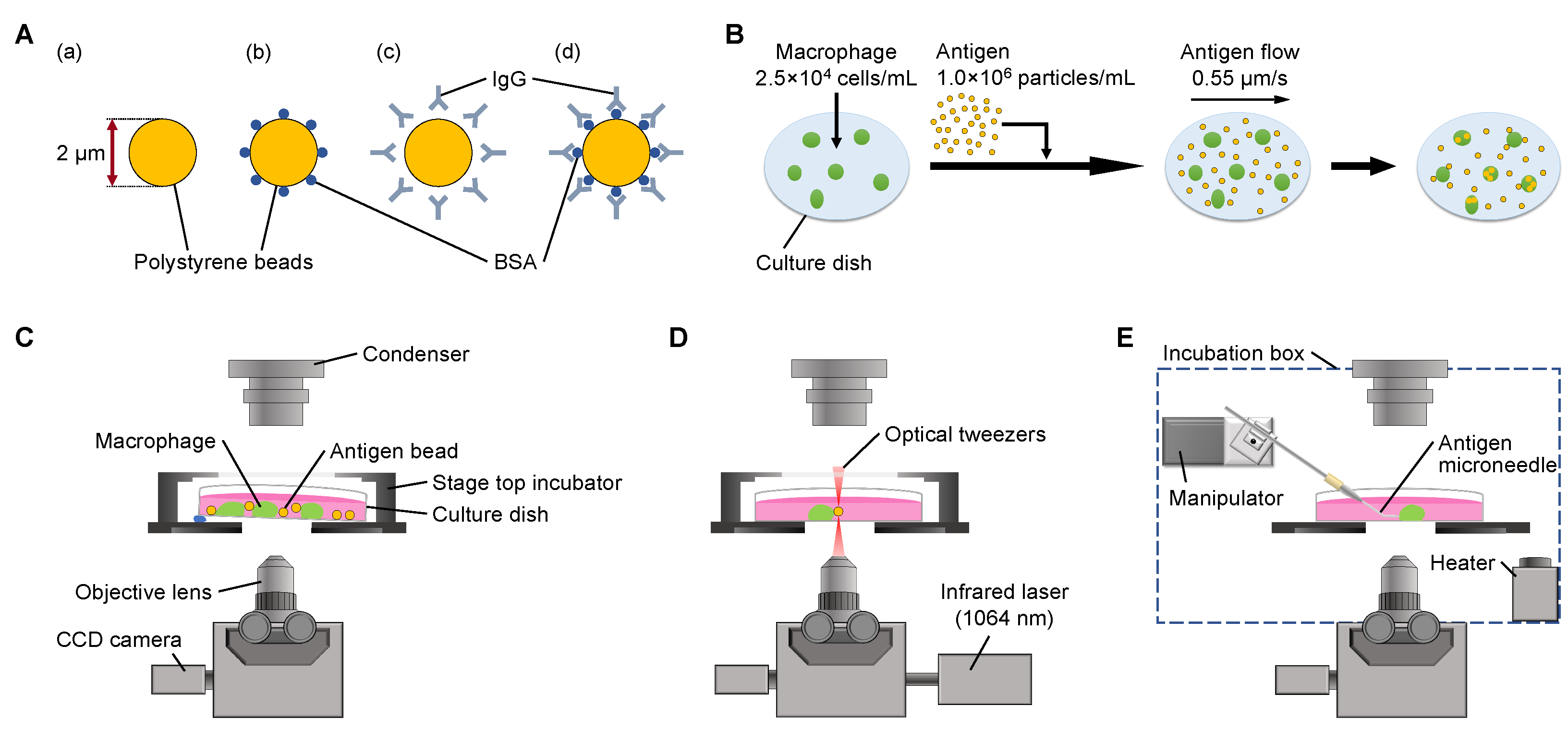
3.1. Evaluation of Free-Flow Method for Stationary Supply of Antigens for Phagocytosis Rate Constant Measurement
In the conventional dish cultivation method, in which macrophages and antigen beads were co-cultivated, the concentration of antigen beads around the macrophages decreased during their phagocytosis. As macrophages might become unable to phagocytose before they reach their actual limit of phagocytosis numbers due to the depletion of the surrounding beads, we might underestimate their phagocytic capacity and phagocytosis rate constant.
To overcome this problem, we developed a simple method to maintain the concentration of antigen beads constantly surrounding the macrophages during the experiments by flowing the antigen beads with a constant slow speed as the on-chip free-flow method (
Figure 1B). Using this method, we were able to keep the concentration of antigens around each macrophage constant to determine the saturated maximum limit of phagocytosis and their efficiencies.
First, we evaluated the ability of the on-chip free-flow method to supply antigens constantly to macrophages.
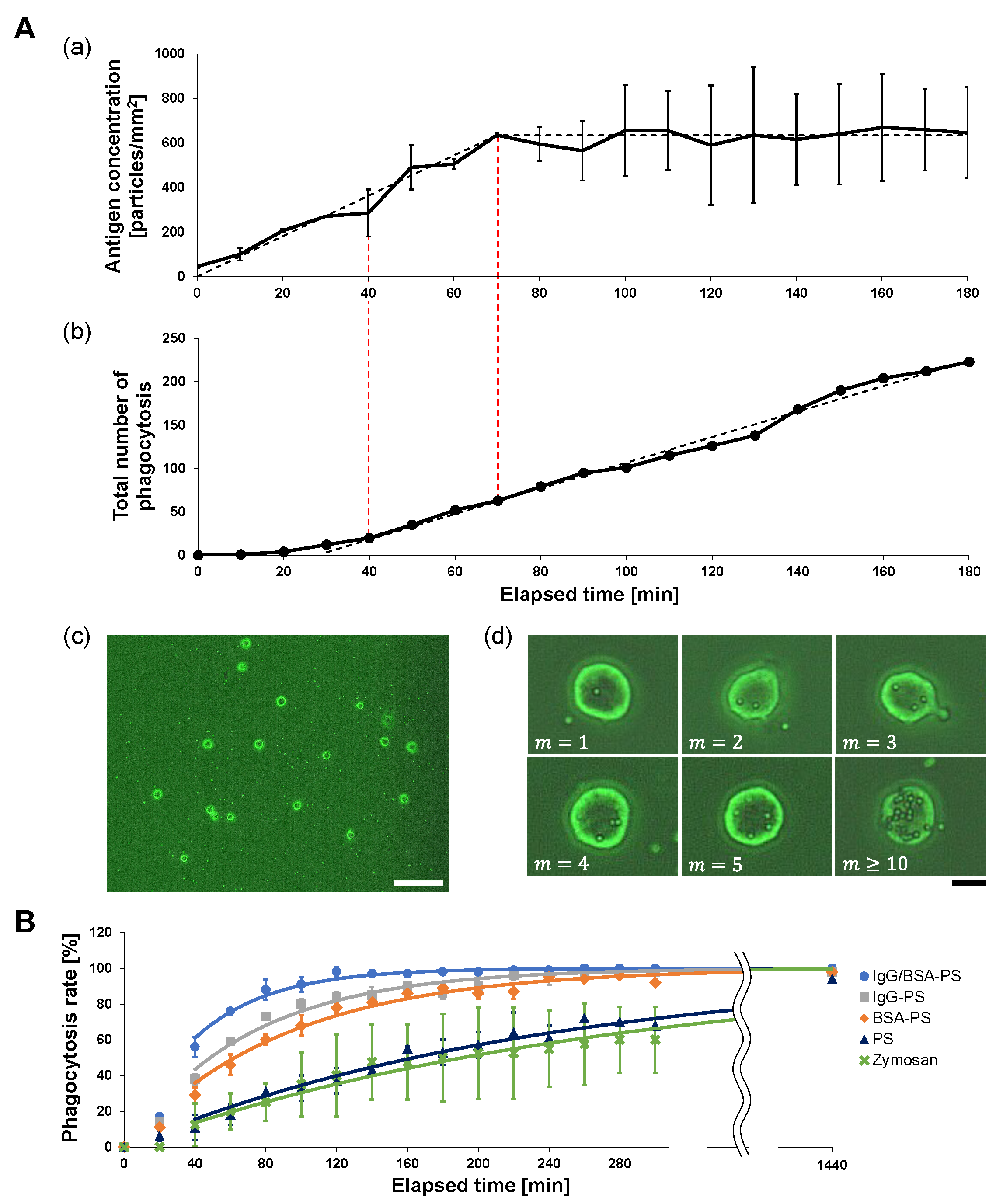
Figure 2A(a) shows an example of the time-course change of the 2
m IgG/BSA-PS bead concentration on the bottom of the dish measured every 10 min from the start of the experiment. Under the
m/s flow of antigens, the concentration increased linearly with a
particles/mm
2 · min ratio to the stable concentration of 635 particles/mm
2 during the initial 70 min. The dashed line is the reference line assuming that the antigen concentration increased with
particles/mm
2 · min during the initial 70 min toward the stable concentration (623 particles/mm
2) and then remained constant.
Figure 2A(b) shows the time-course increase in the total number of antigens engulfed by macrophages with 52 cells/mm
2 on the dish (n = 33). The total number of engulfed antigen beads increased and finally turned into a linear increase with
particles/cell/min or
particles/mm
2/min or 40 min after measurement started, which was earlier than the time of the antigen concentration constant. This engulfment efficiency was maintained until all macrophages reached their limit of engulfment. The graph’s dashed line is the fitting reference line for the constant rate increase in phagocytozed beads.
Compared with
Figure 2A(a), the supply of antigens with a
m/s flow was enough to maintain the surrounding antigen constant at 623 particles/mm
2 and was also beyond the phagocytosis rate constant
particles/cell/min of 52 cells/mm
2 macrophages. Hence, we can regard that the on-chip free-flow system can supply enough antigens for macrophages to phagocytoze with the highest phagocytosis rate constants with the saturated number of surrounding antigens during this experiment.
3.2. Time-Course Phagocytosis Number Measurement of Antigens with On-Chip Free-Flow Method: Sample Surface Decoration Dependence of Phagocytosis Efficiency
Next, we observed macrophage phagocytosis in the on-chip free-flow method for each of the four kinds of antigen bead and Zymosan to evaluate the difference in phagocytosis efficiency depending on the surface decoration of antigens. The bottom of the dish was observed at the fixed area every 20 min after antigen flow was started, and the percentage of phagocytic cells, macrophages that phagocytozed at least one antigen, to the total number of cells in the observation area was measured. We named this rate the “phagocytosis rate” and used it as an index of the phagocytosis efficiency of the macrophages. By comparing the phagocytosis rate and its time change among antigens, we were able to quantitatively evaluate the phagocytozed efficiency of each antigen.
Figure 2B shows the time-course increase of the phagocytozed macrophage number for five types of antigens: IgG/BSA-PS (blue line, n = 100), IgG-PS (gray, n = 100), BSA-PS (red, n = 100), PS (yellow, n = 100), and Zymosan (green, n = 40). The phagocytic efficiency of those five types of antigens for macrophages was estimated as the ratio at each time representing the percentage of macrophages that phagocytozed at least one antigen relative to the total number of macrophages in the observation region. The results show that the increase in the phagocytozed macrophage number was larger in the order of IgG/BSA-PS bead > IgG-PS bead > BSA-PS bead > PS bead > Zymosan, indicating that the phagocytosis rate constant of the four types of PS beads for macrophages should be different depending on the surface decoration of antigens. The fastest was for the IgG/BSA-PS beads because these rate constants should be the same as the increase in the phagocytozed macrophage numbers. As the four types of indigestible PS beads remained in macrophages after phagocytosis, the total number of phagocytozed macrophages reached 100% after 1440 min cultivation, regardless of the decoration on the PS surfaces. In contrast, since Zymosan is a digestible biological sample, the observed number of phagocytozed macrophages reached 60% and was saturated because Zymosan phagocytozed in the cell was digested.
3.3. Rate Constant Estimation of Phagocytosis in Five Types of Antigens
To quantitatively estimate the time-course rate of the first phagocytosis in five types of antigens observed in
Figure 2B, we assumed a simple chemical-reaction-like model in phagocytosis and calculated the phagocytosis rate constant of macrophages to be able to compare the differences between the five types of antigens.
In the model, the cell concentration and the surrounding antigen concentration determined the reaction rate constant between the cells and each antigen. The number ratio of macrophages engulfed in at least one antigen
against the total number of intact macrophages in the observation area
N at time
t is described as,
where
m is the number of engulfed antigens in a macrophage,
is the maximum phagocytosis rate constant of macrophages surrounded with a saturated antigen concentration, and
N is the total number of macrophages. In this model, we applied the concentration condition of the surrounding antigens being constant as
c because of the continuous supply of antigens. We also supposed that the phagocytosis rate constant
is independent of the engulfed antigen number
m. As we set the initial conditions
, the time course of the engulfed macrophage number is described in Equation (4). We can also replot Equation (2) in the differential equation. When we use
, the equation is simplified in typical rate equation formula as,
Hence, the increase curve of the phagocytozed macrophage number ratio is exponentially described in,
We used
of the five types of antigens as a quantitative index of phagocytosis efficiency. When we used the above Equation (
6) for the fitting with the acquired data in
Figure 2B under the constant increase time region from 40 min after measurement started, the acquired maximum phagocytosis rate constant for each type of antigen is summarized in
Table 1. As shown in this table, the efficiency of phagocytosis in IgG/BSA-PS beads had a maximum speed of engulfment and was about two times faster than IgG-PS or BSA-PS and also ten times larger than non-coated PS and Zymosan. This means that macrophages engulfed indigestible IgG/BSA-PS beads with the highest efficiency of phagocytosis rather than engulfing Zymosan if the rate constant of those particles was independent of the engulfed number of indigestible antigens in each macrophage, as we assumed for the above estimation.
It should be noted that the possibility of underestimation of the rate constant of Zymosan because of the efficiency of engulfment was based on the number counting of Zymosan particles in macrophages. Hence, the digestion of Zymosan reduced the estimation of total phagocytosis intervals.
3.4. Phagocytosis Number Dependence of Phagocytosis Efficiency: One-By-One Feeding Evaluation
In the above phagocytosis rate constant estimation, we supposed that the ability and efficiency of phagocytosis were independent of the phagocytosis number. To confirm this hypothesis, we examined their difference during serial one-by-one feeding of antigens for single macrophages using optical tweezers, as shown in
Figure 1D. For this experiment, IgG/BSA-PS beads were chosen as antigen beads because phagocytosis experiments using the free-flow method described above showed that IgG/BSA-PS beads are phagocytozed by macrophages most efficiently among the four kinds of antigen beads.
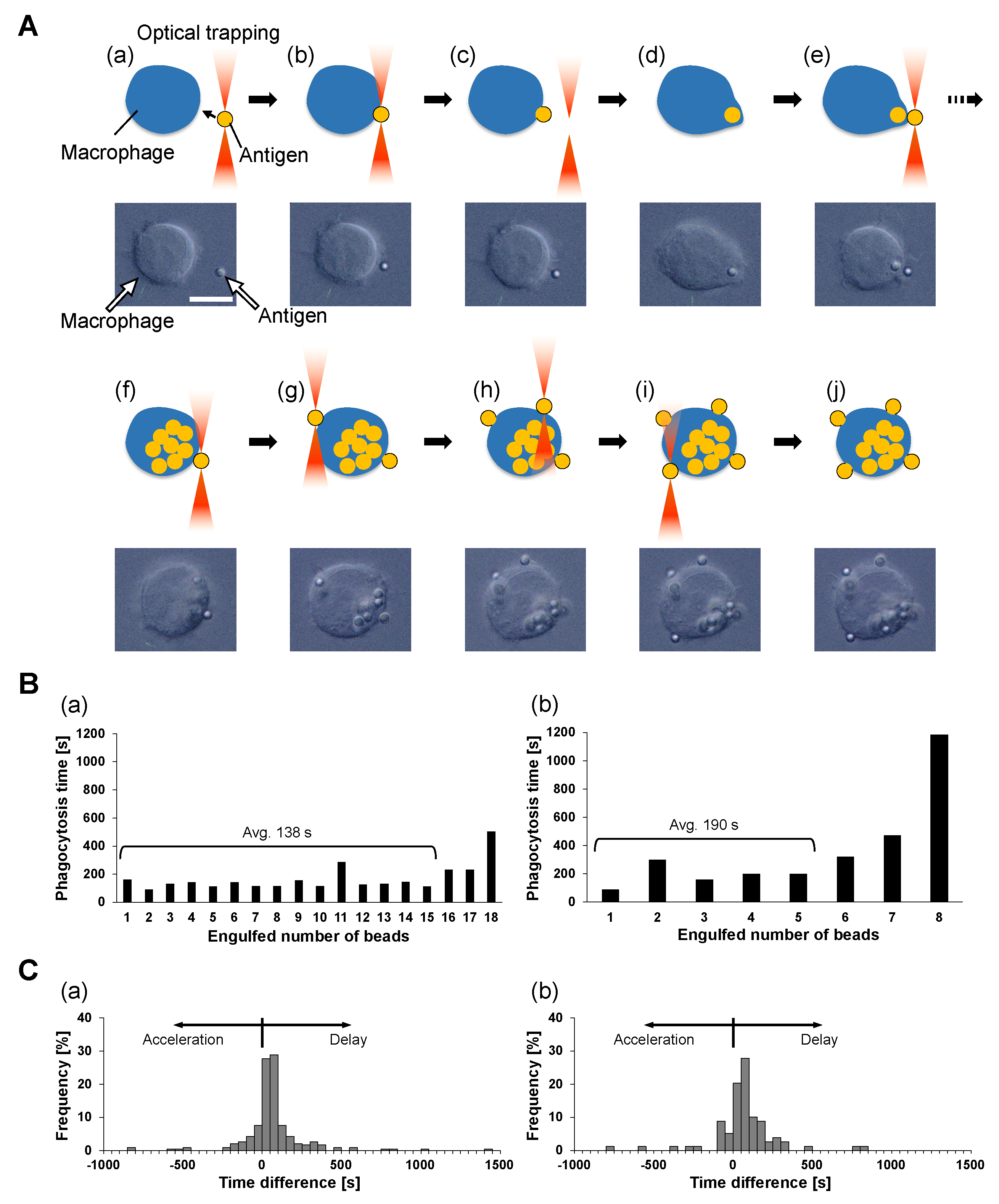
As shown in
Figure 3A, the one-by-one feeding process of antigen beads was operated as follows. First, an antigen bead, the first bead, was captured and brought into contact with the cell surface with optical tweezers (a and b). After confirming the adhesion of the first bead to the cell, the bead was released from the optical tweezers (c) and internalized into the cell (d). Similarly, the second bead was captured and brought to the same place on the cell surface (e). There was a short lag time for the phagocytosis interval between the completion of the first bead phagocytosis and the contact of the second bead. In the same way, contact and internalization were carried out for the third and subsequent beads until the cell reached its maximum limit of phagocytosis. We confirmed the mineralization of the last antigen bead by the following procedure. First, an antigen bead was attached to the same point on the cell at which the previous beads were brought into contact (f), and we confirmed that this bead was not phagocytozed for more than 30 min. Then, an additional bead was brought into contact with an opposite point from the previous one (g), and no response for 30 min was confirmed again. Finally, we added two more antigens to the other two points between these two beads similarly and checked for no responses for 30 min (h–j).
These one-by-one feeding results also indicate the interesting characteristics of engulfment. As shown in the above results, once the macrophage reaches the maximum limit of engulfment of non-digestive antigens on the part of the cell surface, the macrophage does not start the next engulfment in the place of the last phagocytosis or on any other part of the cell surface. This means the regulation of the maximum limit of phagocytosis is not caused by the local membrane capacity limit or local receptor concentration but is caused by the information of the whole cell volume increase.
Figure 3B shows typical examples of the phagocytosis number dependence of the time required for the completion of phagocytozing of 2
m and 4.5
m beads during the series one-by-one attachment. During the interval of phagocytosis of each bead by macrophages, the time from adhesion of the beads to the cell membrane (
Figure 3A(c)) to completion of bead uptake into the cell (
Figure 3A(d)) was measured and defined as the phagocytosis time. The two bar graphs show that the phagocytosis time was similar with fluctuations independent of the phagocytosis number except for the final few phagocytoses, which took longer than the earlier ones.
To confirm this consistency of phagocytosis time, we replotted the difference in phagocytosis times between adjacent phagocytoses as the histograms in
Figure 3C. These histograms show that phagocytosis times tend to be slightly delayed, on average, 17.6 s and 18.7 s in (a) and (b), respectively. However, in (a), 68.1% of the total serial phagocytosis shows a time difference of 77.5 s (half of the median of the phagocytosis time) or less, and in (b), 63.3% of the total cases show a phagocytosis time difference of 102.5 s or less.
Hence, we concluded that the required time for phagocytosis is independent of their number in the series phagocytosis. One-by-one feeding results show that the phagocytosis rate of macrophages remains constant, independent of their previous phagocytosis history. Therefore, the assumption in the previous section suggests that the phagocytosis rate constant of indigestible polystyrene beads is independent of the engulfed antigen number m as long as the macrophages do not reach their phagocytosis number limit.
3.5. Evaluation of Independence of Local Engulfments in Space: Simultaneous Two-Microneedle Phagocytosis Assay
As shown above, each phagocytosis was independent of their number during the series of one-by-one phagocytoses. Then, we might ask, what about the difference in simultaneous phagocytosis? We applied the IgG-coated two-microneedle phagocytosis experiment to examine the difference in two simultaneous phagocytoses on a single macrophage.
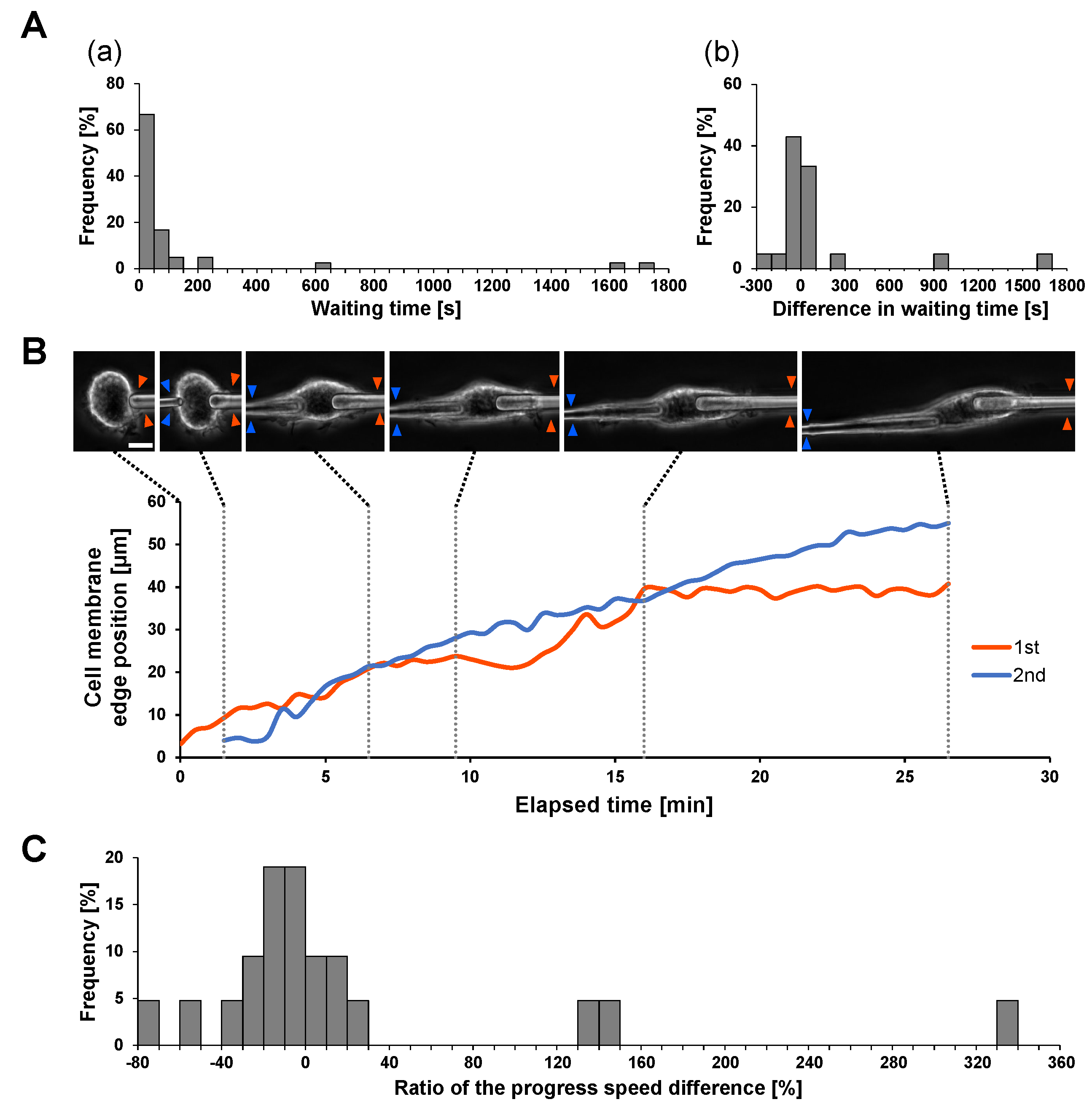
Figure 4A shows the required time for initiation of engulfment of two microneedles (A) and their differences (b) in 21 experiments. As shown in the bar graphs, the local responses of macrophages in different places showed no significant differences. Then, we compared the difference in internalization efficiency in simultaneous engulfment by comparing the speed of the cell membrane extension on the IgG-coated microneedle.
Figure 4B shows an example of two microneedle experiments. As shown in the micrographs and the graph, the velocities of cell membrane extensions on the two microneedles were similar, at 0.0390
/s (1st) and 0.0338
/s (2nd), respectively. This similarity is also supported in
Figure 4C. These results also indicate that the internalization efficiency in simultaneous phagocytosis is similar and the results are independent of each other.
Therefore, the estimation of the phagocytosis rate constant in the on-chip free flow does not need to consider the engulfment intervals or the possibility of simultaneous occurrence.
3.6. Possible Depletion of Macrophages by PM2.5
In this study, we demonstrated that indigestible 2 m polystyrene spheres with surface decorations of blood components such as BSA and IgG showed a much higher phagocytosis rate constant than natural digestible antigens like Zymosan. Significantly, the beads decorated with IgG and BSA maintained a stable high phagocytosis rate constant until the macrophages almost reached their saturation, which was more than six times the efficiency of non-coated polystyrene spheres and Zymosan.
Our results in one-by-one feeding also showed the deactivation of macrophages when they engulfed nondigestible IgG/BSA-PS beads to their maximum number limit.
These two pieces of evidence suggest the possibility of depletion of macrophages when we apply sufficient nondigestible PM2.5 into the vascular system. As the macrophages engulfed IgG/BSA-coated nondigestible microparticles with high frequency, a shortage of active macrophages might occur in our immune system.
When foreign matter enters the body, it does not remain at a static site but moves through bodily fluids, such as lymph and blood circulations. According to the affinity of those microparticle surfaces to the blood components, a reasonable amount of particulate matter should be opsonized to become antigens. Therefore, macrophages should engulf those microparticles, and most of the saturated macrophages proceed to apoptosis.
Many reports have shown the effect of PM2.5 on the functions of macrophages. For example, macrophages exposed to PM2.5 have impaired viability [
9,
10,
16,
17], ability to respond to antigens or phagocytic capacity [
12,
16,
18], production of cytokines and nitric oxide, which induce inflammation and increase the bactericidal activity of immune cells [
12], and ability to activate lymphocytes [
16]. These impaired functions can cause a significant reduction in host defense against infection since they result in impaired mobilization and activation of other immune cells, as well as clearance of antigens by the macrophages themselves. This reduction of host defenses can continue for as long as a week after exposure to PM2.5 [
16].
All the above evidence is consistent with our results. Because PM2.5 particles are indigestible particles, macrophages saturated with phagocytosis by PM2.5 are unable to digest the particles they have taken in and regain their phagocytic capacity, which eventually leads to apoptosis, resulting in the impairment of cell viability and phagocytic function. In addition, since antigen presentation, which is the process of lymphocyte activation, and secretion of inflammation-inducing substances are both based on antigen information phagocytozed by macrophages, if the phagocytic function is impaired, they will be impaired accordingly. In the present study we showed that phagocytic saturation of macrophages by PM2.5 is completed in the shortest time when PM2.5 is modified by BSA or IgG. In other words, macrophages that respond to large amounts of PM2.5 at certain local sites in the body, for example, in the alveoli, can be depleted before calling for the support of other immune cells. The time required for the host to recognize this local depletion of macrophages and to clear depleted cells and replenish new macrophages will be more than that for normal inflammation with pathogens as antigens, and thereby the host’s defenses will stagnate for some time; for example, one week shown by previous studies.
However, PM2.5 can also cause diseases and disorders due to immune overactivity. For example, it has been reported that PM2.5 can cause an increase in M1 macrophages, which leads to immune hyperactivity [
11], and exacerbates lipid accumulation in macrophage foam cells, which causes atherosclerosis [
17]. In addition, adverse effects not only on macrophages but also on the immune system as a whole (immunotoxicity) and damage to the cardiovascular system have been pointed out [
19,
20]. For example, a study measuring CD4(+)T cell counts and HIV viral load in the blood of HIV/AIDS patients exposed to PM2.5 showed that CD4(+)T cells decreased and HIV viral load increased as the concentration of PM2.5 in the air increased [
19]. Although this study focused on macrophage dysfunction and inactivity, the application of the free-flow method devised in this study is expected to elucidate the occurrence mechanisms of these reported disorders of polarity, an effector of mitochondrial damage on phagocytosis, and the hyperactivity of macrophages [
21].