A Pipette for High-Resolution Sampling and Delivery of pL Bio-Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagent and Consumables
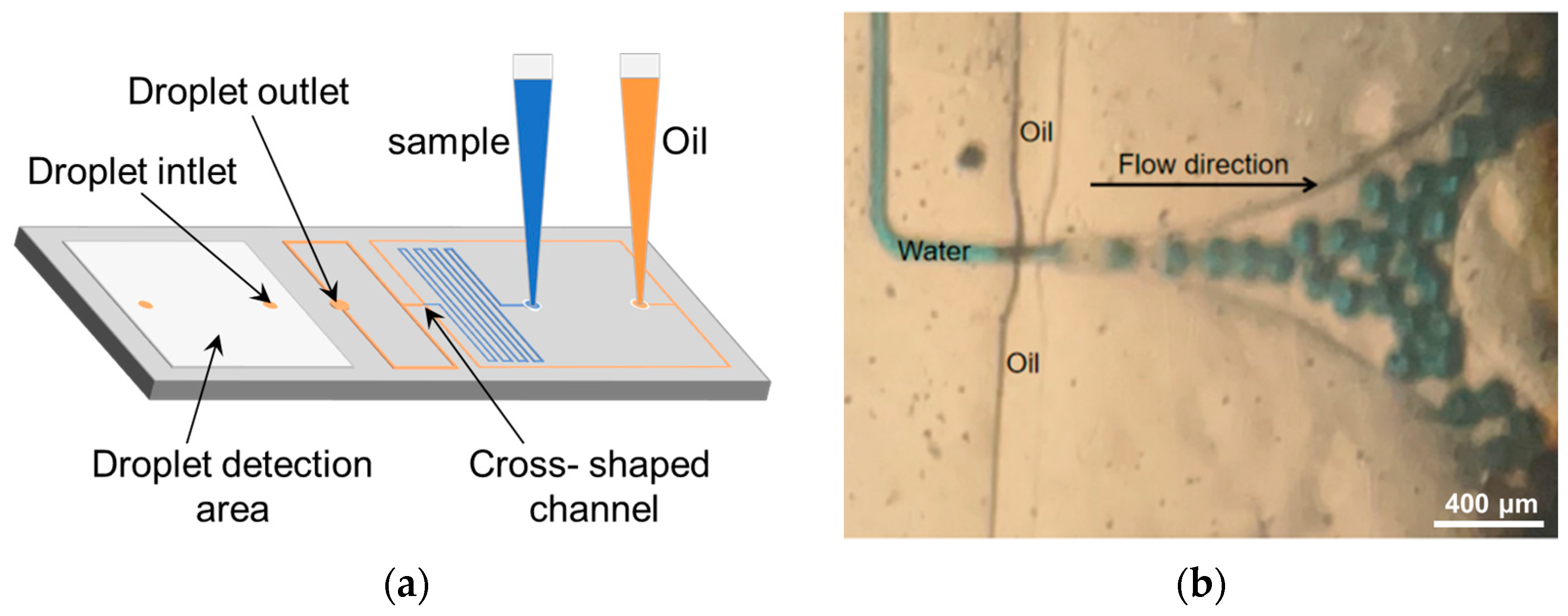
2.2. Design and Microfabrication of Droplet-Based Microfluidic Chip
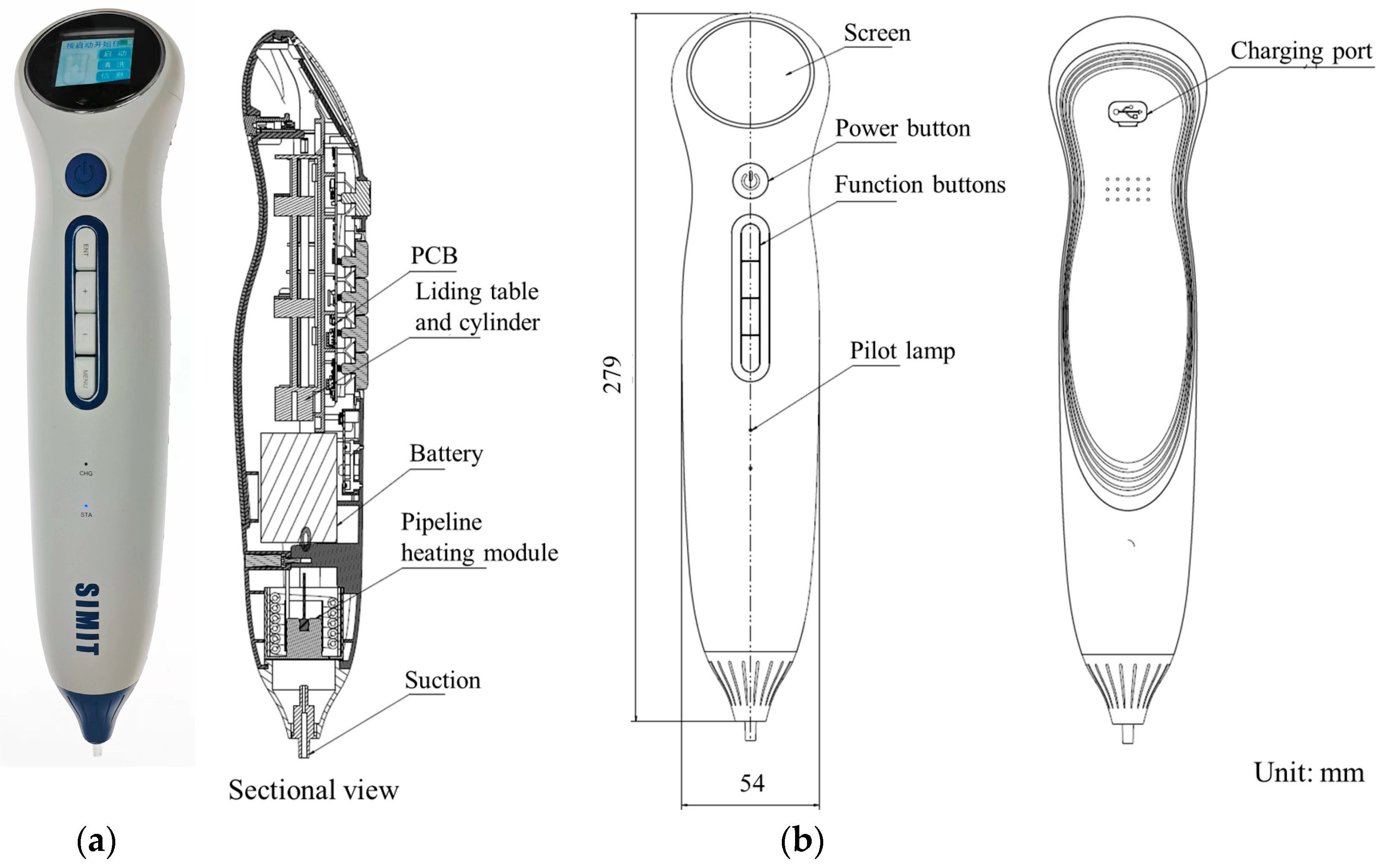
2.3. Design and Manufacture of Sampling Pipette
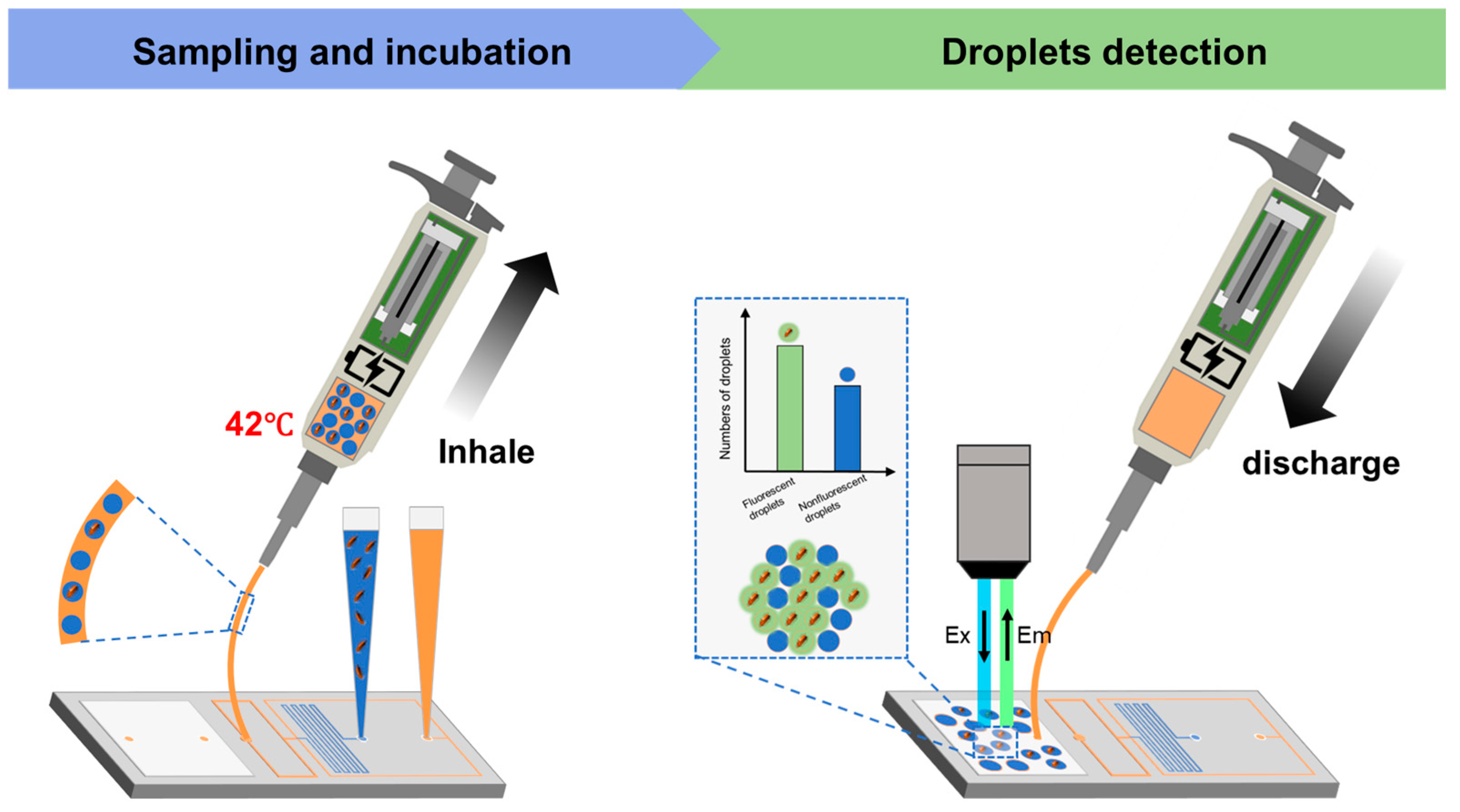
2.4. Droplet Formation and Biological Monitoring Process
3. Results
3.1. Simulation of Droplet Formation
3.2. Assembly and Display of Sampling Pipette
3.3. Droplet Sampling Pipette–Chip Connection and E. coli Detection
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| E. coli | Escherichia coli |
| pL | Picoliter |
| POCT | Point-of-Care Testing |
| FDG | Fluorescein di(β-D-galactopyranoside) |
| GAL | β-Galactosidase |
| PCR | Polymerase chain reaction |
| LAMP | Loop-mediated Isothermal Amplification |
| PDMS | Polydimethylsiloxane |
References
- Hansen, G.; Marino, J.; Wang, Z.X.; Beavis, K.G.; Rodrigo, J.; Labog, K.; Westblade, L.F.; Jin, R.; Love, N.; Ding, K.; et al. Clinical Performance of the Point-of-Care cobas Liat for Detection of SARS-CoV-2 in 20 Minutes: A Multicenter Study. J. Clin. Microbiol. 2021, 59, e02811-20. [Google Scholar] [CrossRef] [PubMed]
- Sah, A.K.; Joshi, B.; Khadka, D.K.; Gupta, B.P.; Adhikari, A.; Singh, S.K.; Rai, G.; Vaidya, G.S.; Rajbhandari, R.; Pant, B.; et al. Comparative Study of GeneXpert MTB/RIF Assay and Multiplex PCR Assay for Direct Detection of Mycobacterium tuberculosis in Suspected Pulmonary Tuberculosis Patients. Curr. Microbiol. 2017, 74, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ma, C.; Wu, M.; Jia, C.; Feng, S.; Zhao, J.; Liang, L. Sensitivity of photoelctrocehmical aptasensor using spiral nanorods for detecting antiobiotic levels in experimental and real samples. Talanta 2022, 237, 122930. [Google Scholar] [CrossRef]
- Kusonpan, P.; Kunpatee, K.; Chailapakul, O.; Kalcher, K.; Ortner, A.; Chaiyo, S.; Samphao, A. A simple manually rotated paper-based analytical device with electrochemical sensors for the determination of nitrite and nitrate. Talanta 2025, 292, 127919. [Google Scholar] [CrossRef] [PubMed]
- Siri, Y.; Sthapit, N.; Malla, B.; Raya, S.; Haramoto, E. Comparative performance of electronegative membrane filtration and automated concentrating pipette for detection of antibiotic resistance genes and microbial markers in river water samples. Sci. Total Environ. 2024, 953, 176109. [Google Scholar] [CrossRef]
- Blums, K.; Herzog, J.; Costa, J.; Quirico, L.; Turber, J.; Weuster-Botz, D. Automation of RNA-Seq Sample Preparation and Miniaturized Parallel Bioreactors Enable High-Throughput Differential Gene Expression Studies. Microorganisms 2025, 13, 849. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Deng, Q.; Li, J.; Yang, L.; Li, H.; Zhao, Z.; Wang, Z.; Pang, C.; Zhang, Y.; Lui, V.C.; et al. Sound-controlled fluidic processor. Sci. Adv. 2025, 11, eadv6314. [Google Scholar] [CrossRef]
- Mau, R.; Seitz, H. Influence of the Volatility of Solvent on the Reproducibility of Droplet Formation in Pharmaceutical Inkjet Printing. Pharmaceutics 2023, 15, 367. [Google Scholar] [CrossRef]
- Jacobs, C.B.; Peairs, M.J.; Venton, B.J. Review: Carbon nanotube based electrochemical sensors for biomolecules. Anal. Chim. Acta 2010, 662, 105–127. [Google Scholar] [CrossRef]
- Bontidean, I.; Ahlqvist, J.; Mulchandani, A.; Chen, W.; Bae, W.; Mehra, R.K.; Mortari, A.; Csöregi, E. Novel synthetic phytochelatin-based capacitive biosensor for heavy metal ion detection. Biosens. Bioelectron. 2003, 18, 547–553. [Google Scholar] [CrossRef]
- Datsenko, K.A.; Wanner, B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [Google Scholar] [CrossRef]
- Huang, C.M.; Zhu, Y.; Jin, D.Q.; Kelly, R.T.; Fang, Q. Direct Surface and Droplet Microsampling for Electrospray Ionization Mass Spectrometry Analysis with an Integrated Dual-Probe Microfluidic Chip. Anal. Chem. 2017, 89, 9009–9016. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Shirani, E.; Inglis, D.W. Droplets for Sampling and Transport of Chemical Signals in Biosensing: A Review. Biosensors 2019, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Fang, Q. Analytical detection techniques for droplet microfluidics—A review. Anal. Chim. Acta 2013, 787, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhou, Y.; Chen, Y.; Zhou, L.; Yang, Y.; Xiao, J.; Liu, G.L.; Wang, J.; Huang, L.; Li, Y. High-Electron-Mobility MXene-Enhanced Metasurface Biosensors Integrated with Microfluidics for Real-Time Multifunctional Monitoring. ACS Nano 2025, 19, 12007–12020. [Google Scholar] [CrossRef]
- Wang, Y.; Xue, Y.; Wang, H.; Qu, Y.; Zhang, K.; Shang, L.; Liang, P.; Chen, F.; Tang, X.; Luo, W.; et al. Automated Laser-Assisted Single-Cell Sorting for Cell Functional and RNA Sequencing. ACS Sens. 2025, 10, 846–856. [Google Scholar] [CrossRef]
- Gong, P.; Gao, Z.; Sun, R.; He, B.; Yuan, F.; Chen, C.; Zhao, J.; Su, H.; Wang, L.; Liu, B.; et al. A microfluidic system for rapid enrichment and sensitive detection of E. coli based on bilayer membrane and high flux droplets. Talanta 2025, 293, 128005. [Google Scholar] [CrossRef]
- Sun, M.; Fang, Q. High-throughput sample introduction for droplet-based screening with an on-chip integrated sampling probe and slotted-vial array. Lab Chip 2010, 10, 2864–2868. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, R.; Gong, P.; Al-Olayan, E.; Abukhadra, M.R.; Liu, B.; Su, P.; Zhang, D.; Feng, S. A Large-field droplets for high-throughput Escherichia coli identification within one field of view. Talanta 2025, 284, 127242. [Google Scholar] [CrossRef]
- Nan, L.; Lai, M.Y.A.; Tang, M.Y.H.; Chan, Y.K.; Poon, L.L.M.; Shum, H.C. On-Demand Droplet Collection for Capturing Single Cells. Small 2020, 16, e1902889. [Google Scholar] [CrossRef]
- Song, H.; Ismagilov, R.F. Millisecond kinetics on a microfluidic chip using nanoliters of reagents. J. Am. Chem. Soc. 2003, 125, 14613–14619. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Huang, Y.; Huang, Y.; Wang, H.; Li, M.; Zhou, Y.; Zhao, H.; Lan, Y.; Wu, Z.; Jia, C.; et al. Droplet magnetic-controlled microfluidic chip integrated nucleic acid extraction and amplification for the detection of pathogens and tumor mutation sites. Anal. Chim. Acta 2023, 1271, 341469. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yong, Z.; Leung, C.W.; Zhang, X.; Chen, Y.; Chan, H.L.; Wang, Y. Time-variant 1D photonic crystals using flowing microdroplets. Opt. Express 2012, 20, 24330–24341. [Google Scholar] [CrossRef] [PubMed]
- Yadavali, S.; Jeong, H.H.; Lee, D.; Issadore, D. Silicon and glass very large scale microfluidic droplet integration for terascale generation of polymer microparticles. Nat. Commun. 2018, 9, 1222. [Google Scholar] [CrossRef]
- Zhu, P.; Wang, L. Passive and active droplet generation with microfluidics: A review. Lab Chip 2016, 17, 34–75. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Z.; Gong, P.; Su, H.; Gao, Z.; Feng, S.; Zhao, J. A Pipette for High-Resolution Sampling and Delivery of pL Bio-Samples. Micromachines 2025, 16, 630. https://doi.org/10.3390/mi16060630
Han Z, Gong P, Su H, Gao Z, Feng S, Zhao J. A Pipette for High-Resolution Sampling and Delivery of pL Bio-Samples. Micromachines. 2025; 16(6):630. https://doi.org/10.3390/mi16060630
Chicago/Turabian StyleHan, Ziyang, Pengfei Gong, Hengxiang Su, Zehang Gao, Shilun Feng, and Jianlong Zhao. 2025. "A Pipette for High-Resolution Sampling and Delivery of pL Bio-Samples" Micromachines 16, no. 6: 630. https://doi.org/10.3390/mi16060630
APA StyleHan, Z., Gong, P., Su, H., Gao, Z., Feng, S., & Zhao, J. (2025). A Pipette for High-Resolution Sampling and Delivery of pL Bio-Samples. Micromachines, 16(6), 630. https://doi.org/10.3390/mi16060630





