Modulating Reaction Kinetics Using an Electrolytic Method to Achieve Efficient Vehicle Identification Number Reappearance
Abstract
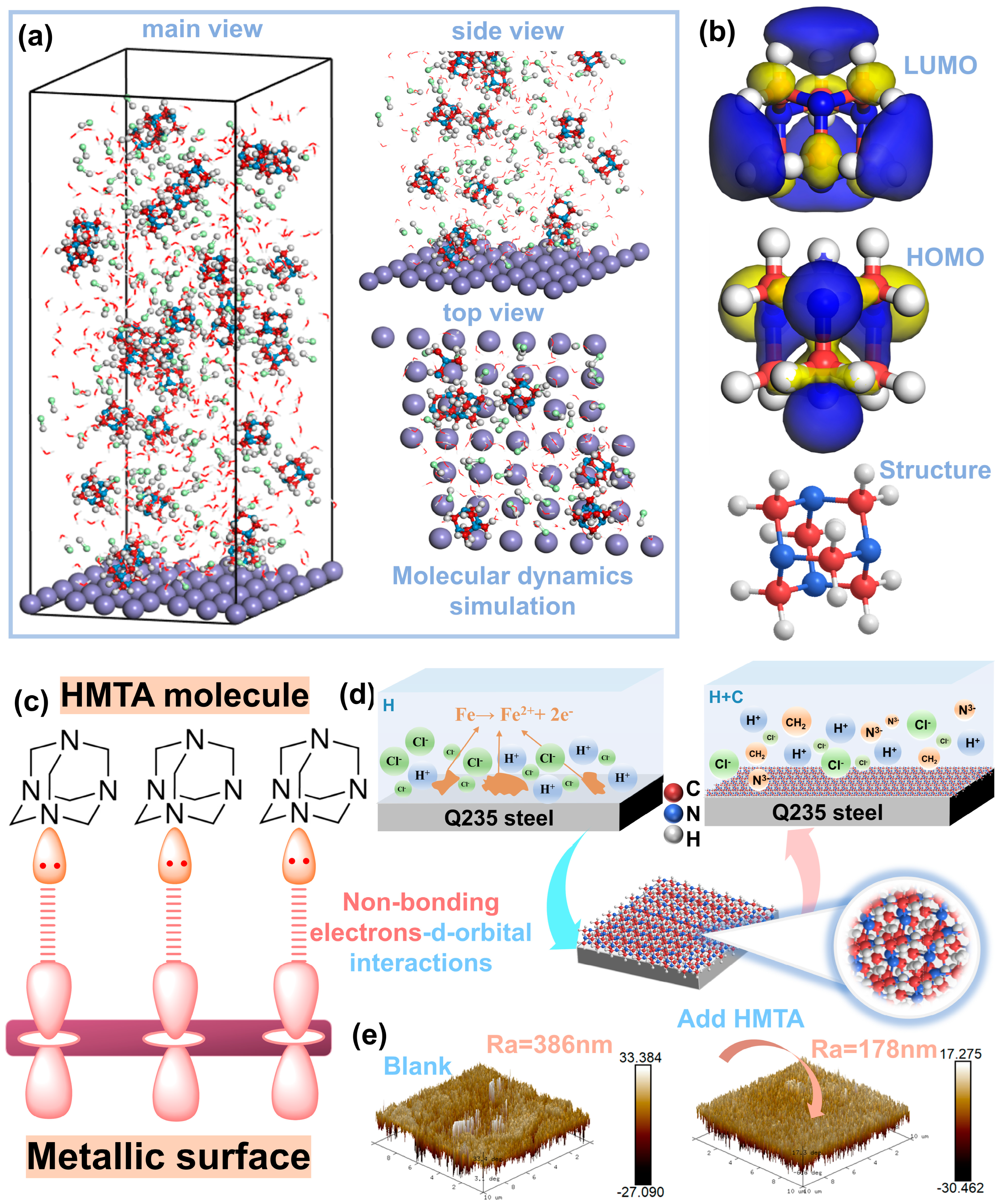
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Sample Preparation
2.3. Reappearance of Engraved Marks
2.4. Surface Analysis
2.5. Electrochemical Characterization
2.6. Computational Details
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Yuan, G.; Li, L.; Kang, J.; Yan, F.; Du, P.; Raabe, D.; Wang, G. Ductile 2-GPa steels with hierarchical substructure. Science 2023, 379, 168–173. [Google Scholar] [CrossRef]
- Kim, S.-H.; Kim, H.; Kim, N.J. Brittle intermetallic compound makes ultrastrong low-density steel with large ductility. Nature 2015, 518, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Lopez, F.A.; Lauinger, D.; Vuille, F.; Müller, D.B. On the potential of vehicle-to-grid and second-life batteries to provide energy and material security. Nat. Commun. 2024, 15, 4179. [Google Scholar] [CrossRef] [PubMed]
- Levine, N.; Ceccato, V. Malignant mixes: The overlap of motor vehicle crashes and crime in Stockholm. Anal. Prev. 2021, 161, 106361. [Google Scholar] [CrossRef]
- Stauffer, E.; Bonfanti, M. Forensic Investigation of Stolen-Recovered and Other Crime-Related Vehicles; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Moala, A.; Ho, L.; Quinino, R. Multivariate control charts to monitor the monthly frequency of vehicle robberies in São Paulo city. Spatial Stat. 2019, 29, 49–65. [Google Scholar] [CrossRef]
- Tseng, P.; Lin, P.; Kristianto, E. Vehicle theft detection by generative adversarial networks on driving behavior. Eng. Appl. Artif. Intell. 2023, 117, 105571. [Google Scholar] [CrossRef]
- Dao, T.; Thill, J. CrimeScape: Analysis of socio-spatial associations of urban residential motor vehicle theft. Soc. Sci. Res. 2022, 101, 102618. [Google Scholar] [CrossRef]
- Uysal, S.; Mercan, M.; Uzun, L. Serial number restoration on polymer surfaces: A survey of recent literature. Forensic Chem. 2020, 20, 100267. [Google Scholar] [CrossRef]
- Uysal, S.; Yildiz, E.; Armutcu, C.; Sarıbey, A.Y.; Uzun, L. Development of etching paste for serial number restoration on aluminum engine block. Forensic Sci. 2023, 68, 1325–1329. [Google Scholar] [CrossRef]
- Yin, S.H.; Kuppuswamy, R. On the sensitivity of some common metallographic reagents to restoring obliterated marks on medium carbon (0.31% C) steel surfaces. Forensic Sci. Int. 2009, 183, 50–53. [Google Scholar] [CrossRef]
- Wahab, M.F.A.; Ghani, N.I.M.; Kuppuswamy, R. An investigation into the suitability of some etching reagents to restoring obliterated stamped numbers on cast iron engine blocks of cars. Forensic Sci. Int. 2012, 223, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Uli, N.; Kuppuswamy, R.; Amran, M.F.C. A survey of some metallographic etching reagents for restoration of obliterated engraved marks on aluminium–silicon alloy surfaces. Forensic Sci. Int. 2011, 208, 66–73. [Google Scholar] [CrossRef]
- Lima, M.G.B.; Pacheco, C.J.; Nóbrega, M.C.S.; Pereira, G.R.J. Sensitivity analysis of nondestructive magnetic techniques for the restoration of stamped marks on low carbon steel. J. Mater. Res. Technol. 2020, 9, 162–167. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, R.; Zhao, J.; Zou, Z.; Huang, Q.; Sui, Y.; Shi, J.; Aslam, R.; Sun, Y.; Yan, Z. A Corrosion inhibition performance of protein derived carbon quantum dots as corrosion inhibitors on low carbon steel in sulfuric acid solution. Microchem. J. 2024, 207, 111957. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, S.; Long, J.; Liu, H.; Han, P.; Qiao, Y.; Zheng, K. Revealing the influence of zirconium content on the cavitation erosion-corrosion of a wear-resistant steel in sodium chloride solution. Tribol. Int. 2023, 189, 108942. [Google Scholar] [CrossRef]
- Wang, B.; Xue, W.; Wu, Z.; Jin, X.; Wu, J.; Du, J. Influence of discharge time on properties of plasma electrolytic borocarburized layers on Q235 low-carbon steel. Mater. Chem. Phys. 2015, 168, 10–17. [Google Scholar] [CrossRef]
- Luo, X.; Li, C.; Liu, P.; Fu, Y.; Du, J.; Chen, X.; Liu, J.; Zuo, Z.; Zhao, L. Optimization and evaluation of corrosion inhibitors for N80 steel in sulfamic acid solutions. Electrochem. Sci. 2024, 19, 100759. [Google Scholar] [CrossRef]
- Wang, B.; Xue, W.; Jin, X.; Zhang, Y.; Wu, Z.; Li, Y. Combined treatment plasma electrolytic carburizing and borocarburizing on Q235 low-carbon steel. Mater. Chem. Phys. 2019, 221, 232–238. [Google Scholar] [CrossRef]
- Shwetha, K.M.; Praveen, B.M.; Devendra, B.K. A review on corrosion inhibitors: Types, mechanisms, electrochemical analysis, corrosion rate and efficiency of corrosion inhibitors on mild steel in an acidic environment. Res. Surf. Interfaces 2024, 16, 100258. [Google Scholar] [CrossRef]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP); Bampidis, V.; Azimonti, G.; de Lourdes Bastos, M.; Christensen, H.; Dusemund, B.; Kouba, M.; Durjava, M.K.; López-Alonso, M.; Puente, S.L.; et al. Safety of hexamethylene tetramine for pigs, poultry, bovines, sheep, goats, rabbits and horses. EFSA J. 2020, 18, e06012. [Google Scholar] [CrossRef]
- Tan, Y.; Azizuddin, A.; Câmpian, M.; Haiduc, I.; Tiekink, E. Serendipitous compositional and structural diversity in urotropine adducts of binary cadmium xanthates. Z. Krist.—Cryst. Mater. 2016, 231, 155–165. [Google Scholar] [CrossRef][Green Version]
- Huang, J.; Xu, J.; Guan, B.; Fu, R.; Hu, Q.; Liu, W.; Hu, Z. Effect of Al addition on mechanical and corrosion behavior of B10 alloy. J. Mater. Res. Technol. 2025, 36, 13–25. [Google Scholar] [CrossRef]
- Havlík, R.; Drienovský, M.; Gerhátová, Ž.; Babincová, P.; Kusý, M.; Gogola, P.; Palcut, M. Phase constitution, microstructure and corrosion performance of binary Sn–Bi alloys. J. Mater. Res. Technol. 2025, 36, 173–181. [Google Scholar] [CrossRef]
- Wang, X.; Yang, J.; Chen, X. 2-benzylsulfanyl-1H-benzoimidazole and its mixture as highly efficient corrosion inhibitors for carbon steel under dynamic supercritical CO2 flow conditions. Corros. Sci. 2024, 235, 112170. [Google Scholar] [CrossRef]
- Mahdavi, M.; Abdollah-Zadeh, A.; Elmkhah, H. A comparative study on time-dependent corrosion properties of Ti-BN films applied by PACVD method. J. Mater. Res. Technol. 2025, 36, 80–97. [Google Scholar] [CrossRef]
- Doumane, G.; Bensalah, J.; Ouakki, M.; Aribou, Z.; Boussalem, O.; Mzioud, K.; Safi, Z.S.; Berisha, A.; Bourhia, M.; Gaafar, A.-R.Z.; et al. Alkaloid extract of seed Citrullus colocynthis as novel green inhibitor for mild steel corrosion in one molar HCl acid solution: DFT and MC/MD approaches. Sci. Rep. 2024, 14, 16857. [Google Scholar] [CrossRef]
- Nguyen, T.; Arrighi, C.; Thai, T.; Dangreau, L.; Gonon, M.; Trinh, A.; Olivier, M.-G. Inhibitive effect of the Ce (III) chloride and nitrate on the corrosion resistance of Zn alloyed sacrificial coatings: Effect of alloying compounds of the sacrificial layer. Electrochim. Acta 2023, 452, 142296. [Google Scholar] [CrossRef]
- Chu, Q.; Yang, H.; Liu, Z.; Li, X.; Niu, G.; Zhang, J.; Zhang, X.; Li, Y.; Ye, Y. Thiol–ene Click Chemistry Synthesis of L-Cysteine-Grafted Graphene Oxide as a New Corrosion Inhibitor for Q235 Steel in Acidic Environment. Langmuir 2024, 40, 12526–12538. [Google Scholar] [CrossRef]
- Tang, H.; Zhou, C.; Li, J.; Xiong, W.; Chen, B.; Peng, J.; Pan, X.; Guo, M.; Xiao, Z.; Dai, H.; et al. In-depth insight into corrosion inhibition performance of sweet potato leaf extract as a green and efficient inhibitor for 6N01 Al alloy in the seawater: Experimental and theoretical perspectives. Langmuir 2024, 40, 9543–9555. [Google Scholar] [CrossRef]
- De Ketelaere, E.; Moed, D.; Vanoppen, M.; Depover, T.; Verbeken, K. Investigation of an eco-friendly sodium alginate-sodium silicate inhibitor blend for carbon steel in a dynamic salt water environment. Corros. Sci. 2024, 231, 111991. [Google Scholar] [CrossRef]
- Feng, L.; Zheng, S.; Ma, X.; Zhu, H.; Hu, Z.; Sun, Y. Enhancing corrosion protection in acidic environments through biomass-derived carbon quantum dots. Microchem. J. 2024, 199, 110003. [Google Scholar] [CrossRef]
- El-Hout, S.; Radwan, A.; Salem, A.; Alrashdi, K.; Al-Qahtani, S.; Chen, C. Hierarchical nanostructures and their implications in pushing the boundaries of chemiresistive gas sensing. Microchem. J. 2024, 199, 111643. [Google Scholar] [CrossRef]
- Jia, H.; Jia, H.; Wang, Q.; Xu, Y.; Wang, B.; Wang, Q.; Li, X.; Wang, Z.; Lv, K.; Huang, P. Imidazolium-based polymeric ionic liquids with short alkyl chains as green corrosion inhibitors for mild steel in 1 M HCl: Experimental and theoretical investigations. Langmuir 2024, 40, 14141–14152. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Wang, X.; Liu, H.; Luo, H.; Zhang, Z. Explore the corrosion behavior of Al-Mg-Si-Cu alloy with various microstructure characteristics through controlling Zener-Hollomon parameter. Mater. Charact. 2024, 217, 114390. [Google Scholar] [CrossRef]
- Li, C.; Zhang, P.; Yang, B.; Li, Y. Effect of carbon content on the corrosion behavior of Ni-Co-Cr-Mo-Cu alloys in both sulfuric and hydrochloric acids. Mater. Charact. 2024, 215, 114200. [Google Scholar] [CrossRef]
- Xiang, G.; Yin, J.; Zhang, X.; Hou, P.; Xu, X. Booting the electrochemical properties of Fe-based anode by the formation multiphasic nanocomposite for lithium-ion batteries. Chin. Chem. Lett. 2021, 32, 2169–2173. [Google Scholar] [CrossRef]
- Wang, Y.; Goh, B.; Sridharan, K.; Couet, A. In situ corrosion monitoring of the T91 alloy in a molten chloride salt using a miniaturized electrochemical probe for high-throughput applications. Anal. Chem. 2022, 94, 4012–4020. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, F.; Ma, M.; Liu, Z. Fabrication of Highly Ordered Macropore Arrays in p-Type Silicon by Electrochemical Etching: Effect of Wafer Resistivity and Other Etching Parameters. J. Micromachines 2025, 16, 154. [Google Scholar] [CrossRef]
- Li, Z.; Yang, W.; Yu, Q.; Wu, Y.; Wang, D.; Liang, J.; Zhou, F. New method for the corrosion resistance of AZ31 Mg alloy with a porous micro-arc oxidation membrane as an ionic corrosion inhibitor container. Langmuir 2018, 35, 1134–1145. [Google Scholar] [CrossRef]
- Dauphin-Ducharme, P.; Mauzeroll, J. Surface analytical methods applied to magnesium corrosion. J. Anal. Chem. 2015, 87, 7499–7509. [Google Scholar] [CrossRef]
- Pan, L.; Li, G.; Wang, Z.; Liu, D.; Zhu, W.; Tong, C.; Zhu, R.; Hu, S. Carbon dots as environment-friendly and efficient corrosion inhibitors for Q235 steel in 1 M HCl. Langmuir 2021, 37, 14336–14344. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhu, M.; He, Z.; Zhang, R.; Kaya, S.; Lin, Y.; Saji, V.S. One-pot hydrothermal synthesized nitrogen and sulfur codoped carbon dots for acid corrosion inhibition of Q235 steel. Langmuir 2022, 38, 3984–3992. [Google Scholar] [CrossRef] [PubMed]
- Grandy, L.; Yassine, S.R.; Lacasse, R.; Mauzeroll, J. Selective Initiation of Corrosion Pits in Stainless Steel Using Scanning Electrochemical Cell Microscopy. Anal. Chem. 2024, 96, 7394–7400. [Google Scholar] [CrossRef]
- Yan, W.; Xue, S.; Zhao, X.; Zhang, W.; Li, J. Hexagonal boron nitride based slippery liquid infused porous surface with anti-corrosion, anti-contaminant and anti-icing properties for protecting magnesium alloy. J. Chin. Chem. Lett. 2024, 35, 109224. [Google Scholar] [CrossRef]
- Zheng, T.; Liu, J.; Wang, M.; Liu, Q.; Wang, J.; Chong, Y.; Jia, G. Synergistic corrosion inhibition effects of quaternary ammonium salt cationic surfactants and thiourea on Q235 steel in sulfuric acid: Experimental and theoretical research. Corros. Sci. 2022, 199, 110199. [Google Scholar] [CrossRef]
- Su, H.; Liu, Y.; Gao, X.; Qian, Y.; Li, W.; Ren, T.; Wang, L.; Zhang, J. Corrosion inhibition of magnesium alloy in NaCl solution by ionic liquid: Synthesis, electrochemical and theoretical studies. J. Alloys Compd. 2019, 791, 681–689. [Google Scholar] [CrossRef]
- Silva, G.; Morgado, P.; Filipe, E. Towards compartmentalized micelles: Mixed perfluorinated and hydrogenated ionic surfactants in aqueous solution. J. Colloid. Interface Sci. 2024, 654, 906–914. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, R.; Tan, B.; Li, W.; Liu, H.; Wu, S. Locust Bean Gum as a green and novel corrosion inhibitor for Q235 steel in 0.5 M H2SO4 medium. J. Mol. Liq. 2020, 310, 113239. [Google Scholar] [CrossRef]
- Zhang, Q.; Xing, S.; Han, J.; Feng, L.; Li, J.; Qian, Z.; Zhou, J. Organic pollutant sensing for human health based on carbon dots. Chin. Chem. Lett. 2024, 36, 110117. [Google Scholar] [CrossRef]
- Ardakani, E.; Kowsari, E.; Ehsani, A.; Ramakrishna, S. Performance of all ionic liquids as the eco-friendly and sustainable compounds in inhibiting corrosion in various media: A comprehensive review. Microchem. J. 2021, 165, 106049. [Google Scholar] [CrossRef]
- Liu, C.; Wang, B.; Wang, X.; Liu, J.; Gao, G.; Zhou, J. Effect of Alkyl Chain Length on the Corrosion Inhibition Performance of Imidazolium-Based Ionic Liquids for Carbon Steel in 1 M HCl Solution: Experimental Evaluation and Theoretical Analysis. Langmuir 2024, 40, 8806–8819. [Google Scholar] [CrossRef] [PubMed]

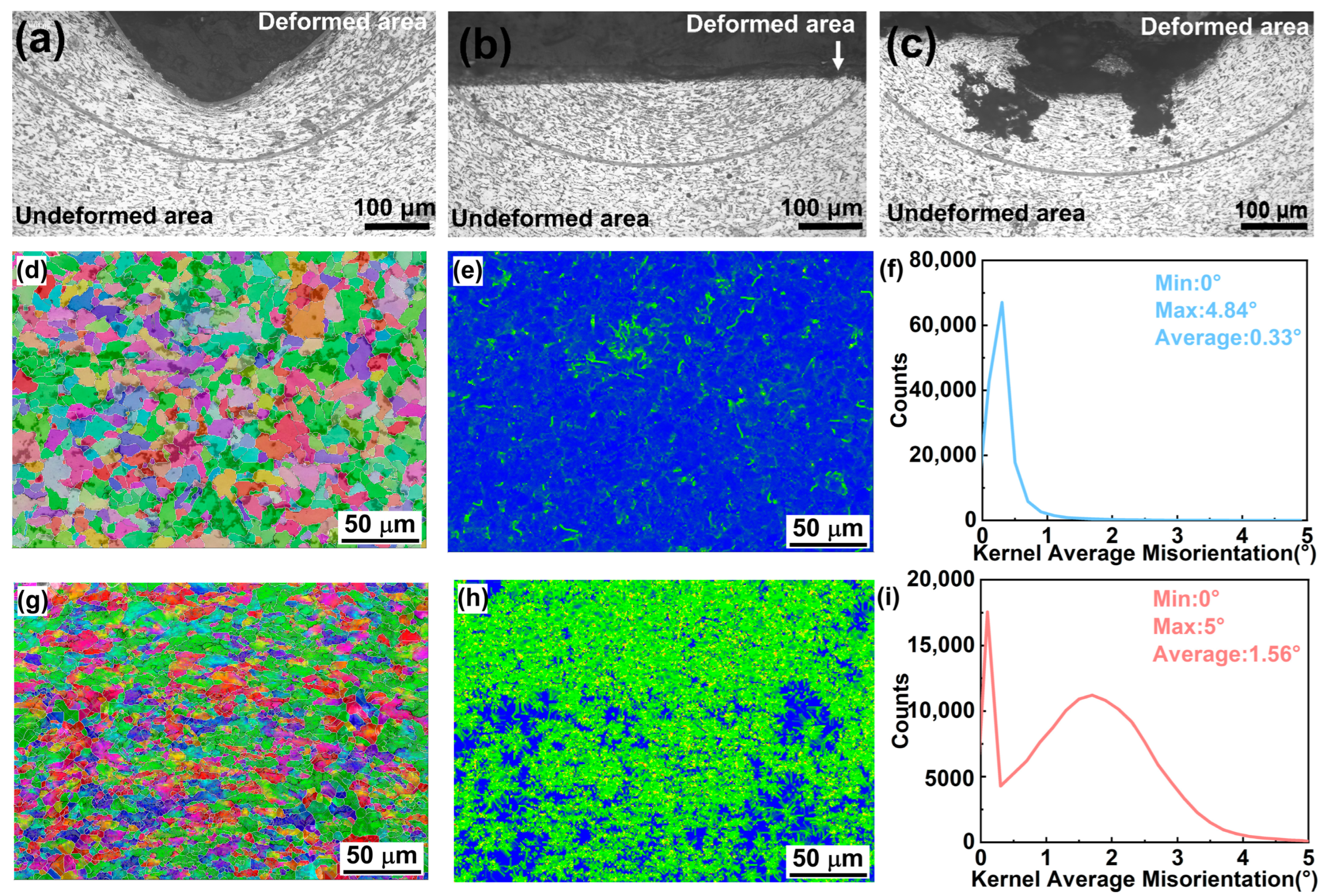

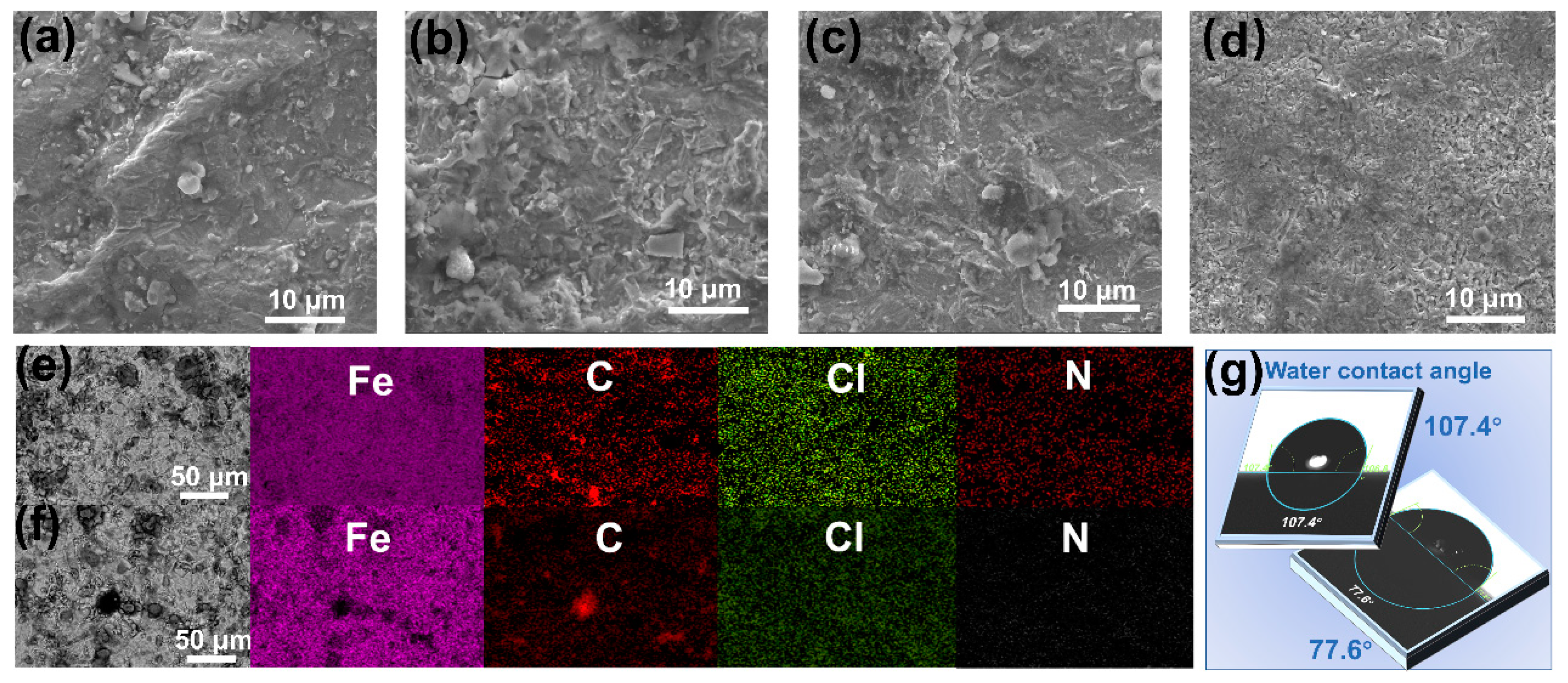
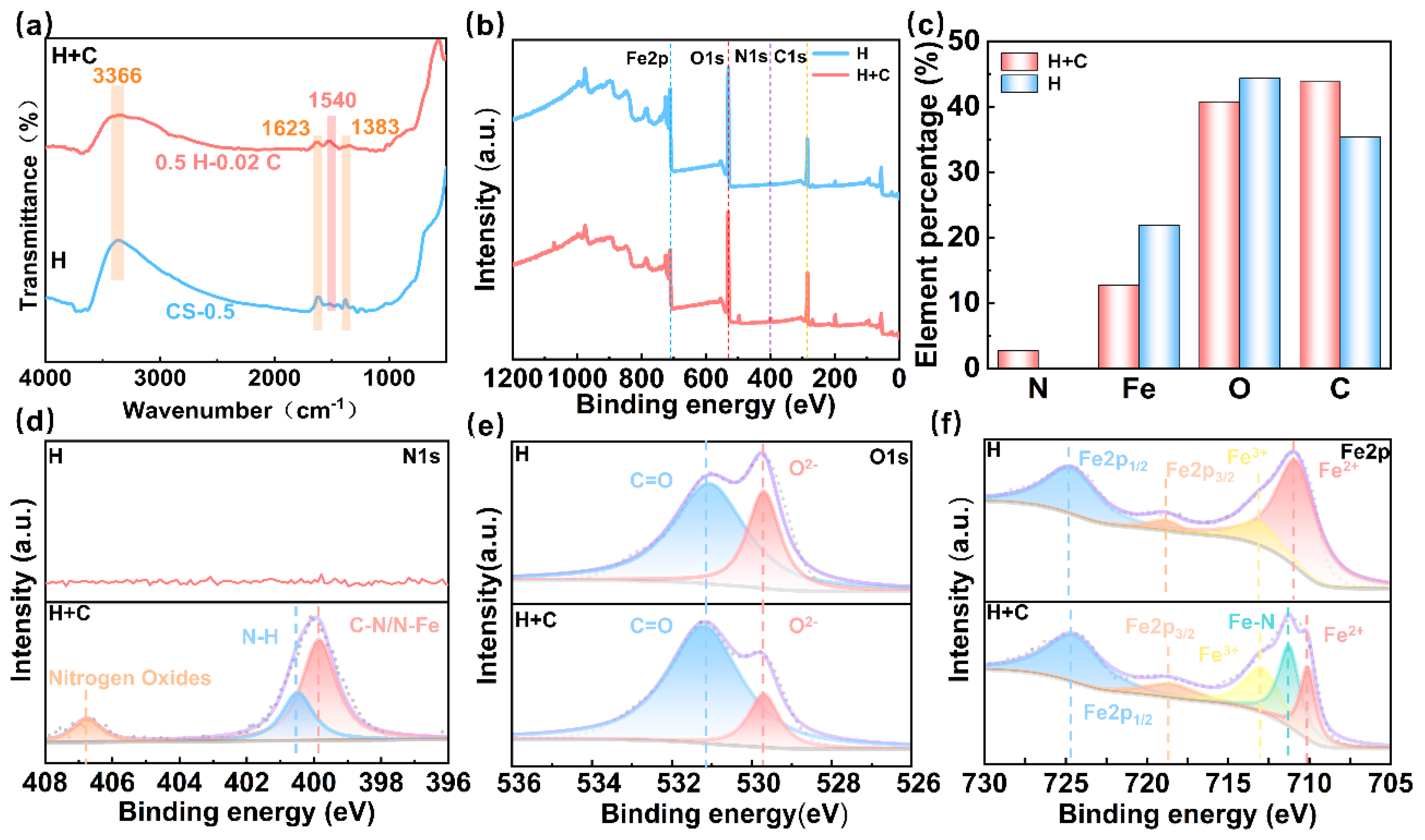
| Reagent (M) | Current (A) | Average Time (S) | Experimental Results |
|---|---|---|---|
| 0 | 0.25~0.53 | - | Failure to reappear |
| 0.01 | 0.29~0.45 | 174 | Recognizable but blurry |
| 0.02 | 0.29~0.47 | 198 | Clear contrast is obvious |
| 0.03 | 0.33~0.57 | 200 | Clear contrast is obvious |
| 0.04 | 0.33~0.59 | 207 | Recognizable but blurry |
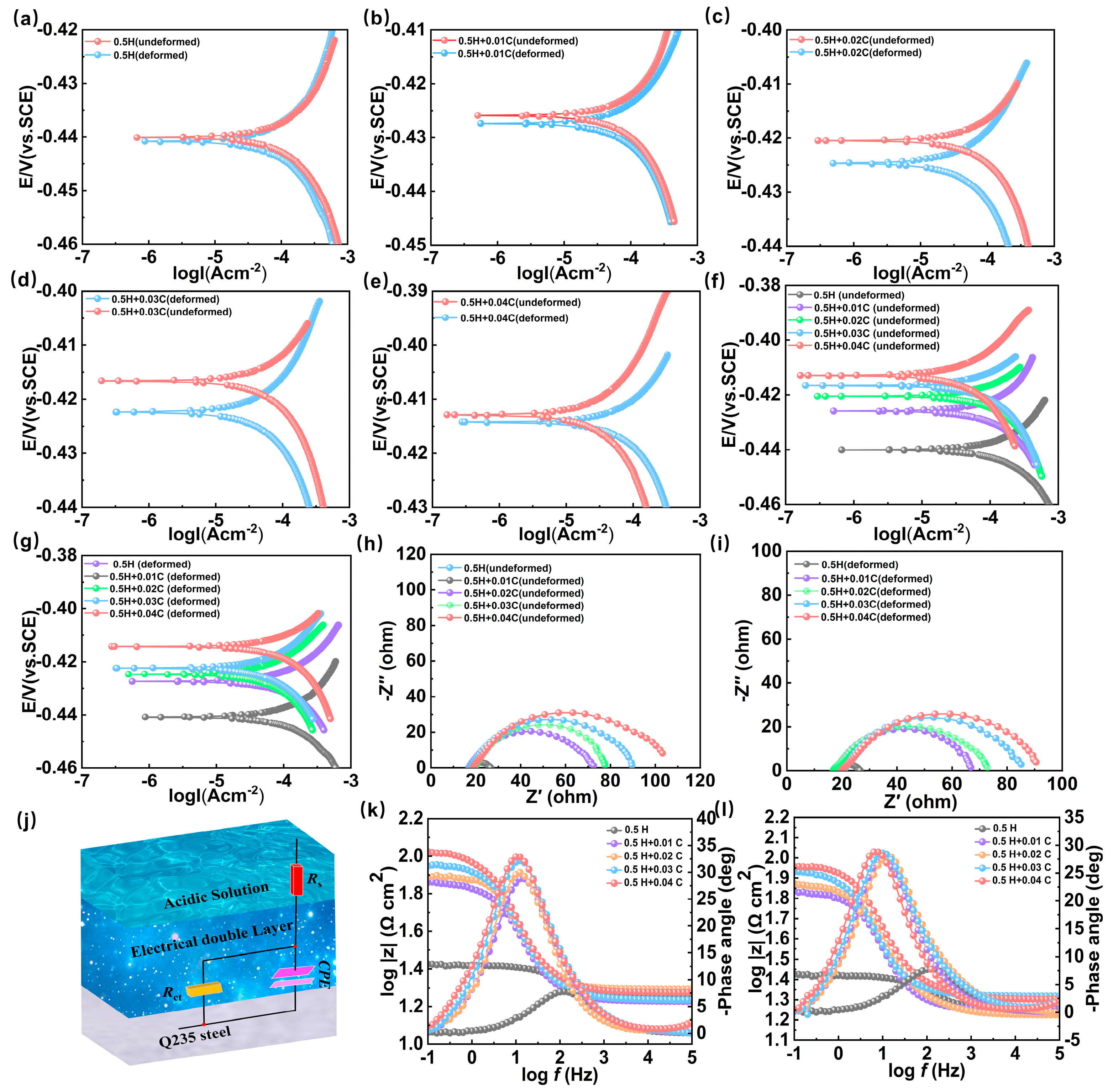
| Metallic Condition | Concentration (M) | −Ecorr (mV) | Icorr × 10−3 (A/cm−2) | Δ (mV) | θ |
|---|---|---|---|---|---|
| Undeformed | 0 | 439 | 0.664 | 1 | - |
| 0.01 | 426 | 0.506 | 2 | 0.237 | |
| 0.02 | 420 | 0.293 | 5 | 0.558 | |
| 0.03 | 416 | 0.196 | 6 | 0.704 | |
| 0.04 | 412 | 0.165 | 2 | 0.751 | |
| Deformed | 0 | 440 | 0.887 | 1 | - |
| 0.01 | 427 | 0.56 | 2 | 0.368 | |
| 0.02 | 425 | 0.493 | 5 | 0.444 | |
| 0.03 | 422 | 0.332 | 6 | 0.625 | |
| 0.04 | 414 | 0.278 | 2 | 0.686 |
| Metallic Condition | Concentration (M) | Rs (Ω cm2) | Rct (Ω cm2) | Y0 × 10−6 (S sn cm−2) | n | η (%) |
|---|---|---|---|---|---|---|
| undeformed | 0 | 17.97 | 8.37 | 856.73 | 0.7908 | - |
| 0.01 | 16.97 | 55.41 | 799.73 | 0.7997 | 84.89 | |
| 0.02 | 19.73 | 59.29 | 765.87 | 0.8563 | 85.88 | |
| 0.03 | 17.35 | 74.07 | 792.55 | 0.7925 | 88.69 | |
| 0.04 | 18.94 | 87.62 | 780.98 | 0.7809 | 90.44 | |
| deformed | 0 | 17.98 | 8.29 | 1322 | 0.7967 | - |
| 0.01 | 17.88 | 50.15 | 1198 | 0.8214 | 83.46 | |
| 0.02 | 17 | 57.61 | 1148 | 0.7628 | 85.61 | |
| 0.03 | 20.93 | 64.61 | 1159 | 0.8235 | 87.16 | |
| 0.04 | 20.34 | 73.12 | 1086 | 0.7664 | 88.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhang, X.; Chen, M.; Zhang, X.; Zhang, Z.; Liu, J. Modulating Reaction Kinetics Using an Electrolytic Method to Achieve Efficient Vehicle Identification Number Reappearance. Micromachines 2025, 16, 578. https://doi.org/10.3390/mi16050578
Wang J, Zhang X, Chen M, Zhang X, Zhang Z, Liu J. Modulating Reaction Kinetics Using an Electrolytic Method to Achieve Efficient Vehicle Identification Number Reappearance. Micromachines. 2025; 16(5):578. https://doi.org/10.3390/mi16050578
Chicago/Turabian StyleWang, Jintao, Xiaoshun Zhang, Mengfan Chen, Xihao Zhang, Zhongliang Zhang, and Jianguo Liu. 2025. "Modulating Reaction Kinetics Using an Electrolytic Method to Achieve Efficient Vehicle Identification Number Reappearance" Micromachines 16, no. 5: 578. https://doi.org/10.3390/mi16050578
APA StyleWang, J., Zhang, X., Chen, M., Zhang, X., Zhang, Z., & Liu, J. (2025). Modulating Reaction Kinetics Using an Electrolytic Method to Achieve Efficient Vehicle Identification Number Reappearance. Micromachines, 16(5), 578. https://doi.org/10.3390/mi16050578





