High-Efficiency Drug Loading in Lipid Vesicles by MEMS-Driven Gigahertz Acoustic Streaming
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of GUVs
2.3. MEMS Resonator Fabrication
2.4. Acoustic Experimental Setup
2.5. Preparation of DOX-Encapsulated SUVs
2.6. Absorbance Measurements
2.7. TEM Measurements
2.8. Statistical Analysis
3. Results and Discussion
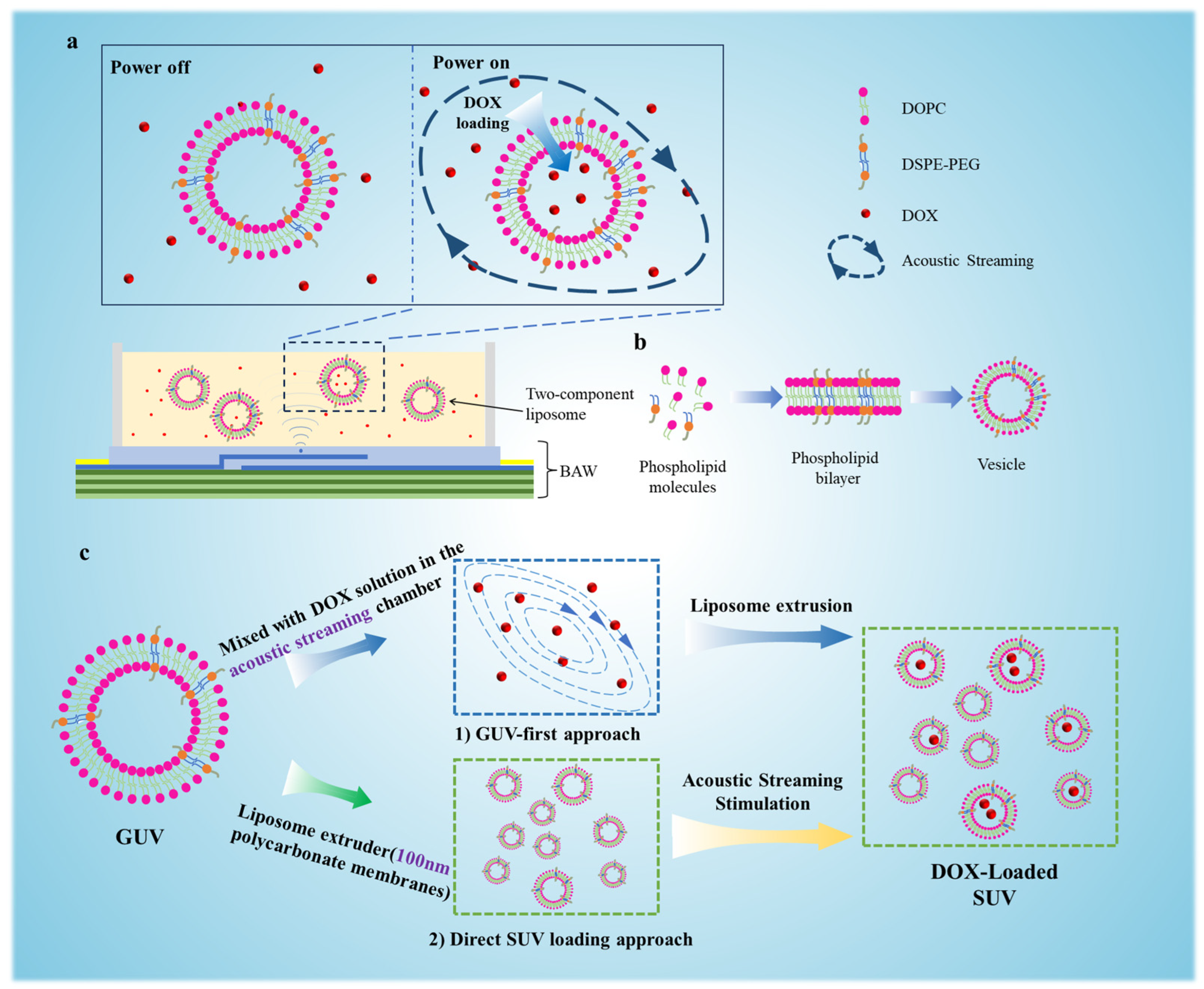
3.1. Acoustic Streaming-Driven Drug Loading Platform
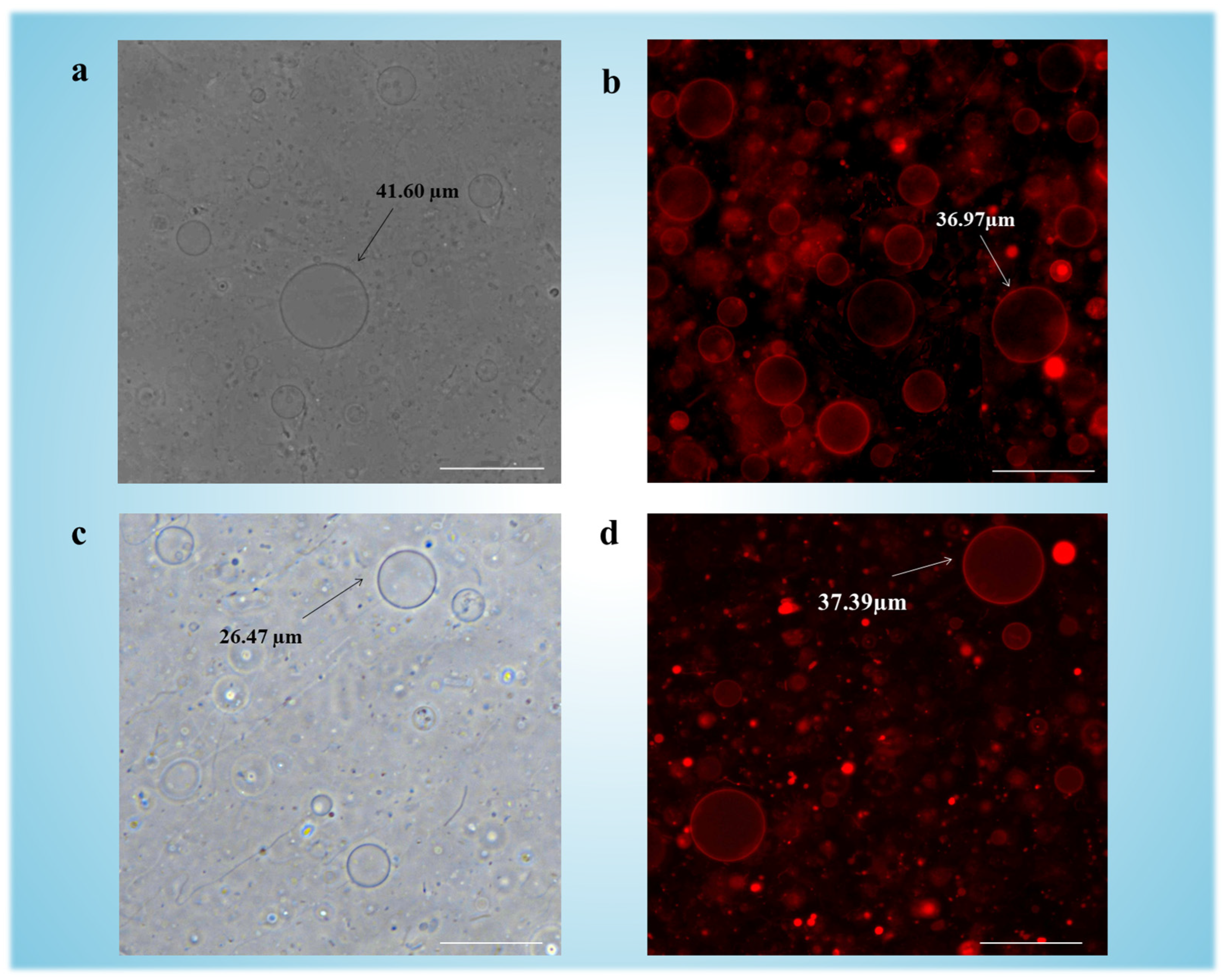
3.2. Synthesis and Structural Characterization of GUVs
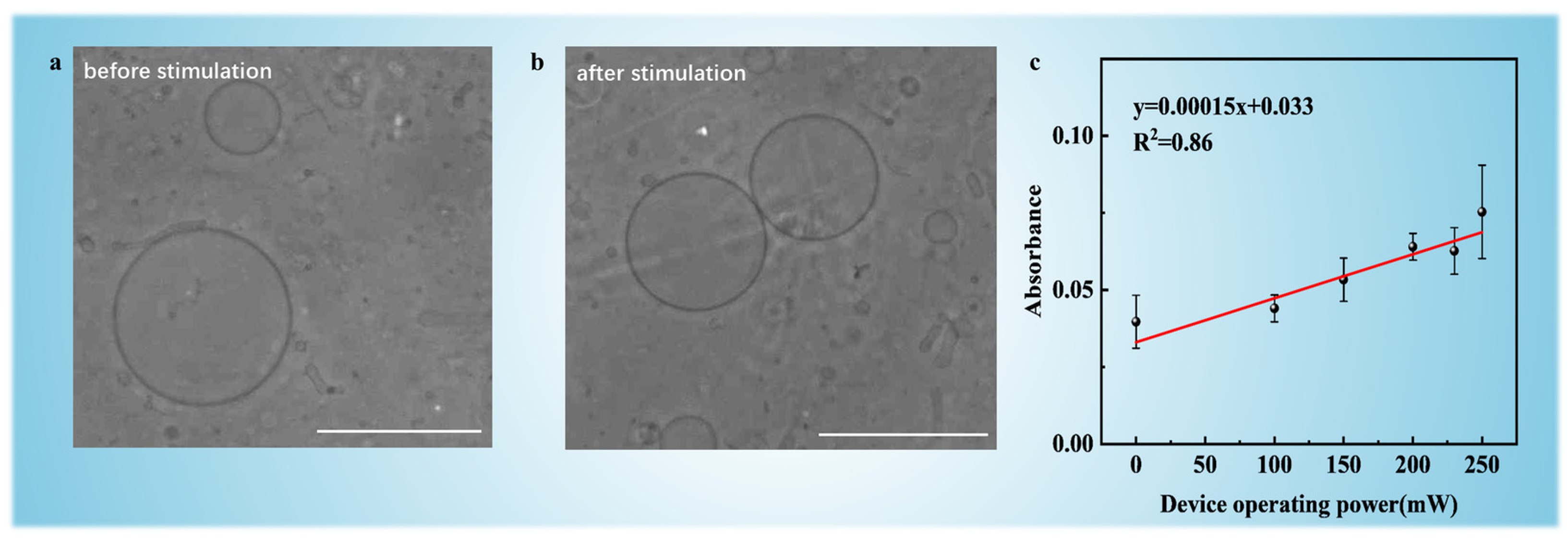
3.3. Power-Dependent DOX Loading in GUVs
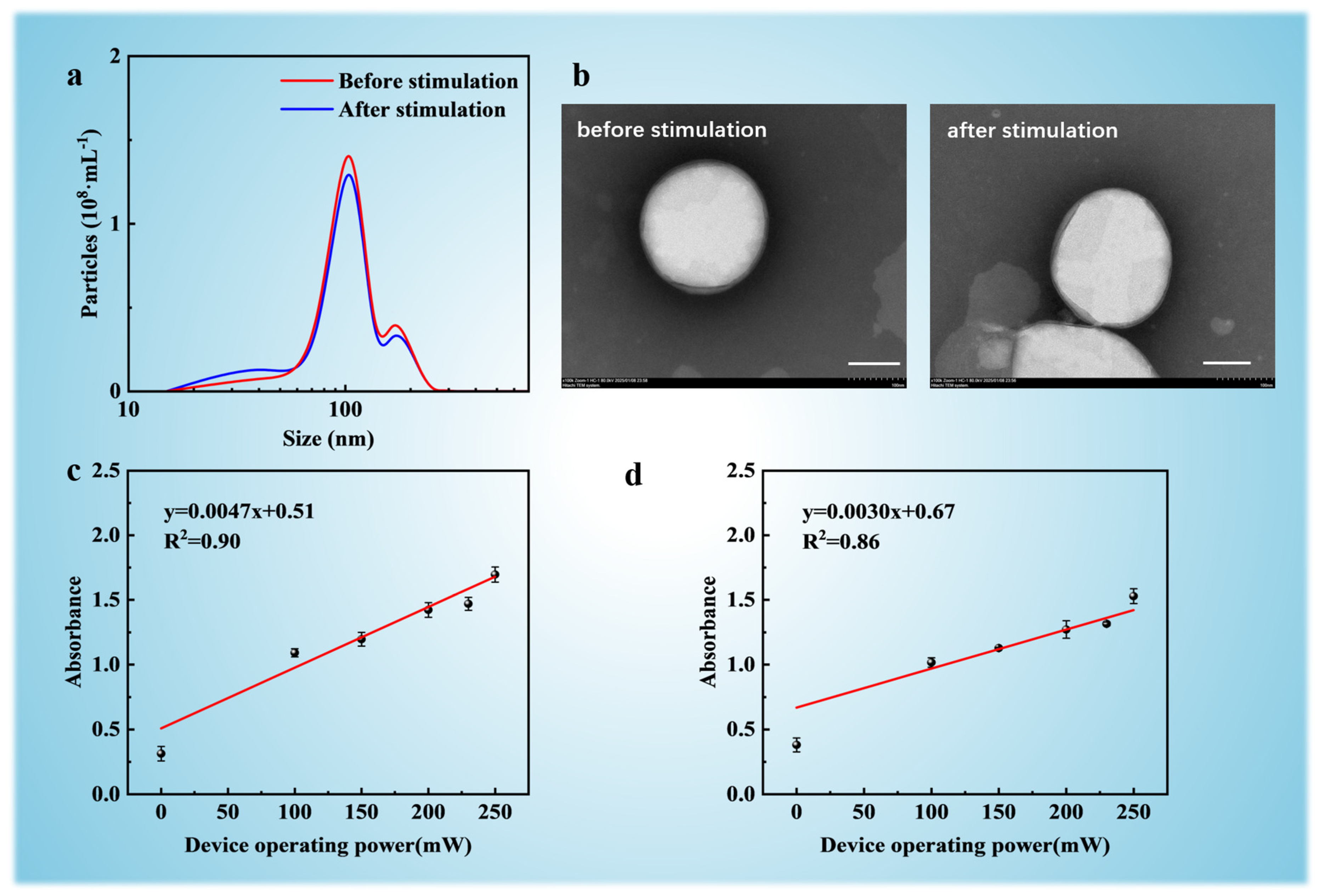
3.4. SUV Loading Efficiency Comparison
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waheed, S.; Li, Z.; Zhang, F.; Chiarini, A.; Armato, U.; Wu, J. Engineering nano-drug biointerface to overcome biological barriers toward precision drug delivery. J. Nanobiotechnol. 2022, 20, 395. [Google Scholar] [CrossRef] [PubMed]
- Moholkar, D.N.; Kandimalla, R.; Gupta, R.C.; Aqil, F. Advances in lipid-based carriers for cancer therapeutics: Liposomes, exosomes and hybrid exosomes. Cancer Lett. 2023, 565, 216220. [Google Scholar] [CrossRef] [PubMed]
- Al-Jipouri, A.; Almurisi, S.H.; Al-Japairai, K.; Bakar, L.M.; Doolaanea, A.A. Liposomes or Extracellular Vesicles: A Comprehensive Comparison of Both Lipid Bilayer Vesicles for Pulmonary Drug Delivery. Polymers 2023, 15, 318. [Google Scholar] [CrossRef] [PubMed]
- Farooque, F.; Wasi, M.; Mughees, M.M. Liposomes as Drug Delivery System: An Updated Review. J. Drug Deliv. Ther. 2021, 11, 149–158. [Google Scholar] [CrossRef]
- Haghiralsadat, F.; Amoabediny, G.; Sheikhha, M.H.; Zandieh-Doulabi, B.; Naderinezhad, S.; Helder, M.N.; Forouzanfar, T. New liposomal doxorubicin nanoformulation for osteosarcoma: Drug release kinetic study based on thermo and pH sensitivity. Chem. Biol. Drug Des. 2017, 90, 368–379. [Google Scholar] [CrossRef]
- De Leo, V.; Milano, F.; Agostiano, A.; Catucci, L. Recent Advancements in Polymer/Liposome Assembly for Drug Delivery: From Surface Modifications to Hybrid Vesicles. Polymers 2021, 13, 1027. [Google Scholar] [CrossRef]
- Cavalcante, C.H.; Fernandes, R.S.; Silva, J.d.O.; Oda, C.M.R.; Leite, E.A.; Cassali, G.D.; Charlie-Silva, I.; Fernandes, B.H.V.; Ferreira, L.A.M.; de Barros, A.L.B. Doxorubicin-loaded pH-sensitive micelles: A promising alternative to enhance antitumor activity and reduce toxicity. Biomed. Pharmacother. 2021, 134, 111076. [Google Scholar] [CrossRef]
- Wei, X.-Q.; Zhu, J.-F.; Wang, X.-B.; Ba, K. Improving the Stability of Liposomal Curcumin by Adjusting the Inner Aqueous Chamber pH of Liposomes. ACS Omega 2020, 5, 1120–1126. [Google Scholar] [CrossRef]
- Naziris, N.; Skandalis, A.; Forys, A.; Trzebicka, B.; Pispas, S.; Demetzos, C. A thermal analysis and physicochemical study on thermoresponsive chimeric liposomal nanosystems. J. Therm. Anal. Calorim. 2020, 141, 751–766. [Google Scholar] [CrossRef]
- Hashimoto, T.; Hirai, Y.; Yuba, E.; Harada, A.; Kono, K. Temperature-Responsive Molecular Assemblies Using Oligo(Ethylene Glycol)-Attached Polyamidoamine Dendron Lipids and their Functions as Drug Carriers. J. Funct. Biomater. 2020, 11, 16. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, F.; Zhang, W.; He, L.; Li, Y.; Zheng, S.; Wang, Y.; Yu, T.; Du, L.; Shen, Y.; et al. Biosynthetic Gas Vesicles Combined with Focused Ultrasound for Blood–Brain Barrier Opening. Int. J. Nanomed. 2022, 17, 6759–6772. [Google Scholar] [CrossRef] [PubMed]
- Amador, G.J.; van Dijk, D.; Kieffer, R.; Aubin-Tam, M.-E.; Tam, D. Hydrodynamic shear dissipation and transmission in lipid bilayers. Proc. Natl. Acad. Sci. USA 2021, 118, e2100156118. [Google Scholar] [CrossRef] [PubMed]
- Pritzl, S.D.; Morstein, J.; Kahler, S.; Konrad, D.B.; Trauner, D.; Lohmüller, T. Postsynthetic Photocontrol of Giant Liposomes via Fusion-Based Photolipid Doping. Langmuir 2022, 38, 11941–11949. [Google Scholar] [CrossRef]
- Caramazza, L.; Nardoni, M.; De Angelis, A.; Paolicelli, P.; Liberti, M.; Apollonio, F.; Petralito, S. Frontiers|Proof-of-Concept of Electrical Activation of Liposome Nanocarriers: From Dry to Wet Experiments. Available online: https://www.frontiersin.org/journals/bioengineering-and-biotechnology/articles/10.3389/fbioe.2020.00819/full (accessed on 23 July 2020).
- Caramazza, L.; Paffi, A.; Liberti, M.; Apollonio, F. Modeling Liposome Electroporation by nsPEF: Towards Realism. In Proceedings of the 2023 IEEE MTT-S International Microwave Biomedical Conference (IMBioC), Leuven, Belgium, 11–13 September 2023; pp. 136–138. [Google Scholar]
- Saha, S.; Ishtiaque, M.S.; Karal, M.A.S.; Alam, M.K. Simulation and Intelligent Data Mining of Molecular Transport Through Multiple Nanopores in an Electroporated Giant Unilamellar Vesicle. In Proceedings of the 2023 International Conference on Information and Communication Technology for Sustainable Development (ICICT4SD), Dhaka, Bangladesh, 21–23 September 2023; pp. 7–11. [Google Scholar]
- Cardoso, B.D.; Cardoso, V.F.; Lanceros-Méndez, S.; Castanheira, E.M.S. Solid Magnetoliposomes as Multi-Stimuli-Responsive Systems for Controlled Release of Doxorubicin: Assessment of Lipid Formulations. Biomedicines 2022, 10, 1207. [Google Scholar] [CrossRef]
- Broek, B.V.D.; Wuyts, C.; Irobi, J. Extracellular vesicle-associated small heat shock proteins as therapeutic agents in neurodegenerative diseases and beyond. Adv. Drug Deliv. Rev. 2021, 179, 114009. [Google Scholar] [CrossRef]
- Vaishnav, J.K.; Mukherjee, T.K. Selective uptake and modulation of nanometal surface energy transfer from quantum dot to Au nanoparticle across lipid bilayer of liposomes. J. Photochem. Photobiol. A Chem. 2020, 401, 112773. [Google Scholar] [CrossRef]
- Urakami, N.; Sakuma, Y.; Chiba, T.; Imai, M. Vesicle deformation and division induced by flip-flops of lipid molecules. Soft Matter 2021, 17, 8434–8445. [Google Scholar] [CrossRef]
- Tian, M.; Keshavarz, M.; Demircali, A.A.; Han, B.; Yang, G. Localized Microrobotic Delivery of Enzyme-Responsive Hydrogel-Immobilized Therapeutics to Suppress Triple-Negative Breast Cancer. Small 2024, 20, e2408813. [Google Scholar] [CrossRef]
- Yuana, Y.; Jiang, L.; Lammertink, B.H.; Vader, P.; Deckers, R.; Bos, C.; Schiffelers, R.M.; Moonen, C.T. Microbubbles-Assisted Ultrasound Triggers the Release of Ex-Tracellular Vesicles. Available online: https://www.mdpi.com/1422-0067/18/8/1610 (accessed on 2 May 2025).
- Lu, Y.; de Vries, W.C.; Overeem, N.J.; Duan, X.; Zhang, H.; Zhang, H.; Pang, W.; Ravoo, B.J.; Huskens, J. Controlled and Tunable Loading and Release of Vesicles by Using Gigahertz Acoustics. Angew. Chem. 2018, 131, 165–169. [Google Scholar] [CrossRef]
- Sedaghatkish, A.; Rezaeian, M.; Heydari, H.; Ranjbar, A.M.; Soltani, M. Acoustic streaming and thermosensitive liposomes for drug delivery into hepatocellular carcinoma tumor adjacent to major hepatic veins; an acoustics-thermal-fluid-mass transport coupling model. Int. J. Therm. Sci. 2020, 158, 106540. [Google Scholar] [CrossRef]
- Lu, Y.; Huskens, J.; Pang, W.; Duan, X. Hypersonic poration of supported lipid bilayers. Mater. Chem. Front. 2019, 3, 782–790. [Google Scholar] [CrossRef]
- Mahendra, A.; James, H.P.; Jadhav, S. PEG-grafted phospholipids in vesicles: Effect of PEG chain length and concentration on mechanical properties. Chem. Phys. Lipids 2018, 218, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Najlah, M.; Said Suliman, A.; Tolaymat, I.; Kurusamy, S.; Kannappan, V.; Elhissi, A.M.; Wang, W. Development of Injectable PEGylated Liposome Encapsulating Disulfiram for Colorectal Cancer Treatment. Available online: https://www.mdpi.com/1999-4923/11/11/610 (accessed on 14 November 2019).
- Zhang, W.; Wang, Z.; Wu, C.; Jin, Y.; Liu, X.; Wu, Z.; Liu, J. The effect of DSPE-PEG2000, cholesterol and drug incorporated in bilayer on the formation of discoidal micelles. Eur. J. Pharm. Sci. 2018, 125, 74–85. [Google Scholar] [CrossRef]
- Ishima, Y.; Yamazaki, N.; Chuang, V.T.; Shimizu, T.; Ando, H.; Ishida, T. A Maleimide-Terminally Modified PEGylated Liposome Induced the Accelerated Blood Clearance Independent of the Production of Anti-PEG IgM Antibodies. Available online: https://www.jstage.jst.go.jp/article/bpb/45/10/45_b22-00389/_article/-char/ja/ (accessed on 1 October 2022).
- Faizi, H.A.; Frey, S.L.; Steinkühler, J.; Dimova, R.; Vlahovska, P.M. Bending rigidity of charged lipid bilayer membranes. Soft Matter 2019, 15, 6006–6013. [Google Scholar] [CrossRef]
- Svandova, L.; Groleau, A.; Dorval, A.; Hamdan, A.; Kelar, J.; Laroche, G.; Profili, J. Surface Tuning of Transparent Electrodes with Atmospheric Pressure Plasma for Third Generation Photovoltaics. ECS Meet. Abstr. 2024, MA2024-01, 1411. [Google Scholar] [CrossRef]
- Kim, S.; Chang, R. Structure, Dynamics, and Phase Behavior of DOPC/DSPC Mixture Membrane Systems: Molecular Dynamics Simulation Studies. Bull. Korean Chem. Soc. 2016, 37, 1076–1085. [Google Scholar] [CrossRef]
- Steffes, V.M.; Zhang, Z.; MacDonald, S.; Crowe, J.; Ewert, K.K.; Carragher, B.; Potter, C.S.; Safinya, C.R. PEGylation of Paclitaxel-Loaded Cationic Liposomes Drives Steric Stabilization of Bicelles and Vesicles thereby Enhancing Delivery and Cytotoxicity to Human Cancer Cells. ACS Appl. Mater. Interfaces 2020, 12, 151–162. [Google Scholar] [CrossRef]
- Bi, H.; Yang, B.; Wang, L.; Cao, W.; Han, X. Electroformation of giant unilamellar vesicles using interdigitated ITO electrodes. J. Mater. Chem. A 2013, 1, 7125–7130. [Google Scholar] [CrossRef]
- Liu, D.; Mori, A.; Huang, L. Role of liposome size and RES blockade in controlling biodistribution and tumor uptake of GM1-containing liposomes. Biochim. Biophys. Acta (BBA)—Biomembr. 1992, 1104, 95–101. [Google Scholar] [CrossRef]
- Mora, N.L.; Boyle, A.L.; Kolck, B.J.V.; Rossen, A.; Pokorná, Š; Koukalová, A.; Šachl, R.; Hof, M.; Kros, A. Controlled Peptide-Mediated Vesicle Fusion Assessed by Simultaneous Du-al-Colour Time-Lapsed Fluorescence Microscopy|Scientific Reports. Available online: https://www.nature.com/articles/s41598-020-59926-z (accessed on 20 February 2020).
- Bhatia, T.; Robinson, T.; Dimova, R. Membrane permeability to water measured by microfluidic trapping of giant vesicles. Soft Matter 2020, 16, 7359–7369. [Google Scholar] [CrossRef]
- Kowalska, M.; Broniatowski, M.; Mach, M.; Płachta, Ł.; Wydro, P. The effect of the polyethylene glycol chain length of a lipopolymer (DSPE-PEGn) on the properties of DPPC monolayers and bilayers. J. Mol. Liq. 2021, 335, 116529. [Google Scholar] [CrossRef]
- Kocun, M.; Lazzara, T.D.; Steinem, C.; Janshoff, A. Preparation of Solvent-Free, Pore-Spanning Lipid Bilayers: Modeling the Low Tension of Plasma Membranes. Langmuir 2011, 27, 7672–7680. [Google Scholar] [CrossRef] [PubMed]
- Brandl, M. Liposomes as drug carriers: A technological approach. Biotechnol. Annu. Rev. 2004, 7, 59–85. [Google Scholar] [CrossRef]
- Massing, U.; Fuxius, S. Liposomal formulations of anticancer drugs: Selectivity and effectiveness. Drug Resist. Updat. 2000, 3, 171–177. [Google Scholar] [CrossRef]
- Hu, W.; Mao, A.; Wong, P.; Larsen, A.; Yazaki, P.J.; Wong, J.Y.; Shively, J.E. Characterization of 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine–N-[Methoxy(polyethylene glycerol)-2000] and Its Complex with Doxorubicin Using Nuclear Magnetic Resonance Spectroscopy and Molecular Dynamics. Bioconjugate Chem. 2017, 28, 1777–1790. [Google Scholar] [CrossRef]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: Ithaca, NY, USA, 1953. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Wang, H.; Wang, Z.; Xie, H.; Lu, Y. High-Efficiency Drug Loading in Lipid Vesicles by MEMS-Driven Gigahertz Acoustic Streaming. Micromachines 2025, 16, 562. https://doi.org/10.3390/mi16050562
Li B, Wang H, Wang Z, Xie H, Lu Y. High-Efficiency Drug Loading in Lipid Vesicles by MEMS-Driven Gigahertz Acoustic Streaming. Micromachines. 2025; 16(5):562. https://doi.org/10.3390/mi16050562
Chicago/Turabian StyleLi, Bingxuan, Haopu Wang, Zhen Wang, Huikai Xie, and Yao Lu. 2025. "High-Efficiency Drug Loading in Lipid Vesicles by MEMS-Driven Gigahertz Acoustic Streaming" Micromachines 16, no. 5: 562. https://doi.org/10.3390/mi16050562
APA StyleLi, B., Wang, H., Wang, Z., Xie, H., & Lu, Y. (2025). High-Efficiency Drug Loading in Lipid Vesicles by MEMS-Driven Gigahertz Acoustic Streaming. Micromachines, 16(5), 562. https://doi.org/10.3390/mi16050562






