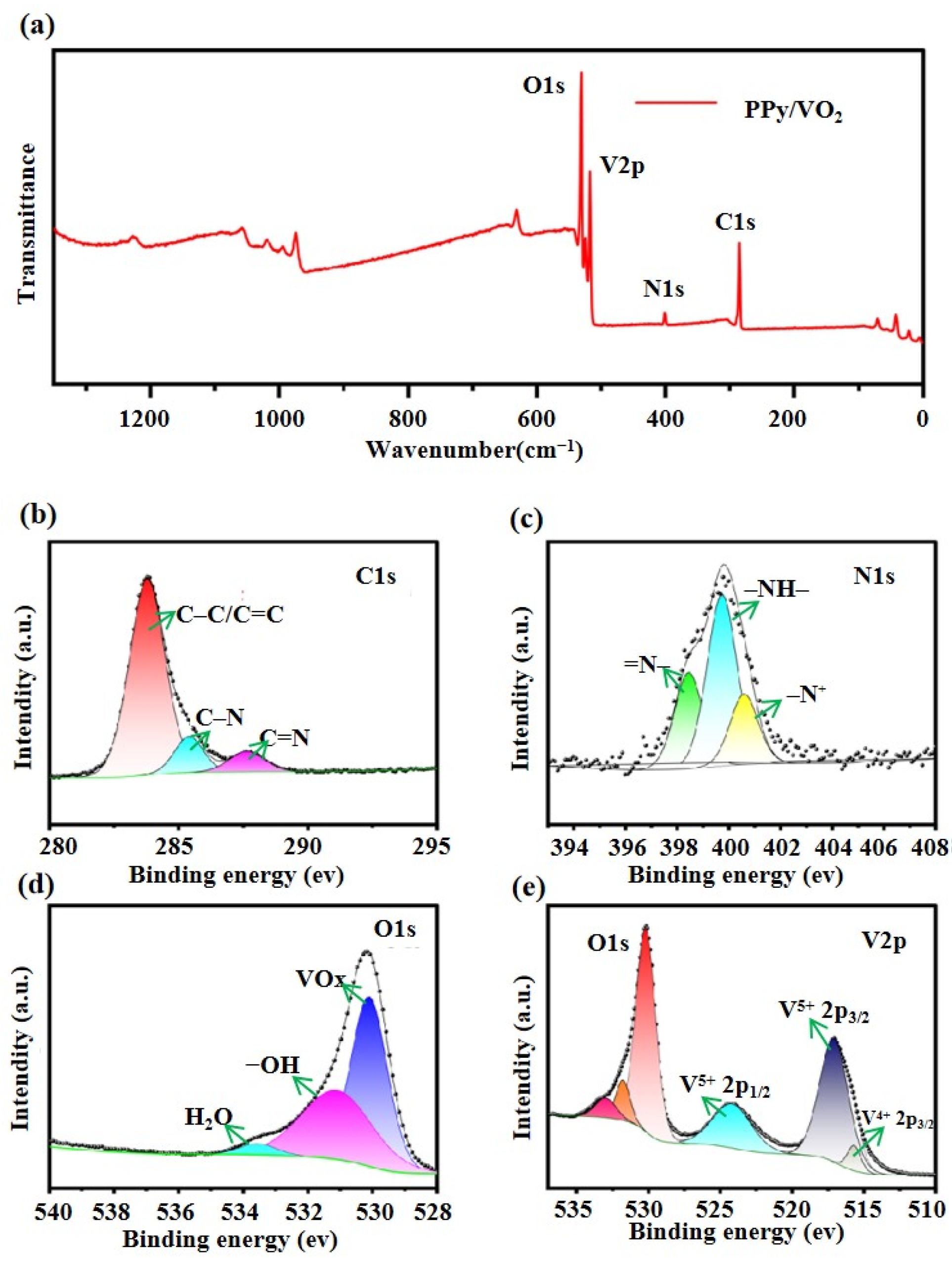
3.1.5. X-Ray Photoelectron Spectroscopy (XPS) Analysis
3.2. Electrochemical Performance
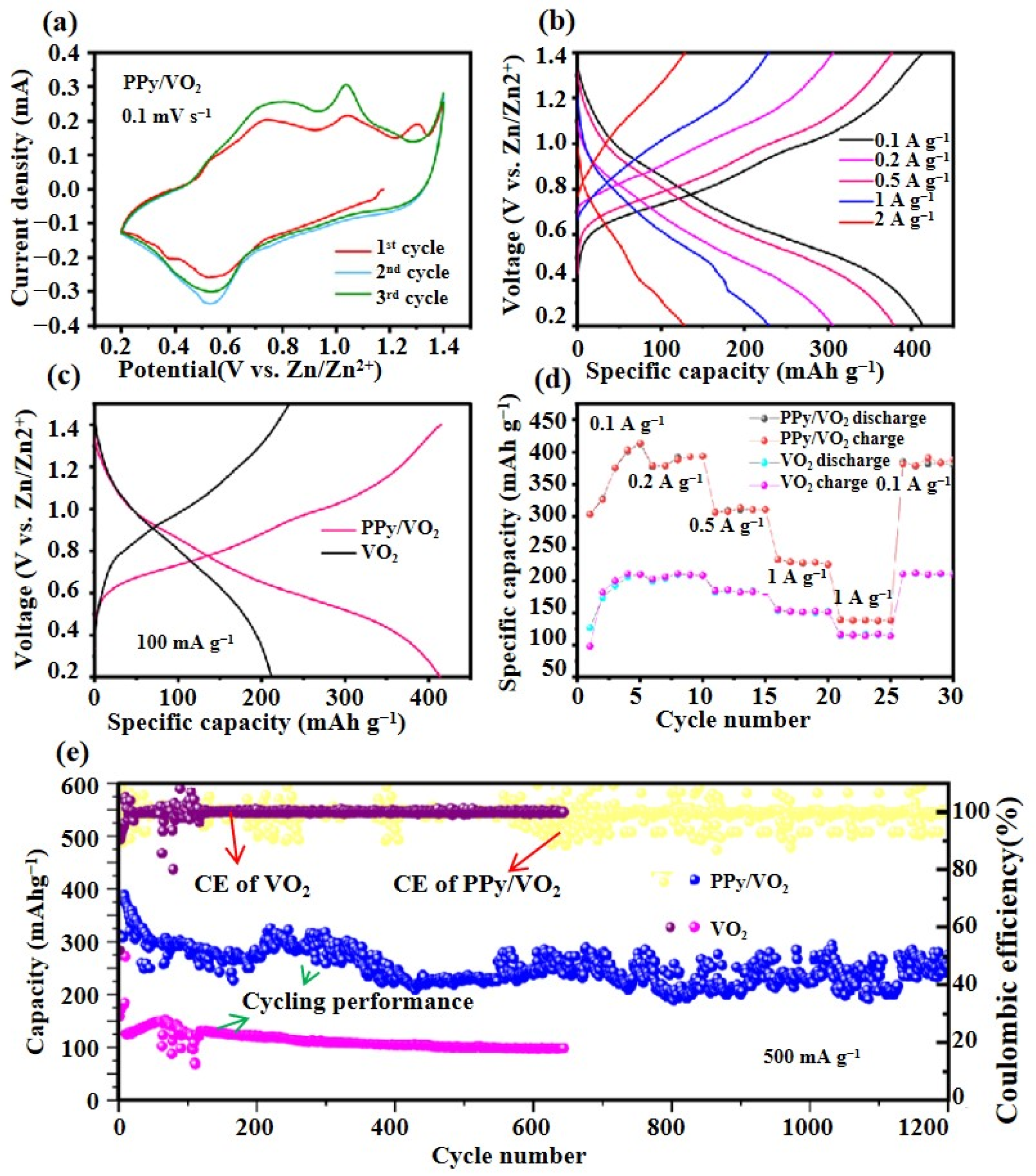
3.2.1. Cyclic Voltammetry (CV) Test
3.2.2. Electrochemical Kinetics Analysis
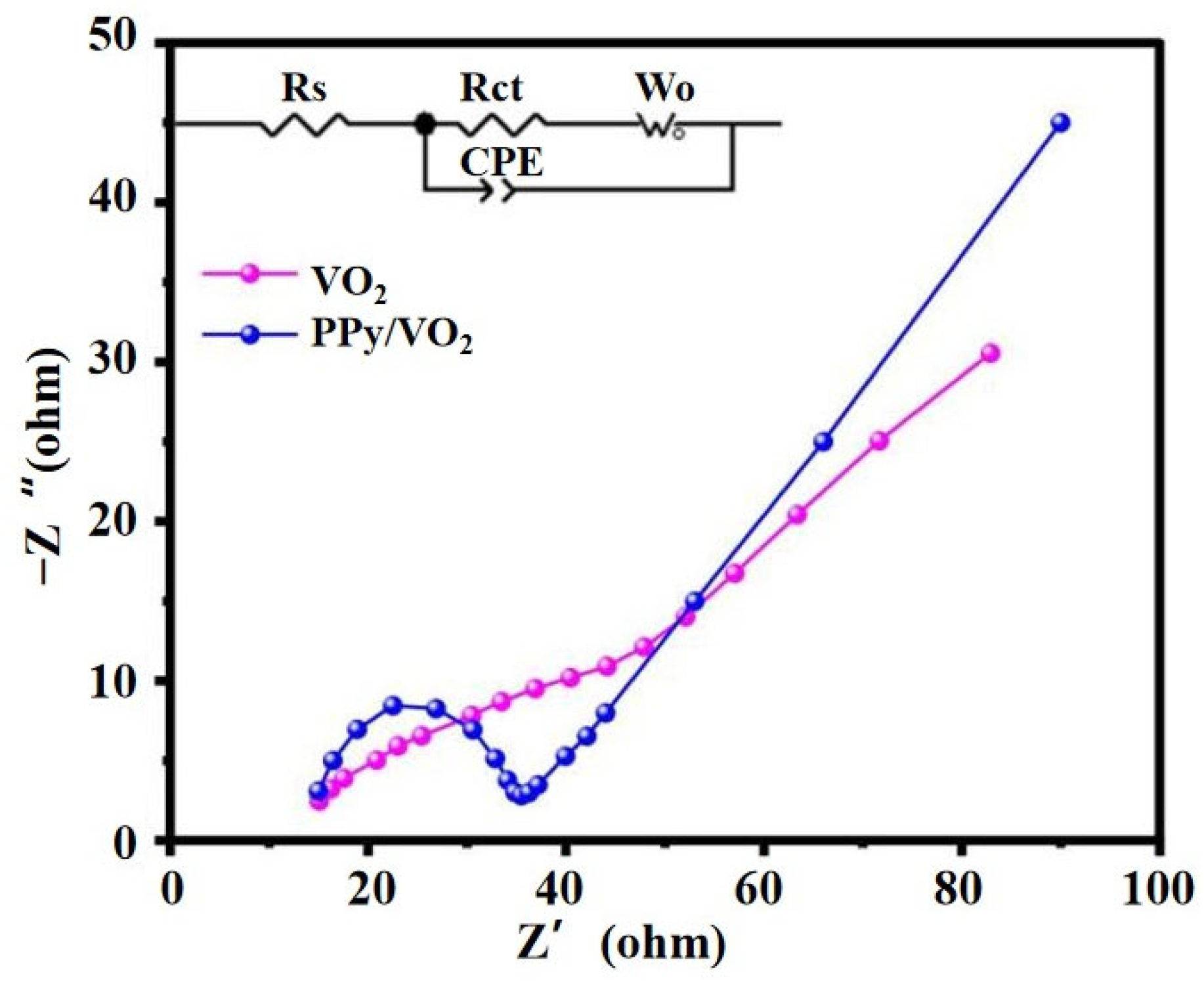
3.2.3. Electrochemical Impedance Spectroscopy (EIS) Analysis
3.3. Performance Analysis
- (1)
- Specific capacity at comparable current densities
- (2)
- Cycling stability
- (3)
- Rate performance and high-rate adaptability
- (4)
- Interfacial charge transfer and electrolyte compatibility
- (5)
- Scalability and practicality electrode processing
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hu, A.; Chen, W.; Li, F.; He, M.; Chen, D.; Li, Y.; Zhu, J.; Yan, Y.; Long, J.; Hu, Y. Nonflammable Polyfluorides-Anchored Quasi-Solid Electrolytes for Ultra-safe Anode-free Lithium Pouch Cells without Thermal Runaway. Adv. Mater. 2023, 35, 202304762. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Li, X.; Tang, T.; Cheng, Y.; Wang, Y.; Yang, Y.; Liu, L.; Li, Y.; Li, M.; Xiao, J.; et al. Modularized Cathode with Neural Network Topology for High Rate and Fault-Tolerant Lithium–Sulfur Batteries. Adv. Mater. 2025, 2504908. [Google Scholar] [CrossRef]
- Zhao, C.; Yan, Z.; Zhou, B.; Pan, Y.; Hu, A.; He, M.; Liu, J.; Long, J. Identifying the Role of Lewis-Base Sites for the Chemistry in Lithium-Oxygen Batteries. Angew. Chem. Int. Ed. 2023, 62, 202302746. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Lan, Y.; Lu, G.; Liu, L.; Tang, T.; Li, M.; Cheng, Y.; Xiao, J.; Li, X. Integrated Design for Discrete Sulfur @ Polymer Nanoreactor with Tandem Connection as Lithium–Sulfur Battery Cathodes. Angew. Chem. Int. Ed. 2024, 63, e202406693. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Gong, Z.; Wang, D.; Hu, R.; Ye, K.; Gao, Y.; Zhu, K.; Yan, J.; Wang, G.; Cao, D. Facile Fabrication of F-doped Biomass Carbon as High-Performance Anode Material for Potassium-ion Batteries. Electrochim. Acta 2021, 389, 138799. [Google Scholar] [CrossRef]
- Quilty, C.; Wu, D.; Li, W.; Bock, D.; Wang, L.; Housel, L.; Abraham, A.; Takeuchi, K.; Marschilok, A.; Takeuchi, E. Electron and Ion Transport in Lithium and Lithium-Ion Battery Negative and Positive Composite Electrodes. Chem. Rev. 2023, 123, 1327–1363. [Google Scholar] [CrossRef]
- Zhu, Y.; Ge, M.; Lai, H. Multifunctional Electrolyte Additives for Better Metal Batteries. Adv. Funct. Mater. 2024, 34, 2301964. [Google Scholar] [CrossRef]
- Yi, X.; Feng, Y.; Rao, A.; Zhou, J.; Wang, C.; Lu, B. Quasi-Solid Aqueous Electrolytes for Low-Cost Sustainable Alkali-metal Batteries. Adv. Mater. 2023, 35, 2302280. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Rao, A.; Lu, Z. Selective Potassium Deposition Enables Dendrite-resistant Anodes for Ultrastable Potassium-Metal Batteries. Adv. Mater. 2023, 35, 2300886. [Google Scholar] [CrossRef]
- Wang, T.; Sun, J.; Hua, Y.; Krishna, B.; Xi, Q.; Ai, W.; Yu, J. Planar and Dendrite-Free Zinc Deposition Enabled by Exposed Crystal Plane Optimization of Zinc Anode. Energy Storage Mater. 2022, 53, 273–304. [Google Scholar] [CrossRef]
- Yi, Z.; Chen, G.; Hou, F. Strategies for the Stabilization of Zn Metal Anodes for Zn-Ion Batteries. Adv. Energy Mater. 2021, 11, 2003065. [Google Scholar] [CrossRef]
- Xiao, Y.; Ren, J.; Li, M.; Xiao, K.; Wang, Y. Charge-Tuning Mediated Rapid Kinetics of Zinc Ions in Aqueous Zn-Ion Battery. Chem. Eng. J. 2023, 474, 145801. [Google Scholar] [CrossRef]
- Huang, J.; Li, Y.; Xie, R.; Li, J.; Tian, Z.; Chai, G.; Zhang, Y.; Lai, F.; He, G.; Liu, C. Structural Engineering of Cathodes for Improved Zn-Ion Batteries. J. Energy Chem. 2021, 58, 147–155. [Google Scholar] [CrossRef]
- Tian, Y.; An, Y.; Liu, C.; Xiong, S.; Feng, J.; Qian, Y. Reversible Zinc-based Anodes Enabled by Zincophilic Antimony Engineered MXene for Stable and Dendrite-free Aqueous Zinc Batteries. Energy Storage Mater. 2021, 41, 343–353. [Google Scholar] [CrossRef]
- Fu, X.; Li, G.; Wang, X.; Wang, J.; Yu, W.; Dong, X.; Liu, D. The Etching Strategy of Zinc Anode to Enable high Performance Zinc-Ion Batteries. J. Energy Chem. 2024, 88, 125–143. [Google Scholar] [CrossRef]
- Blanc, L.; Kundu, D.; Nazar, L. Scientific Challenges for the Implementation of Zn-Ion Batteries. Joule 2020, 4, 771–799. [Google Scholar] [CrossRef]
- Xia, J.; Zhou, Y.; Zhang, J.; Lu, T.; Gong, W.; Zhang, D.; Wang, X.; Di, J. Triggering High Capacity and Superior Reversibility of Manganese Oxides Cathode via Magnesium Modulation for Zn//MnO2 Batteries. Small 2023, 19, 2301906. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Wu, X. Enhanced Electrochemical Performance of Zn/VO-x Batteries by a Carbon-Encapsulation Strategy. ACS Appl. Mater. Interfaces 2022, 14, 11654–11662. [Google Scholar] [CrossRef]
- Sambandam, B.; Kim, S.; Pham, D.; Mathew, V.; Lee, J.; Lee, S.; Soundharrajan, V.; Kim, S.; Alfaruqi, M.; Hwang, J. Hyper oxidized V6O13+x·nH2O Layer Oxide for Aqueous Rechargeable Zn Battery: Effect on Dual Carriers Transportation and Parasitic Reactions. Energy Storage Mater. 2020, 35, 47–61. [Google Scholar] [CrossRef]
- Deng, W.; Li, Z.; Ye, Y.; Zhou, Z.; Li, R. Zn2+ Induced Phase Transformation of K2MnFe(CN)6 Boosts Highly Stable Zinc-Ion Storage. Adv. Energy Mater. 2021, 11, 2003639. [Google Scholar] [CrossRef]
- Dai, Y.; Kong, X.; Wang, L.; Gu, Y.; Guo, J. Al-induced Fast Phase Transition in Vanadium Oxide Cathode Materials for High-performance Aqueous Zinc-ion Batteries. CrystEngComm 2025, 27, 801–808. [Google Scholar] [CrossRef]
- Yan, M.; He, P.; Chen, Y.; Wang, S.; Wei, Q.; Zhao, K.; Xu, X.; An, Q.; Shuang, Y.; Shao, Y. Water-Lubricated Intercalation in V2O5·nH2O for High-Capacity and High-Rate Aqueous Rechargeable Zinc Batteries. Adv. Mater. 2018, 30, 1703725. [Google Scholar] [CrossRef]
- Li, Z.; Ganapathy, S.; Xu, Y.; Zhou, Z.; Sarilar, M.; Wagemaker, M. Mechanistic Insight into the Electrochemical Performance of Zn/VO2 Batteries with an Aqueous ZnSO4 Electrolyte. Adv. Energy Mater. 2019, 9, 1900237. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, Y.; Zuo, C.; Tang, W.; Liu, G.; Wang, S.; Cai, W.; Dong, S.; Luo, P. Adjusting the Valence State of Vanadium in VO2(B) by Extracting Oxygen Anions for High-Performance Aqueous Zinc-Ion Batteries. Chem. Sustain. Energy Mater. 2021, 14, 971–978. [Google Scholar] [CrossRef]
- Qi, Y.; Jin, X.; Xu, L.; Ren, X.; Xia, Y. Ultra-high Mass-Loading V5+-VO2@PPy Cathode for Aqueous Zinc-Ion Battery. J. Alloys Compd. 2023, 967, 171750. [Google Scholar] [CrossRef]
- Ping, M.; Zhang, Q.; Sun, J.; Guo, J.; Wang, X.; Zhou, Z.; He, B.; Li, Q.; Xie, L.; Zhao, J. Hierarchically Structured VO2@PPy Core-Shell Nanowire Arrays Grown on Carbon Nanotube Fibers as Advanced Cathodes for High-Performance Wearable Asymmetric Supercapacitors. Carbon 2018, 139, 21–28. [Google Scholar]
- Deng, W.; Li, C.; Zou, W.; Xu, Y.; Chen, Y.; Li, R. Understanding the Super-Theoretical Capacity Behavior of VO2 in Aqueous Zn Batteries. Small 2023, 20, 2309527. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Zhou, X. Chemical Modulation of Metal–Insulator Transition toward Multifunctional Applications in Vanadium Dioxide Nanostructures. Small 2023, 19, 2305234. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, X. High Durable Aqueous Zinc Ion Batteries by Synergistic Effect of V6O13/VO2 Electrode Materials. J. Energy Chem. 2023, 87, 334–341. [Google Scholar] [CrossRef]
- Song, Z.; Zhao, Y.; Wang, H.; Zhou, A.; Jin, X.; Huang, Y.; Li, L.; Wu, F.; Chen, R. Dual mechanism with graded energy storage in long-term aqueous zinc-ion batteries achieved using a polymer/vanadium dioxide cathode. Energy Environ. Sci. 2024, 17, 6666–6675. [Google Scholar] [CrossRef]
- Chu, F.; Cheng, S.; Ye, Z.; Wu, F.; Zhuang, H.; Dong, W.; Xie, A. In Situ Etching by Released Proton in Aniline Polymerization to form Network VO2 Doped Polyaniline Composites with Variable Infrared Emissivity for Electromagnetic Absorption Application. Adv. Compos. Hybrid Mater. 2022, 5, 2760–2771. [Google Scholar] [CrossRef]
- Liu, H.; Yang, L.; Ting, S.; Li, C.; Kang, T.; Niu, H.; Huang, W.; Chang, C.; Yang, M.; Cao, G.; et al. Distorting Local Structures to Modulate Ligand Fields in Vanadium Oxide for High-Performance Aqueous Zinc-Ion Batteries. ACS Nano 2025, 19, 9132–9143. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Li, X.; Su, S.; Li, J.; Fang, J.; Liang, B.; Hou, J.; Luo, M. Hydrated Lithium Ions Intercalated V2O5 with Dual-ion Synergistic Insertion Mechanism for High-Performance Aqueous Zinc-Ion Batteries. J. Colloid Interface Sci. 2022, 606, 645–653. [Google Scholar] [CrossRef]
- Xie, B.; Wu, X.; Wang, J.; Wang, R.; Dong, Y.; Hou, J.; Lv, R.; Chen, M.; Diao, G. Confinement Sacrifice Template Synthesis of Size Controllable Heterogeneous Double-layer Hollow Spheres SnO2@Void@HCSs as Anode for Li+/Na+ Batteries. J. Electroanal. Chem. 2022, 923, 116830. [Google Scholar] [CrossRef]
- Xu, Z.; Sun, Z.; Shan, J.; Jin, S.; Cui, J.; Deng, Z.; Seo, M.; Wang, X. O, N-Codoped, Self-Activated, Holey Carbon Sheets for Low-Cost And High-Loading Zinc-Ion Supercapacitors. Adv. Funct. Mater. 2024, 34, 2302818. [Google Scholar] [CrossRef]
- Wang, J.; Polleux, J.; Lim, J.; Dunn, B. Pseudocapacitive contributions to electrochemical energy storage in TiO2 (anatase) nanoparticles. J. Phys. Chem. 2007, 111, 14925–14931. [Google Scholar] [CrossRef]
- Xia, Y.; Mathis, T.; Zhao, M.; Anasori, B.; Dang, A.; Zhou, Z.; Cho, H.; Gogotsi, Y.; Yang, S. Thickness-independent Capacitance of Vertically Aligned Liquid-crystalline MXenes. Nature 2018, 557, 409–412. [Google Scholar] [CrossRef]
- Zhou, Y.; Fu, Y.; Zhang, T.; Hu, C.; Qiao, F.; Wang, J.; Noh, H.; Baek, J. Synthesis of Size-controllable, Yolk-shell Metal Sulfide Spheres for Hybrid Supercapacitors. Chem. Eng. J. 2023, 476, 146377. [Google Scholar] [CrossRef]
- Dhanda, M.; Arora, R.; Saini, M. Nehrac and Suman LataProlific Intercalation of VO2 (D)/polypyrrole/g-C3N4 as an Energy Storing Electrode with Remarkable Capacitance. New J. Chem. 2022, 46, 14251. [Google Scholar] [CrossRef]
- Li, S.; Zhou, T.; Liu, M.; Zhao, Q.; Liu, Y.; Cheng, Y.; Li, X. Electrode Design Based on Porous MnO2/PPy Hybrid Nanocomposite and Its Application in Zinc-Ion Batteries. Micromachines 2025, 16, 536. [Google Scholar] [CrossRef]

| Technique | Instrument & Manufacturer | Key Parameters | Software |
|---|---|---|---|
| XRD | Rigaku D/MAX-2500 (Rigaku, Tokyo, Japan) | Cu Kα (λ = 1.5406 Å); 40 kV, 30 mA; 5°/min; 0.02° step Cu Kα (λ = 1.5406 Å), 40 kV, 30 mA, 2θ: 10~70°, 5°/min, 0.02° step | MDI Jade 6.5 |
| SEM & EDS | Hitachi S-4800 (Hitachi, Tokyo, Japan) + Oxford EDS (Oxford, UK) | 5–20 kV accelerating voltage | Quartz PCI 8.0, INCA 5.05 |
| TEM | JEOL JEM-2100 (JEOL, Tokyo, Japan) | 200 kV accelerating voltage | Digital Micrograph 3.30.2004 |
| XPS | Thermo ESCALAB 250Xi (Thermo Fisher, Waltham, MA, USA) | Al Kα (1486.6 eV), pass energy: 20 eV, resolution: 0.5 eV, base pressure < 5 × 10−9 mbar | Thermo Avantage 5.991 |
| Raman Spectroscopy | LabRAM HR Evolution (Horiba, Kyoto, Japan) | Excitation: 512 nm, room temperature | LabSpec 6.5 |
| BET/BJH | ASAP 2460 (Micromeritics, Norcross, GA, USA) | −196 °C, N2 adsorption–desorption, BET for SSA, BJH for pore size | MicroActive 6.07 |
| TGA/DTA | SDT Q600 (TA Instruments, New Castle, DE, USA) | Air atmosphere, RT–800 °C, heating rate: 10 °C/min | Universal Analysis 5.5 |
| GCD | Neware CT4008 (Neware, Shenzhen, China) | Voltage range: 0.2~1.4 V (vs. Zn/Zn2+), room temperature | Neware BTSDA 7.6 |
| CV, EIS | CHI760E (Chenhua Instruments, Shanghai, China) | CV: 0.1–1.0 mV/s; EIS: 100 kHz–0.01 Hz, 5 mV AC amplitude, two-electrode system, room temperature | CHI Software 14.01 |
| Sample | Rs (Ω) | Rct (Ω) | CPE-T | CPE-P | Wo-R (Ω) | Wo-T (s) | Wo-P |
|---|---|---|---|---|---|---|---|
| PPy/VO2 | 18.0 | 16.4 | 2.50 × 10−5 | 0.88 | 6.0 | 55.0 | 0.450 |
| VO2 | 35.0 | 45.2 | 1.50 × 10−5 | 0.85 | 10.0 | 70.0 | 0.450 |
| Materials | Current Density | Discharge Capacity | Cycles | Capacity Retention | Electrolyte | System Type | Ref. |
|---|---|---|---|---|---|---|---|
| V5+-VO2@PPy-180 | 0.5 A/g | 314.2 mAh/g | 1500 | 95.99% | 3 M Zn(CF3SO3)2 | Aqueous ZIB | [25] |
| VO2@PPy NW Arrays | — | — | 4000 | 88.9% | — | Supercapacitor (FASC) | [26] |
| VO2(D)/PPy/g-C3N4 | 0.5 A/g | — | 2000 | 99.71% | 1 M H2SO4(3-electrode system) | 3-electrode system (CV only) | [39] |
| MnO2/PPy | 0.1 A/g | 205.2 mAh/g | 1000 | 75% | 2 M ZnSO4 + 0.1 M MnSO4 | Aqueous ZIB | [40] |
| PPy/VO2 | 0.1 A/g | 413 mAh/g | 1200 | 87.2% | 3 M Zn(CF3SO3)2 | Aqueous ZIB | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, T.; Li, S.; Xie, D.; Liu, Y.; Cheng, Y.; Li, X. Hydrothermally Synthesized PPy/VO2 Nanorod Composites for High-Performance Aqueous Zinc-Ion Battery Cathodes. Micromachines 2025, 16, 705. https://doi.org/10.3390/mi16060705
Zhou T, Li S, Xie D, Liu Y, Cheng Y, Li X. Hydrothermally Synthesized PPy/VO2 Nanorod Composites for High-Performance Aqueous Zinc-Ion Battery Cathodes. Micromachines. 2025; 16(6):705. https://doi.org/10.3390/mi16060705
Chicago/Turabian StyleZhou, Taoyun, Shilin Li, Dong Xie, Yi Liu, Yun Cheng, and Xinyu Li. 2025. "Hydrothermally Synthesized PPy/VO2 Nanorod Composites for High-Performance Aqueous Zinc-Ion Battery Cathodes" Micromachines 16, no. 6: 705. https://doi.org/10.3390/mi16060705
APA StyleZhou, T., Li, S., Xie, D., Liu, Y., Cheng, Y., & Li, X. (2025). Hydrothermally Synthesized PPy/VO2 Nanorod Composites for High-Performance Aqueous Zinc-Ion Battery Cathodes. Micromachines, 16(6), 705. https://doi.org/10.3390/mi16060705





