1. Introduction
MEMS (Microelectromechanical System) energy harvesting has long been a focus for biomedical applications, offering the appealing prospect of eliminating the need for internal batteries within the human body. The potential reduction in device size and the elimination of the need for repeated surgical interventions for prolonged device use are significant advantages [
1,
2,
3]. Consequently, researchers have explored various mechanisms to power in vivo devices. Some harness the movement of the human body, converting mechanical energy from bodily movements such as those of the lungs or heart into electrical power [
4]. Others rely on external energy sources for transmission, employing methods such as radio frequency (RF) power transmission [
5], electromagnetic inductive power transmission [
6], and acoustic power transmission. Among these, acoustic wave transmission experiences less attenuation in many cases compared to RF and inductive methods, allowing it to reach deeper within the human body [
7]. Additionally, there is a trend of reduced attenuation in human tissue as frequencies decrease, making it advantageous to design energy-harvesting devices within the audible range [
8].
Acoustic energy harvesting generally uses a piezoelectric material to transform acoustically excited mechanical movements to electrical energy. Piezoelectric material characterization commonly employs the following equation:
where
i,
j, and
k represent the three spatial dimensions;
D denotes the electrical displacement;
d represents the piezoelectric stress coefficient tensor;
T is the mechanical stress vector;
ε is the permittivity tensor; and
E represents the electric field vector [
9]. Consequently, increasing the stress vector of the material enhances the power output. Piezoelectric materials typically function under two modes: the
d31 mode, where the applied stress and harvested electric field are perpendicular to each other, and the
d33 mode, where they are parallel. Another crucial parameter to consider is the electromechanical coupling coefficient,
k, which defines the conversion ratio between mechanical energy and electrical energy. This coefficient acknowledges that a more elastically compliant material will undergo more strain under a load, thereby increasing conversion efficiency compared to a material with the same piezoelectric coefficient,
d [
3].
Various thin-film piezoelectric materials have been developed for energy-harvesting applications, including PZT, BaTiO3, ZnO, PVDF, ALN, etc. Among these, PZT ceramics stands out as one of the most popular choices [
10] for their typical advantage of a high piezoelectric coefficient. To enhance this coefficient, microstructures of materials are often refined, or high-piezoelectric constant materials can be integrated into composite materials. However, despite continuous improvements and efforts over the years, the power output of these devices remains relatively small, necessitating ongoing efforts to enhance performance. One common approach involves exploring alternative structural designs beyond conventional diaphragm or membrane configurations. Different designs aim to improve efficiency by either inducing larger strain under a given applied load, such as with the cymbal design [
11] and a bi-stable beam [
12], or by lowering the resonant frequency to target ambient vibrations, as seen in the arc-based cantilever [
13]. These are just a few examples of the diverse design possibilities being explored. It is important to note that while PZT offers favorable properties for energy harvesting, such as a high piezoelectric coefficient, it contains lead and is not biocompatible, making it unsuitable for many implantable devices.
Another commonly used piezoelectric material is PVDF (polyvinylidene fluoride) and its copolymers. Their advantages typically include ease of fabrication, biocompatibility, and flexibility. Although these materials exhibit much lower
d33 and
d31 coefficients compared to ceramics, their greater flexibility results in still lower but more comparable
k31 and
k33 coefficients [
14]. Another important consideration is the acoustic impedance mismatch. The acoustic impedance of water is 1.5 MRayl. For human tissue, it widely ranges (e.g., 0.2 MRayl for lungs, ~1.6 MRayl for soft tissue, and 8 MRayl for bone). Piezoelectric polymers offer an acoustic impedance of around 4.5 MRayl. Although this is not an optimal match and can cause wave reflection and reduce power efficiency, polymer materials still outperform ceramics, which typically have acoustic impedances greater than 30 MRayl [
10]. In this research, water was used and regarded as an acoustic transmission medium for human tissue due to its similar acoustic impedance level.
Given that the energy level attainable with PVDF polymer is lower compared to PZT ceramic material, utilizing PVDF for low-acoustic power applications can present challenges. In this regard, in macroscale applications, acoustic resonators have been employed to increase the level of harvested acoustic energy. Typical acoustic resonators include tube resonators [
15], Helmholtz resonators [
16], and, more recently, sonic crystal resonators [
17]. Resonator devices are typically significantly larger than the PVDF actuator, usually by one or two orders of magnitude when comparing their largest dimensions and several orders of magnitude larger when comparing their smallest dimensions (e.g., thickness). Consequently, there has been limited exploration in integrating microscale resonators to enhance the energy-harvesting efficiency of PVDF materials. Nevertheless, developing a small-scale resonator capable of amplifying harvested energy while maintaining biocompatibility, flexibility, and ease of fabrication is paramount, particularly for applications in medical implantable devices [
18,
19].
In the meantime, an acoustic bubble serves as a widely utilized microfluidic tool across various applications. When subjected to an acoustic field, a bubble undergoes volumetric oscillation, surface wave formation on its interface, and/or atomization [
20]. This phenomenon has been leveraged for diverse purposes, such as object manipulation [
21], rotational control [
22], microfluidic channel mixing enhancement [
23], liquid pumping [
24], drug delivery [
25], and propulsion generation [
26]. An earlier study has demonstrated the capacity of bubble oscillation to serve as an energy source for harvesting on a cantilever beam [
27].
In this article, we endeavored to develop a small-scale acoustic bubble-based resonator operating within a low-frequency range. The harvesting material employed was PVDF-TrFE. This resonator was inspired by our preliminary experimental observations of significant oscillation of polymer bodies when used to encase bubbles, leading to a natural curiosity regarding the potential utilization of this polymer oscillation for energy-harvesting applications. A comparative study of harvesters with and without a bubble resonator was conducted to ascertain whether acoustically resonating bubbles enhance the oscillation amplitude, thereby augmenting the electrical energy. This study confirms the functionality of the acoustic bubble-based resonator.
2. Device Design
2.1. Design Procedure
To integrate a bubble adjacent to a piezoelectric diaphragm, a pre-designed cavity must be created to securely trap the bubble. Given the frequency-dependent nature of acoustic bubble oscillation, the bubble must be actuated at its resonance frequency to maximize the diaphragm’s performance. Furthermore, as the polarity of electrical charges induced in the piezoelectric material directly depends on whether the applied stress is tension or compression, maintaining the first mode of oscillation is critically important to minimize the cancellation of induced charges and thus to maximize the device performance. To this end, the resonance behavior was initially predicted using analytical models, and the oscillation mode was subsequently validated through preliminary experiments on a PDMS diaphragm observed under a high-speed camera. Finally, a piezoelectric diaphragm was integrated atop the PDMS diaphragm for energy-harvesting studies.
2.2. Resonance Prediction for the Device
The individual components of the harvesting system have their own resonances contributing to the overall system performance. Therefore, our goal was to predict and match the resonance frequencies of the associated components, including the diaphragm, bubble, and acoustic input actuator.
We first chose a diaphragm with a diameter of 1 mm and a 20 μm thickness; the first mode in the resonance frequency was 5.7 kHz, which was predicted by an ANSYS
® 2022 R1 modal analysis. This frequency was selected for the resonance of the whole device. Consequently, the piezo input actuator was also selected with a resonance peak at 5.7 kHz. Next, the resonance of bubbles trapped in a cylindrical cavity was evaluated. Minnaert derived the acoustic resonance of an air bubble in liquid [
28]:
where
f0 is the resonance frequency of the air bubble,
γ is the polytropic coefficient,
P0 is the pressure the bubble is under,
ρ is the density of the surrounding liquid, and
R0 is the radius of the spherical bubble.
The equation was subsequently extended to the Minnaert–Strasberg equation [
29] to accommodate various shapes of non-spherical air cavities. Here, the resonance of the cylindrical bubble trapped in the cavity was first estimated using the Minnaert equation, using the equivalent diameter based on the volume. Then, the shape effect was evaluated using a prolate spheroid bubble with an eccentricity, which was previously developed and utilized for predicting the resonance of PDMS diaphragm oscillations [
30], showing that the resonance frequencies exhibit minimal dependence on the bubble shape. For example, even when the major semi-axis of the spheroid bubble is twice as large as the minor semi-axis, the change in the resonance frequency is approximately limited to within 2% from the equivalent spherical bubble [
29]. Note that the Minnaert equation was originally developed for an air bubble in liquid. However, its application for diaphragm-covered bubbles that do not have any liquid–air interface was demonstrated to be adequate [
30].
In the present study, for a cylindrical bubble with a diameter of 1 mm and a depth of 1 mm, the radius of the spherical bubble for the equivalent volume was 572 μm, which predicted a resonance frequency of 5.7 kHz. The half-depth of the cavity approximately corresponds to the major axis of the spheroid shape, while the radius of the cavity represents the minor axis. Given that in our designs investigated in this study, the ratio of the axes in our design varied from 1 to 2, the shape-induced variation in bubble resonance was estimated to be within 0.1 kHz. Therefore, shape influence could be neglected when predicting resonance.
2.3. Diaphragm Oscillation Visualization
To experimentally validate the aforementioned predictions, the oscillation modes of the PDMS diaphragms were evaluated under the observation of a high-speed camera. In this context, an air cavity with a diaphragm but devoid of a piezoelectric layer was initially fabricated utilizing PDMS. A PDMS diaphragm was spin-coated and subsequently bonded with a PDMS cylindrical cavity, as depicted in
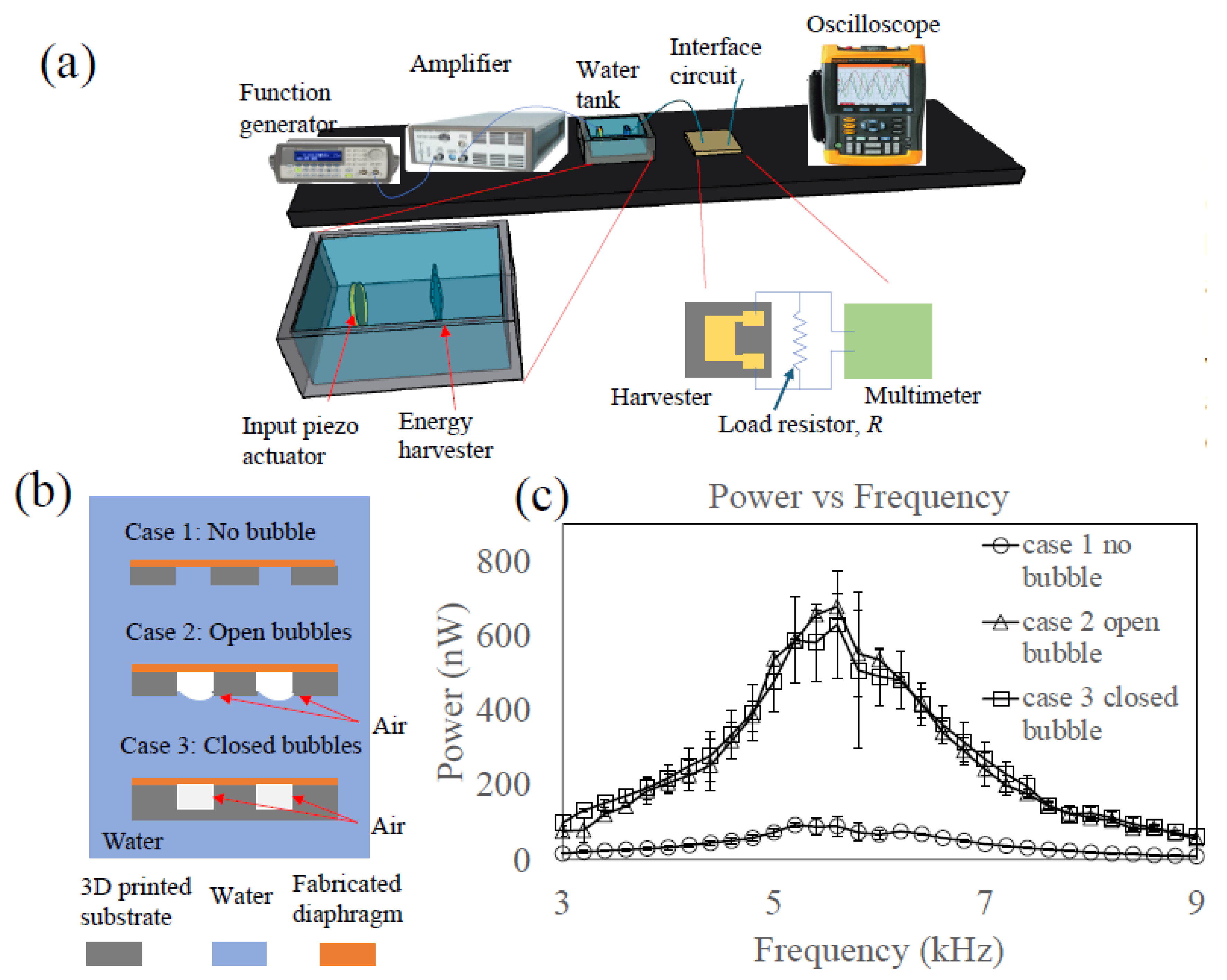
Figure 1a. Multiple designs of the cavity were fabricated, varying its diameter and height to investigate their impact on resonant frequency and mode shapes. For all designs, the membrane thickness remained constant at 20 μm. A PDMS diaphragm device was positioned within a water tank (20 cm by 20 cm by 10 cm), to which a piezo actuator for acoustic wave generation was attached. High-speed camera footage was captured to observe the oscillation mode of the PDMS diaphragm as the frequency of the input signal to the piezo actuator was swept.
The results presented in
Figure 1b,c demonstrate that the mode number in oscillation increases with frequency. It is challenging to discern the oscillation modes from the static images, although mode shapes were more readily identifiable in the high-speed video clips (
Supplementary Videos S1 and S2). To address this limitation, individual frames from the video were extracted and converted into RGB images. Subtraction of corresponding pixel values between frames was subsequently performed to visually illustrate the mode shapes. Notably, the resulting images in
Figure 1b,c contain regions of black or white, indicating that the pixel values after subtraction fell outside the defined RGB range. These regions typically corresponded to the peaks and valleys of the oscillation mode shapes of the diaphragm.
Figure 1b (
Supplementary Video S1) demonstrates the impact of cavity diameter (1, 1.5, and 2 mm) on the resonance mode when the cavity height is fixed at 1 mm and the applied frequency is fixed at 5.7 kHz. As the cavity diameter increases, the mode (wave) number in the oscillation mode also increases.
Figure 1c,d (
Supplementary Video S2) illustrate the effect of cavity height (0.5 and 1 mm) on the resonance while the diameter is fixed. Both cases exhibit the first mode, but the resonance frequency shifts from 5.7 kHz to 7.4 kHz when the cavity height changes from 1 mm to 0.5 mm. Based on these findings, the current energy-harvesting device utilizes a cavity with a diameter of 1 mm and a depth of 1 mm.
2.4. Fabrication
The fabrication of the energy-harvesting device was divided into two distinct phases (
Figure 2a). The first phase involved the assembly of a piezoelectric layer sandwiched between the top and bottom electrodes on the PDMS diaphragm. The second phase entailed the fabrication of a 3D-printed cavity array substrate. The first phase was executed utilizing conventional microfabrication techniques, while the second phase was accomplished through stereolithography (SLA) 3D printing.
Figure 2b shows the detailed microfabrication steps of the PDMS diaphragm with the piezoelectric layer and electrodes. PDMS (Sylgard 184) was chosen as the diaphragm material due to its low Young’s modulus and ease of fabrication. First, a silicon wafer was treated with silane for the easy detachment of PDMS later. PDMS solution was subsequently poured onto the molds and spun using a spin-coater (Laurell Technologies, North Wales, PA, USA) to achieve a 20 μm diaphragm thickness, which could be detached from the silicon wafer later (
Figure 2b(1)). To enhance the adhesion between the PDMS and the bottom gold electrode, the PDMS surface was treated with O
2 plasma to roughen the surface. Subsequently, a 10 nm adhesion titanium layer was applied before the 30 nm gold layer was deposited for the bottom electrode (
Figure 2b(2)). PVDF-TrFE (Solvene 300/P300 from Sigma Aldrich, Burlington, MA, USA) was procured and dissolved in an MEK solution (78-93-3 from Sigma Aldrich) at a 1:10 mass ratio. The solution was then heated at 80 °C and stirred on a hotplate for 30 min to ensure complete dissolution. Subsequently, the solution was spin-coated at 1500 rpm to achieve a uniform thickness of 3 μm. Another layer of 30 nm of gold was then deposited for the top electrode. Photolithography was employed to pattern the top electrode (
Figure 2b(3)). However, after etching the metal layer, the residual photoresist on top of the electrode had to be removed. However, since acetone also removed the PVDF-TrFE layer, O
2 plasma RIE (50 sccm O
2, 200 mW power) was instead employed to strip both the PVDF-TrFE and the photoresist residue (
Figure 2b(4)). To ensure water insulation, the entire device was uniformly vapor-deposited with a 3 μm layer of parylene (
Figure 2b(5)).
Following fabrication, the electrodes of the device were connected to output wires using silver paste for electrical connection. Then, electric poling was carried out for the PVDF-TrFE layer to determine its piezoelectric properties. Since piezoelectricity exhibits a Curie temperature of 115 °C, the device was heated on a 120 °C hotplate. Subsequently, a direct current voltage of 180 V was applied across the device electrodes for a duration of 30 min. Subsequently, the heat was gradually removed before the voltage was deactivated. This procedure ensured that the formed crystalline structure remained fixed in a uniform direction.
Finally, the cavity array (5 × 5) part was then fabricated using 3D SLA printing and glass laser cutting. The PDMS diaphragms detached from the silicon wafer were bonded onto the top of the cavity array part (
Figure 2b(6)).
Figure 2c presents a photo of the fabricated device.
4. Discussion
The device presented in this study has the potential to be a cost-effective solution for generating higher power outputs when exposed to acoustic waves. Although microfabrication was used to fabricate the electrodes, it may not be necessary, as neither the electrode dimensions nor the bubble dimensions require microscale fabrication. The materials used in this study, such as the piezoelectric polymer and the PDMS, are readily available and inexpensive, such that the fabricated devices are cost-effective.
Three other studies [
15,
16,
17] were selected for comparison, summarized in
Table 1, where the harvested powers and the resonator sizes from these studies were compared with those of the present work. The present harvester and the MEMS harvester [
15] showed greater power densities than the other macroscale energy harvesters [
16,
17]. In particular, the present harvester showed the greatest power density.
Obviously, there is room for improvement of the current design to further increase the harvested energy. Currently, the piezo diaphragm is incorporated only on the front side of the cavities. An additional piezo diaphragm could be added to the backside of the cavities to double the harvested power. Another improvement would be the utilization of higher-order oscillation modes, where a certain portion of the induced electric potential is generally cancelled out when a single piece of electrode is used. To eliminate this cancellation, separated multiple electrodes could be used with polarity regulation in the interface circuit. This method may potentially increase the stress level in the diaphragm and eventually enhance the harvested energy level.