Time-Dependent Growth of Sputtered MoS2 Films on ZnO Nanorods for Enhanced NO2 Sensing Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of ZnO Nanorods, MoS2 Films, and MoS2/ZnO Heterostructures
2.2. Gas-Sensing Measurements
2.3. Characterization
3. Results
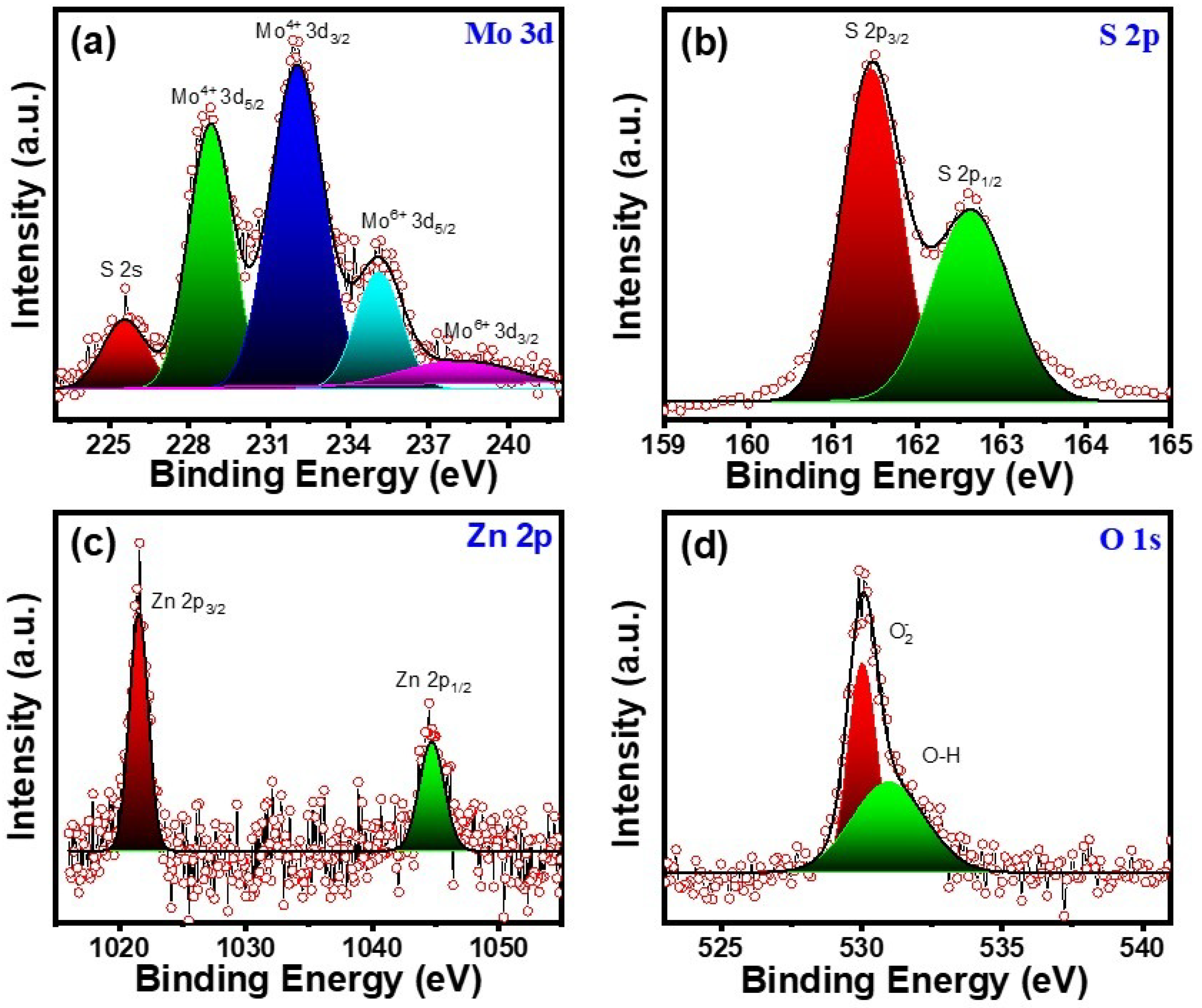
3.1. Structural Properties
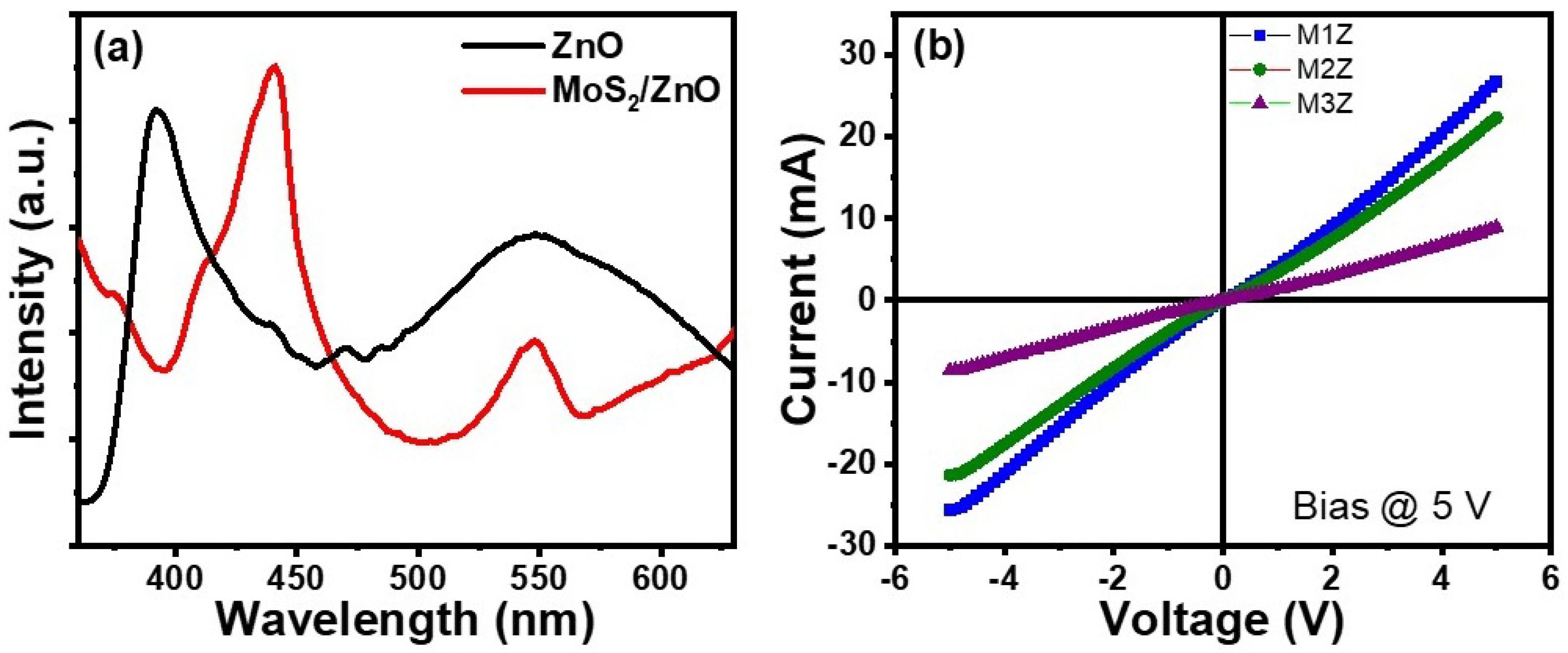
3.2. Gas-Sensing Characteristics
3.3. Gas-Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kelly, F.J.; Fussell, J.C. Air pollution and public health: Emerging hazards and improved understanding of risk. Environ. Geochem. Health 2015, 37, 631–649. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, A.H.; Patz, J.A. Reactive nitrogen and human health: Acute and long-term implications. Ambio A J. Hum. Environ. 2002, 31, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and health impacts of air pollution: A review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Song, W.; Zhan, H.; Zhang, Y.; Zhang, X.; Li, W.; Tong, S.; Pei, C.; Wang, Y.; Chen, Y. Contribution of vehicle emission and NO2 surface conversion to nitrous acid (HONO) in urban environments: Implications from tests in a tunnel. Environ. Sci. Technol. 2021, 55, 15616–15624. [Google Scholar] [CrossRef]
- Shen, J.; Rajalakshmi, K.; Muthusamy, S.; Ahn, D.H.; Song, J.W.; Choi, K.Y.; Xi, C.; Dai, J.; Zhou, Z.; Kannan, P.; et al. Multifunctional Sensors for Successive Detection of Endogenous ONOO–and Mitochondrial Viscosity: Discriminating Normal to Cancer Models. Anal. Chem. 2024, 96, 16289–16297. [Google Scholar] [CrossRef]
- Ramadan, M.N.; Ali, M.A.; Khoo, S.Y.; Alkhedher, M.; Alherbawi, M. Real-time IoT-powered AI system for monitoring and forecasting of air pollution in industrial environment. Ecotoxicol. Environ. Saf. 2024, 283, 116856. [Google Scholar] [CrossRef]
- Krishna, K.G.; Parne, S.; Pothukanuri, N.; Kathirvelu, V.; Gandi, S.; Joshi, D. Nanostructured metal oxide semiconductor-based gas sensors: A comprehensive review. Sens. Actuators A Phys. 2022, 341, 113578. [Google Scholar] [CrossRef]
- Galstyan, V.; Comini, E.; Baratto, C.; Faglia, G.; Sberveglieri, G. Nanostructured ZnO chemical gas sensors. Ceram. Int. 2015, 41, 14239–14244. [Google Scholar] [CrossRef]
- kumar Anbalagan, A.; Gupta, S.; Kumar, R.R.; Tripathy, A.R.; Chaudhary, M.; Haw, S.-C.; Murugesan, T.; Lin, H.-N.; Chueh, Y.-L.; Tai, N.-H. Gamma-ray engineered surface defects on zinc oxide nanorods towards enhanced NO2 gas sensing performance at room temperature. Sens. Actuators B Chem. 2022, 369, 132255. [Google Scholar] [CrossRef]
- Wang, S.; Yang, J.; Zhang, H.; Wang, Y.; Gao, X.; Wang, L.; Zhu, Z. One-pot synthesis of 3D hierarchical SnO2 nanostructures and their application for gas sensor. Sens. Actuators B Chem. 2015, 207, 83–89. [Google Scholar] [CrossRef]
- Garzella, C.; Comini, E.; Tempesti, E.; Frigeri, C.; Sberveglieri, G. TiO2 thin films by a novel sol–gel processing for gas sensor applications. Sens. Actuators B Chem. 2000, 68, 189–196. [Google Scholar] [CrossRef]
- Paleczek, A.; Grochala, D.; Staszek, K.; Gruszczynski, S.; Maciak, E.; Opilski, Z.; Kałużyński, P.; Wójcikowski, M.; Cao, T.-V.; Rydosz, A. An NO2 sensor based on WO3 thin films for automotive applications in the microwave frequency range. Sens. Actuators B Chem. 2023, 376, 132964. [Google Scholar] [CrossRef]
- Kumar, R.R.; Gupta, S.; Huang, H.-F.; Murugesan, T.; Tai, N.-H.; Lin, H.-N. Light intensity effects on the performance of In2O3 gas sensors: Insights into adsorption and desorption dynamics. Mater. Chem. Phys. 2025, 337, 130639. [Google Scholar] [CrossRef]
- Fazio, E.; Spadaro, S.; Corsaro, C.; Neri, G.; Leonardi, S.G.; Neri, F.; Lavanya, N.; Sekar, C.; Donato, N.; Neri, G. Metal-Oxide Based Nanomaterials: Synthesis, Characterization and Their Applications in Electrical and Electrochemical Sensors. Sensors 2021, 21, 2494. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.R.; Murugesan, T.; Chang, T.-W.; Lin, H.-N. Defect controlled adsorption/desorption kinetics of ZnO nanorods for UV-activated NO2 gas sensing at room temperature. Mater. Lett. 2021, 287, 129257. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Yang, Y.; Huang, Q.; Li, D.; Zeng, D. A review on two-dimensional materials for chemiresistive-and FET-type gas sensors. Phys. Chem. Chem. Phys. 2021, 23, 15420–15439. [Google Scholar] [CrossRef]
- Liu, Y.; Duan, X.; Huang, Y.; Duan, X. Two-dimensional transistors beyond graphene and TMDCs. Chem. Soc. Rev. 2018, 47, 6388–6409. [Google Scholar] [CrossRef]
- Gupta, A.; Sakthivel, T.; Seal, S. Recent development in 2D materials beyond graphene. Prog. Mater. Sci. 2015, 73, 44–126. [Google Scholar] [CrossRef]
- Ganatra, R.; Zhang, Q. Few-Layer MoS2: A Promising Layered Semiconductor. ACS Nano 2014, 8, 4074–4099. [Google Scholar] [CrossRef]
- Cho, B.; Hahm, M.G.; Choi, M.; Yoon, J.; Kim, A.R.; Lee, Y.-J.; Park, S.-G.; Kwon, J.-D.; Kim, C.S.; Song, M.; et al. Charge-transfer-based Gas Sensing Using Atomic-layer MoS2. Sci. Rep. 2015, 5, 8052. [Google Scholar] [CrossRef]
- Rajalakshmi, K.; John, S.A. Highly sensitive determination of nitrite using FMWCNTs-conducting polymer composite modified electrode. Sens. Actuators B Chem. 2015, 215, 119–124. [Google Scholar] [CrossRef]
- Jiang, C.-H.; Yao, C.-B.; Wang, L.-Y.; Wang, X.; Wang, Z.-M.; Yin, H.-T. Interface engineering of modified ZnO@MoS2 heterostructure to efficiently enhance charge transfers and carrier regulation. J. Lumin. 2023, 255, 119546. [Google Scholar] [CrossRef]
- Huang, M.; Wang, L.; Liu, Q.; You, W.; Che, R. Interface compatibility engineering of Multi-shell Fe@C@TiO2@MoS2 heterojunction expanded microwave absorption bandwidth. Chem. Eng. J. 2022, 429, 132191. [Google Scholar] [CrossRef]
- Kumar, R.R.; Habib, M.R.; Khan, A.; Chen, P.-C.; Murugesan, T.; Gupta, S.; Anbalagan, A.k.; Tai, N.-H.; Lee, C.-H.; Lin, H.-N. Sulfur Monovacancies in Liquid-Exfoliated MoS2 Nanosheets for NO2 Gas Sensing. ACS Appl. Nano Mater. 2021, 4, 9459–9470. [Google Scholar] [CrossRef]
- Kumar, A.; Chandra, R.; Malik, V.K. MoS2 nanoworm thin films for NO2 gas sensing application. Thin Solid Films 2021, 725, 138625. [Google Scholar] [CrossRef]
- Tang, H.; Li, Y.; Sokolovskij, R.; Sacco, L.; Zheng, H.; Ye, H.; Yu, H.; Fan, X.; Tian, H.; Ren, T.-L.; et al. Ultra-High Sensitive NO2 Gas Sensor Based on Tunable Polarity Transport in CVD-WS2/IGZO p-N Heterojunction. ACS Appl. Mater. Interfaces 2019, 11, 40850–40859. [Google Scholar] [CrossRef]
- Anbalagan, A.K.; Hu, F.C.; Chan, W.K.; Gandhi, A.C.; Gupta, S.; Chaudhary, M.; Chuang, K.W.; Ramesh, A.K.; Billo, T.; Sabbah, A.; et al. Gamma-ray irradiation induced ultrahigh room-temperature ferromagnetism in MoS2 sputtered few-layered thin films. ACS Nano 2023, 17, 6555–6564. [Google Scholar] [CrossRef]
- Anbalagan, A.k.; Gupta, S.; Kumar, A.; Haw, S.-C.; Kulkarni, S.S.; Tai, N.-H.; Tseng, F.-G.; Hwang, K.C.; Lee, C.-H. Gamma Ray Irradiation Enhances the Linkage of Cotton Fabrics Coated with ZnO Nanoparticles. ACS Omega 2020, 5, 15129–15135. [Google Scholar] [CrossRef]
- Kumar, R.R.; Yu, W.-C.; Murugesan, T.; Chen, P.-C.; Ranjan, A.; Lu, M.-Y.; Lin, H.-N. Formation of large-scale MoS2/Cu2O/ZnO heterostructure arrays by in situ photodeposition and application for ppb-level NO2 gas sensing. J. Alloys Compd. 2023, 952, 169984. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, G.; Liao, J.; Lv, S.; Zhu, Z.; Li, Z. Vertically aligned MoS2/ZnO nanowires nanostructures with highly enhanced NO2 sensing activities. Appl. Surf. Sci. 2018, 456, 808–816. [Google Scholar] [CrossRef]
- Ikram, M.; Lv, H.; Liu, Z.; Shi, K.; Gao, Y. Hydrothermally derived p–n MoS2–ZnO from p–p MoS2-ZIF-8 for an efficient detection of NO2 at room temperature. J. Mater. Chem. A 2021, 9, 14722–14730. [Google Scholar] [CrossRef]
- Shahid, A.; Hussain, S.; Liaqat, M.J.; Begi, A.N.; Ibrahim, T.K.; Amu-Darko, J.N.O.; Fouda, A.M.; Manavalan, R.K.; Zhang, X.; Liu, G.; et al. Nano architecture of MoS2/ZnO nanocomposite for efficient NO2 gas-sensing properties. Mater. Today Commun. 2025, 43, 111577. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, C.; Guo, Y. UV assisted ultrasensitive trace NO2 gas sensing based on few-layer MoS2 nanosheet–ZnO nanowire heterojunctions at room temperature. J. Mater. Chem. A 2018, 6, 10286–10296. [Google Scholar] [CrossRef]





| Material | Nanocomposite Synthesis Method | Temperature | Concentration | Response (%) | Ref. | |
|---|---|---|---|---|---|---|
| MoS2 | ZnO | |||||
| MoS2/CuxO/ZnO | Photo deposition | Hydrothermal method | RT | 500 ppb | 890 | [29] |
| MoS2/ZnO nanowires | Mo—Sputtering S—Sulfurization | Hydrothermal method | 200 °C | 50 ppm | 31.2 | [30] |
| MoS2/ZnO nanorods | Hydrothermal method | Hydrothermal method | RT | 50 ppm | 34.91 | [31] |
| MoS2/ZnO composites | Hydrothermal method | Hydrothermal method | 200 °C | 10 ppm | 33.77 | [32] |
| MoS2/ZnO | Liquid exfoliation | Hydrothermal method | RT | 200 ppb | 188 | [33] |
| MoS2/ZnO | MoS2 as target—Sputtering | Hydrothermal method | RT | 500 ppb | 20.9 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, R.R.; Gupta, S.; Anbalagan, A.k.; Khan, A.; Tai, N.-H.; Lee, C.-H.; Lin, H.-N. Time-Dependent Growth of Sputtered MoS2 Films on ZnO Nanorods for Enhanced NO2 Sensing Performance. Micromachines 2025, 16, 659. https://doi.org/10.3390/mi16060659
Kumar RR, Gupta S, Anbalagan Ak, Khan A, Tai N-H, Lee C-H, Lin H-N. Time-Dependent Growth of Sputtered MoS2 Films on ZnO Nanorods for Enhanced NO2 Sensing Performance. Micromachines. 2025; 16(6):659. https://doi.org/10.3390/mi16060659
Chicago/Turabian StyleKumar, Rishi Ranjan, Shivam Gupta, Aswin kumar Anbalagan, Afzal Khan, Nyan-Hwa Tai, Chih-Hao Lee, and Heh-Nan Lin. 2025. "Time-Dependent Growth of Sputtered MoS2 Films on ZnO Nanorods for Enhanced NO2 Sensing Performance" Micromachines 16, no. 6: 659. https://doi.org/10.3390/mi16060659
APA StyleKumar, R. R., Gupta, S., Anbalagan, A. k., Khan, A., Tai, N.-H., Lee, C.-H., & Lin, H.-N. (2025). Time-Dependent Growth of Sputtered MoS2 Films on ZnO Nanorods for Enhanced NO2 Sensing Performance. Micromachines, 16(6), 659. https://doi.org/10.3390/mi16060659









