Abstract
Microfluidics is a multidisciplinary science that includes physics, chemistry, engineering, and biotechnology. Such microscale systems are receiving growing interest in applications such as analysis, diagnostics, and biomedical research. Thermoplastic polymers have emerged as one of the most attractive materials for microfluidic device fabrication owing to advantages such as being optically transparent, biocompatible, cost-effective, and mass producible. However, thermoplastic bonding is a key challenge for sealing microfluidic devices. Given the wide range of bonding methods, the appropriate bonding approach should be carefully selected depending on the thermoplastic material and functional requirements. In this review, we aim to provide a comprehensive overview of thermoplastic fabricating and bonding approaches, presenting their advantages and disadvantages, to assist in finding suitable microfluidic device bonding methods. In addition, we highlight current applications of thermoplastic microfluidics to analyses and diagnostics and introduce future perspectives on thermoplastic bonding strategies.
1. Introduction
Microfluidics is a multidisciplinary technology that is used in various applications including analyses, diagnostics, and biomedical research [1,2,3,4]. Historically, silicon and glass substrates were used for fabricating microfluidic devices, then, the rapid advancement in soft lithography technology allowed using polydimethylsiloxane (PDMS) [5,6]. However, PDMS is limited by its hydrophobic absorption and low mechanical rigidity. Moreover, PDMS device fabrication is relatively complex and has a low throughput [7,8]. Subsequently, thermoplastic materials have been widely applied because of their good mechanical rigidity and high-throughput fabrication [9]. Typical thermoplastic materials for microfluidics include polystyrene (PS), polycarbonate (PC), poly (methyl methacrylate) (PMMA), cyclic olefin copolymer (COC), poly (ethylene terephthalate) (PET), polypropylene, and polyvinyl chloride (PVC). These materials are optically clear, rigid, compatible with many organic solvents, and show low absorption of small molecules [9,10]. These advantages make thermoplastics ideal for analytical microfluidics. However, because these materials are barely permeable to gas, their sealed microchannels are inappropriate for long-term cell culture. Nevertheless, in contrast to traditional materials such as silicon and glass, thermoplastics are recommended for microfluidics due to their low fabrication costs and easy manipulation.
Apart from material selection and microchannel fabrication, microdevice bonding is a concern in the development of thermoplastic microfluidics. Although thermoplastics share common characteristics, each material possesses unique properties including chemical composition, glass transition temperature, mechanical rigidity, and solvent compatibility [11]. Therefore, there are diverse bonding methods including common strategies such as thermal bonding, solvent bonding, adhesive bonding, and physical/chemical-assisted bonding (Figure 1). Several key factors determine the effective bonding in thermoplastic microdevices. Particularly, bond strength is a critical factor that determines robust and stable bonding without leakage during microdevice operation. Moreover, optical transmittance and biocompatibility should be considered when selecting a bonding method for optical detection and cell research [12]. In this review, we discuss the advantages and limitations of each thermoplastic bonding method. Further, we reviewed multidisciplinary applications of thermoplastic microfluidic devices, such as nucleic acid-based diagnosis, cell manipulation, and organ-on-a-chip (Figure 1).
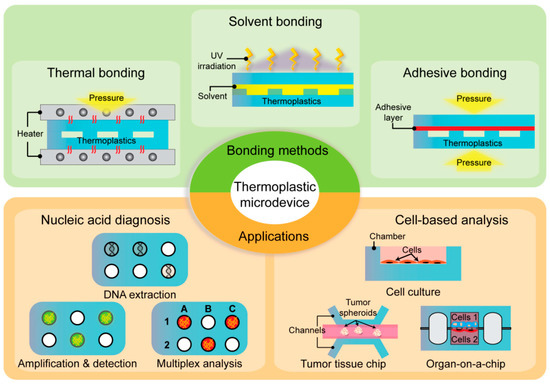
Figure 1.
Summary of representative bonding methods (thermal bonding, solvent bonding, and adhesive bonding) and the applications of thermoplastic microfluidics in nucleic acid diagnosis and cell-based analysis.
2. Thermoplastic Materials for Microfluidic Fabrications
The fabrication of microfluidic devices with thermoplastics involves a variety of replication methods such as hot embossing, injection molding, imprinting, and thermoforming [13,14,15,16]. Injection molding involves the injection of the molten thermoplastic polymer in a high-precision mold under high pressure. Then, the mold is cooled below the glass transition temperature (Tg) of the plastic material and the microfluidic structure is formed. This technique enables the fabrication of complex structures, but the fabrication cost is high and it is difficult to assemble separate layers for the construction of closed microfluidic devices [17]. The hot embossing technique is based on pressing a microstructure mold with a force onto a thermoplastic substrate while the substrate is heated slightly above the Tg. The thermoplastic substrate contains the microstructures after cooling and releasing from the mold. Hot embossing has many advantages, including lower manufacturing costs and easy operation, but parameters such as temperature and force need to be critically controlled to achieve maximum accuracy [18]. Imprinting involves embossing a hard mold into a soft material of thermoplastic polymers to yield small features on large area substrates. A crucial factor to qualify for successful molding is material flowability, since flow resistance can impede the creation of smaller structures [19]. In thermoforming, the thermoplastic sheets are heated and softened, maintaining a solid state (thermoelastic state) without losing material coherence. Thermoforming enables the formation of 3D structures; however, the processing time is relatively long [20]. Recently, thermoplastics have been engraved by direct machining methods including laser ablation and mechanical micromilling [21,22]. Laser ablation relies on an ultraviolet (UV) pulse of laser radiation onto a material to break bonds within a polymeric molecule, which enables the creation of microfluidic structures. Parameters such as wavelength, power, pulse duration, and repetition rate are precisely controlled for the high volume replication of microfluidic devices. A smaller wavelength and beam quality can create finer features. The laser ablation process is realized in a relatively short time, but requires expensive equipment and a high cost for the manufacture [23,24]. Alternatively, micromilling uses a mill with high-speed rotation to create microfluidic structures. Usually, the tool speed and position are automatically controlled by computer numerical control (CNC) programming from a computer-aided design file. CNC milling is used to fabricate prototypes or to create structured molds for the rapid generation of microchips via PDMS casting. Micromilling can easily and rapidly manufacture structures with high aspect ratios and can be an effective and relatively low-cost strategy for prototyping microfluidic devices. Nevertheless, micromilling has the limitations of poor microstructure resolution. The CNC machines may be combined with microscope to monitor the desired sizes and features, improving the accurate tolerances on milled structures [25,26].
Thermoplastics are densely crosslinked polymers that soften when heated to their Tg but solidify upon cooling while maintaining their original chemical bonds. Thermoplastics are generally durable due to their chemical and dimensional stability, which makes them highly adaptable for a wide range of microfluidic applications. Depending on the application, both thermoplastic materials and fabrication methods are appropriately selected according to their physical and chemical properties. PS is optically transparent, biocompatible, and inert, making it suitable for cell culture research [27]. The PS surface can be easily functionalized via physical and chemical modifications such as irradiation, gas plasma, and corona discharge to increase surface hydrophilicity [28]. Although PS is an inexpensive material, expensive equipment is required to produce complex chips. Injection molding and hot embossing are commonly used as molding methods for PS [27]. Next, PC is a durable and transparent thermoplastic polymer for microfluidics used in bioanalytic applications such as nucleic acid isolation and pathogen detection. Particularly, PC is appropriate for a range of enzymatic amplifications such as continuous flow polymerase chain reaction (CF-PCR) because its Tg is very high (Tg = 145–155 °C) [29,30]. However, the fabrication of the PC microstructure depends on hot embossing; thus, the bonding method is limited by thermal bonding since the thermal bonding of PC requires high temperatures which can damage microchannels [31]. PMMA is a cheap and easy-to-fabricate polymer; it is the most common thermoplastic. In addition to its optical transparent and rigid properties, PMMA is biologically compatible with cells and useful for cell research [32,33]. PMMA microfluidic devices are also applied in extraction and electrophoresis separation systems [34]. PMMA patterns can be formed through hot embossing and injection molding. Moreover, this material can easily engrave microchannels by CO2 laser or micromachining [9]. COC is an amorphous thermoplastic copolymer made from cyclic monomer polymerization [35]. The COC surface is hydrophobic, causing nonspecific adsorption of analytes. Therefore, its surface chemical modification is necessary. COC is resistant to acids and several organic polar solvents; thus, COC microfluidic systems are attractive for on-chip chromatography [36]. COC exhibits highly optical transparency and low background fluorescence, making it interesting for lab-on-a-chip systems designed for fluorescent detection using integrated circuits [37]. The Tg of COC ranges from 70 to 170 °C, depending on polymer content. Moreover, molding methods such as injection molding, compression molding, thermoforming, and many others can be applied to COC materials [38]. Additionally, there are other less common thermoplastics used for microfluidics such as PET and PVC. These materials have a low Tg of around 80 °C and good resistance to solvents [39], and both materials can be molded by hot embossing, imprinting, and laser ablation [40].
3. Thermoplastic Bonding
3.1. Thermal Bonding
Thermal bonding is a bonding process that uses heat and pressure to seal microfluidic devices. In thermal bonding, two thermoplastic substrates are heated near or above their Tg, and the substrates become rubbery fusing at the interface under the pressure. This leads to a robust bond between the surfaces due to the crosslinked polymers at the interface, as shown in Figure 2a. Therefore, under optimal bonding conditions, not only similar but also dissimilar thermoplastic substrates are easily sealed using the thermal bonding method. Thus, this bonding method is also called thermal fusion bonding. Thermal bonding is simple and robust, making it the most common method for sealing microfluidic chips [41]. Under ideal conditions, high bond strength can be achieved through a simple process. Moreover, given the direct bonding without intermediate materials, these microchannels present homologous surfaces after bonding which maintain the initial properties of thermoplastic materials [40]. During thermal bonding, the thermoplastic substrates are aligned between supporting plates and a hot press machine applies heat and pressure [42]. Interestingly, the hot press machine can be utilized for both hot embossing microchannel fabrication and thermal bonding. Chen et al. designed a spring-driven press device for hot embossing and thermal bonding of PMMA microfluidic chips. This simple press device consisted of press heads, compression springs, and screw nuts to fix the PMMA plates before heating them in a convection oven for embossing or bonding [43]. Later, the press device had a positive temperature coefficient ceramic heater inside [44].
The Tg of the material determines the bonding temperature; therefore, the bonding of thermoplastics with the same or similar Tg is recommended to prevent channel deformation. For instance, PMMA bonding was achieved by heating at 90–95 °C for 10 min under a pressure of 1–2 MPa [43,45,46]. In another study, the thermal bonding required heating at over 120 °C for 1 h by a pressure cooker since a specific PMMA with high Tg was used [47]. A temperature of 105 °C and a pressure of 0.4 MPa is required to produce low-deformation thermal bonding of PS nanostructured microfluidic chips [48]. Due to the high Tg property, PC (Tg = 147 °C) and polyimide (PI) (Tg > 300 °C) thermal bonding has been performed at 134 °C for 10 min and 380 °C for 5 min, respectively [49,50]. However, thermal bonding poses some drawbacks such as thermal deformation or microchannel collapse during PET, acrylonitrile butadiene styrene (ABS), and PC chip manufacturing (Figure 2b) [51]. In another study, to decrease the bonding temperature, Liu et al. presented plasma-assisted thermal bonding for sealing PMMA microfluidic chips integrated with metal microelectrodes (Figure 2c) [52]. With the use of plasma, the bonding temperature was decreased from 100 to 85 °C due to a lower Tg at the surface of polymers after plasma treatment, and the fracture of copper microelectrodes was eliminated. Adapting to the same concept, Immanuel et al. also introduced surface activation through H2O plasma treatment linked with low-temperature annealing for bonding PMMA devices for blood tests [53].
Additionally, thermal bonding can be also applied for sealing hybrid thermoplastic materials such as PMMA–COC and PMMA–TPE (thermoplastic elastomer). The bonding parameters for these materials are usually 70–80 °C for 15 min under a pressure < 1.6 MPa [54,55,56]. Apart from optimizing the bonding parameters (temperature, pressure, and time), surface pretreatments such as gas plasma, UV, and chemical treatments can improve the bonding. Indeed, treating the PMMA substrates with isopropyl alcohol for 75 s in a boiling bath before thermal bonding, improved a four-fold increase in bond strength, with full favorable optical clarity [57]. In addition, a surface treatment can help lower the bonding temperature, reducing the risk of microchannel deformation due to high temperature and force application. The activation of UV/O3 light helps PMMA and COC sealing at 70 °C, a temperature significantly below the Tg of the substrates [54]. Moreover, plasma-assisted thermal bonding has been shown to be beneficial in increasing bond strength and decreasing bonding temperature [56,58].
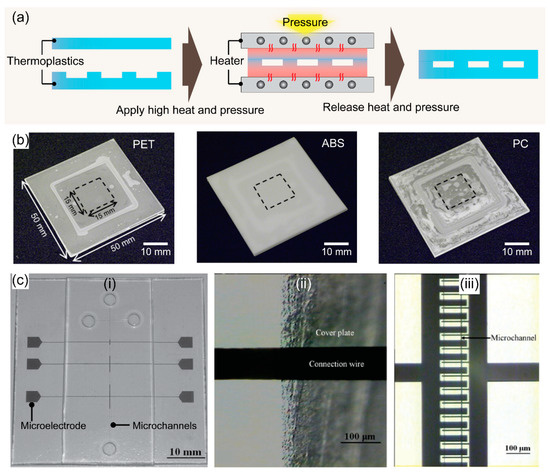
Figure 2.
(a) Schematics showing the representative procedures for thermal bonding for fabricating thermoplastic devices. (b) Photographs of PET, ABS, and PC substrates bonded under the optimal heating conditions at 107, 152, and 202 °C using the thermal bonding method, respectively, the squares indicated by black dotted lines show the parts cut out using the picosecond laser to measure bonding strength. Adapted with permission from Ref. [51]. Copyright 2018, Elsevier. (c) PMMA microfluidic device with integrated metal microelectrodes bonded at 85 °C using a plasma-assisted thermal bonding method: (i) photograph of the chip with copper IDMAs, (ii) connection wire at the edge of the cover plate without cracks, (iii) copper IDMAs in the microchannel. Adapted with permission from Ref. [52]. Copyright 2009, Elsevier.
3.2. Solvent Bonding
Solvent bonding is a versatile process commonly used for the permanent joining of thermoplastic materials. In solvent bonding, a solvent is applied to dissolve and break down polymer chains at the contact surface, and then the polymer chains of two substrates are crosslinked to create a permanent bond. After the solvent evaporates, a strong thermoplastic-to-thermoplastic bond is formed even at low temperatures with less requirement of equipment (Figure 3a). Solvent bonding enables robust bonding at a relatively low temperature. The process is fast and inexpensive. Notably, high bond strength of 11.75 and 14.95 MPa can be achieved when applying acetic acid for microwave-assisted or UV-assisted solvent bonding of PMMA microdevices, respectively [59,60]. These bonding strengths are higher than the limits of typical thermal and adhesive bonding. Different types of solvents can be applied for thermoplastic bonding depending on thermoplastic materials. For example, PMMA devices can be bonded using ethanol [61,62], chloroform [63], isopropyl alcohol [64], and acetic acid [36,59,60], while cyclic olefin polymer (COP) sealing can be performed using cyclohexane and toluene [65].
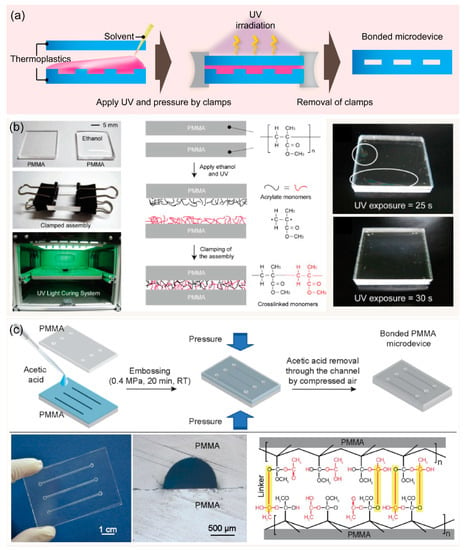
Figure 3.
(a) Schematics showing the procedures for solvent bonding for fabricating thermoplastic devices. (b) The overall procedure for bonding two PMMA substrates via ethanol treatment followed by UV irradiation, a chemical reaction is anticipated to take place on the surfaces of two PMMAs substrates after ethanol and UV treatment. Adapted with permission from Ref. [61]. Copyright 2013, Elsevier. (c) The overall procedure for bonding two PMMA substrates at room temperature by acetic acid under pressure. The photographs show bonded PMMA microdevice and cross-section of the microchannels after the bonding, chemical bonds are anticipated to form between two PMMA substrates after acetic acid and pressure treatment. Adapted with permission from Ref. [33]. Copyright 2019, Elsevier.
Lukashenko et al. investigated a chemical solvent bonding technique for manufacturing nondetachable PMMA substrates using different solvents such as ethyl acrylate, n-butylacrylate, and vinyl acetate. In particular, vinyl acetate was selected since it exhibited the solvent-bonded seam with smaller change in the working volume of microstructures after bonding [66]. An optimized solvent composition for bonding is a key for good solvent bonding performance. For instance, a weak solvent or one at low concentrations does not allow the substrates to fully bond. Otherwise, a solvent excessively strong or at high concentration has the risk of microchannel clogging and distortion by excessively dissolving thermoplastic polymers. Trinh et al. introduced acetic acid as a solvent for clog-free bonding of PMMA microdevices at room temperature within 20 min [36]. Moreover, increased acetic acid concentration (10–100%) showed expansion of the bonding area; 50% acetic acid was the optimal concentration for completely bonding PMMA substrates [59]. In another study, UV exposure for 30 s and ethanol <50% showed reversible bonding, while ethanol >50% supported the irreversible bonding of PMMA assemblies [61]. Moreover, UV irradiation reinforces the activation of the thermoplastic surfaces in the presence of a solvent, thus, the monomers of two surfaces are rapidly activated and re-crosslinked to realize a permanent bond under relatively low pressure condition. For instance, 50% of acetic acid has been used to seal two PMMA substrates at room temperature for 20 min under a pressure of 0.4 MPa using a press machine [33]. UV-assisted acetic acid bonding required only 30 s of UV irradiation when assisted with clamps [59].
Effective and rapid thermoplastic bonding can be achieved by applying a mixture of different solvents. PMMA substrates were treated with acetone and ethanol (v:v, 8:2) for 30 s to fabricate a microfluidic chip without microchannel deformation [67]. COC chips were exposed to a mixture of 60% cyclohexane and 40% acetone (v:v) for 120 s to achieve high bond strength and good channel integrity [68]. Moreover, three critical components, i.e., acetone, n-pentane, and 1H,1H,2H,2H-perfluorooctyl trichlorosilane, assisted in one-step bonding of PC microfluidic chips within only 10 s [69]. One of the challenges of solvent bonding is the rapid evaporation of solvent near the free edges of microdevices due to the inherent volatility of solvent, which causes poor bonding and leakage. This phenomenon can be mitigated by adding grooves near the edges of microfluidic devices [61,70]. The addition of peripheral grooves is supported to retain the solvent, preventing evaporation during microwaving, and significantly improving the bonding coverage [71]. Further, the additional feature grooves substantially decreased the unbounded area surrounding individual microchannels [70]. A surface modification also suggests an improvement in solvent bonding. Ethanol and UV exposure of internal surfaces produces excellent bonding, increasing the bond strength between PMMA and acrylonitrile butadiene styrene [71]. In addition, surface chemical and plasma modification followed by solvent bonding suggest reproducible bonding in PMMA microfluidic devices [72,73].
3.3. Adhesive Bonding
Adhesive bonding is a rapid and simple method for sealing thermoplastics where substrates are bonded at their interface by an adhesive (Figure 4c). Due to its simplicity, adhesive bonding is widely used for thermoplastics and for other materials. Liquid and dry adhesives can be used for specific requirements of thermoplastic bonding. A liquid adhesive usually requires a photo or thermal activation to form the bonded interface between two pieces of thermoplastic substrates. For example, microchannel and micropillar PMMA systems were bonded using a commercial UV adhesive (Slink 80801) with UV irradiation for 60 s [74]. Kratz et al. characterized four biomedical-grade pressure-sensitive adhesives (ARcare 92712, ARcare 90445, ARcare 90106, and ARseal 90880) for rapid prototyping of lab-on-a-chip systems; ARcare 90445 exhibited good bonding strength and gas tightness combined with satisfactory cell adhesion and viability [75]. In addition to commercial adhesives, several biopolymers can function as adhesion agents for bonding thermoplastic materials. Trinh et al. introduced the chitosan (CS)–polydopamine (pDA) hydrogel complex as an adhesion agent for reversible thermoplastic bonding assisted by UV irradiation [76]. Similarly, poly(acrylic acid) was adopted as UV-assisted adhesion promoter for fabricating thermoplastic microdevices [77]. A major challenge of adhesive bonding is channel clogging due to excessive liquid adhesive inside the microchannels. Therefore, several strategies such as adhesive printing, spin coating, and capillarity-driven adhesive delivery have been developed to prevent adhesive clogging [74,78].
Contrary to liquid adhesives, the simplest form of dry adhesive bonding is directly applying an adhesive tape onto thermoplastic substrates [79]. Tsao and Syu reported dry adhesive tape bonding of inflexible and flexible substrates using a manual scraper press and a hot press machine [80]. ORDYL dry film photoresist was used for packaging of COC microstructures; subsequently, oxygen plasma was used for adhesion improvement [81]. A thick adhesive film was applied for bonding multilayers of PMMA to form micropump with actuation chambers [82,83]. One advantage of adhesive bonding is the sealing of hybrid thermoplastic materials such as PMMA–PC, PMMA–PS, PMMA–PI, PMMA–PET, and PMMA–PVC [76,77] or a thermoplastic and elastomer (PMMA–PDMS) [83,84]. Song and Park used a 2.5% (w/w) PMMA solution as an adhesive layer to bond heterogeneous PMMA–PC polymers, by enclosing the PMMA microfluidic channels with PC [85]. Notably, the adhesive interface plays an important role in reversible bonding. Yao et al. reported a new reversible bonding strategy to seal conventional and hybrid reversible bonding (PMMA–PMMA or glass–PMMA) using UV release tape [86]. Thermoplastic bonding with a CS–pDA adhesion agent slightly decreased bond strength after four reversible bonding cycles [77].
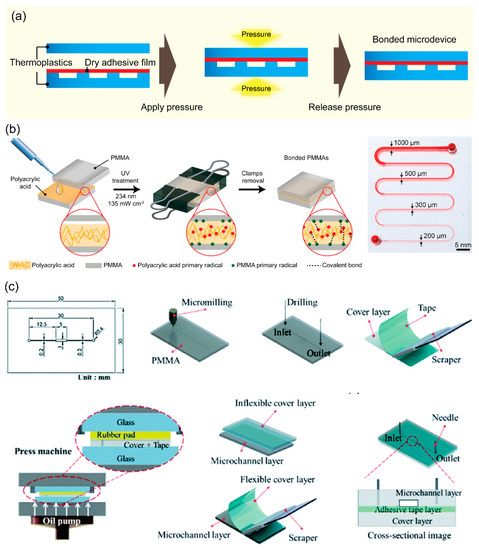
Figure 4.
(a) Schematics showing the representative procedures for adhesive bonding using a dry adhesive film for fabricating thermoplastic microdevices. (b) Schematic illustration of the overall procedure for bonding PMMA device using PAA assisted by UV, the photograph of a clog-free PMMA microdevice including a serpentine microchannel with various channel dimensions. Adapted with permission from Ref. [77]. Copyright 2021, Elsevier. (c) Schematics showing the procedures of PMMA microdevice fabrication using an adhesive tape and press machine for bonding microdevice. Adapted with permission from Ref. [80]. Copyright 2020, Royal Society of Chemistry.
3.4. Other Bonding Methods
In addition to the three major methods above, thermoplastic polymers can be sealed by other effective bonding methods such as physical-assisted bonding, chemical-assisted bonding, ultrasonic/laser welding, and microwave bonding. Physical modification of the surfaces can increase the surface energy, promoting bonding and enhancing the bonding strength between the two substrates. On the one hand, plasma and UV radiation are common physical agents for physical-assisted bonding. Plasma processing used deep O2 plasma etching on PMMA and a photosensitive PDMS as resist for the high-throughput mass production of polymeric microfluidic fabrication [87]. Vacuum UV (VUV) light irradiation, VUV irradiation in the presence of oxygen gas (VUV/O3), or O2 plasma treatment were used for direct bonding of two COP plates [88]. For example, Wen et al. employed a photo-bonding process with VUV light to fabricate microfluidic devices without using any solvent for cell culture applications [89]. To investigate the effects of residual solvent, the decrease in apoptosis was observed and compared with a device bonded using solvent. On the other hand, chemical-assisted bonding uses chemical reagents for the activation of thermoplastic surfaces for bonding. Surface modification-assisted bonding has been performed by plasma oxidation followed by tetraethyl orthosilicate treatment to facilitate siloxane bonding between the two polymer substrates (PMMA–PMMA and PMMA–PC) [90]. Surface modification of (3-aminopropyl)triethoxysilane has been shown to promote chemical bonding and robust irreversible bonding between PDMS and thermoplastics such as PS, PC, PMMA, and PET [91,92,93]. Moreover, Nguyen et al. reported a method for bonding PMMA to PET membranes using (3-glycidyloxypropyl)trimethoxysilane followed by air plasma and heating at 100 °C [94].
Ultrasonic and laser welding accelerate bonding through local melting and welding. Ultrasonic bonding involves bonding through local melting by the propagation of ultrasonic sound; in contrast, laser bonding involves localized heating at the interface of two thermoplastic substrates [95,96]. Ultrasonic actuation has been applied for 10 s to preheated COC substrates to accelerate thermal compression bonding [97]. A diode laser has been used for micro-welding two PMMA substrates together using an intermediate thin film metal as a localized absorber [98]. Kim et al. introduced a new photothermal bonding of a PMMA device using copper sulfide/reduced graphene oxide-poly(ethylene glycol) (CuS/rGO-PEG) nanocomposites and near-infrared (NIR) laser irradiation (Figure 5) [99]. However, both ultrasonic and laser welding have limitations, including difficult adjustment of energy distribution and excessive fusion due to ultrasonic sound or laser pulses [100]. Alternatively, microwave bonding uses a microwave to heat the interface layer during bonding to produce bonding between thermoplastic substrates. Microwaving allows localized heating, which avoids excessive heat and prevents channel deformation. The thin film metal deposited on a PMMA substrate surface is designed to absorb microwave power, causing localized melting and improving adhesion at the interface for PMMA bonding [101]. Microwave bonding is a good alternative and user-friendly bonding method for thermoplastic microfluidic devices using a household microwave oven [59,101].
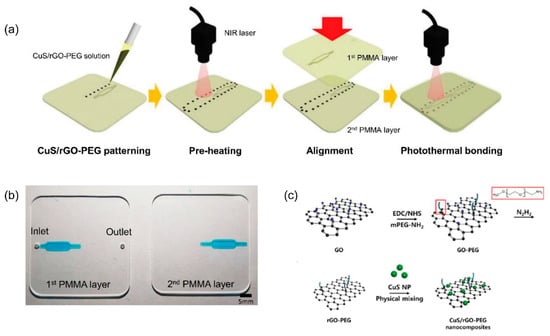
Figure 5.
(a) Schematics showing PMMA bonding process using CuS/rGO-PEG nanocomposite and the photothermal effect. (b) Photographs of the PMMA device. (c) The synthesis process of the CuS/rGO-PEG nanocomposite [99].
The typical bonding requirements, advantages, and disadvantages of several bonding methods are summarized in Table 1. Generally, several types of equipment such as a heater, press machine, ultrasonic/laser/microwave sources, and plasma/UV machine are required for thermoplastic bonding. With respect to reagents, these bonding methods require a variety of solvents, chemicals, and dry or liquid adhesives. Moreover, each approach has advantages and limitations.

Table 1.
Comparison of bonding methods for thermoplastic devices.
4. Analytical and Diagnostic Applications
4.1. Nucleic Acid Diagnosis
Nucleic acid (DNA and RNA) analysis is important for genetic research, disease diagnosis, and pathogen detection. PCR is one of the most robust nucleic acid amplification tools. Microfluidic PCR or CF-PCR permits rapid testing and identification of genetic samples with high throughput and high efficiency [103]. Since thermal bonding presents heat and chemical resistant ability, numerous PCR thermoplastic microchips have been developed for various applications [33,92,104]. A PS microdevice has been fabricated by micromilling replication and thermal bonding for pre-concentration and CF-PCR amplification of E. coli DNA [104]. Trinh et al. reported on an integrated monolithic PMMA microfluidic device for on-site detection of major foodborne pathogens in a continuous flow. The reported device consisted of a serpentine microchannel for on-chip amplification and a detection chamber for end-point fluorescence signal [105]. Zhang and co-workers presented a glass-like sol-gel (bis[3-(trimethoxysilyl)propyl]aminosilane) coating on the PC surface to facilitate one-step bonding of two PC substrates at a mild temperature under atmospheric pressure within 30 min [106]. In this case, sol-gel coated PC microchannel was employed for DNA purification, and integrated with a flow-through PCR to realize seamless DNA purification and amplification for rapid detection of E. coli using a monolithic PC device realized in 70 min (Figure 6a). Moreover, thermoplastic bonding is compatible with integrated surface plasmon resonance (SPR) fiber sensors. Solvent bonding exhibits a strong permanent bond of two substrates under mild pressure, allowing for the integration of sensor system into a microfluidic device. For instance, the integration of a microfluidic PCR device and SPR fiber sensor into one PMMA platform fabricated by ethanol solvent bonding was previously reported. This all-in-one system allowed DNA amplification-to-detection within 30 min through a digital SPR sensor signal [107]. Low pressure required in UV-assisted acetic acid bonding supported the integration of a platinum electrode array into a PMMA microfluidic device [59]. Another novel one-step method has great potential for manufacturing PC microfluidic chips for digital droplet PCR using ultrafast solvent bonding (acetone, n-pentane, and 1H,1H,2H,2H-perfluorooctyl trichlorosilane). Fortunately, 1H,1H,2H,2H-perfluorooctyl trichlorosilane plays a key role in the hydrophobic modification of the PC channel, significantly promoting the generation of monodisperse droplets [64]. In addition to PCR devices, thermoplastic microfluidics can apply novel isothermal amplification techniques for rapid and early pathogen detection [108,109]. Furthermore, adhesive bonding applies to fabricating point-of-care platforms since it is simple and low-cost, which meets the requirement of the point-of-care application. Centrifugal or foldable microdevices were fabricated by using thin PC and adhesive tape for multiple bacteria detections. These thermoplastic chips were integrated with DNA extraction, an isothermal amplification called loop-mediated isothermal amplification (LAMP), and colorimetric detections for multiplex point-of-care testing [110,111].
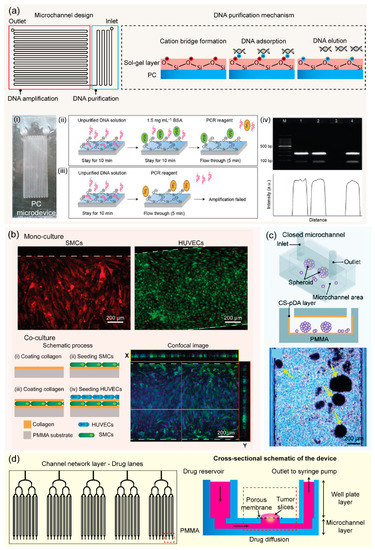
Figure 6.
(a) Schemes illustrating a sol-gel coated polycarbonate (PC) microdevice for DNA purification and amplification, results of capturing of DNA using sol-gel coating layers used for DNA purification: (i) a photo of the purification microdevice, (ii,iii) schematic showing DNA elution using PCR reagent inside the microchannel with and without BSA treated, respectively, (iv) results of the PCR performed from the on-chip purification. Adapted with permission from Ref. [106]. Copyright 2014, Elsevier. (b) Results showing successful culture of SMCs and HUVECs inside a bonded PMMA microdevice using poly(acrylic acid) as an adhesion agent, schemes illustrating a layered co-culture model of SMCs and HUVECs using a poly(methyl methacrylate) (PMMA) microdevice. Adapted with permission from Ref. [77]. Copyright 2021, Elsevier. (c) Schematic representation of the MSC spheroids formed inside a closed-microchannel fabricated using the CS–pDA hydrogel complex, optical image showing MSC spheroids formed after five days of cell culture inside the microchannel, reproduced from [76]. (d) Schemes illustrating the PMMA platform for the drug-response testing system. Adapted with permission from Ref. [112]. Copyright 2020, Royal Society of Chemistry.
4.2. Cell-Based Analysis
Microfluidic platforms allow cell culturing and effective cell capturing, positioning, and analysis. Due to its biocompatibility, thermoplastic microfluidic devices have been widely applied for cell research. Several studies have reported various biocompatible and eco-friendly solvent bonding methods using acetic acid for PMMA microdevices. These bonded microdevices were successfully applied for culturing human cells such as human umbilical vein endothelial cells (HUVECs) and mesenchymal stromal cells (MSCs). This has provided good alternative platforms to perform on-chip viability assays [33,59,60]. Young et al. described the fabrication of PS microfluidic devices (hot embossing replication and thermal bonding) for two different cell-based applications including HUVECs activation and neutrophil chemotaxis [28]. Moreover, biopolymers have great potential as green materials for adhesive bonding of cell-based microfluidic devices due to their biocompatibility. Poly(acrylic acid) has been used as an adhesion promoter for UV-assisted bonding of thermoplastic microfluidic platforms in an in vitro blood vessel wall model. Smooth muscle cells (SMCs) and HUVECs have been cultured inside bonded microdevices in a co-culture model mimicking human blood vessels, applicable for organ-on-a-chip experiment (Figure 6b) [77]. A PMMA microdevice fabricated using the CS–pDA hydrogel complex and O2 plasma treatment promoted MSC proliferation and aggregation to form spheroids, allowing research on 3D human cell cultures (Figure 6c) [76]. In addition, several commercial adhesives, optically transparent and biocompatible, are available for fabricating microdevices for cell monolayers and 3D cell culture systems [75]. Thermoplastic devices are also useful for the validation of drug testing. PMMA–PET microfluidic devices sealed by chemical-assisted bonding were used to culture human lung adenocarcinoma cells. The PMMA devices exhibited more reliable cytotoxicity for vincristine (anticancer drug) as compared with conventional PDMS devices [94]. Rodriguez et al. developed a microfluidic platform for multiplexed drug testing of intact tumor slices from a patient’s colorectal tumor. The device was digitally manufactured in PMMA by CO2 laser micromachining and methylene chloride solvent bonding (Figure 6d) [112]. Thermoplastic devices have also been useful for on-chip electroporation of human cells to produce cell-free viruses. Using low-pressure solvent bonding, a PMMA microfluidic device was successfully integrated with microelectrode arrays, which continuously electrolyzed varicella-zoster virus-infected human foreskin fibroblasts for high-throughput production of cell-free viruses [113].
4.3. Other Analytical Applications
Protein and biomarker analyses are crucial in medical diagnostics and laboratory research. Due to the advantages of thermoplastics, ongoing efforts have focused on developing electrophoresis in microfluidic devices. Hot embossing and thermal bonding have been successfully applied to fabricate PMMA microfluidic systems for high-resolution electrophoretic separations of fluorescently labeled amino acids [42]. Similarly, a COC microfluidic device has been manufactured for reversed-phase electrochromatography separation of polycyclic aromatic hydrocarbons. These microstructures were fabricated by hot embossing and the microdevice was sealed by solvent-enhanced thermal bonding [36]. Wouters et al. reported on the use of COC microfluidic chips in high-performance liquid chromatography. The long straight separation channel layout was engraved by using a CNC micromilling robot, and solvent-vapor-assisted bonding was used to irreversibly seal the chips, producing the ideal channel geometry [114]. Moreover, an integrated PMMA microfluidic system has been fabricated to quantitatively determine fluorescently labeled α-fetoprotein (a biomarker for liver cancer) in human serum. The integrated microdevices were successfully applied for immunoaffinity purification, electrophoresis separation, and laser-induced fluorescence detection [115,116].
Table 2 comprehensively summarizes representative thermoplastic microfluidic systems for various applications. Numerous thermoplastic materials (PMMA, PS, PC, and COC) are commonly used for microfluidics. CNC micromilling is often applied for molding replications due to its automated and mass-producible properties apart from hot embossing and injection molding. Thermoplastic microfluidics are widely applicable to various fields such as integrated microfluidic systems, point-of-care devices, 2D/3D cell culture, organ-on-a-chip, drug testing, and microfluidic molecular separation/detection. A variety of bonding strategies such as thermal bonding, solvent bonding, and adhesive bonding are used for sealing microfluidic devices which are applied for various analytical and diagnostic applications. PCR microfluidic devices are operated in high temperature and high pressure conditions. For these reasons, thermal and solvent bonding are selected since the methods are highly resistant to high temperature and high pressure applications. Meanwhile, adhesive bonding using biocompatible materials such as hydrogels is suitable for fabricating cell-based microchips. Moreover, the modifications of the surface improve the functionality of the microfluidic channels including DNA purification, cell adhesion, or selective capture of biomolecules.

Table 2.
Representative thermoplastic bonding strategies and applications.
5. Conclusions and Future Perspective
In this review, we recapitulated the available knowledge of thermoplastic bonding for fabricating microfluidic devices as well as their applications. Numerous thermoplastic bonding approaches such as thermal bonding, solvent bonding, adhesive bonding, chemical/physical bonding, ultrasonic/laser welding, and microwave bonding are available for microfluidic devices. Researchers should select the appropriate bonding technique depending on the specific properties of the thermoplastic substrate and the requirements of the microfluidic chips. Furthermore, post-process applications could help determine the suitable approach for thermoplastic bonding. Typically, on the one hand, thermal bonding and solvent bonding techniques are used for thermal cycling (such as CF-PCR) microchips due to their thermostability and high bond strength. On the other hand, cell-based microdevices require biocompatible materials, preferring adhesive bonding.
With the development of microfluidic technology, thermoplastic microfluidics have great potential for applications such as nucleic acid analysis (DNA/RNA extraction, amplification, and detection), cell-based research (2D/3D cell culture, organ-on-a-chip, and drug-response testing), and electrophoresis. In addition to its robustness, low cost, and high throughput, its commercialization has allowed the research and development of thermoplastic microfluidic chips. In the future, microfluidic devices will become more complex and integrated, promoting all-in-one devices, wherein thermoplastic bonding allows large-scale bonding of multilayers and dissimilar materials.
Author Contributions
Conceptualization, K.T.L.T., D.A.T., and N.Y.L.; writing—original draft preparation, K.T.L.T. and D.A.T.; writing—review and editing, K.T.L.T. and N.Y.L.; supervision, N.Y.L.; project administration, N.Y.L.; funding acquisition, N.Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (no. NRF 2020R1A2B5B01001971) and also by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2021R1A6A1A03038996).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Manz, A.; Graber, N.; Widmer, H.M. Miniaturized total chemical analysis systems: A novel concept for chemical sensing. Sens. Actuators B Chem. 1990, 1, 244–248. [Google Scholar] [CrossRef]
- Mark, D.; Haeberle, S.; Roth, G.; Stetten, F.V.; Zengerle, R. Microfluidic lab-on-a-chip platforms: Requirements, characteristics and applications. Chem. Soc. Rev. 2010, 39, 1153–1182. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M. Recent Advances in Microfluidic-Based Microphysiological Systems. BioChip J. 2021, 16, 13–16. [Google Scholar] [CrossRef]
- Han, J.; Kang, U.; Moon, E.Y.; Yoo, H.; Gweon, B. Imaging technologies for microfluidic biochips. BioChip J. 2022, 1–15. [Google Scholar] [CrossRef]
- Harris, N.R.; Hill, M.; Beeby, S.; Shen, Y.; White, N.M.; Hawkes, J.J.; Coakley, W.T. A silicon microfluidic ultrasonic separator. Sens. Actuators B Chem. 2003, 95, 425–434. [Google Scholar] [CrossRef]
- Fallahi, H.; Zhang, J.; Phan, H.P.; Nguyen, N.T. Flexible microfluidics: Fundamentals, recent developments, and applications. Micromachines 2019, 10, 830. [Google Scholar] [CrossRef]
- Halldorsson, S.; Lucumi, E.; Gómez-Sjöberg, R.; Fleming, R.M. Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosens. Bioelectron. 2015, 63, 218–231. [Google Scholar] [CrossRef]
- Jung, B.J.; Jang, H.; Lee, G.Y.; Kim, J.; Song, Z.; Pyun, J.C.; Lee, W. Surface functionalization and bonding of chemically inert parylene microfluidics using parylene-A adhesive layer. Biochip J. 2022, 16, 168–174. [Google Scholar] [CrossRef]
- Nge, P.N.; Rogers, C.I.; Woolley, A.T. Advances in microfluidic materials, functions, integration, and applications. Chem. Rev. 2013, 113, 2550–2583. [Google Scholar] [CrossRef]
- Ren, K.; Zhou, J.; Wu, H. Materials for microfluidic chip fabrication. Acc. Chem. Res. 2013, 46, 2396–2406. [Google Scholar] [CrossRef]
- Becker, H.; Gärtner, C. Polymer microfabrication technologies for microfluidic systems. Anal. Bioanal. Chem. 2008, 390, 89–111. [Google Scholar] [CrossRef] [PubMed]
- Becker, H.; Locascio, L.E. Polymer microfluidic devices. Talanta 2002, 56, 267–287. [Google Scholar] [CrossRef]
- Abgrall, P.; Low, L.N.; Nguyen, N.T. Fabrication of planar nanofluidic channels in a thermoplastic by hot-embossing and thermal bonding. Lab Chip 2007, 7, 520–522. [Google Scholar] [CrossRef] [PubMed]
- Mair, D.A.; Geiger, E.; Pisano, A.P.; Fréchet, J.M.; Svec, F. Injection molded microfluidic chips featuring integrated interconnects. Lab Chip 2006, 6, 1346–1354. [Google Scholar] [CrossRef] [PubMed]
- Martynova, L.; Locascio, L.E.; Gaitan, M.; Kramer, G.W.; Christensen, R.G.; MacCrehan, W.A. Fabrication of plastic microfluidic channels by imprinting methods. Anal. Chem. 1997, 69, 4783–4789. [Google Scholar] [CrossRef]
- Giselbrecht, S.; Gietzelt, T.; Gottwald, E.; Trautmann, C.; Truckenmüller, R.; Weibezahn, K.F.; Welle, A. 3D tissue culture substrates produced by microthermoforming of pre-processed polymer films. Biomed. Microdevices 2006, 8, 191–199. [Google Scholar] [CrossRef]
- Agrawal, A.R.; Pandelidis, I.O.; Pecht, M. Injection-molding process control—A review. Polym. Eng. Sci. 1987, 27, 1345–1357. [Google Scholar] [CrossRef]
- Deshmukh, S.S.; Goswami, A. Hot embossing of polymers—A review. Mater. Today Proc. 2020, 26, 405–414. [Google Scholar] [CrossRef]
- Li, R.; Chen, Z.; Datye, A.; Simon, G.H.; Ketkaew, J.; Kinser, E.; Liu, Z.; Zhou, C.; Dagdeviren, O.E.; Sohn, S.; et al. Atomic imprinting into metallic glasses. Commun. Phys. 2018, 1, 75. [Google Scholar] [CrossRef]
- Truckenmüller, R.; Giselbrecht, S.; Rivron, N.; Gottwald, E.; Saile, V.; Van den Berg, A.; Wessling, M.; Van Blitterswijk, C. Thermoforming of film-based biomedical microdevices. Adv. Mater. 2011, 23, 1311–1329. [Google Scholar] [CrossRef]
- Shaegh, S.A.M.; Pourmand, A.; Nabavinia, M.; Avci, H.; Tamayol, A.; Mostafalu, P.; Ghavifekr, H.B.; Aghdam, E.N.; Dokmeci, M.R.; Khademhosseini, A.; et al. Rapid prototyping of whole-thermoplastic microfluidics with built-in microvalves using laser ablation and thermal fusion bonding. Sens. Actuators B Chem. 2018, 255, 100–109. [Google Scholar] [CrossRef]
- Lashkaripour, A.; Silva, R.; Densmore, D. Desktop micromilled microfluidics. Microfluid. Nanofluid. 2018, 22, 31. [Google Scholar] [CrossRef]
- Alting, L.; Kimura, F.; Hansen, H.N.; Bissacco, G. Micro engineering. CIRP Ann. 2003, 52, 635–657. [Google Scholar] [CrossRef]
- Liang, C.; Liu, C.; Liu, Z.Y.; Meng, F.J.; Li, J.M. Laser-bulge based ultrasonic bonding method for fabricating multilayer thermoplastic microfluidic devices. J. Micromech. Microeng. 2017, 27, 115012. [Google Scholar] [CrossRef]
- Scott, S.M.; Ali, Z. Fabrication methods for microfluidic devices: An overview. Micromachines 2021, 12, 319. [Google Scholar] [CrossRef]
- Heckele, M.; Schomburg, W.K. Review on micro molding of thermoplastic polymers. J. Micromech. Microeng. 2004, 14, R1. [Google Scholar] [CrossRef]
- Chen, C.S.; Breslauer, D.N.; Luna, J.I.; Grimes, A.; Chin, W.C.; Lee, L.P.; Khine, M. Shrinky-Dink microfluidics: 3D polystyrene chips. Lab Chip 2008, 8, 622–624. [Google Scholar] [CrossRef]
- Young, E.W.; Berthier, E.; Guckenberger, D.J.; Sackmann, E.; Lamers, C.; Meyvantsson, I.; Huttenlocher, A.; Beebe, D.J. Rapid prototyping of arrayed microfluidic systems in polystyrene for cell-based assays. Anal. Chem. 2011, 83, 1408–1417. [Google Scholar] [CrossRef]
- Chen, P.C.; Park, D.S.; You, B.H.; Kim, N.; Park, T.; Soper, S.A.; Nikitopoulos, D.E.; Murphy, M.C. Titer-plate formatted continuous flow thermal reactors: Design and performance of a nanoliter reactor. Sens. Actuators B Chem. 2010, 149, 291–300. [Google Scholar] [CrossRef]
- Anderson, R.C.; Su, X.; Bogdan, G.J.; Fenton, J. A miniature integrated device for automated multistep genetic assays. Nucleic Acids Res. 2000, 28, e60. [Google Scholar] [CrossRef]
- Ogończyk, D.; Węgrzyn, J.; Jankowski, P.; Dąbrowski, B.; Garstecki, P. Bonding of microfluidic devices fabricated in polycarbonate. Lab Chip 2010, 10, 1324–1327. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, L.; Chen, G. Fabrication, modification, and application of poly (methyl methacrylate) microfluidic chips. Electrophoresis 2008, 29, 1801–1814. [Google Scholar] [CrossRef] [PubMed]
- Trinh, K.T.L.; Pham, Q.N.; Lee, N.Y. Clog-free and reliable solvent bonding of poly (methyl methacrylate) microdevice mediated by eco-friendly acetic acid at room temperature and its application for polymerase chain reaction and human cell culture. Sens. Actuators B Chem. 2019, 282, 1008–1017. [Google Scholar] [CrossRef]
- Shamsi, A.; Shamloo, A.; Mohammadaliha, N.; Hajghassem, H.; Mehrabadi, J.F.; Bazzaz, M. High throughput blood plasma separation using a passive PMMA microfluidic device. Microsyst. Technol. 2016, 22, 2447–2454. [Google Scholar] [CrossRef]
- Hong, M.; Cui, L.; Liu, S.; Li, Y. Synthesis of novel cyclic olefin copolymer (COC) with high performance via effective copolymerization of ethylene with bulky cyclic olefin. Macromolecules 2012, 45, 5397–5402. [Google Scholar] [CrossRef]
- Faure, K.; Albert, M.; Dugas, V.; Crétier, G.; Ferrigno, R.; Morin, P.; Rocca, J.L. Development of an acrylate monolith in a cyclo-olefin copolymer microfluidic device for chip electrochromatography separation. Electrophoresis 2008, 29, 4948–4955. [Google Scholar] [CrossRef]
- Jena, R.K.; Yue, C.Y. Cyclic olefin copolymer based microfluidic devices for biochip applications: Ultraviolet surface grafting using 2-methacryloyloxyethyl phosphorylcholine. Biomicrofluidics 2012, 6, 012822. [Google Scholar] [CrossRef]
- Bruijns, B.; Veciana, A.; Tiggelaar, R.; Gardeniers, H. Cyclic olefin copolymer microfluidic devices for forensic applications. Biosensors 2019, 9, 85. [Google Scholar] [CrossRef]
- Ha, C.S.; Kim, Y.; Lee, W.K.; Cho, W.J.; Kim, Y. Fracture toughness and properties of plasticized PVC and thermoplastic polyurethane blends. Polymer 1998, 39, 4765–4772. [Google Scholar] [CrossRef]
- Tsao, C.W.; DeVoe, D.L. Bonding of thermoplastic polymer microfluidics. Microfluid. Nanofluid. 2009, 6, 1–16. [Google Scholar] [CrossRef]
- Lai, S.; Cao, X.; Lee, L.J. A packaging technique for polymer microfluidic platforms. Anal. Chem. 2004, 76, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.T.; Woolley, A.T. Thermal bonding of polymeric capillary electrophoresis microdevices in water. Anal. Chem. 2003, 75, 1941–1945. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, L.; Chen, G. A spring-driven press device for hot embossing and thermal bonding of PMMA microfluidic chips. Electrophoresis 2010, 31, 2512–2519. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, L.; Chen, G. Hot embossing and thermal bonding of poly (methyl methacrylate) microfluidic chips using positive temperature coefficient ceramic heater. Anal. Bioanal. Chem. 2011, 401, 2657–2665. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Chang, H.; Song, M.; Liu, C. A method of water pretreatment to improve the thermal bonding rate of PMMA microfluidic chip. Microsyst. Technol. 2012, 18, 423–428. [Google Scholar] [CrossRef]
- Gong, Y.; Park, J.M.; Lim, J. An interference-assisted thermal bonding method for the fabrication of thermoplastic microfluidic devices. Micromachines 2016, 7, 211. [Google Scholar] [CrossRef]
- Park, T.; Song, I.H.; Park, D.S.; You, B.H.; Murphy, M.C. Thermoplastic fusion bonding using a pressure-assisted boiling point control system. Lab Chip 2012, 12, 2799–2802. [Google Scholar] [CrossRef]
- Kurihara, K.; Hokari, R.; Satoh, T.; Sugiura, S.; Miyake, K.; Kanamori, T. Low-deformation precision thermal bonding of nanostructured microfluidic chips. Jpn. J. Appl. Phys. 2020, 59, SIIJ08. [Google Scholar] [CrossRef]
- Liu, Y.; Ganser, D.; Schneider, A.; Liu, R.; Grodzinski, P.; Kroutchinina, N. Microfabricated polycarbonate CE devices for DNA analysis. Anal. Chem. 2001, 73, 4196–4201. [Google Scholar] [CrossRef]
- Mekaru, H. Thermal bonding of polyimide to form sealed microchannels. Jpn. J. Appl. Phys. 2017, 56, 06GM04. [Google Scholar] [CrossRef]
- Mekaru, H. Thermal and ultrasonic bonding between planar polyethylene terephthalate, acrylonitrile butadiene styrene, and polycarbonate substrates. Int. J. Adhes. Adhes. 2018, 84, 394–405. [Google Scholar] [CrossRef]
- Liu, J.; Qiao, H.; Liu, C.; Xu, Z.; Li, Y.; Wang, L. Plasma assisted thermal bonding for PMMA microfluidic chips with integrated metal microelectrodes. Sens. Actuators B Chem. 2009, 141, 646–651. [Google Scholar] [CrossRef]
- Immanuel, P.N.; Chiang, C.-C.; Yang, C.-R.; Subramani, M.; Lee, T.-H.; Huang, S.-J. Surface activation of poly(methyl methacrylate) for microfluidic device bonding through a H2O plasma treatment linked with a low-temperature annealing. J. Micromech. Microeng. 2021, 31, 055004. [Google Scholar] [CrossRef]
- Uba, F.I.; Hu, B.; Weerakoon-Ratnayake, K.; Oliver-Calixte, N.; Soper, S.A. High process yield rates of thermoplastic nanofluidic devices using a hybrid thermal assembly technique. Lab Chip 2015, 15, 1038–1049. [Google Scholar] [CrossRef]
- Yu, S.; Ng, S.P.; Wang, Z.; Tham, C.L.; Soh, Y.C. Thermal bonding of thermoplastic elastomer film to PMMA for microfluidic applications. Surf. Coat. Technol. 2017, 320, 437–440. [Google Scholar] [CrossRef]
- Busek, M.; Nøvik, S.; Aizenshtadt, A.; Amirola-Martinez, M.; Combriat, T.; Grünzner, S.; Krauss, S. Thermoplastic elastomer (TPE)–poly (methyl methacrylate)(PMMA) hybrid devices for active pumping PDMS-free organ-on-a-chip systems. Biosensors 2021, 11, 162. [Google Scholar] [CrossRef]
- Vargas, M.J.; Nieuwoudt, M.; Yong, R.M.; Vanholsbeeck, F.; Williams, D.E.; Simpson, M.C. Excellent quality microchannels for rapid microdevice prototyping: Direct CO2 laser writing with efficient chemical postprocessing. Microfluid. Nanofluid. 2019, 23, 124. [Google Scholar] [CrossRef]
- Yin, Z.; Qi, L.; Zou, H.; Sun, L.; Xu, S. A novel bonding method for fabrication of PET planar nanofluidic chip with low dimension loss and high bonding strength. J. Micromech. Microeng. 2015, 25, 85015. [Google Scholar] [CrossRef]
- Trinh, K.T.L.; Thai, D.A.; Chae, W.R.; Lee, N.Y. Rapid fabrication of poly (methyl methacrylate) devices for lab-on-a-chip applications using acetic acid and UV treatment. ACS Omega 2020, 5, 17396–17404. [Google Scholar] [CrossRef]
- Trinh, K.T.L.; Chae, W.R.; Lee, N.Y. Pressure-free assembling of poly (methyl methacrylate) microdevices via microwave-assisted solvent bonding and its biomedical applications. Biosensors 2021, 11, 526. [Google Scholar] [CrossRef]
- Tran, H.H.; Wu, W.; Lee, N.Y. Ethanol and UV-assisted instantaneous bonding of PMMA assemblies and tuning in bonding reversibility. Sens. Actuators B Chem. 2013, 181, 955–962. [Google Scholar] [CrossRef]
- Chen, P.C.; Duong, L.H. Novel solvent bonding method for thermoplastic microfluidic chips. Sens. Actuators B Chem. 2016, 237, 556–562. [Google Scholar] [CrossRef]
- Sun, D.; Tweedie, M.; Gajula, D.R.; Ward, B.; Maguire, P.D. High-strength thermoplastic bonding for multi-channel, multi-layer lab-on-chip devices for ocean and environmental applications. Microfluid. Nanofluid. 2015, 19, 913–922. [Google Scholar] [CrossRef]
- Zoupanou, S.; Chiriaco, M.S.; Tarantini, I.; Ferrara, F. Innovative 3D microfluidic tools for on-chip fluids and particles manipulation: From design to experimental validation. Micromachines 2021, 12, 104. [Google Scholar] [CrossRef] [PubMed]
- Wan, A.M.; Moore, T.A.; Young, E.W. Solvent bonding for fabrication of PMMA and COP microfluidic devices. J. Vis. Exp. 2017, 119, e55175. [Google Scholar] [CrossRef]
- Lukashenko, T.A.; Zubik, A.N.; Bulyanitsa, A.L.; Tsymbalov, A.I.; Evstrapov, A.A. Solvents for sealing microfluidic devices made of polymethyl methactylate by chemical solvent bonding. Polym. Sci. Ser. D 2021, 14, 350–355. [Google Scholar] [CrossRef]
- Yin, Z.; Wang, W. A novel bonding method for fabrication of PMMA nanofluidic chip with low deformation of the nano-trenches. Microfluid. Nanofluid. 2018, 22, 99. [Google Scholar] [CrossRef]
- Guan, T.; Yuket, S.; Cong, H.; Carton, D.W.; Zhang, N. Permanent hydrophobic surface treatment combined with solvent vapor-assisted thermal bonding for mass production of cyclic olefin copolymer microfluidic chips. ACS Omega 2022, 7, 20104–20117. [Google Scholar] [CrossRef]
- Su, S.; Jing, G.; Zhang, M.; Liu, B.; Zhu, X.; Wang, B.; Fu, M.; Zhu, L.; Cheng, J.; Guo, Y. One-step bonding and hydrophobic surface modification method for rapid fabrication of polycarbonate-based droplet microfluidic chips. Sens. Actuators B Chem. 2019, 282, 60–68. [Google Scholar] [CrossRef]
- Wan, A.M.D.; Sadri, A.; Young, E.W.K. Liquid phase solvent bonding of plastic microfluidic devices assisted by retention grooves. Lab Chip 2015, 15, 3785–3792. [Google Scholar] [CrossRef]
- Duong, L.H.; Chen, P.C. Simple and low-cost production of hybrid 3D-printed microfluidic devices. Biomicrofluidics 2019, 13, 24108. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Koerner, T.; Horton, J.H.; Oleschuk, R.D. Fabrication and characterization of poly (methylmethacrylate) microfluidic devices bonded using surface modifications and solvents. Lab Chip 2006, 6, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.C.; Immanuel, P.N.; Chiu, Y.H.; Huang, S.J. Heterogeneous bonding of PMMA and double-sided polished silicon wafers through H2O plasma treatment for microfluidic devices. Coatings 2021, 11, 580. [Google Scholar] [CrossRef]
- Chen, P.C.; Liu, Y.M.; Chou, H.C. An adhesive bonding method with microfabricating micro pillars to prevent clogging in a microchannel. J. Micromech. Microeng. 2016, 26, 045003. [Google Scholar] [CrossRef]
- Kratz, S.R.A.; Eilenberger, C.; Schuller, P.; Bachmann, B.; Spitz, S.; Ertl, P.; Rothbauer, M. Characterization of four functional biocompatible pressure-sensitive adhesives for rapid prototyping of cell-based lab-on-a-chip and organ-on-a-chip systems. Sci. Rep. 2019, 9, 9287. [Google Scholar] [CrossRef]
- Trinh, K.T.L.; Le, N.X.T.; Lee, N.Y. Chitosan–polydopamine hydrogel complex: A novel green adhesion agent for reversibly bonding thermoplastic microdevice and its application for cell-friendly microfluidic 3D cell culture. Lab Chip 2020, 20, 3524–3534. [Google Scholar] [CrossRef]
- Le, N.X.T.; Trinh, K.T.L.; Lee, N.Y. Poly (acrylic acid) as an adhesion promoter for UV-assisted thermoplastic bonding: Application for the in vitro construction of human blood vessels. Mater. Sci. Eng. C 2021, 122, 111874. [Google Scholar] [CrossRef]
- Matellan, C.; Del Rio Hernandez, A.E. Cost-effective rapid prototyping and assembly of poly(methyl methacrylate) microfluidic devices. Sci. Rep. 2018, 8, 6971. [Google Scholar] [CrossRef]
- Ren, Y.; Ray, S.; Liu, Y. Reconfigurable acrylic-tape hybrid microfluidics. Sci. Rep. 2019, 9, 4824. [Google Scholar] [CrossRef]
- Tsao, C.W.; Syu, W.C. Bonding of thermoplastic microfluidics by using dry adhesive tape. RSC Adv. 2020, 10, 30289–30296. [Google Scholar] [CrossRef]
- El Fissi, L.; Vandormael, D.; Francis, L.A. Direct assembly of cyclic olefin copolymer microfluidic devices helped by dry photoresist. Sens. Actuators A Phys. 2015, 223, 76–83. [Google Scholar] [CrossRef]
- Li, J.; Liang, C.; Zhang, H.; Liu, C. Reliable and high quality adhesive bonding for microfluidic devices. Micro Nano Lett. 2017, 12, 90–94. [Google Scholar] [CrossRef]
- Tan, H.Y.; Loke, W.K.; Nguyen, N.T. A reliable method for bonding polydimethylsiloxane (PDMS) to polymethylmethacrylate (PMMA) and its application in micropumps. Sens. Actuators B Chem. 2010, 151, 133–139. [Google Scholar] [CrossRef]
- Hassanpour-Tamrin, S.; Sanati-Nezhad, A.; Sen, A. A simple and low-cost approach for irreversible bonding of polymethylmethacrylate and polydimethylsiloxane at room temperature for high-pressure hybrid microfluidics. Sci. Rep. 2021, 11, 4821. [Google Scholar] [CrossRef] [PubMed]
- Song, I.H.; Park, T. PMMA solution assisted room temperature bonding for PMMA–PC hybrid devices. Micromachines 2017, 8, 284. [Google Scholar] [CrossRef]
- Yao, Y.; Li, L.; Jiang, J.; Zhang, Y.; Chen, G.; Fan, Y. Reversible bonding for microfluidic devices with UV release tape. Microfluid. Nanofluid. 2022, 26, 23. [Google Scholar] [CrossRef]
- Vourdas, N.; Tserepi, A.; Boudouvis, A.G.; Gogolides, E. Plasma processing for polymeric microfluidics fabrication and surface modification: Effect of super-hydrophobic walls on electroosmotic flow. Microelectron. Eng. 2008, 85, 1124–1127. [Google Scholar] [CrossRef]
- Shinohara, H.; Mizuno, J.; Shoji, S. Studies on low-temperature direct bonding of VUV, VUV/O3 and O2 plasma pretreated cyclo-olefin polymer. Sens. Actuators A Phys. 2011, 165, 124–131. [Google Scholar] [CrossRef]
- Wen, X.; Takahashi, S.; Hatakeyama, K.; Kamei, K. Evaluation of the effects of solvents used in the fabrication of microfluidic devices on cell cultures. Micromachines 2021, 12, 550. [Google Scholar] [CrossRef]
- Tennico, Y.H.; Koesdjojo, M.T.; Kondo, S.; Mandrell, D.T.; Remcho, V.T. Surface modification-assisted bonding of polymer-based microfluidic devices. Sens. Actuators B Chem. 2010, 143, 799–804. [Google Scholar] [CrossRef]
- Nguyen, T.P.O.; Tran, B.M.; Lee, N.Y. Thermally robust and biomolecule-friendly room-temperature bonding for the fabrication of elastomer–plastic hybrid microdevices. Lab Chip 2016, 16, 3251–3259. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, R.; Trinh, K.T.L.; Lee, N.Y. Heat and pressure-resistant room temperature irreversible sealing of hybrid PDMS thermoplastic microfluidic devices via carbon-nitrogen covalent bonding and its application in a continuous-flow polymerase chain reaction. RSC Adv. 2020, 10, 16502–16509. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, R.; Lee, N.Y. Chemically robust succinimide-group-assisted irreversible bonding of poly (dimethylsiloxane)-thermoplastic microfluidic devices at room temperature. Analyst 2020, 145, 6887–6894. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Jung, S.H.; Lee, M.S.; Park, T.E.; Ahn, S.K.; Kang, J.H. Robust chemical bonding of PMMA microfluidic devices to porous PETE membranes for reliable cytotoxicity testing of drugs. Lab Chip 2019, 19, 3706–3713. [Google Scholar] [CrossRef] [PubMed]
- Kistrup, K.; Poulsen, C.E.; Hansen, M.F.; Wolff, A. Ultrasonic welding for fast bonding of self-aligned structures in lab-on-a-chip systems. Lab Chip 2015, 15, 1998–2001. [Google Scholar] [CrossRef]
- Rodriguez-Vidal, E.; Quintana, I.; Etxarri, J.; Azkorbebeitia, U.; Otaduy, D.; Gonzalez, F.; Moreno, F. Optical design and development of a fiber coupled high-power diode laser system for laser transmission welding of plastics. Opt. Eng. 2012, 51, 124301. [Google Scholar] [CrossRef]
- Yu, H.; Tor, S.B.; Loh, N.H. Rapid bonding enhancement by auxiliary ultrasonic actuation for the fabrication of cyclic olefin copolymer (COC) microfluidic devices. J. Micromech. Microeng. 2014, 24, 115020. [Google Scholar] [CrossRef]
- Jiang, X.; Chandrasekar, S.; Wang, C.H. A laser microwelding method for assembly of polymer based microfluidic devices. Opt. Laser Eng. 2015, 66, 98–104. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lim, J.H.; Lee, J.M.; Choi, J.W.; Choi, H.W.; Seo, W.H.; Lee, K.G.; Lee, S.J.; Chung, B.G. CuS/rGO-PEG nanocomposites for photothermal bonding of PMMA-based plastic lab-on-a-chip. Nanomaterials 2021, 11, 176. [Google Scholar] [CrossRef]
- Li, J.M.; Meng, F.J.; Liang, C.; Liu, C. Energy director structure and self-balancing jig for the ultrasonic bonding of microfluidic chips. Micro Nano Lett. 2017, 12, 453–457. [Google Scholar] [CrossRef]
- Lei, K.F.; Ahsan, S.; Budraa, N.; Li, W.J.; Mai, J.D. Microwave bonding of polymer-based substrates for potential encapsulated micro/nanofluidic device fabrication. Sens. Actuators A Phys. 2004, 114, 340–346. [Google Scholar] [CrossRef]
- Toossi, A.; Moghadas, H.; Daneshmand, M.; Sameoto, D. Bonding PMMA microfluidics using commercial microwave ovens. J. Micromech. Microeng. 2015, 25, 85008. [Google Scholar] [CrossRef]
- Hashimoto, M.; Chen, P.C.; Mitchell, M.W.; Nikitopoulos, D.E.; Soper, S.A.; Murphy, M.C. Rapid PCR in a continuous flow device. Lab Chip 2004, 4, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Trinh, K.T.L.; Chon, N.M.; Lee, N.Y. Fabrication of an integrated polystyrene microdevice for pre-concentration and amplification of Escherichia coli O157: H7 from raw milk. Anal. Methods 2018, 10, 5071–5077. [Google Scholar] [CrossRef]
- Trinh, K.T.L.; Zhang, Y.; Lee, N.Y. One-step DNA purification and amplification on an integrated plastic microdevice for on-site identification of foodborne pathogens. Anal. Chim. Acta 2018, 1040, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Trinh, K.T.L.; Yoo, I.-S.; Lee, N.Y. One-step glass-like coating of polycarbonate for seamless DNA purification and amplification on an integrated monolithic microdevice. Sens. Actuators B Chem. 2014, 202, 1281–1289. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Trinh, K.T.L.; Yoon, W.J.; Lee, N.Y.; Ju, H. Integration of a microfluidic polymerase chain reaction device and surface plasmon resonance fiber sensor into an inline all-in-one platform for pathogenic bacteria detection. Sens. Actuators B Chem. 2017, 242, 1–8. [Google Scholar] [CrossRef]
- Trinh, T.N.D.; Lee, N.Y. Nucleic acid amplification-based microfluidic approaches for antimicrobial susceptibility testing. Analyst 2021, 146, 3101–3113. [Google Scholar] [CrossRef]
- Trinh, T.N.D.; Lee, N.Y. Advances in nucleic acid amplification-based microfluidic devices for clinical microbial detection. Chemosensors 2022, 10, 123. [Google Scholar] [CrossRef]
- Trinh, T.N.D.; Lee, N.Y. A rapid and eco-friendly isothermal amplification microdevice for multiplex detection of foodborne pathogens. Lab Chip 2018, 18, 2369–2377. [Google Scholar] [CrossRef]
- Trinh, T.N.D.; La, H.C.; Lee, N.Y. Fully integrated and foldable microdevice encapsulated with agarose for long-term storage potential for point-of-care testing of multiplex foodborne pathogens. ACS Sens. 2019, 4, 2754–2762. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.D.; Horowitz, L.F.; Castro, K.; Kenerson, H.; Bhattacharjee, N.; Gandhe, G.; Raman, A.; Monnat, R.J., Jr.; Yeung, R.; Rostomily, R.C.; et al. A microfluidic platform for functional testing of cancer drugs on intact tumor slices. Lab Chip 2020, 20, 1658–1675. [Google Scholar] [CrossRef] [PubMed]
- Won, E.J.; Thai, D.A.; Duong, D.D.; Lee, N.Y.; Song, Y.J. Microfluidic electrical cell lysis for high-throughput and continuous production of cell-free varicella-zoster virus. J. Biotechnol. 2021, 335, 19–26. [Google Scholar] [CrossRef]
- Wouters, S.; De Vos, J.; Dores-Sousa, J.L.; Wouters, B.; Desmet, G.; Eeltink, S. Prototyping of thermoplastic microfluidic chips and their application in high-performance liquid chromatography separations of small molecules. J. Chromatogr. A 2017, 1523, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yang, W.; Pan, T.; Woolley, A.T. Affinity monolith-integrated poly (methyl methacrylate) microchips for on-line protein extraction and capillary electrophoresis. Anal. Chem. 2008, 80, 5126–5130. [Google Scholar] [CrossRef][Green Version]
- Yang, W.; Sun, X.; Wang, H.Y.; Woolley, A.T. Integrated microfluidic device for serum biomarker quantitation using either standard addition or a calibration curve. Anal. Chem. 2009, 81, 8230–8235. [Google Scholar] [CrossRef][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).