Advanced Robotics for the Next-Generation of Cardiac Interventions
Abstract
1. Introduction
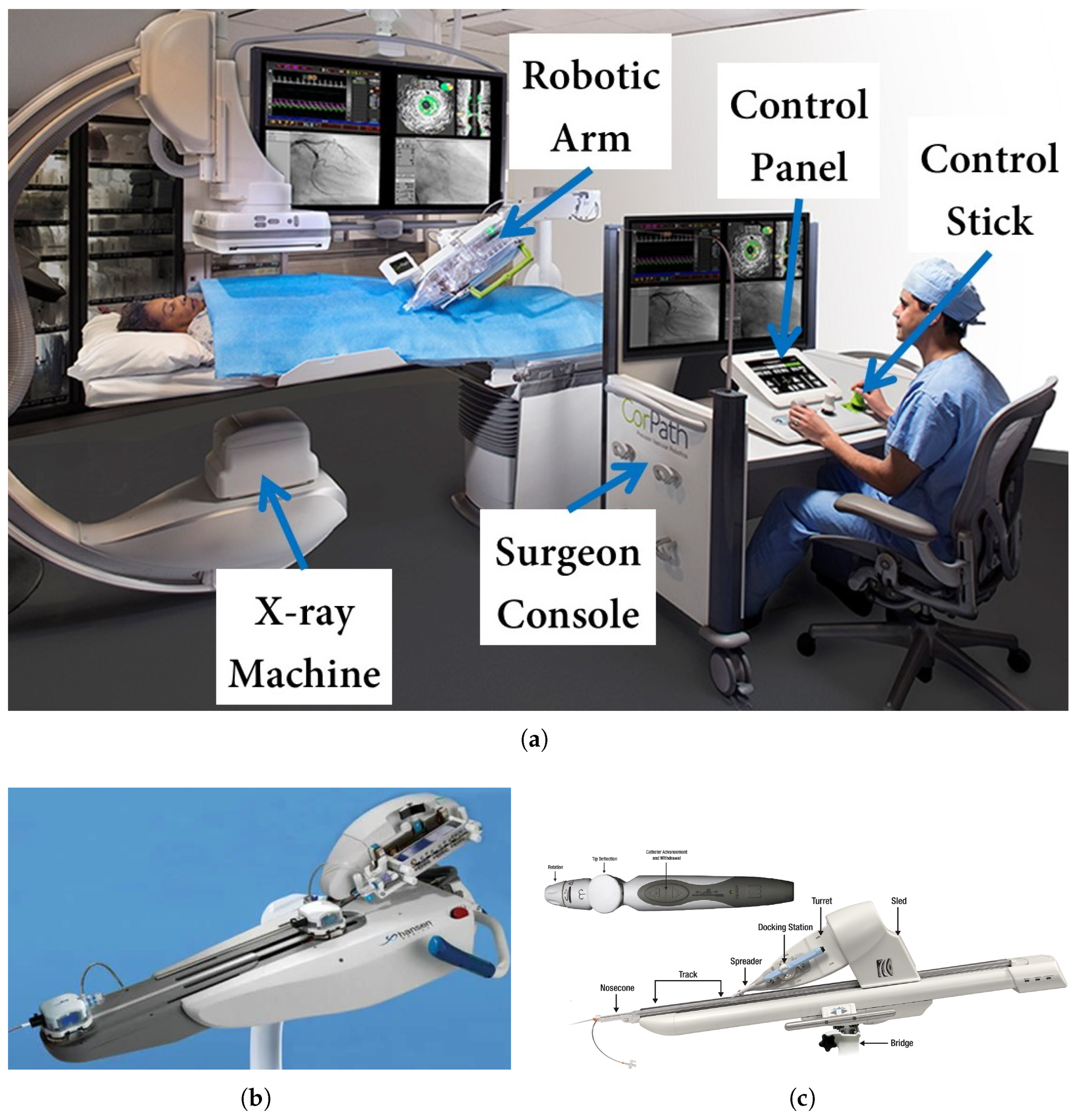
1.1. Robot-Assisted Interventions: System Overview
1.2. Necessity for Advanced Technologies for Cardiac Intervention
1.3. Literature Survey
1.4. Motivations for Next-Generation Systems
2. Advanced Robotics in Cardiac Interventions
2.1. Robot-Assisted Interventions: Clinical Outcomes and Limitations
2.1.1. Control Accuracy
2.1.2. Haptic Feedback and Haptic Devices
2.1.3. Position Control
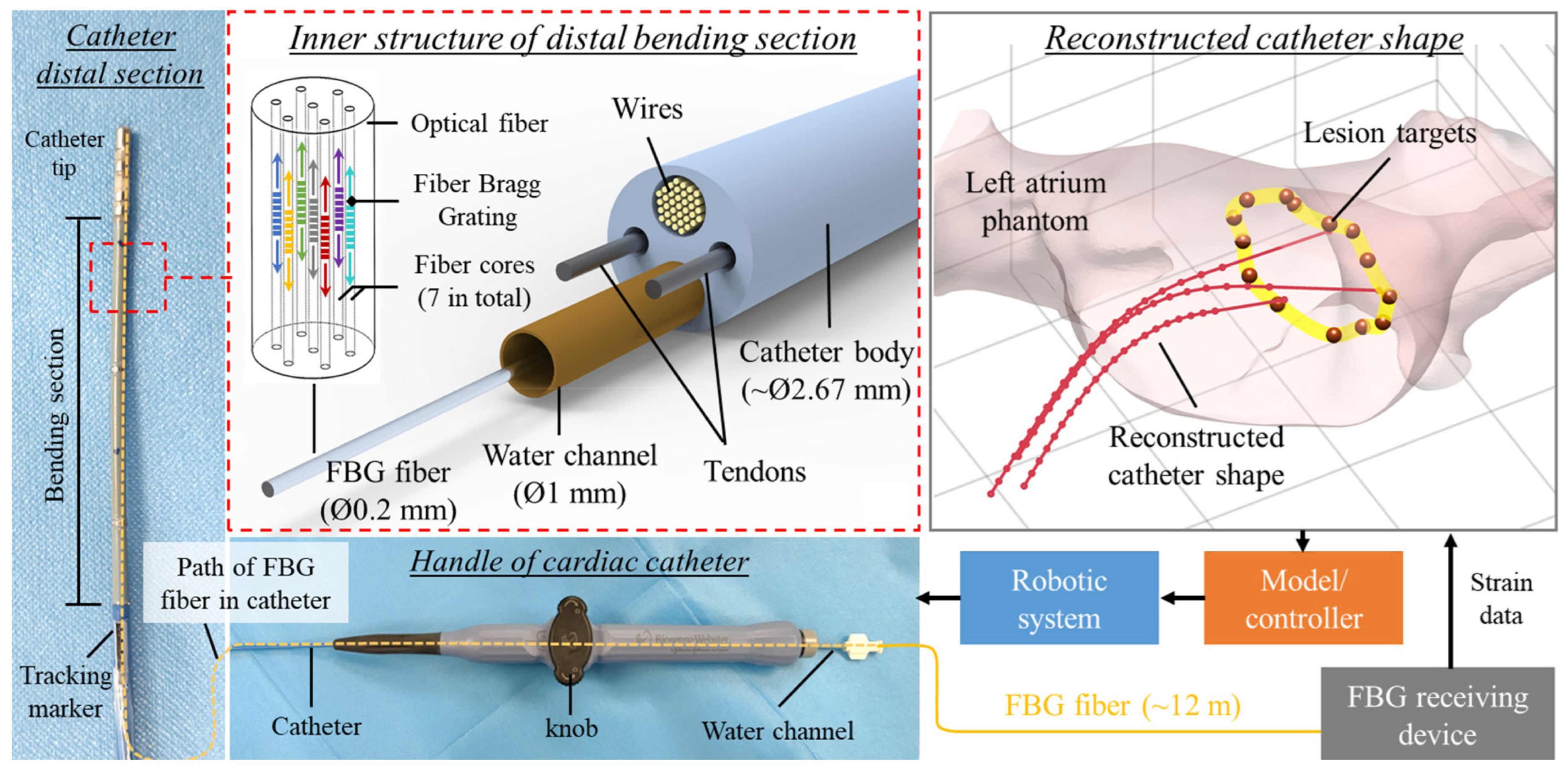
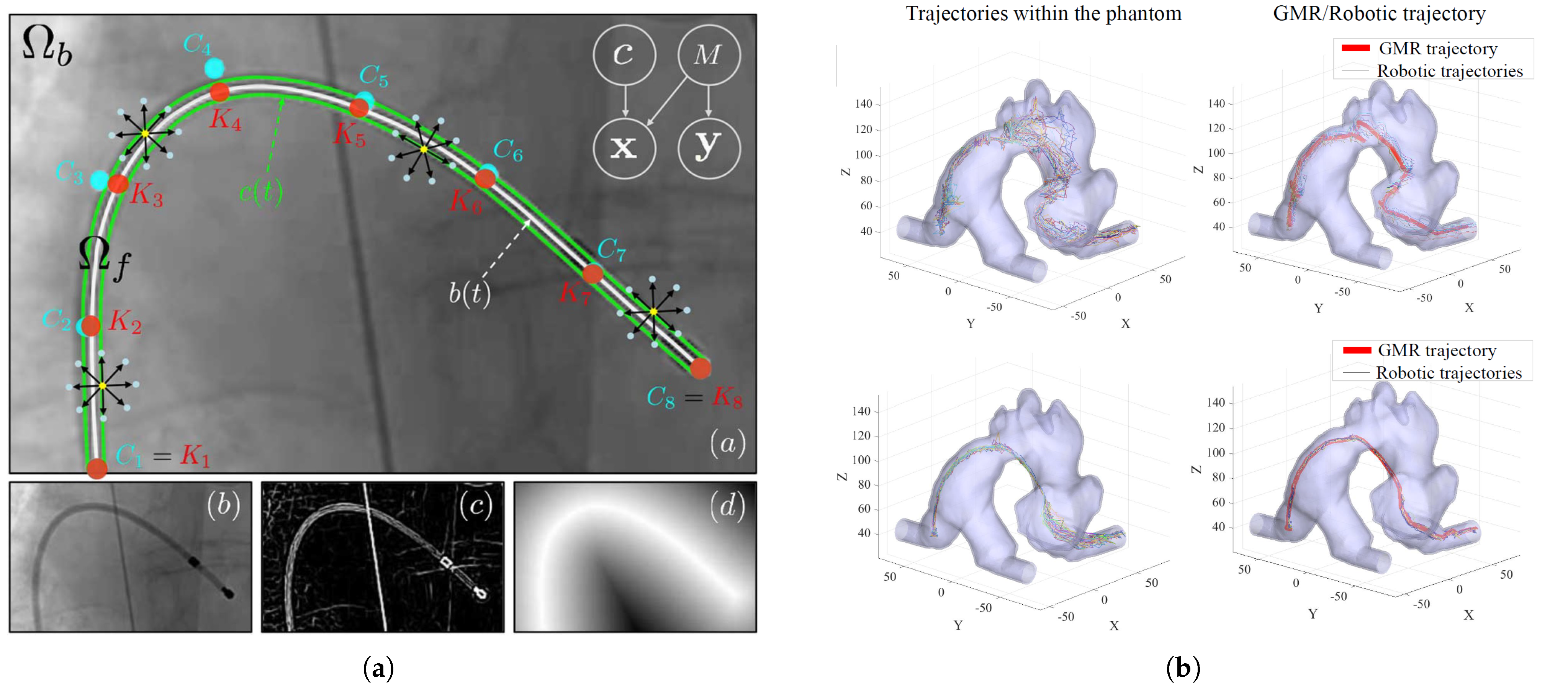
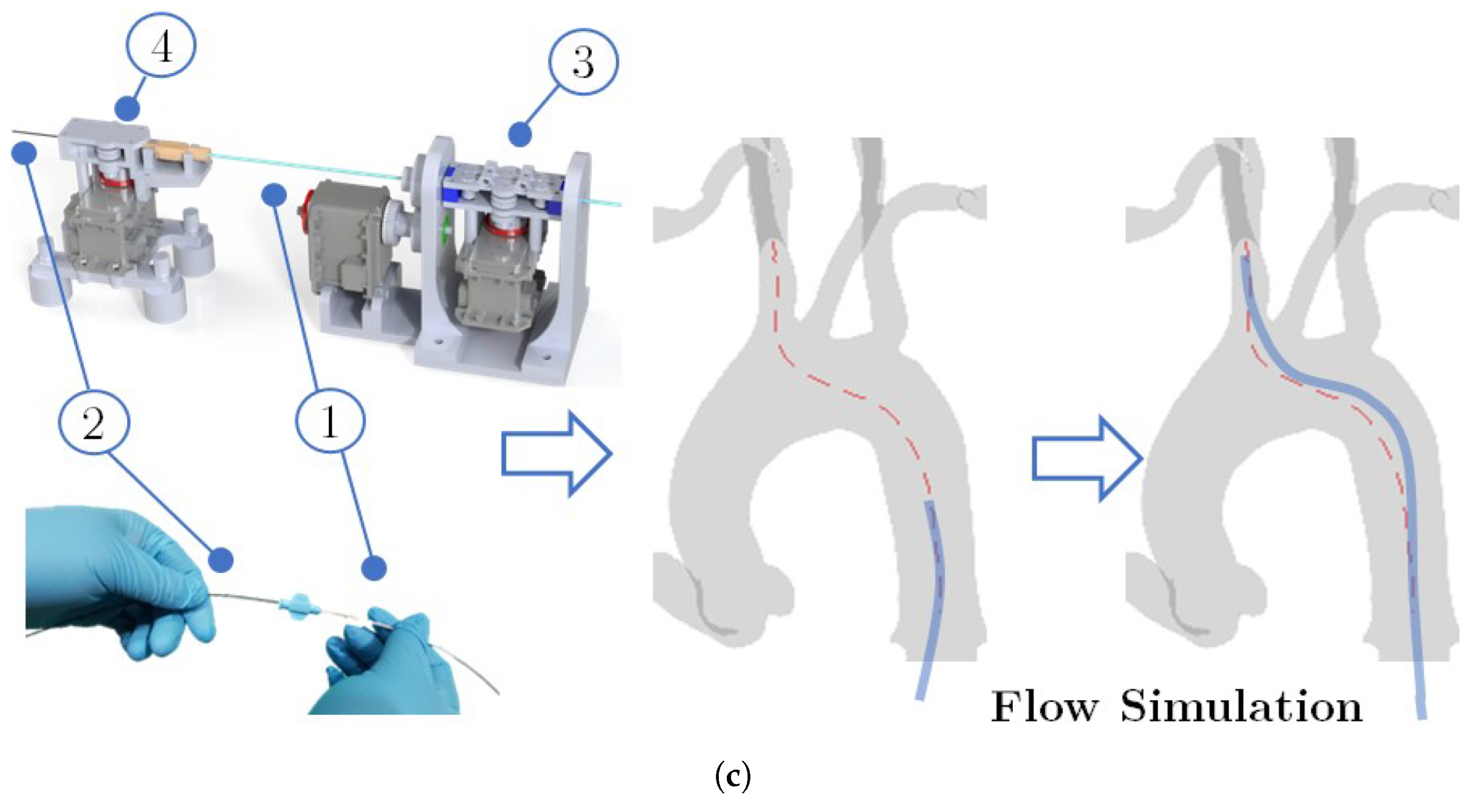
2.1.4. Catheter Tracking and Autonomous Navigation
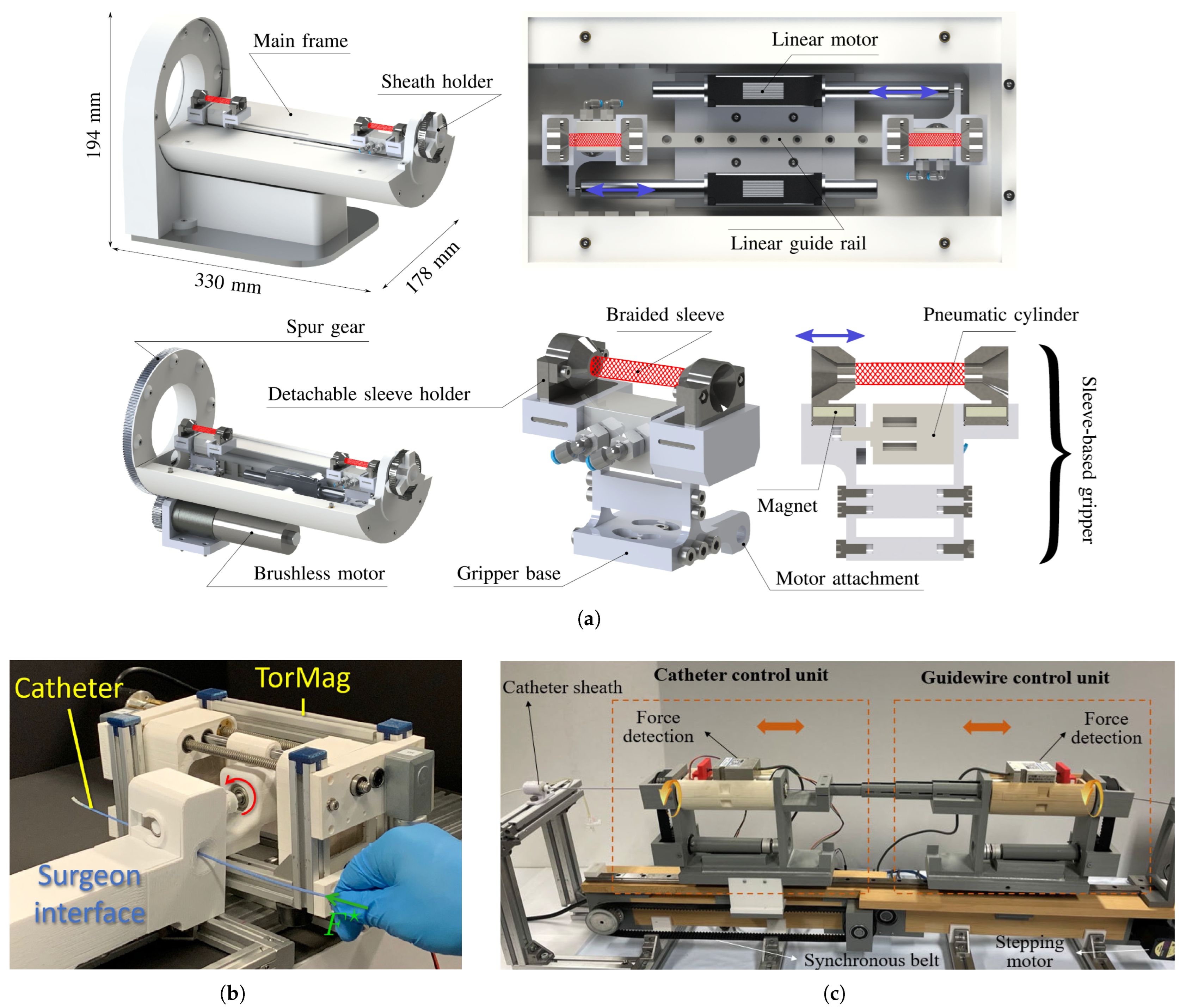
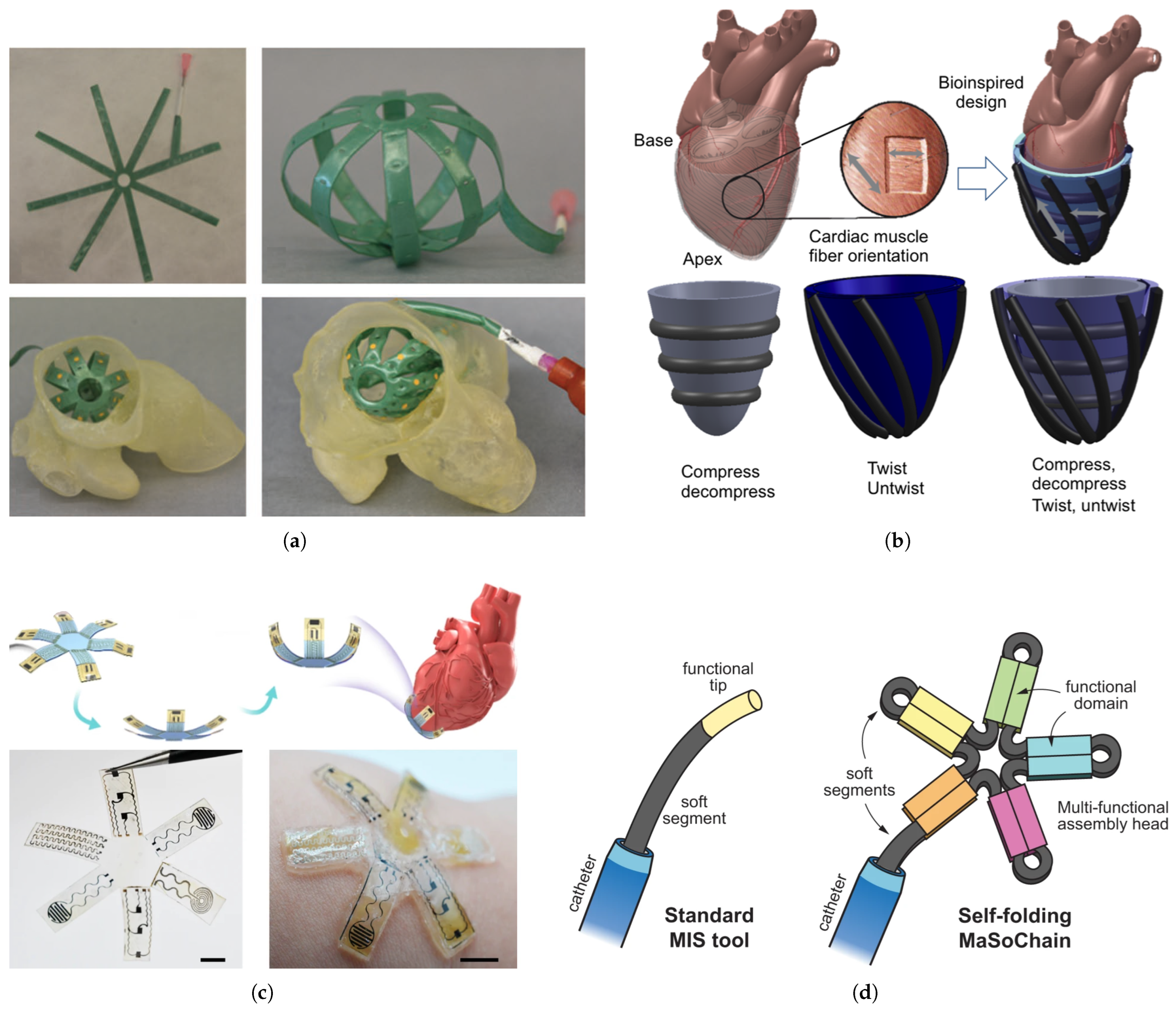
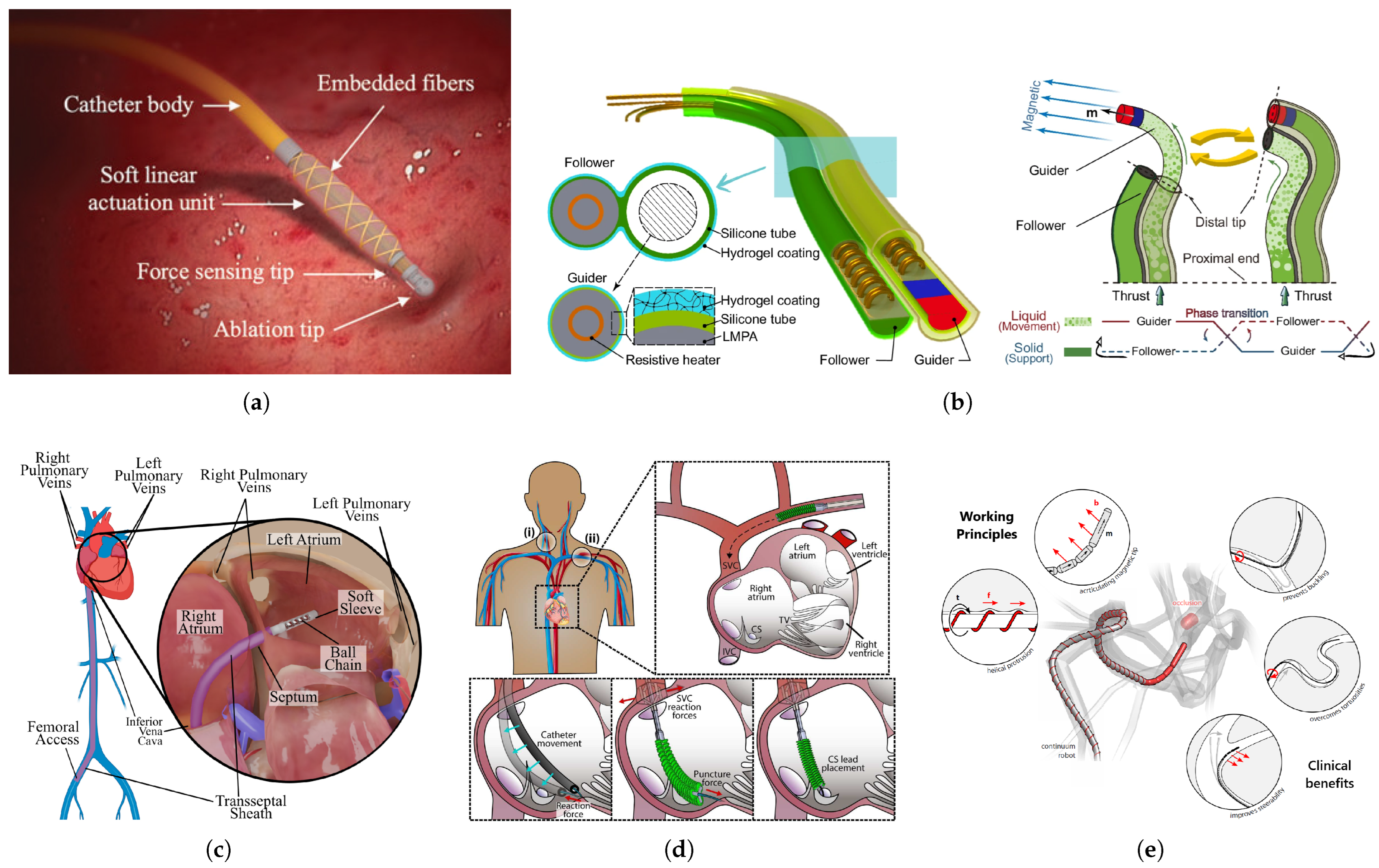
2.2. Soft Robotics and Soft Sensors in Robot-Assisted Cardiac Interventions
3. Discussion of State-of-the-Art Robotic Technologies and Research Gaps
4. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AF | Atrial Fibrillation |
| AI | Artificial Intelligence |
| CAD | Coronary Artery Disease |
| CT | Computed Tomography |
| DoF | Degree of Freedom |
| EM | Electromagnetic |
| FDA | Food and Drug Administration |
| FBG | Fiber Bragg Grating |
| GMM | Gaussian Mixture Model |
| HFAM | Hydraulic Filament Artificial Muscle |
| ICE | Intracardiac Echocardiography |
| ML | Machine Learning |
| MRI | Magnetic Resonance Imaging |
| MIS | Minimally Invasive Surgery |
| MRE | Magnetorheological Elastomer |
| NCDR | National Cardiovascular Data Registry |
| NN | Neural Network |
| PCI | Percutaneous Coronary Intervention |
| PI | Peripheral Interventions |
| PVI | Peripheral Vascular Intervention |
| RCT | Randomized Controlled Trial |
| RL | Reinforcement Learning |
| TEE | Transesophageal Echocardiogram |
| XR | Extended Reality |
References
- Hoole, S.P.; Bambrough, P. Recent advances in percutaneous coronary intervention. Heart 2020, 106, 1380–1386. [Google Scholar] [PubMed]
- Vallabhajosyula, S.; Alasnag, M.; Boudoulas, K.D.; Davidson, L.J.; Pyo, R.T.; Riley, R.F.; Shah, P.B.; Velagapudi, P.; Batchelor, W.B.; Truesdell, A.G.; et al. Future Training Pathways in Percutaneous Coronary Interventions: Interventional Critical Care, Complex Coronary Interventions, and Interventional Heart Failure. JACC Adv. 2024, 3, 101338. [Google Scholar] [PubMed]
- Veillette, J.B.; Carrier, M.A.; Rinfret, S.; Mercier, J.; Arsenault, J.; Paradis, J.M. Occupational Risks of Radiation Exposure to Cardiologists. Curr. Cardiol. Rep. 2024, 26, 601–622. [Google Scholar] [PubMed]
- Harky, A.; Chaplin, G.; Chan, J.S.K.; Eriksen, P.; MacCarthy-Ofosu, B.; Theologou, T.; Muir, A.D. The future of open heart surgery in the era of robotic and minimal surgical interventions. Hear. Lung Circ. 2020, 29, 49–61. [Google Scholar]
- Vento, V.; Kuntz, S.; Lejay, A.; Chakfe, N. Evolutionary trends and innovations in cardiovascular intervention. Front. Med. Technol. 2024, 6, 1384008. [Google Scholar]
- Nogueira, R.G.; Sachdeva, R.; Al-Bayati, A.R.; Mohammaden, M.H.; Frankel, M.R.; Haussen, D.C. Robotic assisted carotid artery stenting for the treatment of symptomatic carotid disease: Technical feasibility and preliminary results. J. NeuroInterventional Surg. 2020, 12, 341–344. [Google Scholar] [CrossRef]
- Koulaouzidis, G.; Charisopoulou, D.; Bomba, P.; Stachura, J.; Gasior, P.; Harpula, J.; Zarifis, J.; Marlicz, W.; Hudziak, D.; Jadczyk, T. Robotic-Assisted solutions for invasive cardiology, cardiac surgery and routine on-ward tasks: A narrative review. J. Cardiovasc. Dev. Dis. 2023, 10, 399. [Google Scholar] [CrossRef]
- Fan, Y.; Xu, L.; Liu, S.; Li, J.; Xia, J.; Qin, X.; Li, Y.; Gao, T.; Tang, X. The State-of-the-Art and Perspectives of Laser Ablation for Tumor Treatment. Cyborg Bionic Syst. 2025, 6, 0062. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Q. Multi-Section Magnetic Soft Robot with Multirobot Navigation System for Vasculature Intervention. Cyborg Bionic Syst. 2025, 6, 0188. [Google Scholar] [CrossRef]
- Ernst, S.; Ouyang, F.; Linder, C.; Hertting, K.; Stahl, F.; Chun, J.; Hachiya, H.; Bansch, D.; Antz, M.; Kuck, K.H. Initial experience with remote catheter ablation using a novel magnetic navigation system: Magnetic remote catheter ablation. Circulation 2004, 109, 1472–1475. [Google Scholar]
- Beyar, R.; Gruberg, L.; Deleanu, D.; Roguin, A.; Almagor, Y.; Cohen, S.; Kumar, G.; Wenderow, T. Remote-control percutaneous coronary interventions: Concept, validation, and first-in-humans pilot clinical trial. J. Am. Coll. Cardiol. 2006, 47, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, E.; Schmid, F.; Kalmar, P.; Deutschmann, H.; Hafner, F.; Rief, P.; Brodmann, M. Feasibility and safety of robotic peripheral vascular interventions: Results of the RAPID trial. Cardiovasc. Interv. 2016, 9, 2058–2064. [Google Scholar]
- Bismuth, J.; Duran, C.; Stankovic, M.; Gersak, B.; Lumsden, A.B. A first-in-man study of the role of flexible robotics in overcoming navigation challenges in the iliofemoral arteries. J. Vasc. Surg. 2013, 57, 14S–19S. [Google Scholar] [CrossRef]
- Khan, E.M.; Frumkin, W.; Ng, G.A.; Neelagaru, S.; Abi-Samra, F.M.; Lee, J.; Giudici, M.; Gohn, D.; Winkle, R.A.; Sussman, J.; et al. First experience with a novel robotic remote catheter system: Amigo™ mapping trial. J. Interv. Card. Electrophysiol. 2013, 37, 121–129. [Google Scholar] [CrossRef]
- Scarà, A.; Sciarra, L.; De Ruvo, E.; Borrelli, A.; Grieco, D.; Palamà, Z.; Golia, P.; De Luca, L.; Rebecchi, M.; Calò, L. Safety and feasibility of atrial fibrillation ablation using Amigo® system versus manual approach: A pilot study. Indian Pacing Electrophysiol. J. 2018, 18, 61–67. [Google Scholar] [CrossRef]
- Shaikh, Z.A.; Eilenberg, M.F.; Cohen, T.J. The Amigo™ remote catheter system: From concept to bedside. J. Innov. Card. Rhythm Manag. 2017, 8, 2795. [Google Scholar] [CrossRef]
- Costa, M.A.; Angiolillo, D.J.; Tannenbaum, M.; Driesman, M.; Chu, A.; Patterson, J.; Kuehl, W.; Battaglia, J.; Dabbons, S.; Shamoon, F.; et al. Impact of stent deployment procedural factors on long-term effectiveness and safety of sirolimus-eluting stents (final results of the multicenter prospective STLLR trial). Am. J. Cardiol. 2008, 101, 1704–1711. [Google Scholar] [CrossRef]
- Hooshiar, A.; Najarian, S.; Dargahi, J. Haptic telerobotic cardiovascular intervention: A review of approaches, methods, and future perspectives. IEEE Rev. Biomed. Eng. 2019, 13, 32–50. [Google Scholar] [CrossRef]
- Tamirisa, K.P.; Alasnag, M.; Calvert, P.; Islam, S.; Bhardwaj, A.; Pakanati, K.; Zieroth, S.; Razminia, M.; Dalal, A.S.; Mamas, M.; et al. Radiation Exposure, Training, and Safety in Cardiology. JACC Adv. 2024, 3, 100863. [Google Scholar] [CrossRef]
- Dugas, C.M.; Schussler, J.M. Advanced technology in interventional cardiology: A roadmap for the future of precision coronary interventions. Trends Cardiovasc. Med. 2016, 26, 466–473. [Google Scholar] [CrossRef]
- Matl, S.; Brosig, R.; Baust, M.; Navab, N.; Demirci, S. Vascular image registration techniques: A living review. Med. Image Anal. 2017, 35, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, P.; Ruijters, D.; Niessen, W.J.; Moelker, A.; van Walsum, T. Fully automatic and real-time catheter segmentation in X-ray fluoroscopy. In Proceedings of the Medical Image Computing and Computer-Assisted Intervention-MICCAI 2017: 20th International Conference, Proceedings, Part II 20, Quebec City, QC, Canada, 11–13 September 2017; pp. 577–585. [Google Scholar]
- Yang, G.Z.; Cambias, J.; Cleary, K.; Daimler, E.; Drake, J.; Dupont, P.E.; Hata, N.; Kazanzides, P.; Martel, S.; Patel, R.V.; et al. Medical robotics—Regulatory, ethical, and legal considerations for increasing levels of autonomy. Sci. Robot. 2017, 2, eaam8638. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Baker, T.S.; Bederson, J.B.; Rapoport, B.I. Levels of autonomy in FDA-cleared surgical robots: A systematic review. NPJ Digit. Med. 2024, 7, 103. [Google Scholar] [CrossRef]
- Pore, A.; Li, Z.; Dall’Alba, D.; Hernansanz, A.; De Momi, E.; Menciassi, A.; Gelpi, A.C.; Dankelman, J.; Fiorini, P.; Vander Poorten, E. Autonomous navigation for robot-assisted intraluminal and endovascular procedures: A systematic review. IEEE Trans. Robot. 2023, 39, 2529–2548. [Google Scholar] [CrossRef]
- Fosch-Villaronga, E.; Khanna, P.; Drukarch, H.; Custers, B. The role of humans in surgery automation: Exploring the influence of automation on human–robot interaction and responsibility in surgery innovation. Int. J. Soc. Robot. 2023, 15, 563–580. [Google Scholar]
- Mennella, C.; Maniscalco, U.; De Pietro, G.; Esposito, M. Ethical and regulatory challenges of AI technologies in healthcare: A narrative review. Heliyon 2024, 10, e26297. [Google Scholar] [CrossRef]
- Dreyfus, R.; Boehler, Q.; Lyttle, S.; Gruber, P.; Lussi, J.; Chautems, C.; Gervasoni, S.; Berberat, J.; Seibold, D.; Ochsenbein-Kölble, N.; et al. Dexterous helical magnetic robot for improved endovascular access. Sci. Robot. 2024, 9, eadh0298. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Z.; Shu, X.; Xie, L. Mechanism design and force sensing of a novel cardiovascular interventional surgery robot. Int. J. Med. Robot. Comput. Assist. Surg. 2022, 18, e2406. [Google Scholar] [CrossRef]
- Karstensen, L.; Behr, T.; Pusch, T.P.; Mathis-Ullrich, F.; Stallkamp, J. Autonomous guidewire navigation in a two dimensional vascular phantom. In Proceedings of the Current Directions in Biomedical Engineering, Hamburg, Germany, 17–19 September 2020; De Gruyter: Berlin, Germany; Volume 6, p. 20200007. [Google Scholar]
- Jianu, T.; Huang, B.; Vo, T.; Vu, M.N.; Kang, J.; Nguyen, H.; Omisore, O.; Berthet-Rayne, P.; Fichera, S.; Nguyen, A. Autonomous Catheterization with Open-source Simulator and Expert Trajectory. arXiv 2024, arXiv:2401.09059. [Google Scholar]
- Wang, Y.; Muthurangu, V.; Wurdemann, H.A. Toward Autonomous Pulmonary Artery Catheterization: A Learning-based Robotic Navigation System. In Proceedings of the 2023 45th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Sydney, Australia, 24–27 July 2023; pp. 1–5. [Google Scholar]
- Nanna, M.G.; Vemulapalli, S.; Fordyce, C.B.; Mark, D.B.; Patel, M.R.; Al-Khalidi, H.R.; Kelsey, M.; Martinez, B.; Yow, E.; Mullen, S.; et al. The prospective randomized trial of the optimal evaluation of cardiac symptoms and revascularization: Rationale and design of the PRECISE trial. Am. Heart J. 2022, 245, 136–148. [Google Scholar] [CrossRef]
- Mahmud, E.; Naghi, J.; Ang, L.; Harrison, J.; Behnamfar, O.; Pourdjabbar, A.; Reeves, R.; Patel, M. Demonstration of the safety and feasibility of robotically assisted percutaneous coronary intervention in complex coronary lesions: Results of the CORA-PCI Study (Complex Robotically Assisted Percutaneous Coronary Intervention). JACC Cardiovasc. Interv. 2017, 10, 1320–1327. [Google Scholar] [PubMed]
- Pourdjabbar, A.; Ang, L.; Behnamfar, O.; Patel, M.P.; Reeves, R.R.; Campbell, P.T.; Madder, R.D.; Mahmud, E. Robotics in percutaneous cardiovascular interventions. Expert Rev. Cardiovasc. Ther. 2017, 15, 825–833. [Google Scholar] [PubMed]
- Deng, Y.; Roshanfar, M.; Mayer, H.; He, C.; Drake, J.; Looi, T.; Diller, E. Towards Bimanual Operation of Magnetically Actuated Surgical Instruments. In Proceedings of the 2024 10th IEEE RAS/EMBS International Conference for Biomedical Robotics and Biomechatronics (BioRob), Heidelberg, Germany, 1–4 September 2024; pp. 1295–1300. [Google Scholar]
- Roshanfar, M.; Dargahi, J.; Hooshiar, A. Design optimization of a hybrid-driven soft surgical robot with biomimetic constraints. Biomimetics 2024, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Roshanfar, M.; Dargahi, J.; Hooshiar, A. Cosserat rod-based dynamic modeling of a hybrid-actuated soft robot for robot-assisted cardiac ablation. Actuators 2023, 13, 8. [Google Scholar] [CrossRef]
- Roshanfar, M.; Sayadi, A.; Dargahi, J.; Hooshiar, A. Stiffness adaptation of a hybrid soft surgical robot for improved safety in interventional surgery. In Proceedings of the 2022 44th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Glasgow, UK, 11–15 July 2022; pp. 4853–4859. [Google Scholar]
- Roshanfar, M.; Dargahi, J.; Hooshiar, A. Toward semi-autonomous stiffness adaptation of pneumatic soft robots: Modeling and validation. In Proceedings of the 2021 IEEE International Conference on Autonomous Systems (ICAS), Montréal, QC, Canada, 11–13 August 2021; pp. 1–5. [Google Scholar]
- Roshanfar, M.; Taki, S.; Sayadi, A.; Cecere, R.; Dargahi, J.; Hooshiar, A. Hyperelastic modeling and validation of hybrid-actuated soft robot with pressure-stiffening. Micromachines 2023, 14, 900. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Z.; Cao, Y.; Zhang, L.; Xie, L. Improved precise guidewire delivery of a cardiovascular interventional surgery robot based on admittance control. Int. J. Comput. Assist. Radiol. Surg. 2024, 19, 209–221. [Google Scholar]
- Dong, Z.; Wang, X.; Fang, G.; He, Z.; Ho, J.D.L.; Cheung, C.L.; Tang, W.L.; Xie, X.; Liang, L.; Chang, H.C.; et al. Shape tracking and feedback control of cardiac catheter using MRI-guided robotic platform—Validation with pulmonary vein isolation simulator in MRI. IEEE Trans. Robot. 2022, 38, 2781–2798. [Google Scholar]
- Akinyemi, T.O.; Omisore, O.M.; Duan, W.; Lu, G.; Al-Handerish, Y.; Han, S.; Wang, L. Fiber Bragg grating-based force sensing in robot-assisted cardiac interventions: A review. IEEE Sens. J. 2021, 21, 10317–10331. [Google Scholar]
- Dagnino, G.; Kundrat, D. Robot-assistive minimally invasive surgery: Trends and future directions. Int. J. Intell. Robot. Appl. 2024, 8, 812–826. [Google Scholar]
- Lahcen, A.A.; Labib, M.; Caprio, A.; Annabestani, M.; Sanchez-Botero, L.; Hsue, W.; Liu, C.F.; Dunham, S.; Mosadegh, B. Design, Testing, and Validation of a Soft Robotic Sensor Array Integrated with Flexible Electronics for Mapping Cardiac Arrhythmias. Micromachines 2024, 15, 1393. [Google Scholar] [CrossRef]
- Razban, M.; Dargahi, J.; Boulet, B. A sensor-less catheter contact force estimation approach in endovascular intervention procedures. In Proceedings of the 2018 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Madrid, Spain, 1–5 October 2018; pp. 2100–2106. [Google Scholar]
- Razban, M.; Dargahi, J.; Boulet, B. Image-based intraluminal contact force monitoring in robotic vascular navigation. arXiv 2020, arXiv:2012.10762. [Google Scholar]
- Hooshiar, A.; Payami, A.; Dargahi, J.; Najarian, S. Magnetostriction-based force feedback for robot-assisted cardiovascular surgery using smart magnetorheological elastomers. Mech. Syst. Signal Process. 2021, 161, 107918. [Google Scholar]
- Tahir, A.; Iqbal, H.; Usman, M.; Ghaffar, A.; Hafeez, A. Cardiac X-ray image-based haptic guidance for robot-assisted coronary intervention: A feasibility study. Int. J. Comput. Assist. Radiol. Surg. 2022, 17, 531–539. [Google Scholar]
- Gerald, A.; Russo, S. Soft sensing and haptics for medical procedures. Nat. Rev. Mater. 2024, 9, 86–88. [Google Scholar]
- Al-Ahmad, O.; Ourak, M.; Vlekken, J.; Vander Poorten, E. Force control with a novel robotic catheterization system based on braided sleeve grippers. IEEE Trans. Med. Robot. Bionics 2023, 5, 602–613. [Google Scholar]
- Li, X.; Guo, S.; Suzuki, K.; Shi, P.; Jin, X. Soft Magnetic-Actuation-Based Haptic Interface for a Robot-Assisted Vascular Interventional System: A Feasibility Study. IEEE Sens. J. 2023, 24, 710–721. [Google Scholar]
- Bao, X.; Guo, S.; Yang, C.; Zheng, L. Haptic interface with force and torque feedback for robot-assisted endovascular catheterization. IEEE/ASME Trans. Mechatron. 2023, 29, 1111–1125. [Google Scholar]
- Jolaei, M.; Hooshiar, A.; Dargahi, J.; Packirisamy, M. Toward task autonomy in robotic cardiac ablation: Learning-based kinematic control of soft tendon-driven catheters. Soft Robot. 2021, 8, 340–351. [Google Scholar]
- Kesner, S.B.; Howe, R.D. Position control of motion compensation cardiac catheters. IEEE Trans. Robot. 2011, 27, 1045–1055. [Google Scholar]
- Zhou, J.J.; Quadri, A.; Sewani, A.; Alawneh, Y.; Gilliland-Rocque, R.; Magnin, C.; Dueck, A.; Wright, G.A.; Tavallaei, M.A. The CathPilot: A novel approach for accurate interventional device steering and tracking. IEEE/ASME Trans. Mechatron. 2022, 27, 5812–5823. [Google Scholar]
- Ren, B.; Zhao, Y.; Zhang, J.; Li, H.; Li, K.; Zhang, J. The critical technologies of vascular interventional robotic catheterization: A Review. IEEE Sens. J. 2023, 23, 30051–30069. [Google Scholar] [CrossRef]
- Scarponi, V.; Duprez, M.; Nageotte, F.; Cotin, S. A zero-shot reinforcement learning strategy for autonomous guidewire navigation. Int. J. Comput. Assist. Radiol. Surg. 2024, 19, 1185–1192. [Google Scholar] [CrossRef]
- Karstensen, L.; Ritter, J.; Hatzl, J.; Ernst, F.; Langejürgen, J.; Uhl, C.; Mathis-Ullrich, F. Recurrent neural networks for generalization towards the vessel geometry in autonomous endovascular guidewire navigation in the aortic arch. Int. J. Comput. Assist. Radiol. Surg. 2023, 18, 1735–1744. [Google Scholar] [CrossRef] [PubMed]
- Robertshaw, H.; Karstensen, L.; Jackson, B.; Granados, A.; Booth, T.C. Autonomous navigation of catheters and guidewires in mechanical thrombectomy using inverse reinforcement learning. Int. J. Comput. Assist. Radiol. Surg. 2024, 19, 1569–1578. [Google Scholar] [CrossRef]
- Annabestani, M.; Sriram, S.; Caprio, A.; Janghorbani, S.; Wong, S.C.; Sigaras, A.; Mosadegh, B. High-fidelity pose estimation for real-time extended reality (XR) visualization for cardiac catheterization. Sci. Rep. 2024, 14, 26962. [Google Scholar] [CrossRef]
- Chang, P.L.; Rolls, A.; De Praetere, H.; Vander Poorten, E.; Riga, C.V.; Bicknell, C.D.; Stoyanov, D. Robust catheter and guidewire tracking using b-spline tube model and pixel-wise posteriors. IEEE Robot. Autom. Lett. 2016, 1, 303–308. [Google Scholar] [CrossRef]
- Chi, W.; Liu, J.; Abdelaziz, M.E.; Dagnino, G.; Riga, C.; Bicknell, C.; Yang, G.Z. Trajectory optimization of robot-assisted endovascular catheterization with reinforcement learning. In Proceedings of the 2018 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Madrid, Spain, 1–5 October 2018; pp. 3875–3881. [Google Scholar]
- Karstensen, L.; Robertshaw, H.; Hatzl, J.; Jackson, B.; Langejürgen, J.; Breininger, K.; Uhl, C.; Sadati, S.; Booth, T.; Bergeles, C.; et al. Learning-Based Autonomous Navigation, Benchmark Environments and Simulation Framework for Endovascular Interventions. arXiv 2024, arXiv:2410.01956. [Google Scholar]
- Fagogenis, G.; Mencattelli, M.; Machaidze, Z.; Rosa, B.; Price, K.; Wu, F.; Weixler, V.; Saeed, M.; Mayer, J.E.; Dupont, P.E. Autonomous robotic intracardiac catheter navigation using haptic vision. Sci. Robot. 2019, 4, eaaw1977. [Google Scholar] [CrossRef]
- Roshanfar, M.; Jang, S.J.; Sinusas, A.; Wong, S.C.; Mosadegh, B. Addressing Peri-Device Leaks in Next-Generation Transcatheter Left Atrial Appendage Occluders: An Open Question. Surgeries 2025, 6, 15. [Google Scholar] [CrossRef]
- Torkaman, T.; Roshanfar, M.; Dargahi, J.; Hooshiar, A. Embedded Six-DoF Force–Torque Sensor for Soft Robots With Learning-Based Calibration. IEEE Sens. J. 2023, 23, 4204–4215. [Google Scholar] [CrossRef]
- Torkaman, T.; Roshanfar, M.; Dargahi, J.; Hooshiar, A. Accurate Embedded Force Sensor for Soft Robots with Rate-dependent Deep Neural Calibration. In Proceedings of the 2022 IEEE International Symposium on Robotic and Sensors Environments (ROSE), Abu Dhabi, United Arab Emirates, 14–15 November 2022; pp. 1–7. [Google Scholar] [CrossRef]
- Torkaman, T.; Roshanfar, M.; Dargahi, J.; Hooshiar, A. Analytical Modeling and Experimental Validation of a Gelatin-based Shape Sensor for Soft Robots. In Proceedings of the 2022 International Symposium on Medical Robotics (ISMR), Atlanta, GA, USA, 13–15 April 2022; pp. 1–7. [Google Scholar] [CrossRef]
- Farokhnia, N.; Caprio, A.; Kashyap, V.; Al’Aref, S.; Baskaran, L.; Mosadegh, B.; Dunham, S. A Catheter-Deployable Soft Robotic Inflatable Basket for Enhanced Conformability to the Left Atrium of the Heart. Adv. Healthc. Mater. 2020, 9, 1900951. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Teng, X.; Qiao, Z.; Yu, H.; Cai, S.; Yang, W. A concentric tube magnetic continuum robot with multiple stiffness levels and high flexibility for potential endovascular intervention. J. Magn. Magn. Mater. 2024, 597, 172023. [Google Scholar] [CrossRef]
- Mac Murray, B.C.; Futran, C.C.; Lee, J.; O’Brien, K.W.; Amiri Moghadam, A.A.; Mosadegh, B.; Silberstein, M.N.; Min, J.K.; Shepherd, R.F. Compliant buckled foam actuators and application in patient-specific direct cardiac compression. Soft Robot. 2018, 5, 99–108. [Google Scholar] [PubMed]
- Roche, E.T.; Horvath, M.A.; Wamala, I.; Alazmani, A.; Song, S.E.; Whyte, W.; Machaidze, Z.; Payne, C.J.; Weaver, J.C.; Fishbein, G.; et al. Soft robotic sleeve supports heart function. Sci. Transl. Med. 2017, 9, eaaf3925. [Google Scholar] [PubMed]
- Weymann, A.; Foroughi, J.; Vardanyan, R.; Punjabi, P.P.; Schmack, B.; Aloko, S.; Spinks, G.M.; Wang, C.H.; Arjomandi Rad, A.; Ruhparwar, A. Artificial muscles and soft robotic devices for treatment of end-stage heart failure. Adv. Mater. 2023, 35, 2207390. [Google Scholar]
- Zhang, L.; Xing, S.; Yin, H.; Weisbecker, H.; Tran, H.T.; Guo, Z.; Han, T.; Wang, Y.; Liu, Y.; Wu, Y.; et al. Skin-inspired, sensory robots for electronic implants. Nat. Commun. 2024, 15, 4777. [Google Scholar] [CrossRef]
- Nguyen, C.C.; Teh, T.; Thai, M.T.; Phan, P.T.; Hoang, T.T.; Low, H.; Davies, J.; Nicotra, E.; Lovell, N.H.; Do, T.N. Bidirectional soft robotic catheter for arrhythmia treatment. In Proceedings of the 2022 International Conference on Robotics and Automation (ICRA), Philadelphia, PA, USA, 23–27 May 2022; pp. 9579–9585. [Google Scholar]
- Gu, H.; Möckli, M.; Ehmke, C.; Kim, M.; Wieland, M.; Moser, S.; Bechinger, C.; Boehler, Q.; Nelson, B.J. Self-folding soft-robotic chains with reconfigurable shapes and functionalities. Nat. Commun. 2023, 14, 1263. [Google Scholar]
- Kim, Y.; Parada, G.A.; Liu, S.; Zhao, X. Ferromagnetic soft continuum robots. Sci. Robot. 2019, 4, eaax7329. [Google Scholar]
- Gopesh, T.; Wen, J.H.; Santiago-Dieppa, D.; Yan, B.; Pannell, J.S.; Khalessi, A.; Norbash, A.; Friend, J. Soft robotic steerable microcatheter for the endovascular treatment of cerebral disorders. Sci. Robot. 2021, 6, eabf0601. [Google Scholar]
- Du, W.; Omisore, O.M.; Duan, W.; Zhou, T.; Lv, X.; Li, Y.; Han, S.; Al-Handarish, Y.; Liu, Q.; Wang, L. Exploration of interventionists’ technical manipulation skills for robot-assisted intravascular PCI catheterization. IEEE Access 2020, 8, 53750–53765. [Google Scholar]
- Kumar, N.; Wirekoh, J.; Saba, S.; Riviere, C.N.; Park, Y.L. Soft miniaturized actuation and sensing units for dynamic force control of cardiac ablation catheters. Soft Robot. 2021, 8, 59–70. [Google Scholar] [PubMed]
- Mao, L.; Yang, P.; Tian, C.; Shen, X.; Wang, F.; Zhang, H.; Meng, X.; Xie, H. Magnetic steering continuum robot for transluminal procedures with programmable shape and functionalities. Nat. Commun. 2024, 15, 3759. [Google Scholar] [PubMed]
- Pittiglio, G.; Leuenberger, F.; Mencattelli, M.; McCandless, M.; O’Leary, E.; Dupont, P.E. Magnetic Ball Chain Robots for Cardiac Arrhythmia Treatment. IEEE Trans. Med. Robot. Bionics 2024, 6, 1322–1333. [Google Scholar] [PubMed]
- Rogatinsky, J.; Recco, D.; Feichtmeier, J.; Kang, Y.; Kneier, N.; Hammer, P.; O’Leary, E.; Mah, D.; Hoganson, D.; Vasilyev, N.V.; et al. A multifunctional soft robot for cardiac interventions. Sci. Adv. 2023, 9, eadi5559. [Google Scholar]
- Thorn, S.L.; Shuman, J.A.; Stacy, M.R.; Purcell, B.P.; Doviak, H.; Burdick, J.A.; Spinale, F.G.; Sinusas, A.J. Matrix metalloproteinase-targeted SPECT/CT imaging for evaluation of therapeutic hydrogels for the early modulation of post-infarct myocardial remodeling. J. Cardiovasc. Transl. Res. 2023, 16, 155–165. [Google Scholar]
- Stendahl, J.C.; Liu, Z.; Boutagy, N.E.; Nataneli, E.; Daghighian, F.; Sinusas, A.J. Prototype device for endoventricular beta-emitting radiotracer detection and molecularly-guided intervention. J. Nucl. Cardiol. 2022, 29, 663–676. [Google Scholar]
- Van der Velden, S.; Kunnen, B.; Koppert, W.J.; Steenbergen, J.H.; Dietze, M.M.; Beijst, C.; Viergever, M.A.; Lam, M.G.; de Jong, H.W. A dual-layer detector for simultaneous fluoroscopic and nuclear imaging. Radiology 2019, 290, 833–838. [Google Scholar]
- Pickell, Z.; Sinusas, A.J. Nuclear Cardiac imaging in the interventional suite. Curr. Cardiol. Rep. 2022, 24, 261–269. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roshanfar, M.; Salimi, M.; Kaboodrangi, A.H.; Jang, S.-J.; Sinusas, A.J.; Wong, S.-C.; Mosadegh, B. Advanced Robotics for the Next-Generation of Cardiac Interventions. Micromachines 2025, 16, 363. https://doi.org/10.3390/mi16040363
Roshanfar M, Salimi M, Kaboodrangi AH, Jang S-J, Sinusas AJ, Wong S-C, Mosadegh B. Advanced Robotics for the Next-Generation of Cardiac Interventions. Micromachines. 2025; 16(4):363. https://doi.org/10.3390/mi16040363
Chicago/Turabian StyleRoshanfar, Majid, Mohammadhossein Salimi, Amir Hossein Kaboodrangi, Sun-Joo Jang, Albert J. Sinusas, Shing-Chiu Wong, and Bobak Mosadegh. 2025. "Advanced Robotics for the Next-Generation of Cardiac Interventions" Micromachines 16, no. 4: 363. https://doi.org/10.3390/mi16040363
APA StyleRoshanfar, M., Salimi, M., Kaboodrangi, A. H., Jang, S.-J., Sinusas, A. J., Wong, S.-C., & Mosadegh, B. (2025). Advanced Robotics for the Next-Generation of Cardiac Interventions. Micromachines, 16(4), 363. https://doi.org/10.3390/mi16040363







