Study on the Multi-Stage Evolution of Thermal Runaway and the Flammability Threshold of Gas Generation in Lithium Iron Phosphate Batteries Based on SOC Gradient
Abstract
1. Introduction
2. Experimental Methodology
2.1. Battery Samples
2.2. Experimental Equipment
2.3. Experimental Procedure
3. Results
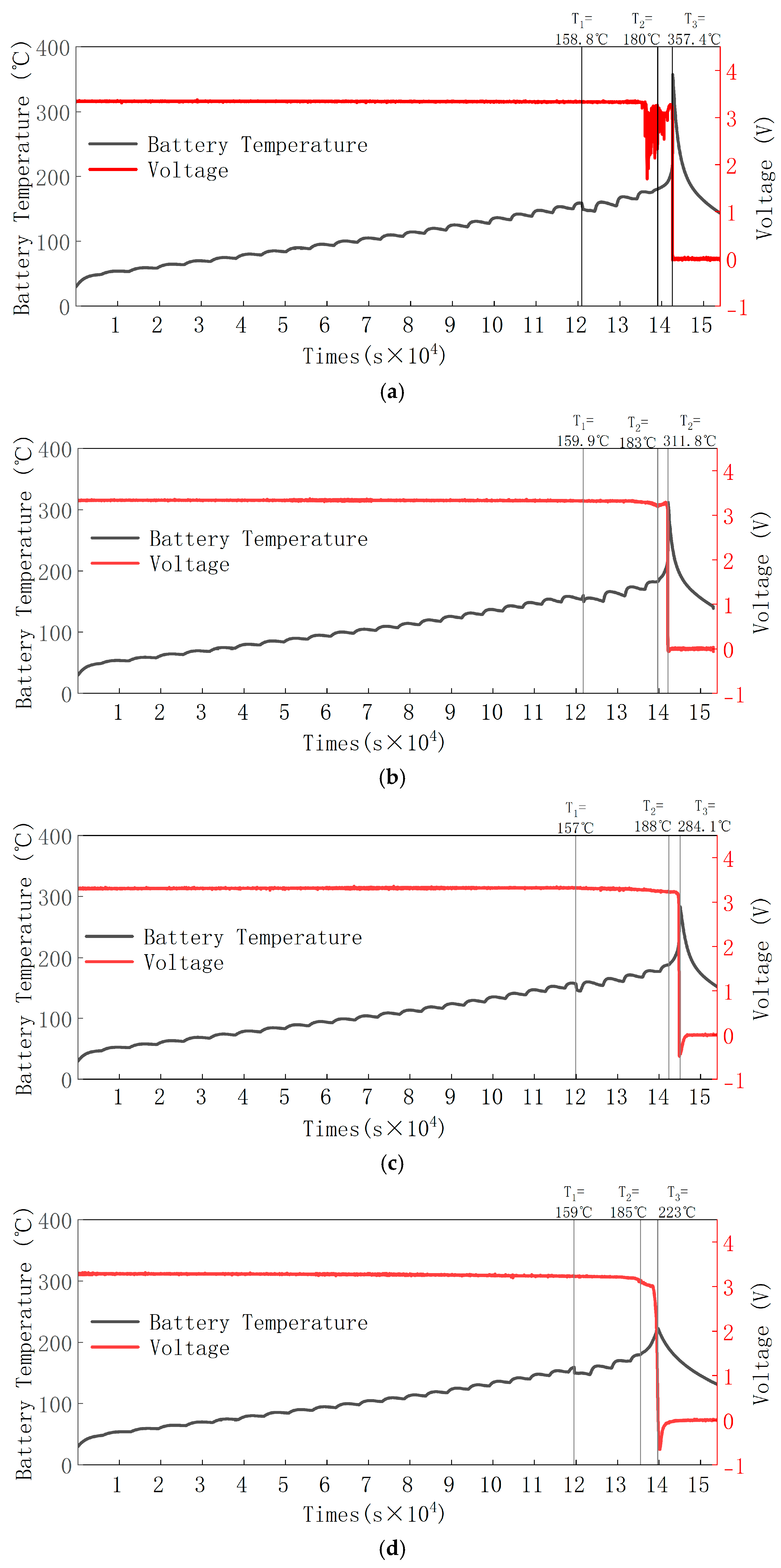
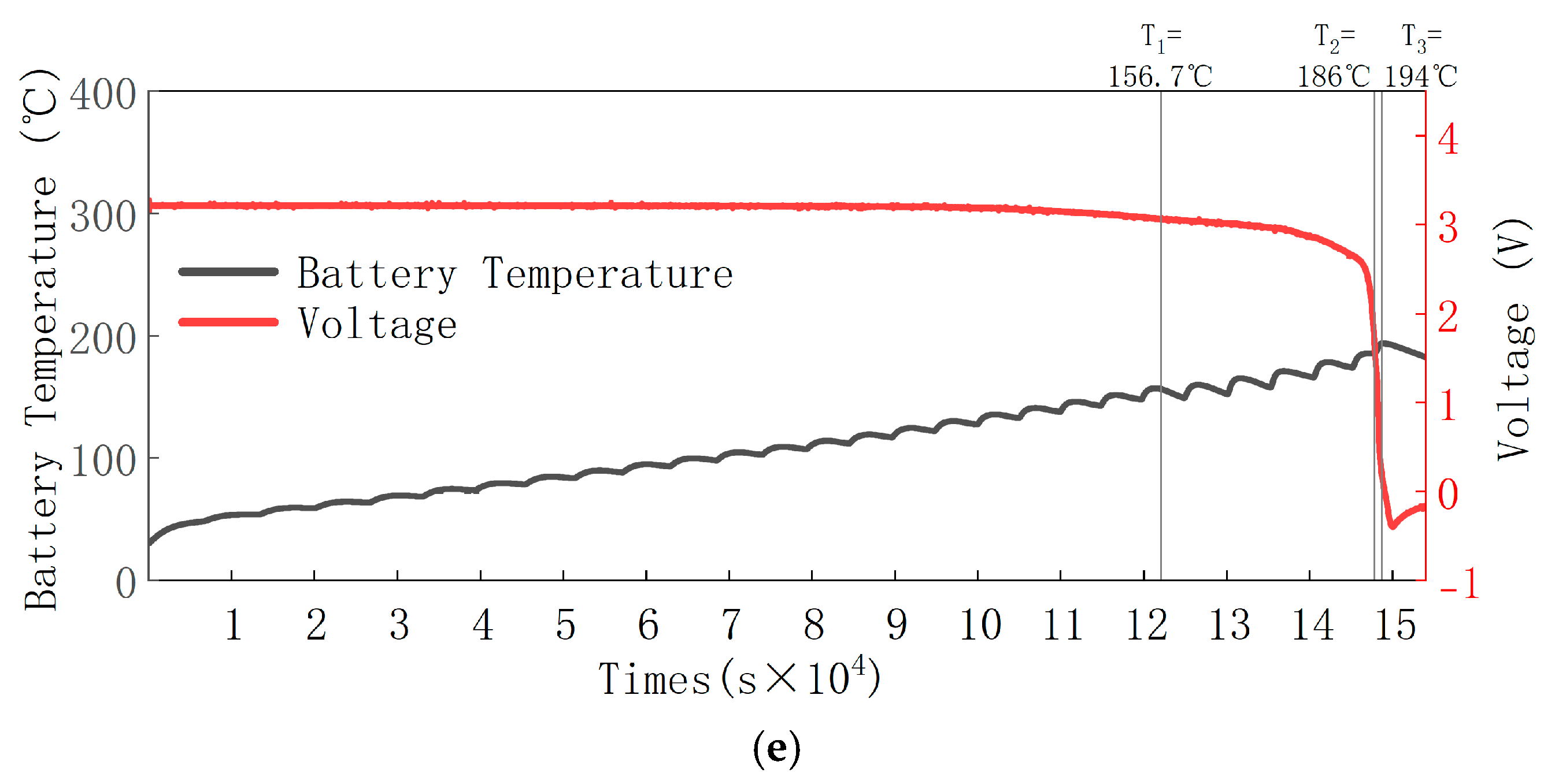
3.1. Battery TR Temperature and Voltage Changes
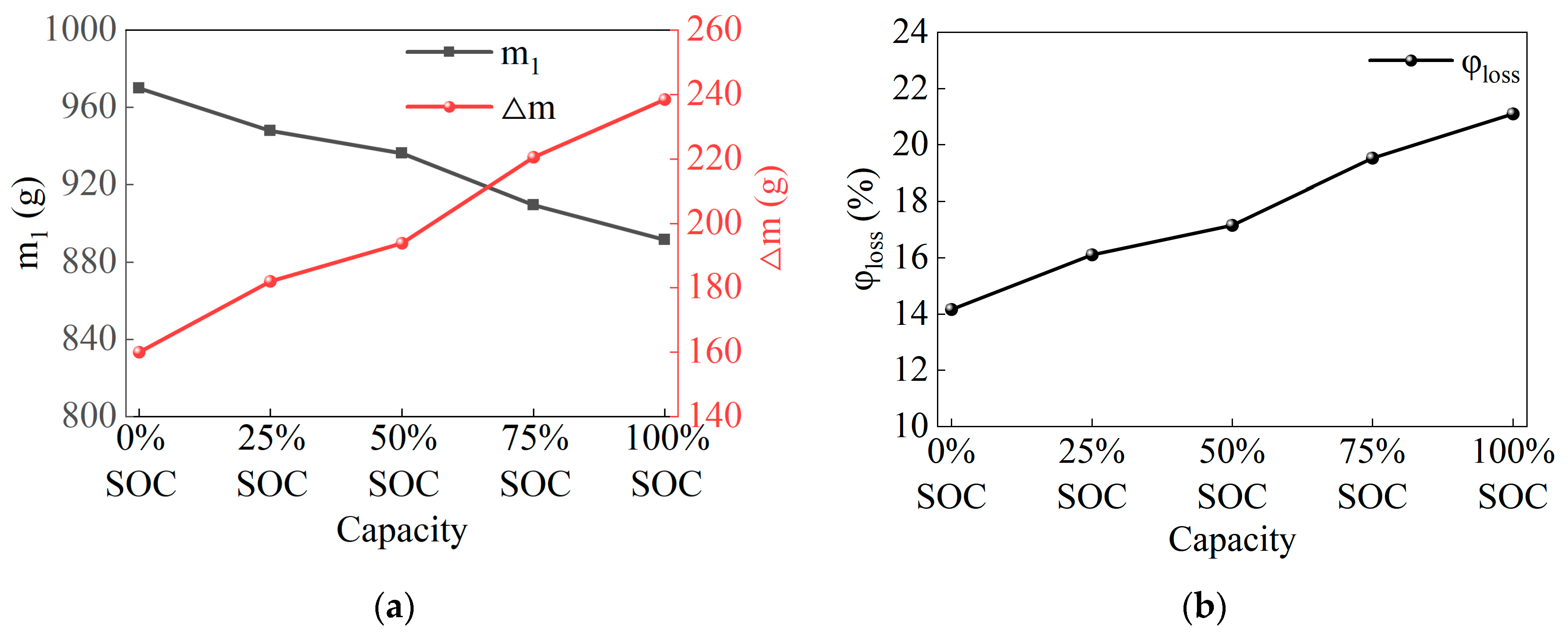
3.2. Mass Loss
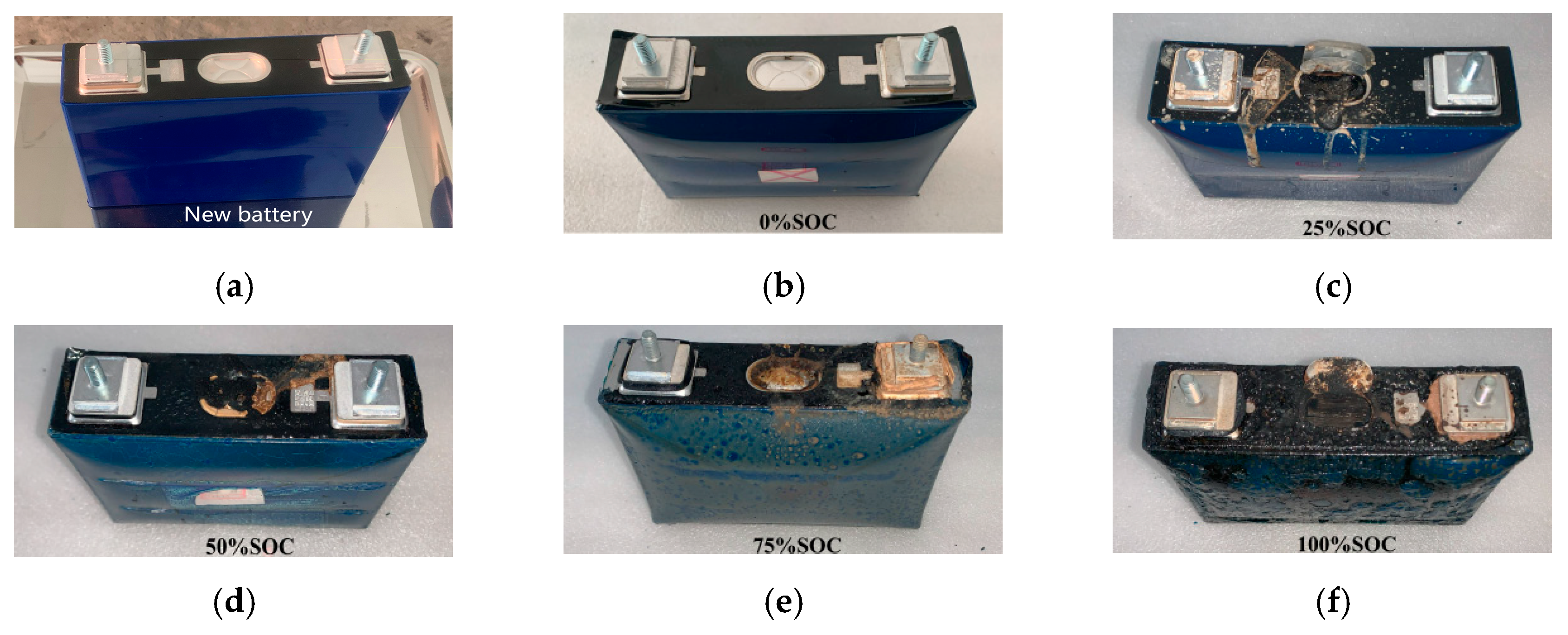
3.3. Appearance Morphology Analysis
3.4. Gas Composition
3.5. Gas Production Volume
- n is the number of moles of gas produced (mol).
- P2 is the pressure inside the sealed heating pressure chamber (SHPC) after thermal runaway (Pa).
- V2 is the volume of the sealed heating pressure chamber (SHPC) (m3).
- R is the ideal gas constant (J/mol·K).
- T2 is the stable environment temperature inside the sealed heating pressure chamber (SHPC) after thermal runaway (K).
- P1 is the pressure inside the sealed heating pressure chamber (SHPC) before thermal runaway (Pa).
- V1 is the volume of gas inside the sealed heating pressure chamber (SHPC) before thermal runaway (m3).
- T1 is the environment temperature inside the sealed heating pressure chamber (SHPC) before the experiment (K).
3.6. Explosive Characteristics
- CCR is the dilution ratio of CO2 to the single flammable gas.
- nCO2 is the number of moles of CO2 (mol).
- ni is the number of moles of a single gas (mol).
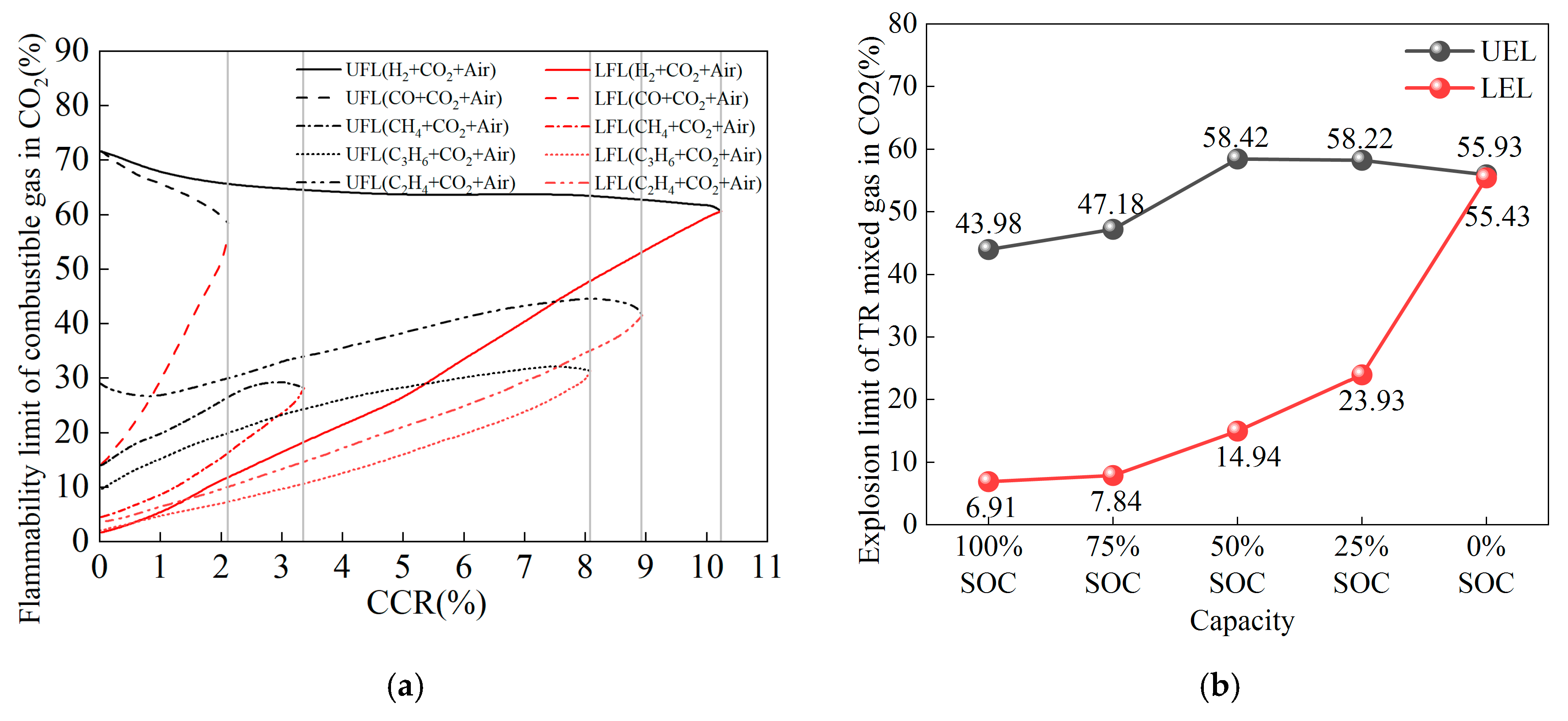
- Lmix represents the flammability limits (both lower and upper) of the thermal runaway gases of the LiFePO4 battery.
- xi is the mole percentage of the i-th gas in the thermal runaway gases of the LiFePO4 battery.
- Li is the flammability limit of the i-th gas in the presence of CO2.
4. Conclusions
- The valve opening temperature T1 during battery thermal runaway remained in the range of 156.7 °C to 159.9 °C across different SOC levels, with minimal fluctuation. This indicates no significant correlation between T1 and SOC. The rapid self-heating onset temperature T2 during thermal runaway was between 180 °C and 186 °C across all SOCs, with little temperature variation, suggesting no significant correlation between T2 and SOC. The maximum temperature T3 during thermal runaway was positively correlated with SOC, meaning that as SOC increases, T3 increases, reaching a maximum of 357.4 °C. A higher SOC leads to a larger internal short-circuit current, higher temperatures, and more intense thermal runaway reactions and eruptions. The side reactions between the anode and cathode materials and the electrolyte generate more gas and electrolyte vapor, which are expelled in greater quantities, resulting in an increased mass loss rate with the increase in SOC. The experiments show that the mass loss rate during thermal runaway triggered by stepwise heating in a 50 Ah LiFePO4 battery is around 14% to 21%.
- For the 50 Ah LiFePO4 battery at five SOC levels, the battery casing experienced similar deformations but did not suffer any rupture or melting. This is because the maximum temperature during thermal runaway was only 357.4 °C, which did not reach the aluminum shell melting point of 660 °C, thus maintaining the integrity of the battery casing. The valve opening temperature T1 was around 158 °C (ranging from 156.7 °C to 159.9 °C), suggesting that the critical point of the battery safety valve is largely unaffected by SOC and is mainly influenced by temperature.
- The volume percentage of gases produced during thermal runaway at five different SOC levels (0%, 25%, 50%, 75%, 100%) was normalized, revealing that as SOC increased, the proportion of H2 produced increased, while the proportion of CO2 decreased. This is because the thermal runaway temperature of higher SOC batteries is higher, leading to more types of side reactions inside the battery, which results in an increase in other types of gases and a decrease in the proportion of CO2 produced.
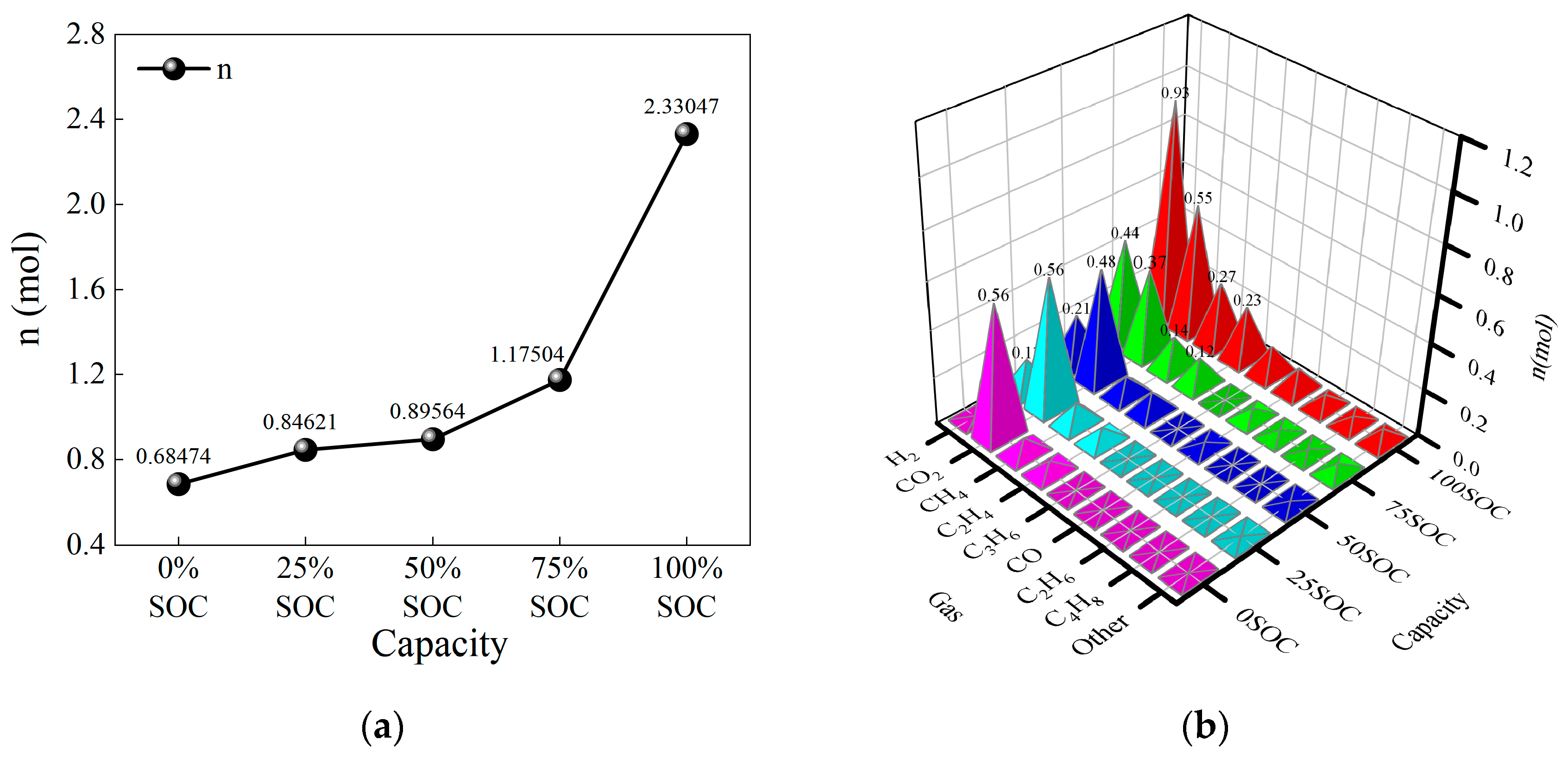
- Below 50% SOC, the total moles of gas produced during thermal runaway were 0.68474 mol (0% SOC), 0.84621 mol (25% SOC), and 0.89564 mol (50% SOC), showing a slow increase in gas production. For SOC levels above 50%, the total moles of gas produced were 1.17504 mol (75% SOC) and 2.33047 mol (100% SOC). As SOC increased, the number and types of side reactions during thermal runaway increased, causing a sharp rise in gas production.
- As SOC increased, the amount of CO2 produced varied little across different SOCs, indicating that the side reaction producing CO2 is independent of SOC. However, the amount of H2 produced increased sharply: 0.01 mol (0% SOC), 0.13 mol (25% SOC), 0.21 mol (50% SOC), 0.43 mol (75% SOC), and 0.93 mol (100% SOC). As the thermal runaway temperature increased, hydrogen production from electrolyte decomposition and binder reactions became dominant among the side reactions. The amounts of CH4 and C2H4 produced gradually increased, suggesting that the side reactions producing these gases were relatively mild, with minimal influence from thermal runaway temperature. Other flammable gases showed little change in production with varying SOC.
- Considering the inerting effect of CO2 in the thermal runaway gas, the recalculated lower flammability limit (LEL) of the thermal runaway mixed gas for the LiFePO4 battery was 6.91% at 100% SOC and 55.43% at 0% SOC. The LEL increased as SOC decreased, indicating that the thermal runaway mixed gas of low SOC batteries has a higher LEL and is harder to ignite, while the thermal runaway mixed gas of high SOC batteries has a lower LEL, making it more susceptible to explosion risks. The flammability range parameter HF for the mixed gas remained relatively stable between 34% and 43% in the SOC range of 25% to 100%, while at 0% SOC, the flammability range was minimal, and the flammability sharply decreased.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chavan, S.; Venkateswarlu, B.; Prabakaran, R.; Salman, M.; Joo, S.W.; Choi, G.S.; Kim, S.C. Thermal runaway and mitigation strategies for electric vehicle lithium-ion batteries using battery cooling approach: A review of the current status and challenges. J. Energy Storage 2023, 72, 108569. [Google Scholar] [CrossRef]
- Hu, G.; Huang, P.; Bai, Z.; Wang, Q.; Qi, K. Comprehensively analysis the failure evolution and safety evaluation of automotive lithium ion battery. ETransportation 2021, 10, 100140. [Google Scholar] [CrossRef]
- Wu, H.; Chen, S.; Hong, Y.; Xu, C.; Zheng, Y.; Jin, C.; Chen, K.; He, Y.; Feng, X.; Wei, X. Thermal safety boundary of lithium-ion battery at different state of charge. J. Energy Chem. 2024, 91, 59–72. [Google Scholar] [CrossRef]
- Huang, P.; Yao, C.; Mao, B.; Wang, Q.; Sun, J.; Bai, Z. The critical characteristics and transition process of lithium-ion battery thermal runaway. Energy 2020, 213, 119082. [Google Scholar]
- Xiao, Y.; Yang, F.; Gao, Z.; Liu, M.; Wang, J.; Kou, Z.; Lin, Y.; Li, Y.; Gao, L.; Chen, Y. Review of mechanical abuse related thermal runaway models of lithium-ion batteries at different scales. J. Energy Storage 2023, 64, 107145. [Google Scholar]
- Li, H.; Zhou, D.; Zhang, M.; Liu, B.; Zhang, C. Multi-field interpretation of internal short circuit and thermal runaway behavior for lithium-ion batteries under mechanical abuse. Energy 2023, 263, 126027. [Google Scholar]
- Wei, D.; Zhang, M.; Zhu, L.; Chen, H.; Huang, W.; Yao, J.; Yuan, Z.; Xu, C.; Feng, X. Study on thermal runaway behavior of Li-ion batteries using different abuse methods. Batteries 2022, 8, 201. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, X.; Huang, W.; He, X.; Wang, L.; Ouyang, M. Challenges and opportunities to mitigate the catastrophic thermal runaway of high-energy batteries. Adv. Energy Mater. 2023, 13, 2203841. [Google Scholar] [CrossRef]
- Kong, D.; Lv, H.; Ping, P.; Wang, G. A review of early warning methods of thermal runaway of lithium ion batteries. J. Energy Storage 2023, 64, 107073. [Google Scholar] [CrossRef]
- Ren, D.; Feng, X.; Liu, L.; Hsu, H.; Lu, L.; Wang, L.; He, X.; Ouyang, M. Investigating the relationship between internal short circuit and thermal runaway of lithium-ion batteries under thermal abuse condition. Energy Storage Mater. 2021, 34, 563–573. [Google Scholar]
- Huang, S.; Du, Z.; Zhou, Q.; Snyder, K.; Liu, S.; Zhang, G. In situ measurement of temperature distributions in a Li-ion cell during internal short circuit and thermal runaway. J. Electrochem. Soc. 2021, 168, 090510. [Google Scholar] [CrossRef]
- Hong, J.; Wang, Z.; Qu, C.; Zhou, Y.; Shan, T.; Zhang, J.; Hou, Y. Investigation on overcharge-caused thermal runaway of lithium-ion batteries in real-world electric vehicles. Appl. Energy 2022, 321, 119229. [Google Scholar]
- Liu, J.; Wang, Z.; Bai, J. Influences of multi factors on thermal runaway induced by overcharging of lithium-ion battery. J. Energy Chem. 2022, 70, 531–541. [Google Scholar]
- Zhang, Q.; Niu, J.; Zhao, Z.; Wang, Q. Research on the effect of thermal runaway gas components and explosion limits of lithium-ion batteries under different charge states. J. Energy Storage 2022, 45, 103759. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, L.; Liu, J.; Wang, J.; Yan, W. Gas Sensing Technology for the Detection and Early Warning of Battery Thermal Runaway: A Review. Energy Fuels 2022, 36, 6038–6057. [Google Scholar] [CrossRef]
- Qiu, M.; Liu, J.; Cong, B.; Cui, Y. Research Progress in Thermal Runaway Vent Gas Characteristics of Li-Ion Battery. Batteries 2023, 9, 411. [Google Scholar] [CrossRef]
- Wei, G.; Huang, R.; Zhang, G.; Jiang, B.; Zhu, J.; Guo, Y.; Han, G.; Wei, X.; Dai, H. A comprehensive insight into the thermal runaway issues in the view of lithium-ion battery intrinsic safety performance and venting gas explosion hazards. Appl. Energy 2023, 349, 121651. [Google Scholar] [CrossRef]
- Willstrand, O.; Pushp, M.; Andersson, P.; Brandell, D. Impact of different Li-ion cell test conditions on thermal runaway characteristics and gas release measurements. J. Energy Storage 2023, 68, 107785. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, T.; Hao, C.; Qu, Y.; Niu, J.; Wang, Q.; Chen, D. In situ Raman investigation on gas components and explosion risk of thermal runaway emission from lithium-ion battery. J. Energy Storage 2022, 56, 105905. [Google Scholar]
- Qi, C.; Liu, Z.; Lin, C.; Liu, X.; Liu, D.; Li, Z.; Yi, A. The gas production characteristics and catastrophic hazards evaluation of thermal runaway for LiNi0. 5Co0. 2Mn0. 3O2 lithium-ion batteries under different SOCs. J. Energy Storage 2024, 88, 111678. [Google Scholar]
- Cui, Y.; Shi, D.; Wang, Z.; Mou, L.; Ou, M.; Fan, T.; Bi, S.; Zhang, X.; Yu, Z.; Fang, Y. Thermal Runaway Early Warning and Risk Estimation Based on Gas Production Characteristics of Different Types of Lithium-Ion Batteries. Batteries 2023, 9, 438. [Google Scholar] [CrossRef]
- Feng, X.; Zheng, S.; Ren, D.; He, X.; Wang, L.; Liu, X.; Li, M.; Ouyang, M. Key Characteristics for Thermal Runaway of Li-ion Batteries. Energy Procedia 2019, 158, 4684–4689. [Google Scholar] [CrossRef]
- Doose, S.; Hahn, A.; Fischer, S.; Müller, J.; Haselrieder, W.; Kwade, A. Comparison of the consequences of state of charge and state of health on the thermal runaway behavior of lithium ion batteries. J. Energy Storage 2023, 62, 106837. [Google Scholar] [CrossRef]
- Chen, J.; Xu, C.; Wang, Q.; Wang, H.; Peng, Y.; Liu, J.; Zhang, J.; Zhang, G.; Lu, L.; Feng, X. The thermal-gas coupling mechanism of lithium iron phosphate batteries during thermal runaway. J. Power Sources 2025, 625, 235728. [Google Scholar] [CrossRef]
- Jia, Z.; Qin, P.; Li, Z.; Wei, Z.; Jin, K.; Jiang, L.; Wang, Q. Analysis of gas release during the process of thermal runaway of lithium-ion batteries with three different cathode materials. J. Energy Storage 2022, 50, 104302. [Google Scholar] [CrossRef]
- Yang, M.; Rong, M.; Ye, Y.; Yang, A.; Chu, J.; Yuan, H.; Wang, X. Comprehensive analysis of gas production for commercial LiFePO4 batteries during overcharge-thermal runaway. J. Energy Storage 2023, 72, 108323. [Google Scholar] [CrossRef]
- Bugryniec, P.J.; Resendiz, E.G.; Nwophoke, S.M.; Khanna, S.; James, C.; Brown, S.F. Review of gas emissions from lithium-ion battery thermal runaway failure—Considering toxic and flammable compounds. J. Energy Storage 2024, 87, 111288. [Google Scholar] [CrossRef]
- Wang, S.; Song, L.; Li, C.; Tian, J.; Jin, K.; Duan, Q.; Wang, Q. Experimental study of gas production and flame behavior induced by the thermal runaway of 280 Ah lithium iron phosphate battery. J. Energy Storage 2023, 74, 109368. [Google Scholar] [CrossRef]
- Su, L.; Yang, F.; Hu, W.; Chen, S.; Lyu, N. Thermal Runaway Gas Generation of Lithium Iron Phosphate Batteries Triggered by Various Abusive Conditions. J. Energy Eng. 2024, 150, 04024017. [Google Scholar] [CrossRef]
- Lin, C.; Yan, H.; Qi, C.; Mao, J.; Lao, L.; Sun, Y.; Ma, T.; Liu, D. Research on thermal runaway and gas generation characteristics of NCM811 high energy density lithium-ion batteries under different triggering methods. Case Stud. Therm. Eng. 2024, 64, 105417. [Google Scholar] [CrossRef]
- Kondo, S.; Takizawa, K.; Takahashi, A.; Tokuhashi, K. Extended Le Chatelier’s formula for carbon dioxide dilution effect on flammability limits. J. Hazard. Mater. 2006, 138, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-K.; Ågren, J.; Hillert, M. Application of the Le Chatelier principle on gas reactions. Fluid Phase Equilibria 1996, 121, 167–177. [Google Scholar] [CrossRef]
- Li, W.; Wang, H.; Zhang, Y.; Ouyang, M. Flammability characteristics of the battery vent gas: A case of NCA and LFP lithium-ion batteries during external heating abuse. J. Energy Storage 2019, 24, 100775. [Google Scholar] [CrossRef]
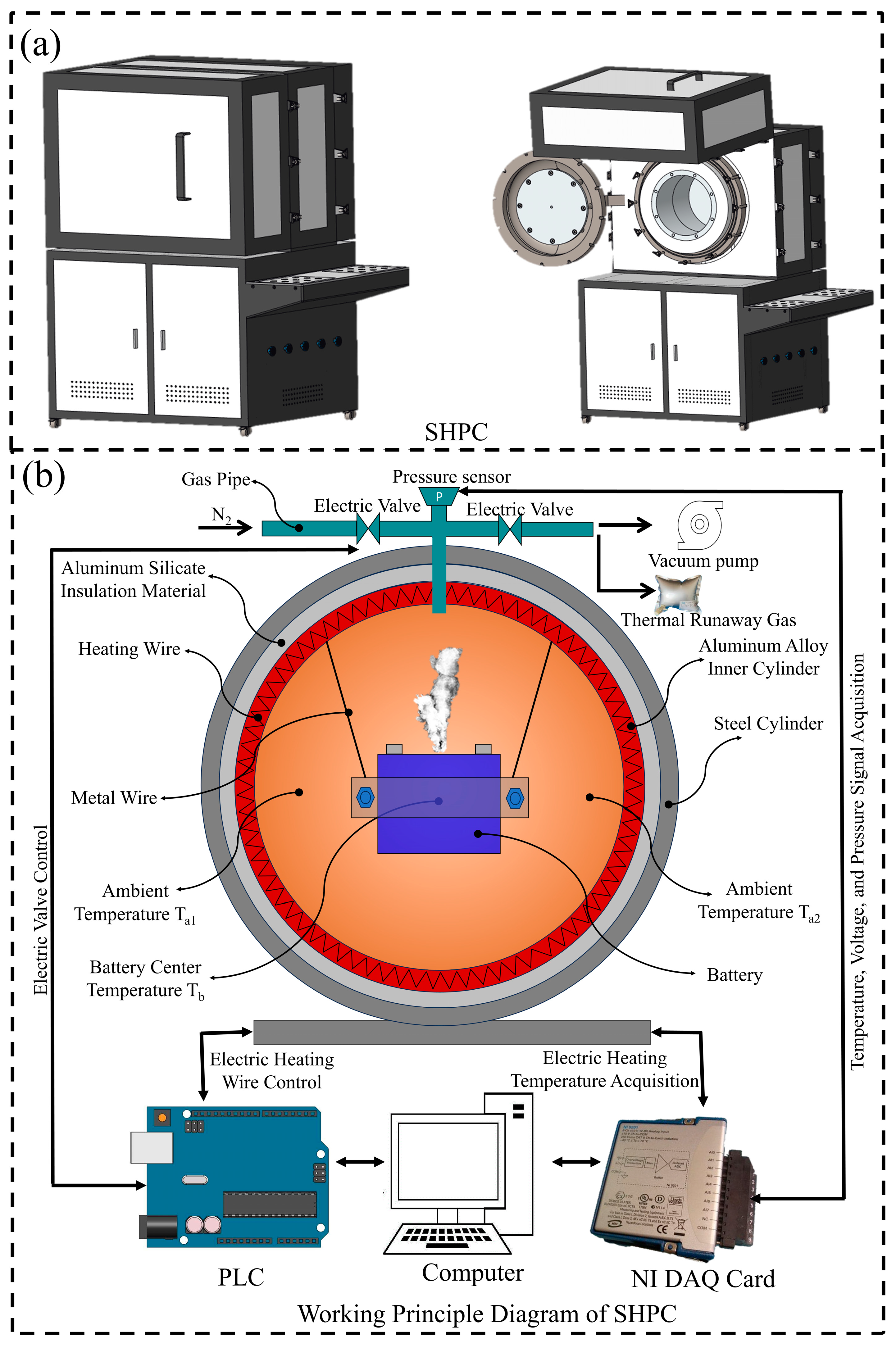
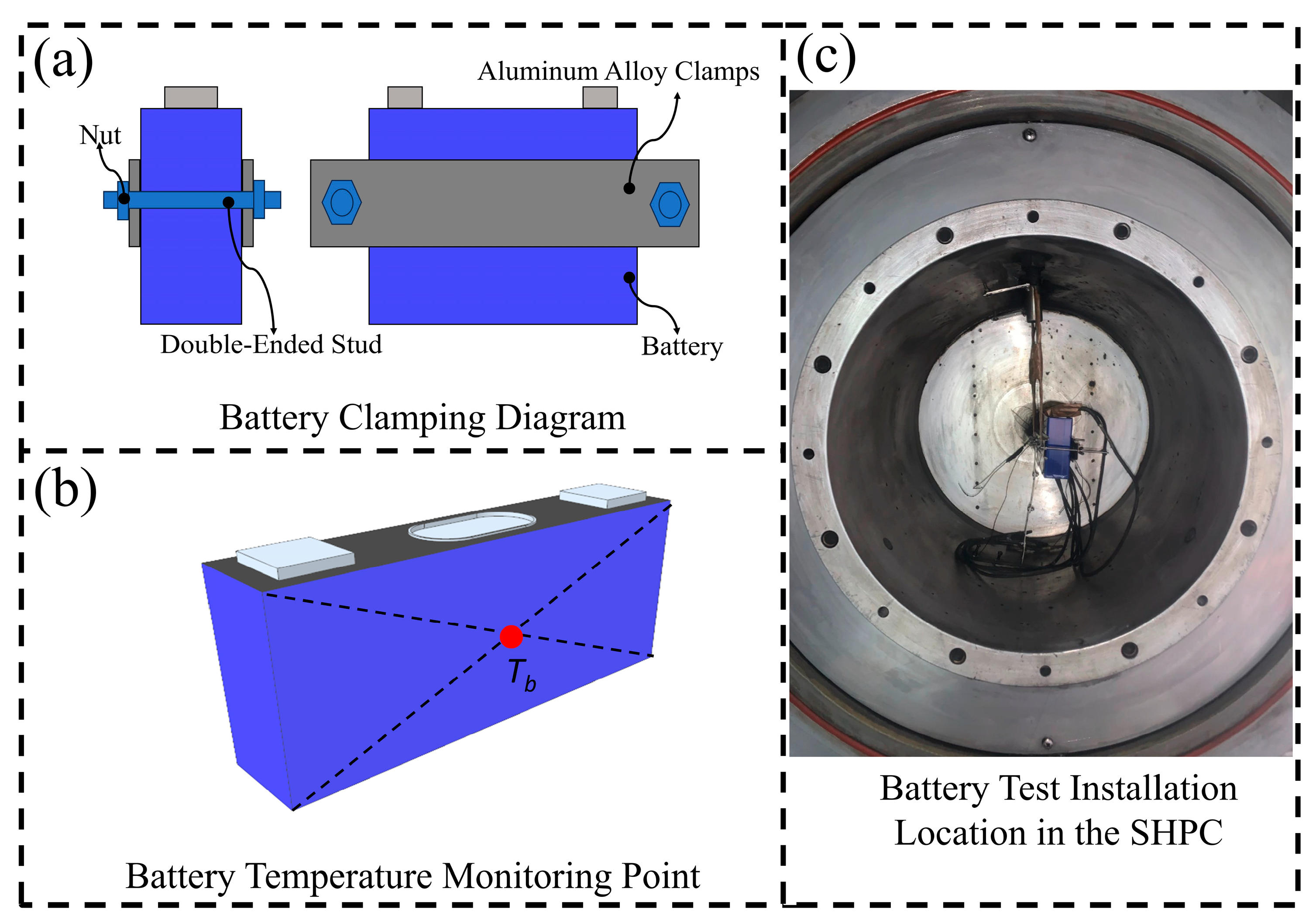
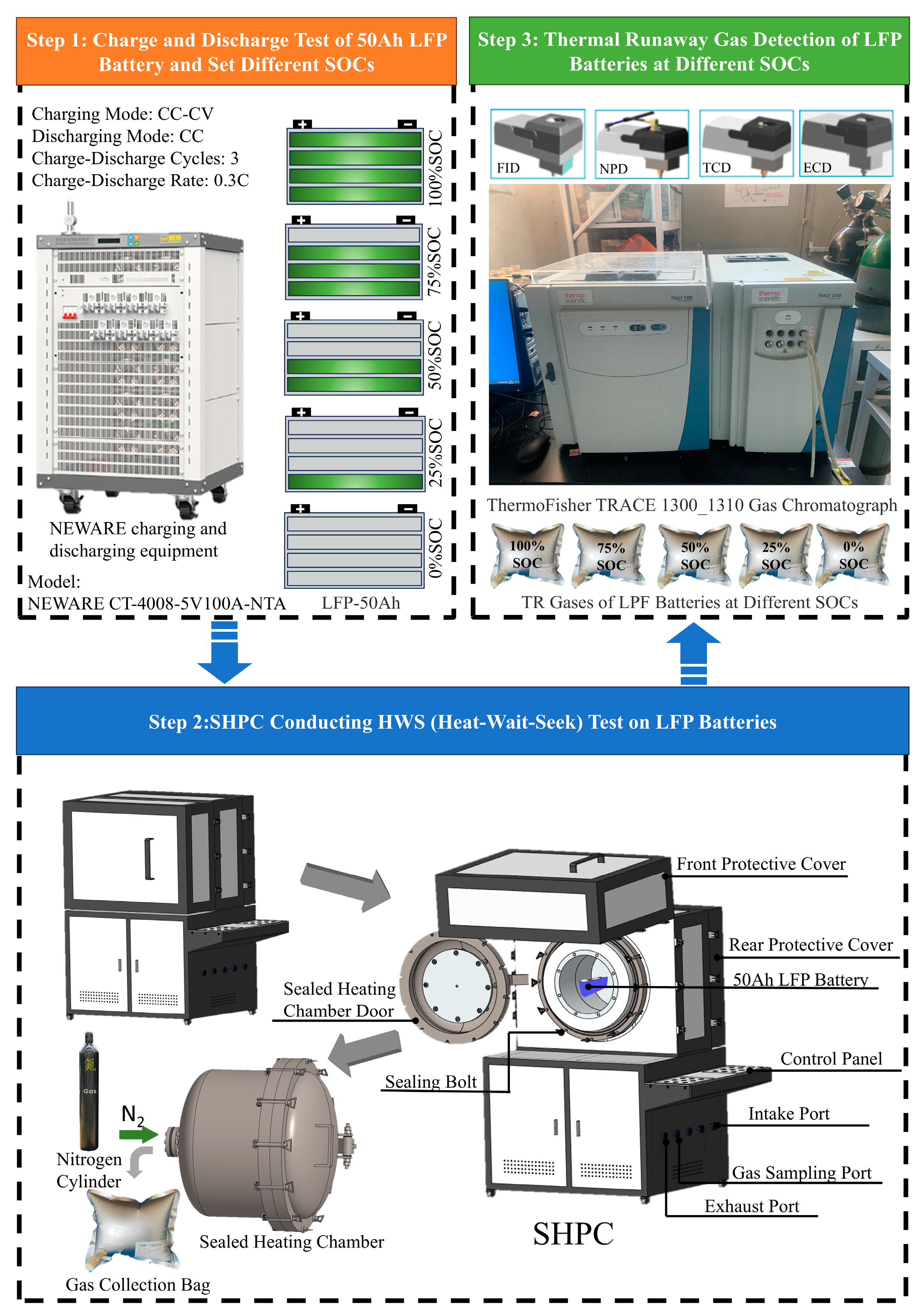




| Parameter | Specification | Condition |
|---|---|---|
| Nominal Capacity | 50 Ah | 25 °C, 0.5 C |
| Minimum Discharge Energy | 160 Wh | 25 °C, 0.5 C |
| Operating Voltage Range | 2.5~3.65 V | >0 °C |
| Cathode | LiFePO4 | N.A. |
| Anode | Graphite | N.A. |
| Standard Voltage | 3.20 V | 25 °C, 0.5 C |
| Charge Operation Temperature Range | −10~55 °C | N.A. |
| Discharge Operation Temperature Range | −30~60 °C | N.A. |
| Alternating Current Resistance | 0.2~0.6 mΩ | 25 °C, BOL, 17% SOC, 1 kHz. |
| Weight | 1130 ± 50 g | N.A. |
| Thickness | 39.7 ± 0.3 mm | N.A. |
| Length | 147.9 ± 0.8 mm | N.A. |
| Height | 95.4 ± 0.5 mm | N.A. |
| Material | Aluminum alloy | N.A. |
| Cathode Current Collector | Aluminum foil | N.A. |
| Anode Current Collector | Copper foil | N.A. |
| Electrolyte Type | Liquid Electrolyte | N.A. |
| Electrolyte Composition | Carbonate + Lithium hexafluorophosphate | N.A. |
| Cycle Life | ≥6000 | Under 150 kgf initial compression force, 25 °C 0.5 C/0.5 C, 2.5 V~3.65 V, cycle to 80%SOH |
| Capacity | 0% SOC | 25% SOC | 50% SOC | 75% SOC | 100% SOC |
|---|---|---|---|---|---|
| Initial Mass m0 (g) | 1130 | 1130 | 1130 | 1130 | 1130 |
| Post-experiment Mass m1 (g) | 970 | 948 | 936.2 | 909.4 | 891.6 |
| Mass Loss Δm (g) | 160 | 182 | 193.8 | 220.6 | 238.4 |
| Mass Loss Rate φloss (%) | 14.159292 | 16.1061947 | 17.1504425 | 19.5221239 | 21.0973451 |
| Capacity | 100% SOC | 75% SOC | 50% SOC | 25% SOC | 0% SOC |
|---|---|---|---|---|---|
| UEL | 43.98 | 47.18 | 58.42 | 58.22 | 55.93 |
| LEL | 6.91 | 7.84 | 14.94 | 23.93 | 55.43 |
| HF | 37.07 | 39.34 | 43.48 | 34.29 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, C.; Wang, H.; Li, M.; Li, C.; Li, Y.; Wei, N.; Zhang, H. Study on the Multi-Stage Evolution of Thermal Runaway and the Flammability Threshold of Gas Generation in Lithium Iron Phosphate Batteries Based on SOC Gradient. Micromachines 2025, 16, 544. https://doi.org/10.3390/mi16050544
Qi C, Wang H, Li M, Li C, Li Y, Wei N, Zhang H. Study on the Multi-Stage Evolution of Thermal Runaway and the Flammability Threshold of Gas Generation in Lithium Iron Phosphate Batteries Based on SOC Gradient. Micromachines. 2025; 16(5):544. https://doi.org/10.3390/mi16050544
Chicago/Turabian StyleQi, Changbao, Hewu Wang, Minghai Li, Cheng Li, Yalun Li, Ningning Wei, and Huipeng Zhang. 2025. "Study on the Multi-Stage Evolution of Thermal Runaway and the Flammability Threshold of Gas Generation in Lithium Iron Phosphate Batteries Based on SOC Gradient" Micromachines 16, no. 5: 544. https://doi.org/10.3390/mi16050544
APA StyleQi, C., Wang, H., Li, M., Li, C., Li, Y., Wei, N., & Zhang, H. (2025). Study on the Multi-Stage Evolution of Thermal Runaway and the Flammability Threshold of Gas Generation in Lithium Iron Phosphate Batteries Based on SOC Gradient. Micromachines, 16(5), 544. https://doi.org/10.3390/mi16050544








