Metal Oxide Nanowire-Based Sensor Array for Hydrogen Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sensors Fabrication
2.2. Gas Sensing Measurements
3. Experimental Results
3.1. Materials Characterization
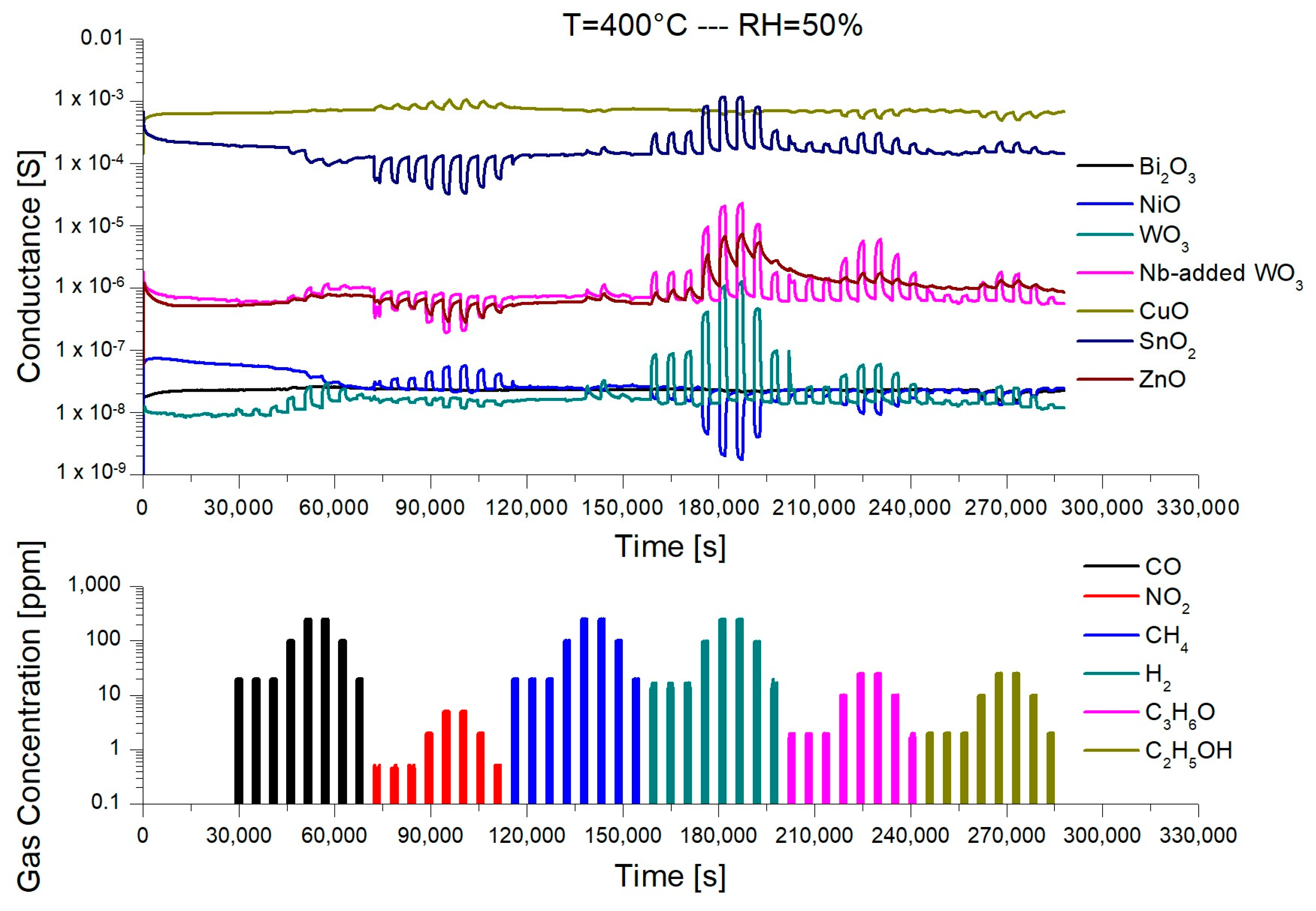
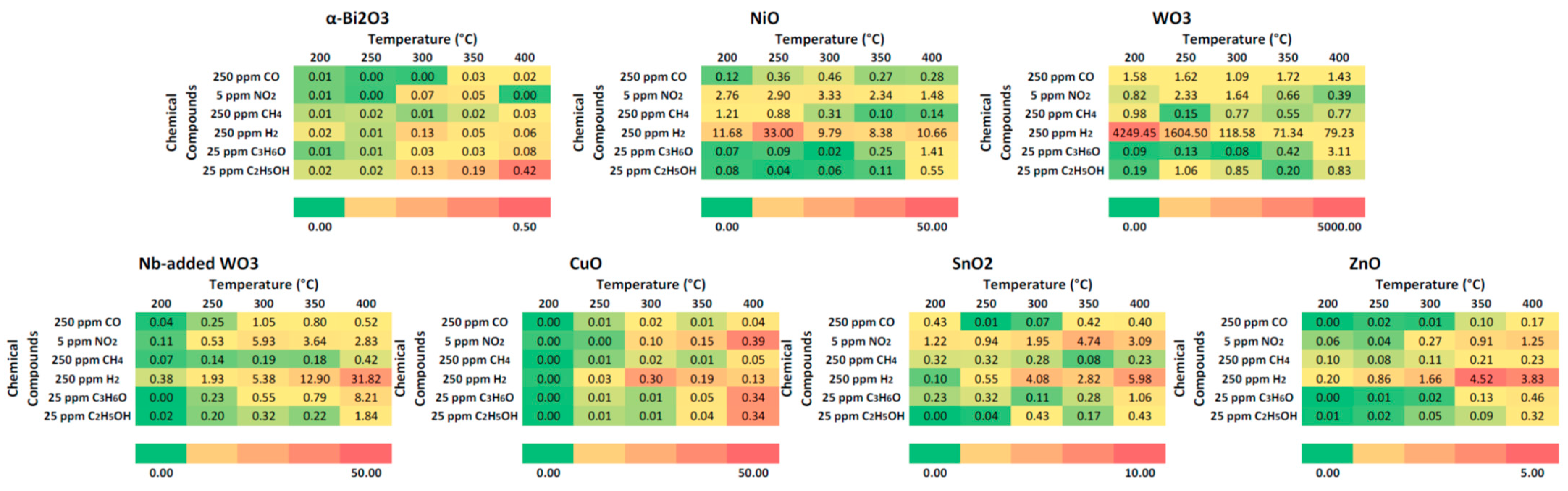
3.2. Gas Sensing Performances
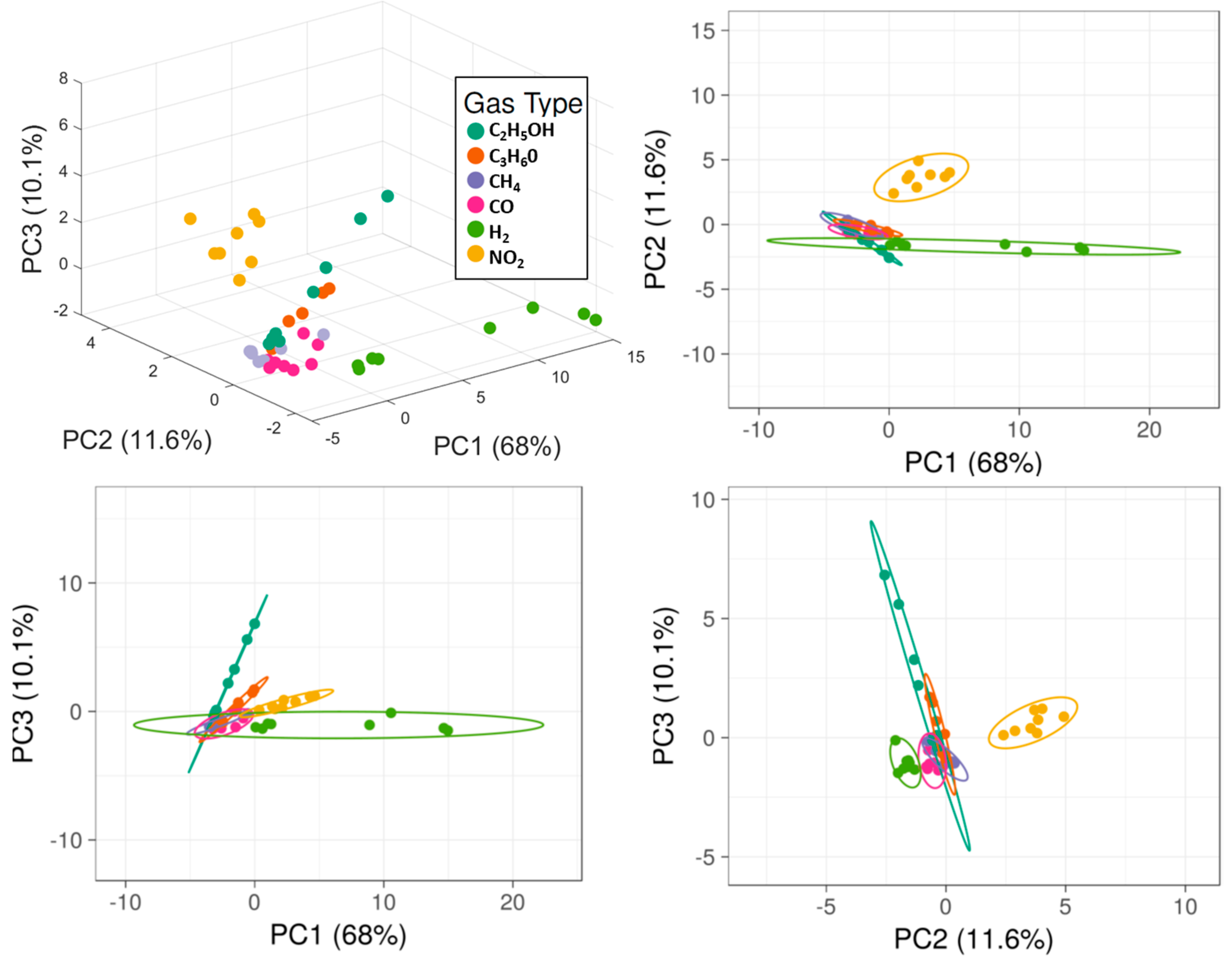
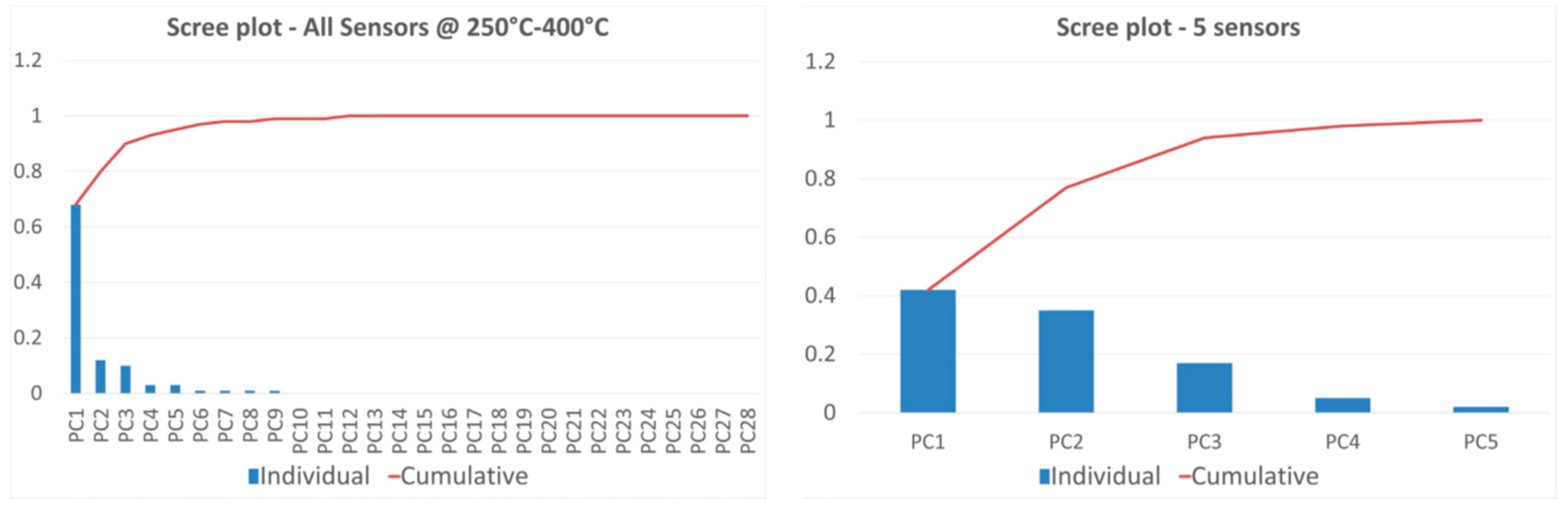
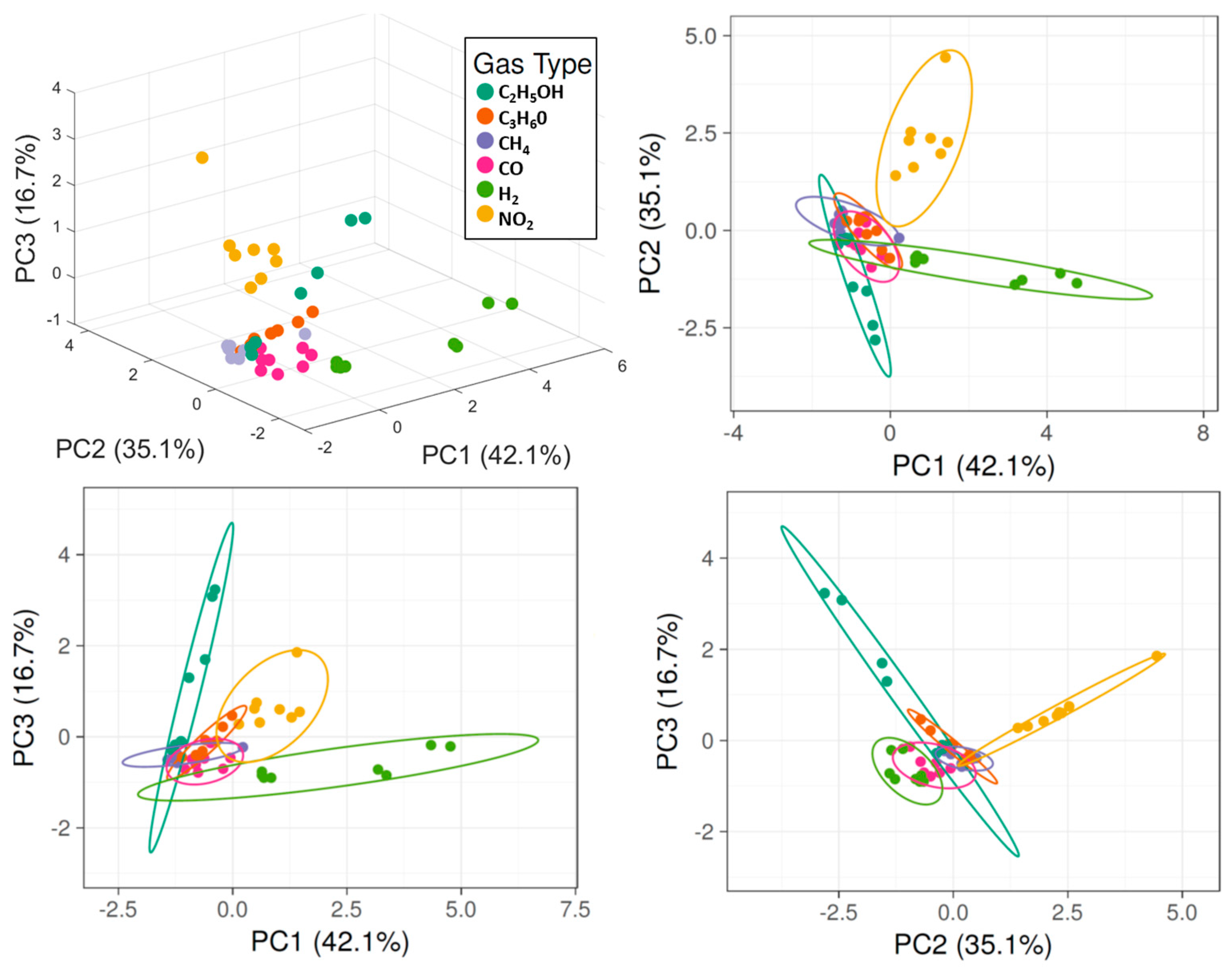
3.3. Data Analysis
4. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sen, D.; Tunç, K.M.M.; Günay, M.E. Forecasting Electricity Consumption of OECD Countries: A Global Machine Learning Modeling Approach. Util. Policy 2021, 70, 101222. [Google Scholar] [CrossRef]
- Key World Energy Statistics 2020—Analysis—IEA. Available online: https://www.iea.org/reports/key-world-energy-statistics-2020 (accessed on 1 December 2021).
- Shao, X.; Zhong, Y.; Liu, W.; Li, R.Y.M. Modeling the Effect of Green Technology Innovation and Renewable Energy on Carbon Neutrality in N-11 Countries? Evidence from Advance Panel Estimations. J. Env. Manag. 2021, 296, 113189. [Google Scholar] [CrossRef]
- Ritchie, H.; Roser, M. Energy. Our World Data. 2020. Available online: https://ourworldindata.org/energy (accessed on 19 October 2022).
- A European Green Deal. European Commission. Available online: https://ec.europa.eu/info/strategy/priorities-2019-2024/european-green-deal_en (accessed on 1 December 2021).
- Rüdisüli, M.; Bach, C.; Bauer, C.; Beloin-Saint-Pierre, D.; Elber, U.; Georges, G.; Limpach, R.; Pareschi, G.; Kannan, R.; Teske, S.L. Prospective Life-Cycle Assessment of Greenhouse Gas Emissions of Electricity-Based Mobility Options. Appl. Energy 2022, 306, 118065. [Google Scholar] [CrossRef]
- Maout, P.L.; Wojkiewicz, J.-L.; Redon, N.; Lahuec, C.; Seguin, F.; Dupont, L.; Mikhaylov, S.; Noskov, Y.; Ogurtsov, N.; Pud, A. Polyaniline Nanocomposites Based Sensor Array for Breath Ammonia Analysis. Portable e-Nose Approach to Non-Invasive Diagnosis of Chronic Kidney Disease. Sens. Actuators B Chem. 2018, 274, 616–626. [Google Scholar] [CrossRef]
- Vergara, A.; Fonollosa, J.; Mahiques, J.; Trincavelli, M.; Rulkov, N.; Huerta, R. On the Performance of Gas Sensor Arrays in Open Sampling Systems Using Inhibitory Support Vector Machines. Sens. Actuators B Chem. 2013, 185, 462–477. [Google Scholar] [CrossRef]
- Patial, P.; Deshwal, M. Systematic Review on Design and Development of Efficient Semiconductor Based Surface Acoustic Wave Gas Sensor. Trans. Electr. Electron. Mater. 2021, 22, 385–393. [Google Scholar] [CrossRef]
- Cavallari, M.R.; Pastrana, L.M.; Sosa, C.D.F.; Marquina, A.M.R.; Izquierdo, J.E.E.; Fonseca, F.J.; de Amorim, C.A.; Paterno, L.G.; Kymissis, I. Organic Thin-Film Transistors as Gas Sensors: A Review. Materials 2020, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Shi, J.; Zhang, M.; Dalapati, R.; Tian, Q.; Chen, S.; Wang, C.; Zang, L. Optical Chemosensors for the Gas Phase Detection of Aldehydes: Mechanism, Material Design, and Application. Mater. Adv. 2021, 2, 6213–6245. [Google Scholar] [CrossRef]
- Ly, N.H.; Son, S.J.; Kim, H.H.; Joo, S.-W. Recent Developments in Plasmonic Sensors of Phenol and Its Derivatives. Appl. Sci. 2021, 11, 10519. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor Metal Oxide Gas Sensors: A Review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Yamazoe, N. New Approaches for Improving Semiconductor Gas Sensors. Sens. Actuators B Chem. 1991, 5, 7–19. [Google Scholar] [CrossRef]
- Abdel-Karim, R.; Reda, Y.; Abdel-Fattah, A. Review—Nanostructured Materials-Based Nanosensors. J. Electrochem. Soc. 2020, 167, 037554. [Google Scholar] [CrossRef]
- Zhu, L.-Y.; Ou, L.-X.; Mao, L.-W.; Wu, X.-Y.; Liu, Y.-P.; Lu, H.-L. Advances in Noble Metal-Decorated Metal Oxide Nanomaterials for Chemiresistive Gas Sensors: Overview. Nano-Micro Letters 2023, 15, 89. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Myung, N.V.; Tran, T.T. 1D Metal Oxide Semiconductor Materials for Chemiresistive Gas Sensors: A Review. Adv. Electron. Mater. 2021, 7, 2100271. [Google Scholar] [CrossRef]
- Lin, J.; Kilani, M.; Mao, G. Recent Advances in Integrating 1D Nanomaterials into Chemiresistive Gas Sensor Devices. Adv. Mater. Tech. 2023, 8, 2202038. [Google Scholar] [CrossRef]
- Moumen, A.; Kaur, N.; Poli, N.; Zappa, D.; Comini, E. One Dimensional ZnO Nanostructures: Growth and Chemical Sensing Performances. Nanomaterials 2020, 10, 1940. [Google Scholar] [CrossRef]
- Kaur, N.; Zappa, D.; Poli, N.; Comini, E. Integration of VLS-Grown WO3 Nanowires into Sensing Devices for the Detection of H2S and O3. ACS Omega 2019, 4, 16336–16343. [Google Scholar] [CrossRef]
- Zappa, D.; Bertuna, A.; Comini, E.; Kaur, N.; Poli, N.; Sberveglieri, V.; Sberveglieri, G. Metal Oxide Nanostructures: Preparation, Characterization and Functional Applications as Chemical Sensors. Beilstein J. Nanotechnol. 2017, 8, 1205–1217. [Google Scholar] [CrossRef]
- Kaur, N.; Comini, E.; Zappa, D.; Poli, N.; Sberveglieri, G. Nickel Oxide Nanowires: Vapor Liquid Solid Synthesis and Integration into a Gas Sensing Device. Nanotechnology 2016, 27, 205701. [Google Scholar] [CrossRef]
- Moumen, A.; Zappa, D.; Poli, N.; Comini, E. Catalyst—Assisted Vapor Liquid Solid Growth of α-Bi2O3 Nanowires for Acetone and Ethanol Detection. Sens. Actuators B Chem. 2021, 346, 130432. [Google Scholar] [CrossRef]
- Zappa, D. The Influence of Nb on the Synthesis of WO3 Nanowires and the Effects on Hydrogen Sensing Performance. Sensors 2019, 19, 2332. [Google Scholar] [CrossRef]
- Choopun, S.; Hongsith, N.; Wongrat, E. Metal-Oxide Nanowires by Thermal Oxidation Reaction Technique. In Nanowires; IntechOpen: London, UK, 2010; pp. 97–116. [Google Scholar]
- Zappa, D.; Comini, E.; Zamani, R.; Arbiol, J.; Morante, J.R.; Sberveglieri, G. Preparation of Copper Oxide Nanowire-Based Conductometric Chemical Sensors. Sens. Actuators B Chem. 2013, 182, 7–15. [Google Scholar] [CrossRef]
- TGS 2616-C00—For the Detection of Hydrogen. Available online: https://www.figarosensor.com/product/docs/tgs2616-c00_product%20information%28fusa%29rev02.pdf (accessed on 30 October 2023).
- GMV-2021B MEMS H2 Sensor Manual. Available online: https://www.winsen-sensor.com/d/files/manual/gmv-2021b.pdf (accessed on 30 October 2023).
- The MiCS-5524 Is a Compact MOS Sensor. Available online: https://www.sgxsensortech.com/content/uploads/2014/07/1084_Datasheet-MiCS-5524-rev-8.pdf (accessed on 30 October 2023).
- Gas Sensor Element GGS 6530 T. Available online: https://www.umweltsensortechnik.de/fileadmin/assets/downloads/gassensoren/single/DataSheet-GGS-6530-T_Rev2203.pdf (accessed on 20 October 2023).
- Shlens, J. A Tutorial on Principal Component Analysis. arXiv 2014, arXiv:1404.1100. [Google Scholar]
- Metsalu, T.; Vilo, J. ClustVis: A Web Tool for Visualizing Clustering of Multivariate Data Using Principal Component Analysis and Heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef] [PubMed]
- MATLAB—Il Linguaggio Del Calcolo Tecnico—MATLAB & Simulink. Available online: https://it.mathworks.com/products/matlab.html (accessed on 2 December 2021).
- Di Natale, C.; Macagnano, A.; Martinelli, E.; Proietti, E.; Paolesse, R.; Castellari, L.; Campani, S.; D’Amico, A. Electronic Nose Based Investigation of the Sensorial Properties of Peaches and Nectarines. Sens. Actuators B Chem. 2001, 77, 561–566. [Google Scholar] [CrossRef]
- Magna, G.; Di Natale, C.; Martinelli, E. Self-Repairing Classification Algorithms for Chemical Sensor Array. Sens. Actuators B Chem. 2019, 297, 126721. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Bharath, S.P.; Mirzaei, A.; Kim, S.S.; Kim, H.W. Identification of gas mixtures using gold-decorated metal oxide based sensor arrays and neural networks. Sens. Actuators B Chem. 2023, 386, 133767. [Google Scholar] [CrossRef]
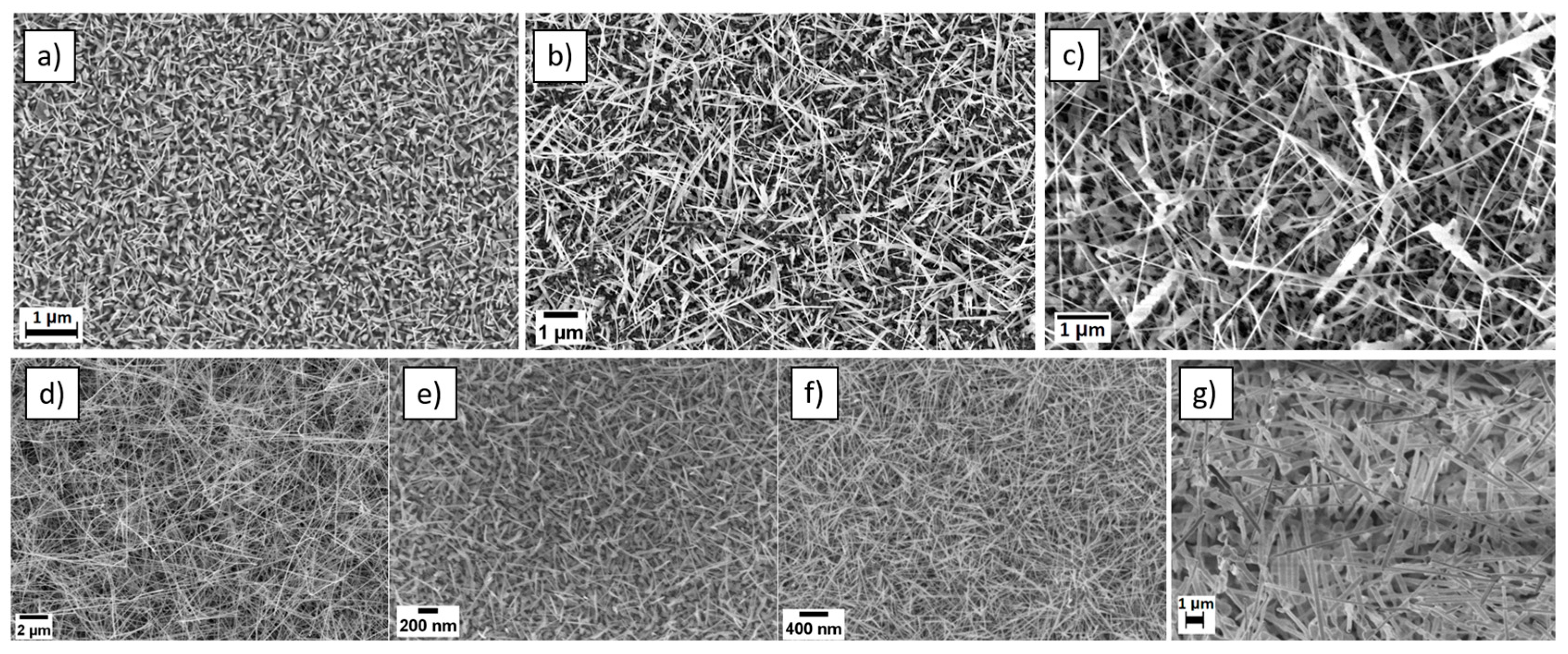
| Material | Technique | Powder Temperature | Substrate Temperature | Time | Pressure | Atmospheric Conditions | Catalyst | Reference |
|---|---|---|---|---|---|---|---|---|
| ZnO | Evaporation-condensation | 1200 °C | 500 °C | 15 min | 10 mbar | Argon flow: 75 sccm | Au | [19] |
| α-Bi2O3 | Evaporation-condensation | 1000 °C | 500 °C | 10 min | 10 mbar | Argon flow: 75 sccm | Au | [23] |
| NiO | Evaporation-condensation | 1450 °C | 930 °C | 12 min | 1 mbar | Argon flow: 100 sccm | Au | [22] |
| SnO2 | Evaporation-condensation | 1370 °C | 860 °C | 2 min | 10 mbar | Argon flow: 100 sccm | Au | [21] |
| WO3 | Evaporation-condensation | 1100 °C | 525 °C | 15 min | 1 mbar | Argon flow: 100 sccm | Au | [20] |
| Nb-WO3 | Thermal oxidation | - | 600 °C | 1 h | 1 mbar | Argon flow: 10 sccm | - | [24] |
| CuO | Thermal oxidation | - | 400 °C | 4 h | 1000 mbar | - | - | [26] |
| Gas Type | Concentrations Injected in Chamber (ppm) |
|---|---|
| CO | 20, 20, 20, 100, 250, 250, 100, 20 |
| NO2 | 0.5, 0.5, 0.5, 2, 5, 5, 2, 0.5 |
| CH4 | 20, 20, 20, 100, 250, 250, 100, 20 |
| H2 | 15, 15, 15, 100, 250, 250, 100, 15 |
| C3H6O | 2, 2, 2, 10, 25, 25, 10, 2 |
| C2H5OH | 2, 2, 2, 10, 25, 25, 10, 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zappa, D.; Kaur, N.; Moumen, A.; Comini, E. Metal Oxide Nanowire-Based Sensor Array for Hydrogen Detection. Micromachines 2023, 14, 2124. https://doi.org/10.3390/mi14112124
Zappa D, Kaur N, Moumen A, Comini E. Metal Oxide Nanowire-Based Sensor Array for Hydrogen Detection. Micromachines. 2023; 14(11):2124. https://doi.org/10.3390/mi14112124
Chicago/Turabian StyleZappa, Dario, Navpreet Kaur, Abderrahim Moumen, and Elisabetta Comini. 2023. "Metal Oxide Nanowire-Based Sensor Array for Hydrogen Detection" Micromachines 14, no. 11: 2124. https://doi.org/10.3390/mi14112124
APA StyleZappa, D., Kaur, N., Moumen, A., & Comini, E. (2023). Metal Oxide Nanowire-Based Sensor Array for Hydrogen Detection. Micromachines, 14(11), 2124. https://doi.org/10.3390/mi14112124










