Towards High-Temperature MEMS: Two-Step Annealing Suppressed Recrystallization in Thin Multilayer Pt-Rh/Zr Films
Abstract
:1. Introduction
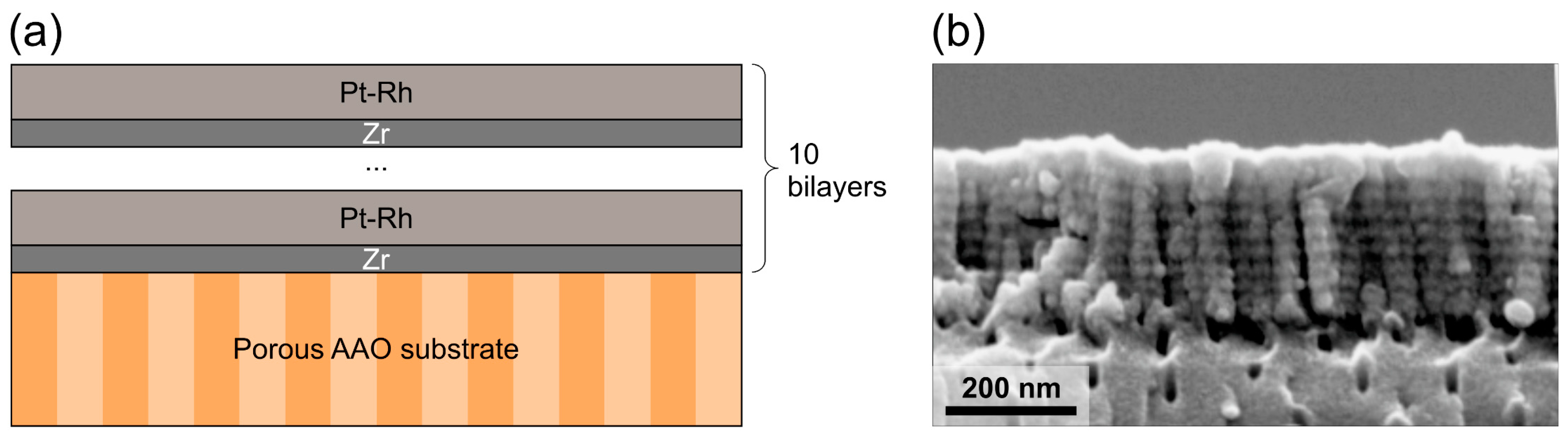
2. Materials and Methods
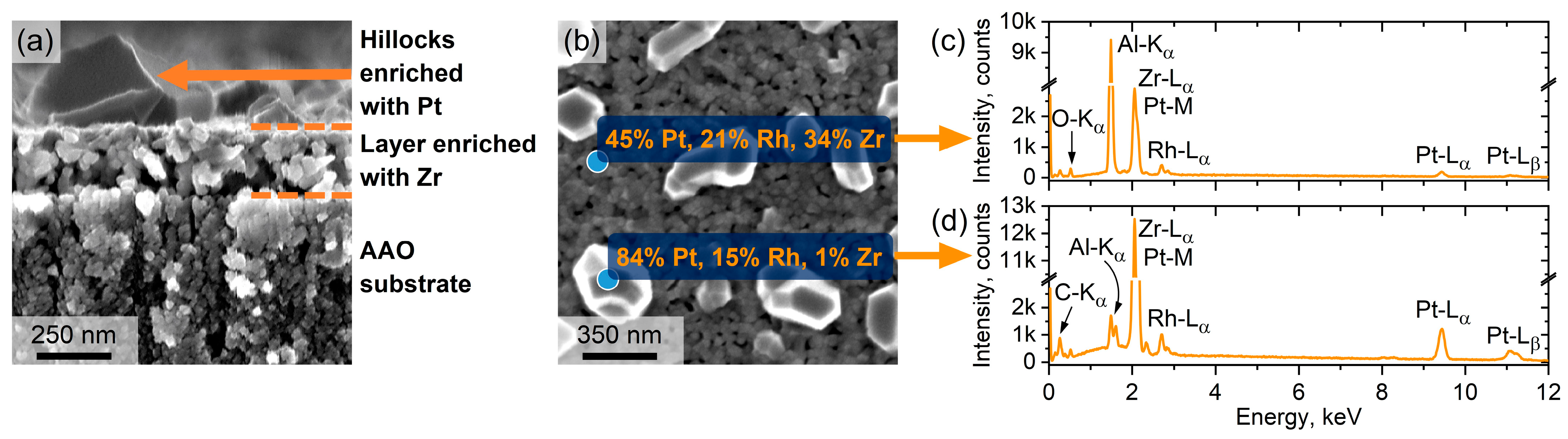
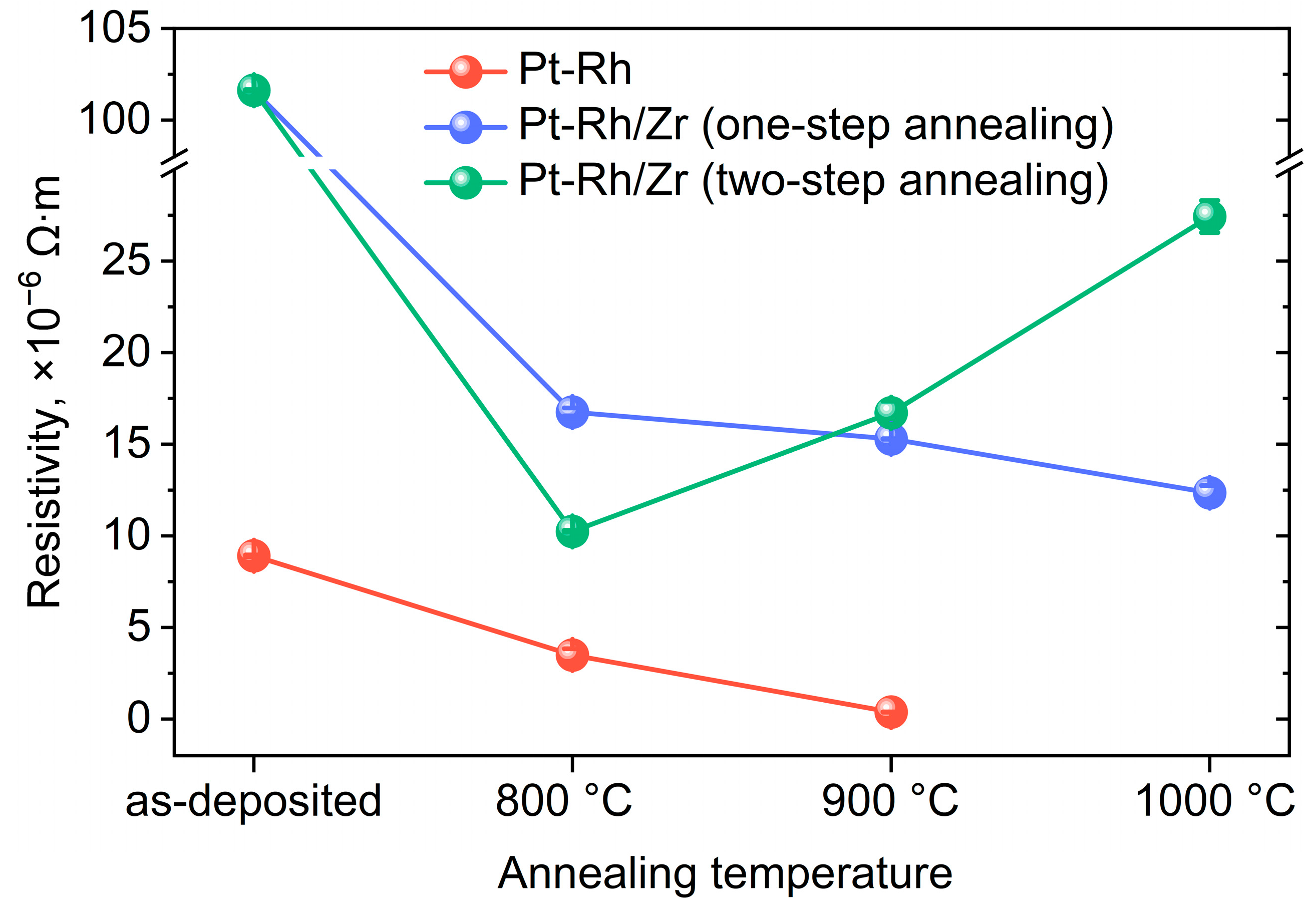
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, H.; Nakamura, M.; Su, P.; Fasching, R.; Saito, Y.; Prinz, F.B. High-Performance Ultrathin Solid Oxide Fuel Cells for Low-Temperature Operation. J. Electrochem. Soc. 2007, 154, B20. [Google Scholar] [CrossRef]
- Kerman, K.; Lai, B.K.; Ramanathan, S. Pt/Y0.16Zr0.84O1.92/Pt Thin Film Solid Oxide Fuel Cells: Electrode Microstructure and Stability Considerations. J. Power Sources 2011, 196, 2608–2614. [Google Scholar] [CrossRef]
- Mailly, F.; Giani, A.; Bonnot, R.; Temple-Boyer, P.; Pascal-Delannoy, F.; Foucaran, A.; Boyer, A. Anemometer with Hot Platinum Thin Film. Sens. Actuators A Phys. 2001, 94, 32–38. [Google Scholar] [CrossRef]
- Mailly, F.; Giani, A.; Martinez, A.; Bonnot, R.; Temple-Boyer, P.; Boyer, A. Micromachined Thermal Accelerometer. Sens. Actuators A Phys. 2003, 103, 359–363. [Google Scholar] [CrossRef]
- Gregory, O.J.; You, T. Ceramic Temperature Sensors for Harsh Environments. IEEE Sens. J. 2005, 5, 833–838. [Google Scholar] [CrossRef]
- Makinwa, K.A.A.; Huijsing, J.H. A Smart Wind Sensor Using Thermal Sigma-Delta Modulation Techniques. Sens. Actuators A Phys. 2002, 97, 15–20. [Google Scholar] [CrossRef]
- Bhattacharyya, P. Technological Journey towards Reliable Microheater Development for MEMS Gas Sensors: A Review. IEEE Trans. Device Mater. Reliab. 2014, 14, 589–599. [Google Scholar] [CrossRef]
- Lee, K.N.; Lee, D.S.; Jung, S.W.; Jang, Y.H.; Kim, Y.K.; Seong, W.K. A High-Temperature MEMS Heater Using Suspended Silicon Structures. J. Micromech. Microeng. 2009, 19, 115011. [Google Scholar] [CrossRef]
- Shaposhnik, A.V.; Moskalev, P.V.; Zviagin, A.A.; Duykova, M.V.; Ryabtsev, S.V.; Ghareeb, D.A.A.; Vasiliev, A.A. Selective Determination of Hydrogen Sulfide Using SnO2–Ag Sensor Working in Non-Stationary Temperature Regime. Chemosensors 2021, 9, 203. [Google Scholar] [CrossRef]
- Baroncini, M.; Placidi, P.; Cardinali, G.C.; Scorzoni, A. Thermal Characterization of a Microheater for Micromachined Gas Sensors. Sens. Actuators A Phys. 2004, 115, 8–14. [Google Scholar] [CrossRef]
- Belmonte, J.C.; Puigcorbé, J.; Arbiol, J.; Vilà, A.; Morante, J.R.; Sabaté, N.; Gràcia, I.; Cané, C. High-Temperature Low-Power Performing Micromachined Suspended Micro-Hotplate for Gas Sensing Applications. Sens. Actuators B Chem. 2006, 114, 826–835. [Google Scholar] [CrossRef]
- Guo, B.; Bermak, A.; Chan, P.C.H.; Yan, G.Z. A Monolithic Integrated 4 × 4 Tin Oxide Gas Sensor Array with On-Chip Multiplexing and Differential Readout Circuits. Solid State Electron 2007, 51, 69–76. [Google Scholar] [CrossRef]
- Hotovy, I.; Rehacek, V.; Mika, F.; Lalinsky, T.; Hascik, S.; Vanko, G.; Drzik, M. Gallium Arsenide Suspended Microheater for MEMS Sensor Arrays. Microsyst. Technol. 2008, 14, 629–635. [Google Scholar] [CrossRef]
- Tanko, J.M. Encyclopedia of Electrochemistry, Volume 8: Organic Electrochemistry Edited by Hans J. Schäfer (Universität Münster). Series Edited by Allen J. Bard and Martin Stratmann. Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany. 2004. ISBN 3-527-30400-2. J. Am. Chem. Soc. 2005, 127, 4538. [Google Scholar] [CrossRef]
- Resnik, D.; Kovač, J.; Vrtačnik, D.; Godec, M.; Pečar, B.; Možek, M. Microstructural and Electrical Properties of Heat Treated Resistive Ti/Pt Thin Layers. Thin Solid Films 2017, 639, 64–72. [Google Scholar] [CrossRef]
- Schössler, T.; Schön, F.; Lemier, C.; Urban, G. Wafer Level Approach for the Investigation of The-Term Stability of Resistive Platinum Devices at elevated Temperatures. In Proceedings of the 2019 IEEE International Reliability Physics Symposium (IRPS), Monterey, CA, USA, 31 March–4 April 2019. [Google Scholar]
- Tiggelaar, R.M.; Sanders, R.G.P.; Groenland, A.W.; Gardeniers, J.G.E. Stability of Thin Platinum Films Implemented in High-Temperature Microdevices. Sens. Actuators A Phys. 2009, 152, 39–47. [Google Scholar] [CrossRef]
- Çiftyürek, E.; Sabolsky, K.; Sabolsky, E.M. Platinum Thin Film Electrodes for High-Temperature Chemical Sensor Applications. Sens. Actuators B Chem. 2013, 181, 702–714. [Google Scholar] [CrossRef]
- Kalinin, I.A.; Roslyakov, I.V.; Khmelenin, D.N.; Napolskii, K.S. Long-Term Operational Stability of Ta/Pt Thin-Film Microheaters: Impact of the Ta Adhesion Layer. Nanomaterials 2023, 13, 94. [Google Scholar] [CrossRef]
- Kim, Y.; Choi, H.Y. Characterization of Ti/W, Ti/Cr, and Ti/Pt Thin Films—part I: Surface Morphologies and Nano-Mechanical Properties. Sens. Actuators A Phys. 2017, 254, 9–19. [Google Scholar] [CrossRef]
- Schmid, P.; Triendl, F.; Zarfl, C.; Schwarz, S.; Artner, W.; Schneider, M.; Schmid, U. Electro-Mechanical Properties of Multilayered Aluminum Nitride and Platinum Thin Films at High Temperatures. Sens. Actuators A Phys. 2019, 293, 128–135. [Google Scholar] [CrossRef]
- Schmid, P.; Zarfl, C.; Triendl, F.; Maier, F.J.; Schwarz, S.; Schneider, M.; Schmid, U. Impact of Adhesion Promoters and Sputter Parameters on the Electro-Mechanical Properties of Pt Thin Films at High Temperatures. Sens. Actuators A Phys. 2019, 285, 149–157. [Google Scholar] [CrossRef]
- Grosser, M.; Schmid, U. The Impact of Annealing Temperature and Time on the Electrical Performance of Ti/Pt Thin Films. Appl. Surf. Sci. 2010, 256, 4564–4569. [Google Scholar] [CrossRef]
- Frankel, D.J.; Moulzolf, S.C.; da Cunha, M.P.; Lad, R.J. Influence of Composition and Multilayer Architecture on Electrical Conductivity of High Temperature Pt-Alloy Films. Surf. Coat. Technol. 2015, 284, 215–221. [Google Scholar] [CrossRef]
- Ma, D.; Mao, S.; Teng, J.; Wang, X.; Li, X.; Ning, J.; Li, Z.; Zhang, Q.; Tian, Z.; Wang, M.; et al. In-Situ Revealing the Degradation Mechanisms of Pt Film over 1000 °C. J. Mater. Sci. Technol. 2021, 95, 10–19. [Google Scholar] [CrossRef]
- Budhani, R.C.; Prakash, S.; Doerr, H.J.; Bunshah, R.F. Summary Abstract: Oxygen Enhanced Adhesion of Platinum Films Deposited on Thermally Grown Alumina Surfaces. J. Vac. Sci. Technol. A Vac. Surf. Films 1986, 4, 3023–3024. [Google Scholar] [CrossRef]
- Firebaugh, S.L.; Jensen, K.F.; Schmidt, M.A. Investigation of high-temperature degradation of platinum thin films with an in situ resistance measurement apparatus. J. Microelectromechanical Syst. 1998, 7, 128–135. [Google Scholar] [CrossRef]
- Schössler, T.; Schön, F.; Lemier, C.; Urban, G. Effect of High Temperature Annealing on Resistivity and Temperature Coefficient of Resistance of Sputtered Platinum Thin Films of SiO2/Pt/SiOx Interfaces. Thin Solid Films 2020, 698, 137877. [Google Scholar] [CrossRef]
- Bíró, F.; Hajnal, Z.; Dücső, C.; Bársony, I. The Role of Phase Changes in TiO2/Pt/TiO2 Filaments. J. Electron. Mater. 2018, 47, 2322–2329. [Google Scholar] [CrossRef]
- Schössler, T.; Schön, F.; Lemier, C.; Urban, G. Reliability Improvements of Thin Film Platinum Resistors on Wafer-Level and Micro-Hotplates at Stress Temperatures in the Range of 140–290 °C. Microelectron. Reliab. 2020, 104, 113557. [Google Scholar] [CrossRef]
- Courbat, J.; Briand, D.; de Rooij, N.F. Reliability Improvement of Suspended Platinum-Based Micro-Heating Elements. Sens. Actuators A Phys. 2008, 142, 284–291. [Google Scholar] [CrossRef]
- Zhao, X.; Liang, X.; Jiang, S.; Zhang, W.; Jiang, H. Microstructure Evolution and Thermoelectric Property of Pt-PtRh Thin Film Thermocouples. Crystals 2017, 7, 96. [Google Scholar] [CrossRef]
- Pereira Da Cunha, M.; Moonlight, T.; Lad, R.; Bernhardt, G.; Frankel, D.J. P4L-1 Enabling Very High Temperature Acoustic Wave Devices for Sensor & Frequency Control Applications. In Proceedings of the 2007 IEEE Ultrasonics Symposium Proceedings, New York, NY, USA, 28–31 October 2007; pp. 2107–2110. [Google Scholar] [CrossRef]
- Kalinin, I.A.; Roslyakov, I.V.; Tsymbarenko, D.M.; Bograchev, D.A.; Krivetskiy, V.V.; Napolskii, K.S. Microhotplates Based on Pt and Pt-Rh Films: The Impact of Composition, Structure, and Thermal Treatment on Functional Properties. Sens. Actuators A Phys. 2021, 317, 112457. [Google Scholar] [CrossRef]
- Moulzolf, S.C.; Frankel, D.J.; Pereira da Cunha, M.; Lad, R.J. Electrically Conductive Pt-Rh/ZrO2 and Pt-Rh/HfO2 Nanocomposite Electrodes for High Temperature Harsh Environment Sensors. Smart Sens. Actuators MEMS VI 2013, 8763, 100–107. [Google Scholar] [CrossRef]
- Moulzolf, S.C.; Frankel, D.J.; Bernhardt, G.P.; Nugent, B.; Lad, R.J. Thin Film Electrodes and Passivation Coatings for Harsh Environment Microwave Acoustic Sensors. Smart Sens. Actuators MEMS V 2011, 8763, 806606. [Google Scholar] [CrossRef]
- Moulzolf, S.C.; Frankel, D.J.; Pereira Da Cunha, M.; Lad, R.J. High Temperature Stability of Electrically Conductive Pt-Rh/ZrO2 and Pt-Rh/HfO2 nanocomposite thin film electrodes. Microsyst. Technol. 2014, 20, 523–531. [Google Scholar] [CrossRef]
- Çiftyürek, E.; McMillen, C.D.; Sabolsky, K.; Sabolsky, E.M. Platinum-Zirconium Composite Thin Film Electrodes for High-Temperature Micro-Chemical Sensor Applications. Sens. Actuators B Chem. 2015, 207, 206–215. [Google Scholar] [CrossRef]
- Tiggelaar, R.M.; Berenschot, J.W.; De Boer, J.H.; Sanders, R.G.; Gardeniers, J.G.; Oosterbroek, R.E.; Elwenspoek, M.C. Fabrication and characterization of high-temperature microreactors with thin film heater and sensor patterns in silicon nitride tubes. Lab A Chip 2005, 5, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Gorokh, G.; Zakhlebayeva, A.; Taratyn, I.; Lozovenko, A.; Zhylinski, V.; Iji, M.; Fedosenko, V.; Taleb, A. A Micropowered Chemoresistive Sensor Based on a Thin Alumina Nanoporous Membrane and SnxBikMoyOz Nanocomposite. Sensors 2022, 22, 3640. [Google Scholar] [CrossRef]
- Hahn, T.A.; Kirby, R.K.; Wolfe, H.C.; Graham, M.G.; Hagy, H.E. Thermal Expansion of Platinum from 293 to 1900 K. AIP Conf. Proc. 1972, 3, 87–95. [Google Scholar] [CrossRef]
- Roslyakov, I.V.; Napol’skii, K.S.; Evdokimov, P.V.; Napolskiy, F.S.; Dunaev, A.V.; Eliseev, A.A.; Lukashin, A.V.; Tret’yakov, Y.D. Thermal properties of anodic alumina membranes. Nanosyst. Phys. Chem. Math. 2013, 4, 120–129. [Google Scholar]
- Jee, S.E.; Lee, P.S.; Yoon, B.J.; Jeong, S.H.; Lee, K.H. Fabrication of Microstructures by Wet Etching of Anodic Aluminum Oxide Substrates. Chem. Mater. 2005, 17, 4049–4052. [Google Scholar] [CrossRef]
- Roslyakov, I.V.; Napolskii, K.S.; Stolyarov, V.S.; Karpov, E.E.; Ivashev, A.V.; Surtaev, V.N. A Thin-Film Platform for Chemical Gas Sensors. Russ. Microelectron. 2018, 47, 226–233. [Google Scholar] [CrossRef]
- Vasiliev, A.A.; Pisliakov, A.V.; Sokolov, A.V.; Samotaev, N.N.; Soloviev, S.A.; Oblov, K.; Guarnieri, V.; Lorenzelli, L.; Brunelli, J.; Maglione, A.; et al. Non-Silicon MEMS Platforms for Gas Sensors. Sens. Actuators B Chem. 2016, 224, 700–713. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Chirakkara, S.; Nanda, K.K.; Krupanidhi, S.B. Pulsed Laser Deposited ZnO: In as Transparent Conducting Oxide. Thin Solid Films 2011, 519, 3647–3652. [Google Scholar] [CrossRef]
- Jones, J.L.; LeBeau, J.M.; Nikkel, J.; Oni, A.A.; Dycus, J.H.; Cozzan, C.; Lin, F.Y.; Chernatynskiy, A.; Nino, J.C.; Sinnott, S.B.; et al. Combined Experimental and Computational Methods Reveal the Evolution of Buried Interfaces during Synthesis of Ferroelectric Thin Films. Adv. Mater. Interfaces 2015, 2, 1500181. [Google Scholar] [CrossRef]
- Ababneh, A.; Al-Omari, A.N.; Dagamseh, A.M.K.; Tantawi, M.; Pauly, C.; Mücklich, F.; Feili, D.; Seidel, H. Electrical and Morphological Characterization of Platinum Thin-Films with Various Adhesion Layers for High Temperature Applications. Microsyst. Technol. 2017, 23, 703–709. [Google Scholar] [CrossRef]
- Abbas, W.; Lin, W.; Kai, J.J.; Ho, D.; Pramanick, A. Critical Effect of Film-Electrode Interface on Enhanced Energy Storage Performance of BaTiO3 -BiScO3 Ferroelectric Thin Films. ACS Appl. Electron. Mater. 2021, 3, 4726–4733. [Google Scholar] [CrossRef]
- Roslyakov, I.V.; Kolesnik, I.V.; Levin, E.E.; Katorova, N.S.; Pestrikov, P.P.; Kardash, T.Y.; Solovyov, L.A.; Napolskii, K.S. Annealing Induced Structural and Phase Transitions in Anodic Aluminum Oxide Prepared in Oxalic Acid Electrolyte. Surf. Coat. Technol. 2020, 381, 125159. [Google Scholar] [CrossRef]
- Mardilovich, P.P.; Govyadinov, A.N.; Mukhurov, N.I.; Rzhevskii, A.M.; Paterson, R. New and modified anodic alumina membranes. Part I. Thermotreatment of anodic alumina membranes. J. Membr. Sci. 1995, 98, 131–142. [Google Scholar] [CrossRef]
- Mata-Zamora, M.E.; Saniger, J.M. Thermal evolution of porous anodic aluminas: A comparative study. Rev. Mex. Fis. 2005, 51, 502–509. [Google Scholar]
- Valvoda, V.; Järvinen, M. On the Harris texture index. Powder Diffr. 1990, 5, 200–203. [Google Scholar] [CrossRef]
- Bocanegra-Bernal, M.H.; De, I.; Torre, L.A. Review Phase Transitions in Zirconium Dioxide and Related Materials for High Performance Engineering Ceramics. J. Mater. Sci. 2002, 37, 4947–4971. [Google Scholar] [CrossRef]
- Garvie, R.C. Stabilization of the Tetragonal Structure in Zirconia Microcrystals. J. Phys. Chem. 1978, 82, 218–224. [Google Scholar] [CrossRef]
- Mitsuhashi, T.; Ichihara, M.; Tatsuke, U. Characterization and Stabilization of Metastable Tetragonal ZrO2. J. Am. Ceram. Soc. 1974, 57, 97–101. [Google Scholar] [CrossRef]
- Osendi, M.I.M.J. Metastability of Tetragonal Zirconia Powders. J. Am. Ceram. Soc. 1985, 68, 135–139. [Google Scholar] [CrossRef]
- Yeh, S.W.; Hsieh, T.Y.; Huang, H.L.; Gan, D.; Shen, P. Annealing Induced Oxidation and Transformation of Zr Thin Film Prepared by Ion Beam Sputtering Deposition. Mater. Sci. Eng. A 2007, 452–453, 313–320. [Google Scholar] [CrossRef]
- Desai, P.D.; James, H.M.; Ho, C.Y. Electrical Resistivity of Vanadium and Zirconium. J. Phys. Chem. Ref. Data 1984, 13, 1097–1130. [Google Scholar] [CrossRef]
- ASM International Handbook Committee. Properties and Selection: Nonferrous Alloys and Special-Purpose Materials; ASM International: Almere, The Netherlands, 1992; Volume 2. [Google Scholar]
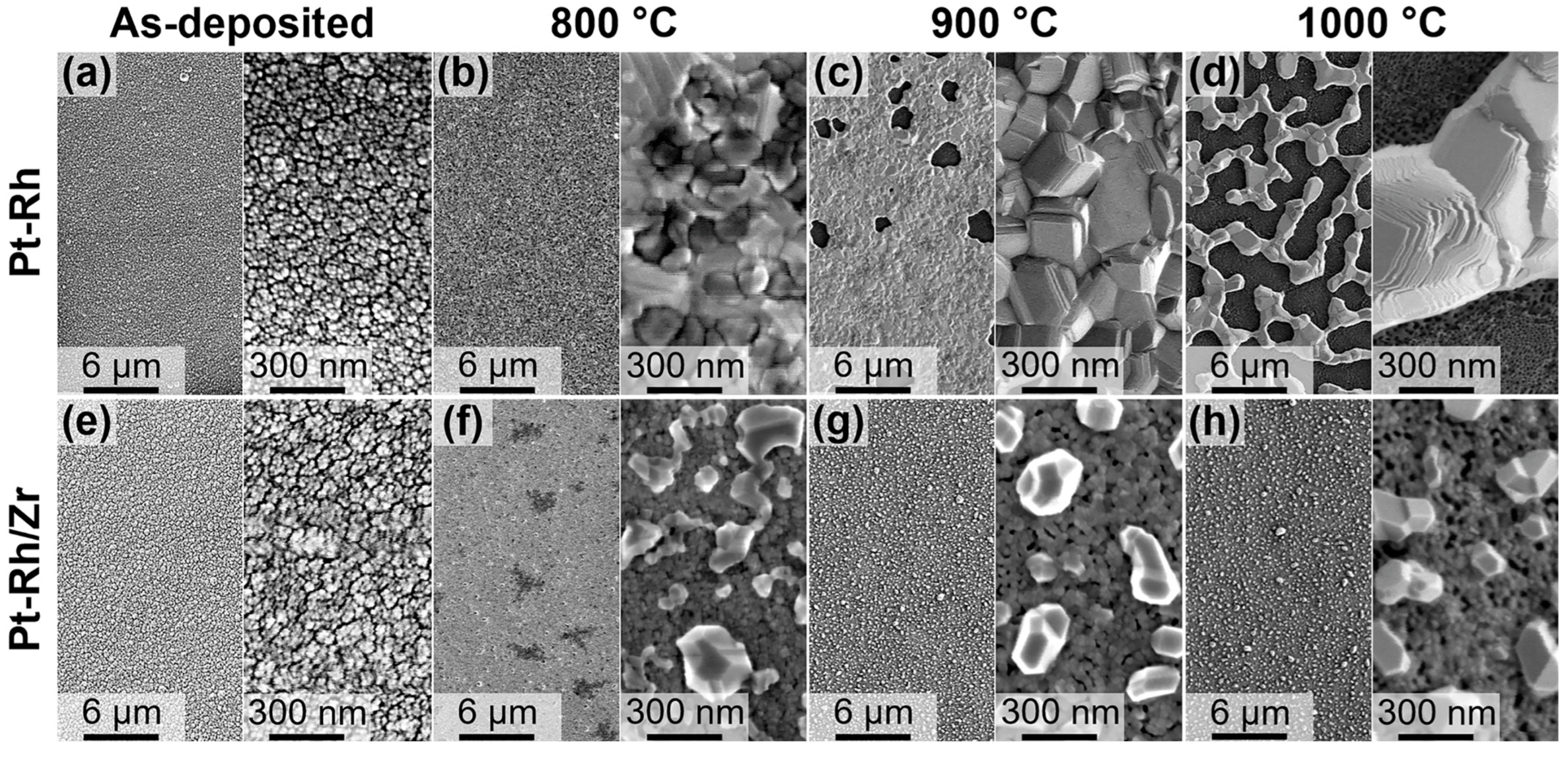

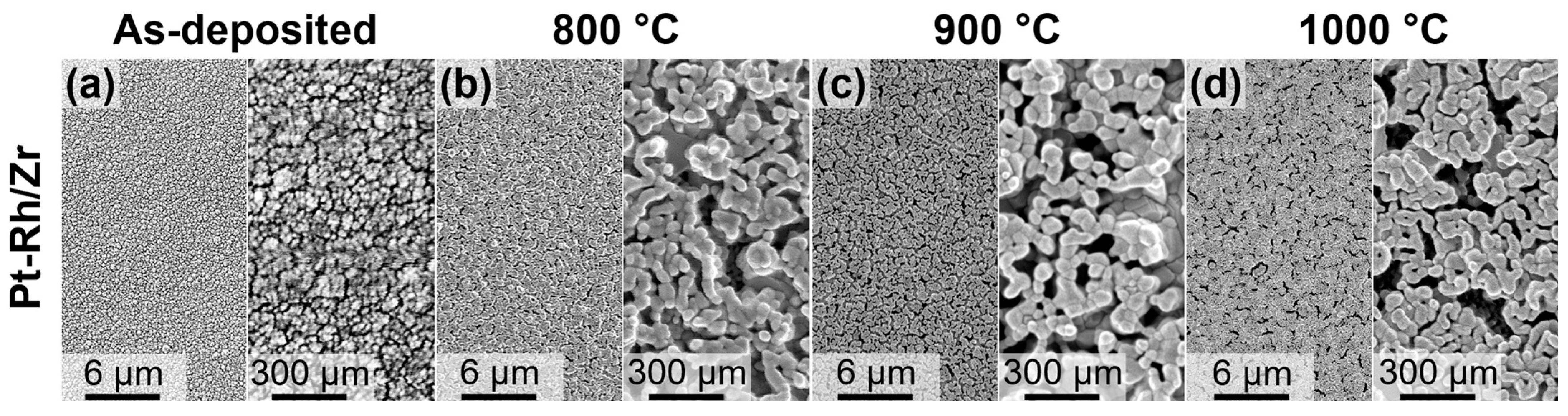

| Annealing Temperature | As-Deposited | 800 °C | 900 °C | 1000 °C | |
|---|---|---|---|---|---|
| Sample | |||||
| Pt-Rh (one-step annealing) | 15 ± 7 nm | 136 ± 48 nm | 496 ± 120 nm | 1496 ± 736 nm | |
| Pt-Rh/Zr (one-step annealing; size of hillocks) | 22 ± 5 nm | 140 ± 89 nm | 202 ± 69 nm | 210 ± 65 nm | |
| Pt-Rh/Zr (two-step annealing) | 44 ± 20 nm | 60 ± 27 nm | 64 ± 20 nm | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pleshakov, G.A.; Kalinin, I.A.; Ivanov, A.V.; Roslyakov, I.V.; Yaminsky, I.V.; Napolskii, K.S. Towards High-Temperature MEMS: Two-Step Annealing Suppressed Recrystallization in Thin Multilayer Pt-Rh/Zr Films. Micromachines 2023, 14, 2003. https://doi.org/10.3390/mi14112003
Pleshakov GA, Kalinin IA, Ivanov AV, Roslyakov IV, Yaminsky IV, Napolskii KS. Towards High-Temperature MEMS: Two-Step Annealing Suppressed Recrystallization in Thin Multilayer Pt-Rh/Zr Films. Micromachines. 2023; 14(11):2003. https://doi.org/10.3390/mi14112003
Chicago/Turabian StylePleshakov, Georgii A., Ivan A. Kalinin, Alexey V. Ivanov, Ilya V. Roslyakov, Igor V. Yaminsky, and Kirill S. Napolskii. 2023. "Towards High-Temperature MEMS: Two-Step Annealing Suppressed Recrystallization in Thin Multilayer Pt-Rh/Zr Films" Micromachines 14, no. 11: 2003. https://doi.org/10.3390/mi14112003
APA StylePleshakov, G. A., Kalinin, I. A., Ivanov, A. V., Roslyakov, I. V., Yaminsky, I. V., & Napolskii, K. S. (2023). Towards High-Temperature MEMS: Two-Step Annealing Suppressed Recrystallization in Thin Multilayer Pt-Rh/Zr Films. Micromachines, 14(11), 2003. https://doi.org/10.3390/mi14112003





