Gas Selectivity Enhancement Using Serpentine Microchannel Shaped with Optimum Dimensions in Microfluidic-Based Gas Sensor
Abstract
:1. Introduction
2. Theory
2.1. Diffusion Model
2.2. Adsorption and Desorption Model
2.3. Gas Response Model
2.4. Selectivity Model
3. Experimental Method
3.1. Microchannel Fabrication
3.2. ZnO Growth
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Montazeri, M.M.; O’Brien, A.; Hoorfar, M. Understanding microfluidic-based gas detectors: A numerical model to investigate fundamental sensor operation, influencing phenomena and optimum geometries. Sens. Actuators B Chem. 2019, 300, 126904. [Google Scholar] [CrossRef]
- Rebordão, G.; Palma, S.I.; Roque, A.C. Microfluidics in gas sensing and artificial olfaction. Sensors 2020, 20, 5742. [Google Scholar] [CrossRef] [PubMed]
- Salim, A.; Lim, S. Review of recent metamaterial microfluidic sensors. Sensors 2018, 18, 232. [Google Scholar] [CrossRef] [PubMed]
- Warden, A.C.; Trowell, S.C.; Gel, M. A miniature gas sampling interface with open microfluidic channels: Characterization of gas-to-liquid extraction efficiency of volatile organic compounds. Micromachines 2019, 10, 486. [Google Scholar] [CrossRef]
- Paknahad, M.; Bachhal, J.S.; Ahmadi, A.; Hoorfar, M. Characterization of channel coating and dimensions of microfluidic-based gas detectors. Sens. Actuators B Chem. 2017, 241, 55–64. [Google Scholar] [CrossRef]
- Kaushik, A.; Kumar, R.; Arya, S.K.; Nair, M.; Malhotra, B.D.; Hansali, S.B. Organic–inorganic hybrid nanocomposite-based gas sensors for environmental monitoring. Chem. Rev. 2015, 115, 4571–4606. [Google Scholar] [CrossRef]
- Martini, V.; Bernardini, S.; Bendahan, M.; Aguir, K.; Perrier, P.; Graur, I. Microfluidic gas sensor with integrated pumping system. Sens. Actuators B Chem. 2012, 170, 45–50. [Google Scholar] [CrossRef]
- Martini-Laithier, V.; Graur, I.; Bernardini, S.; Aguir, K.; Perrier, P.; Bendahan, M. Ammonia detection by a novel Pyrex microsystem based on thermal creep phenomenon. Sens. Actuators B Chem. 2014, 192, 714–719. [Google Scholar] [CrossRef]
- Zhu, L.; Meier, D.; Boger, Z.; Montgomery, C.; Semancik, S.; DeVoe, D.L. Integrated microfluidic gas sensor for detection of volatile organic compounds in water. Sens. Actuators B Chem. 2007, 121, 679–688. [Google Scholar] [CrossRef]
- Ghazi, M.; Janfaza, S.; Tahmooressi, H.; Ravishankara, A.; Earl, E.; Tasnim, N.; Hoorfar, M. Enhanced selectivity of microfluidic gas sensors by modifying microchannel geometry and surface chemistry with graphene quantum dots. Sens. Actuators B Chem. 2021, 342, 130050. [Google Scholar] [CrossRef]
- Kuncová-Kallio, J.; Kallio, P.J. PDMS and its Suitability for Analytical Microfluidic Devices. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; pp. 2486–2489. [Google Scholar]
- Paknahad, M.; Ghafarinia, V.; Hossein-Babaei, F. A microfluidic gas analyzer for selective detection of biomarker gases. In Proceedings of the 2012 IEEE Sensors Applications Symposium Proceedings, Brescia, Itlaly, 7–9 February 2012; pp. 1–5. [Google Scholar]
- Tsao, C.W. Polymer microfluidics: Simple, low-cost fabrication process bridging academic lab research to commercialized production. Micromachines 2016, 7, 225. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Nebuloni, F.; Gao, H.; Zhou, J.; Papautsky, I. Rapid prototyping of soft lithography masters for microfluidic devices using dry film photoresist in a non-cleanroom setting. Micromachines 2019, 10, 192. [Google Scholar] [CrossRef] [PubMed]
- Makamba, H.; Kim, J.H.; Lim, K.; Park, N.; Hahn, J.H. Surface modification of poly (dimethylsiloxane) microchannels. Electrophoresis 2003, 24, 3607–3619. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ellis, A.V.; Voelcker, N.H. Recent developments in PDMS surface modification for microfluidic devices. Electrophoresis 2010, 31, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Paknahad, M.; Mcintosh, C.; Hoorfar, M. Selective detection of volatile organic compounds in microfluidic gas detectors based on ‘like dissolves like. Sci. Rep. 2019, 9, 161. [Google Scholar] [CrossRef] [PubMed]
- Ning, X.; Yang, J.; Zhao, C.L.; Chan, C.C. PDMS-coated fiber volatile organic compounds sensors. Appl. Opt. 2016, 55, 3543–3548. [Google Scholar] [CrossRef]
- Sappati, K.K.; Bhadra, S. Printed acoustic sensor for low concentration volatile organic compound monitoring. IEEE Sens. J. 2021, 21, 9808–9818. [Google Scholar] [CrossRef]
- Shooshtari, M.; Salehi, A. An electronic nose based on carbon nanotube-titanium dioxide hybrid nanostructures for detection and discrimination of volatile organic compounds. Sens. Actuators B Chem. 2022, 357, 131418. [Google Scholar] [CrossRef]
- Kamal, M.S.; Razzak, S.A.; Hossain, M.M. Catalytic oxidation of volatile organic compounds (VOCs)–A review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- Shooshtari, M.; Salehi, A.; Vollebregt, S. Effect of humidity on gas sensing performance of carbon nanotube gas sensors operated at room temperature. IEEE Sens. J. 2020, 21, 5763–5770. [Google Scholar] [CrossRef]
- Zhou, C.; Zhan, Y.; Chen, S.; Xia, M.; Ronda, C.; Sun, M.; Chen, H.; Shen, X. Combined effects of temperature and humidity on indoor VOCs pollution: Intercity comparison. Build. Environ. 2017, 121, 26–34. [Google Scholar] [CrossRef]
- Shooshtari, M.; Pahlavan, S.; Rahbarpour, S.; Ghafoorifard, H. Investigating Organic Vapor Sensing Properties of Composite Carbon Nanotube-Zinc Oxide Nanowire. Chemosensors 2022, 10, 205. [Google Scholar] [CrossRef]
- Rananavare, M.; Shah, R.; Makwana, M.; Rathod, S.S. Design and Simulation of Microfluidic Based Carbon Monoxide Gas Sensor Using COMSOL Multiphysics®. In Proceedings of the 2021 International Conference on Advances in Computing, Communication, and Control (ICAC3), Mumbai, India, 3–4 December 2021; pp. 1–4. [Google Scholar]
- Ghazi, M.; Janfaza, S.; Tahmooressi, H.; Tasnim, N.; Hoorfar, M. Selective detection of VOCs using microfluidic gas sensor with embedded cylindrical microfeatures coated with graphene oxide. J. Hazard. Mater. 2022, 424, 127566. [Google Scholar] [CrossRef] [PubMed]
- Treybal, R.E. Mass Transfer Operations; McGraw-Hill Book: New York, NY, USA, 1980; Volume 466. [Google Scholar]
- Demirel, Y.; Gerbaud, V. Transport and Rate Processes in Physical, Chemical and Biological Systems; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Verros, G.D.; Giovannopoulos, F. On the Maxwell-Stefan Equations for Multi-component Diffusion. In Proceedings of the AIP Conference Proceedings, Hersonissos, Greece, 25–30 September 2009; Volume 1148, pp. 262–266. [Google Scholar]
- Lugg, G.A. Diffusion coefficients of some organic and other vapors in air. Anal. Chem. 1968, 40, 1072–1077. [Google Scholar] [CrossRef]
- Shintake, T. Possibility of Disinfection of SARS-CoV-2 (COVID-19) in Human Respiratory Tract by Controlled Ethanol Vapor Inhalation. arXiv 2003, arXiv:2003.12444. [Google Scholar]
- Shooshtari, M.; Salehi, A.; Vollebregt, S. Effect of temperature and humidity on the sensing performance of TiO2 nanowire-based ethanol vapor sensors. Nanotechnology 2021, 32, 325501. [Google Scholar] [CrossRef]
- Fogler, H.S.; Fogler, S.H. Elements of Chemical Reaction Engineering; Pearson Education: London, UK, 1999. [Google Scholar]
- Adamson, A.W.; Gast, A.P. Physical Chemistry of Surfaces; Interscience publishers: New York, NY, USA, 1967; Volume 150. [Google Scholar]
- Brancher, R.D.; Stefanov, S.; Graur, I.; Frezzotti, A. A kinetic model for gas adsorption-desorption at solid surfaces under non-equilibrium conditions. Vacuum 2020, 174, 109166. [Google Scholar] [CrossRef]
- Shooshtari, M.; Sacco, L.N.; Van Ginkel, J.; Vollebregt, S.; Salehi, A. Enhancement of room temperature Ethanol sensing by optimizing the density of Vertically Aligned Carbon Nanofibers decorated with Gold nanoparticles. Materials 2022, 15, 1383. [Google Scholar] [CrossRef]
- Maji, B.; Achary, L.S.K.; Barik, B.; Sahoo, S.J.; Mohanty, A.; Dash, P. MnCo2O4 decorated (2D/2D) rGO/g-C3N4-based Non-Enzymatic sensor for highly selective and sensitive detection of Chlorpyrifos in water and food samples. J. Electroanal. Chem. 2022, 909, 116115. [Google Scholar] [CrossRef]
- Islam, M.S.; Bhardwaj, A.; Mathur, L.; Kim, I.-H.; Park, J.-Y.; Song, S.-J. Effects of electrolyte variation on ammonia sensing temperature for BiVO4 sensing electrode in mixed potential gas sensor. Sens. Actuators B Chem. 2022, 371, 132504. [Google Scholar] [CrossRef]
- Meisel, N.A.; Elliott, A.M.; Williams, C.B. A procedure for creating actuated joints via embedding shape memory alloys in PolyJet 3D printing. J. Intell. Mater. Syst. Struct. 2015, 26, 1498–1512. [Google Scholar] [CrossRef]
- Chen, C.; Wharton, K. Characterization and failure mode analyses of air plasma oxidized PDMS–PDMS bonding by peeltesting. RSC Adv. 2017, 7, 1286–1289. [Google Scholar] [CrossRef]
- Liu, K.; Sakurai, M.; Aono, M. ZnO-based ultraviolet photodetectors. Sensors 2010, 10, 8604–8634. [Google Scholar] [CrossRef] [PubMed]
- Achary, L.S.K.; Kumar, A.; Barik, B.; Nayak, P.S.; Tripathy, N.; Kar, J.P.; Dash, P. Reduced graphene oxide-CuFe2O4 nanocomposite: A highly sensitive room temperature NH3 gas sensor. Sens. Actuators B Chem. 2018, 272, 100–109. [Google Scholar] [CrossRef]
- Raoufi, D. Synthesis and microstructural properties of ZnO nanoparticles prepared by precipitation method. Renew. Energy 2013, 50, 932–937. [Google Scholar] [CrossRef]
- Maxwell, J.C., IV. On the dynamical theory of gases. Philos. Trans. Royal Soc. Lond. 1867, 157, 49–88. [Google Scholar] [CrossRef]
- He, W.; Lv, W.; Dickerson, J.H. Gas Diffusion Mechanisms and Models. In Gas Transport in Solid Oxide Fuel Cells; Springer: Berlin/Heidelberg, Germany, 2014; pp. 9–17. [Google Scholar]
- Chemin, L.; Braine, J.; Combes, F.; Kam, Z.S.; Carignan, C. Anisotropy of random motions of gas in Messier 33. Astron. Astrophys. 2020, 639, A145. [Google Scholar] [CrossRef]
- Johnson, P.; Papageorgiou, G. Rethinking the introduction of particle theory: A substance-based framework. J. Res. Sci. Teach. 2010, 47, 130–150. [Google Scholar] [CrossRef]
- Niaz, M. A rational reconstruction of the kinetic molecular theory of gases based on history and philosophy of science and its implications for chemistry textbooks. Instr. Sci. 2000, 28, 23–50. [Google Scholar] [CrossRef]
- Bai, Z.; Xie, C.; Hu, M.; Zhang, S.; Zeng, D. Effect of humidity on the gas sensing property of the tetrapod-shaped ZnO nanopowder sensor. Mater. Sci. Eng. B 2008, 149, 12–17. [Google Scholar] [CrossRef]
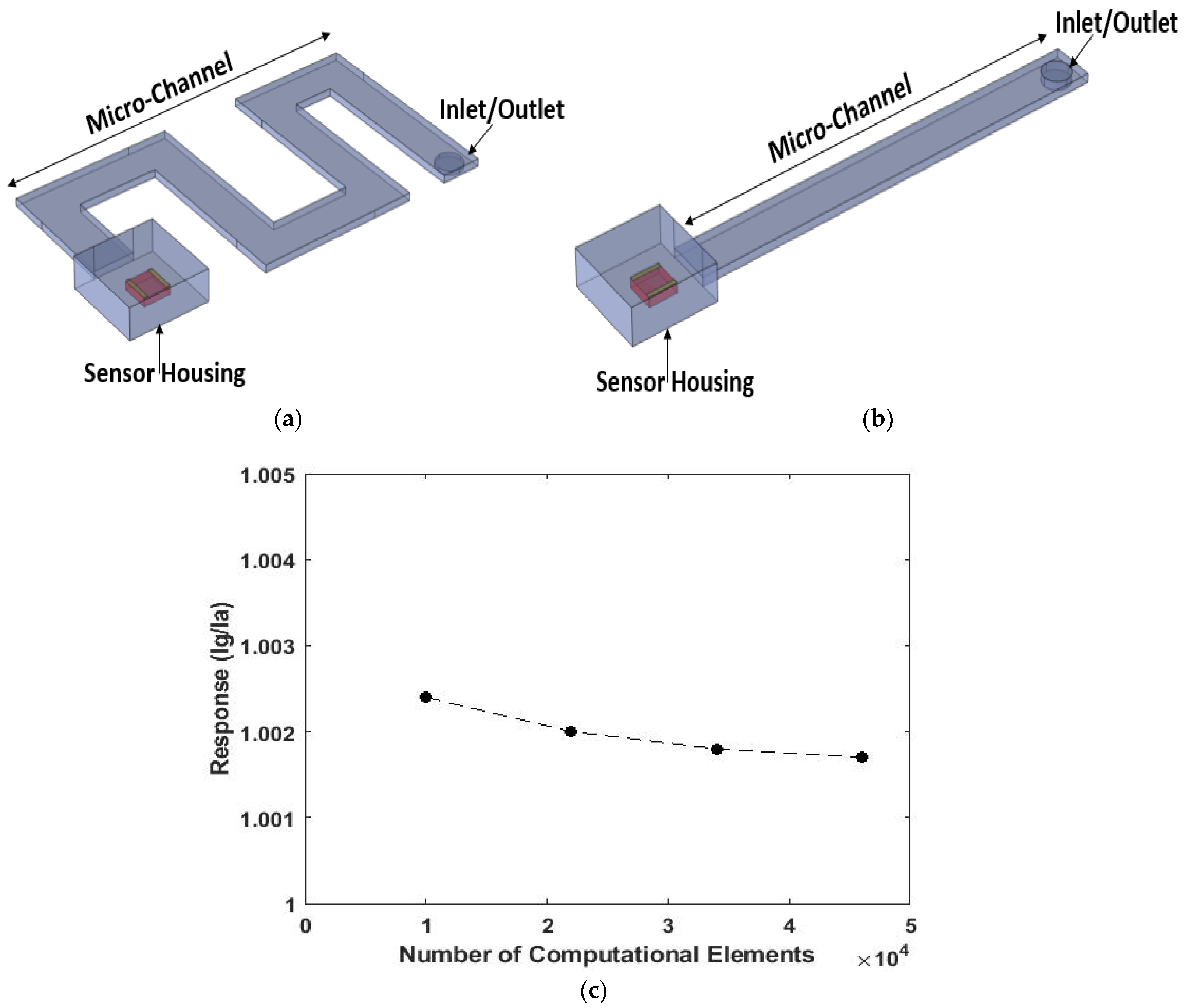
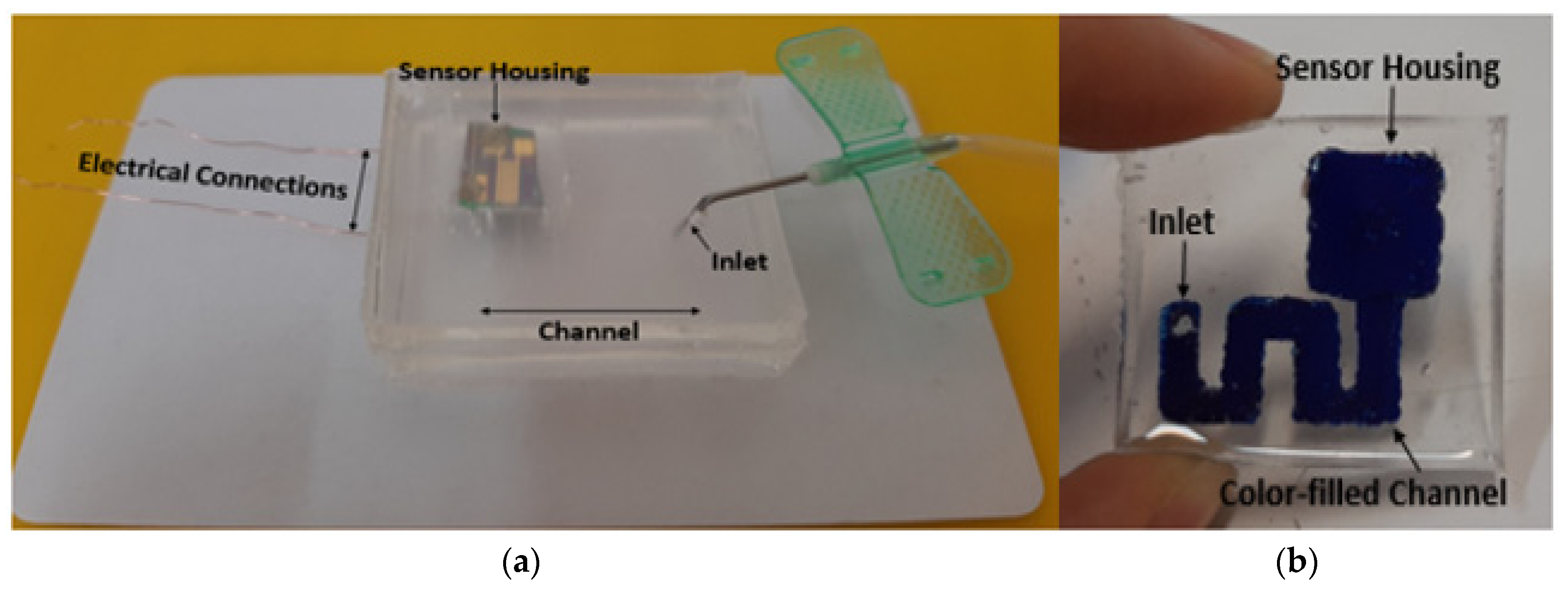
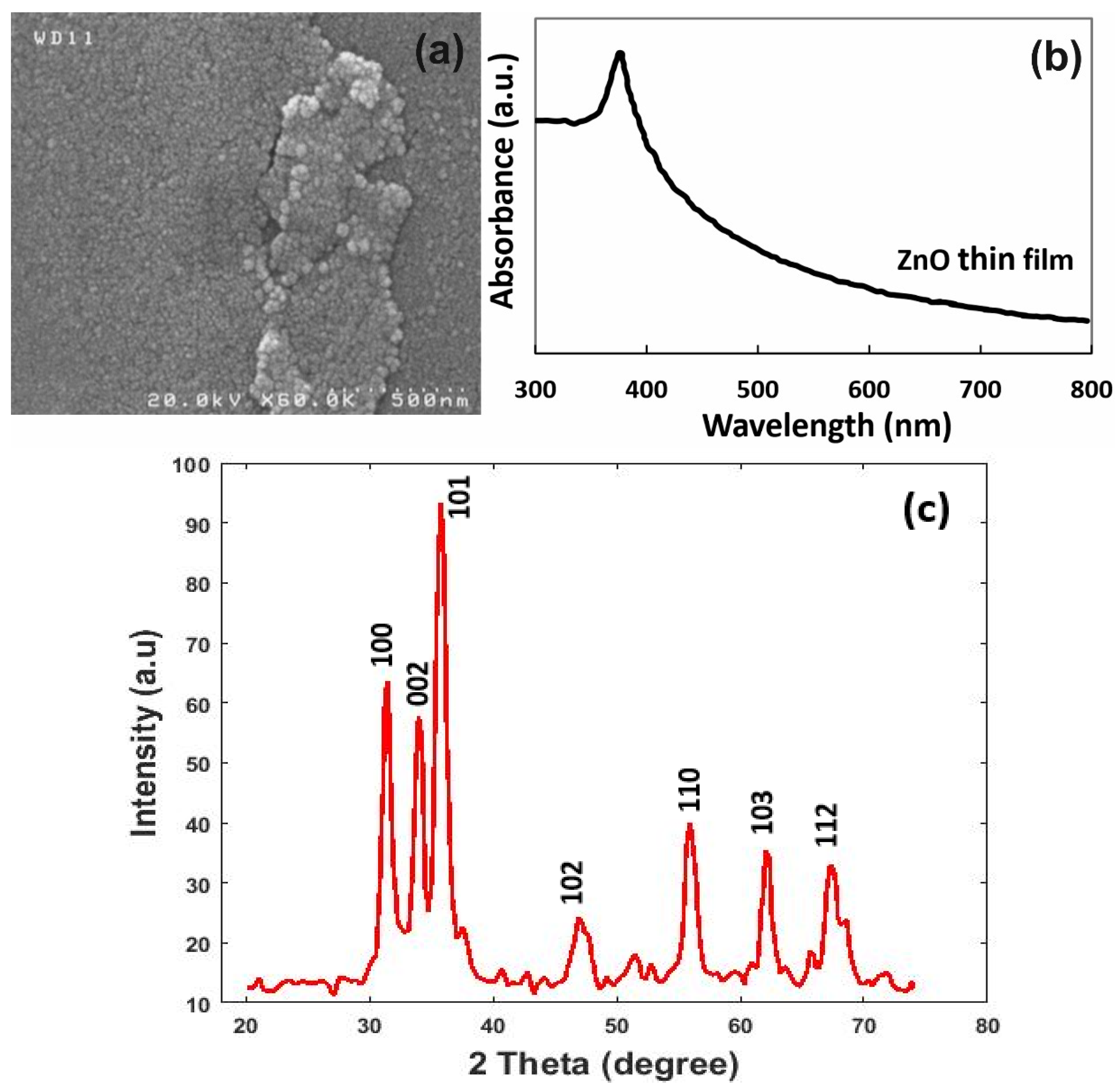
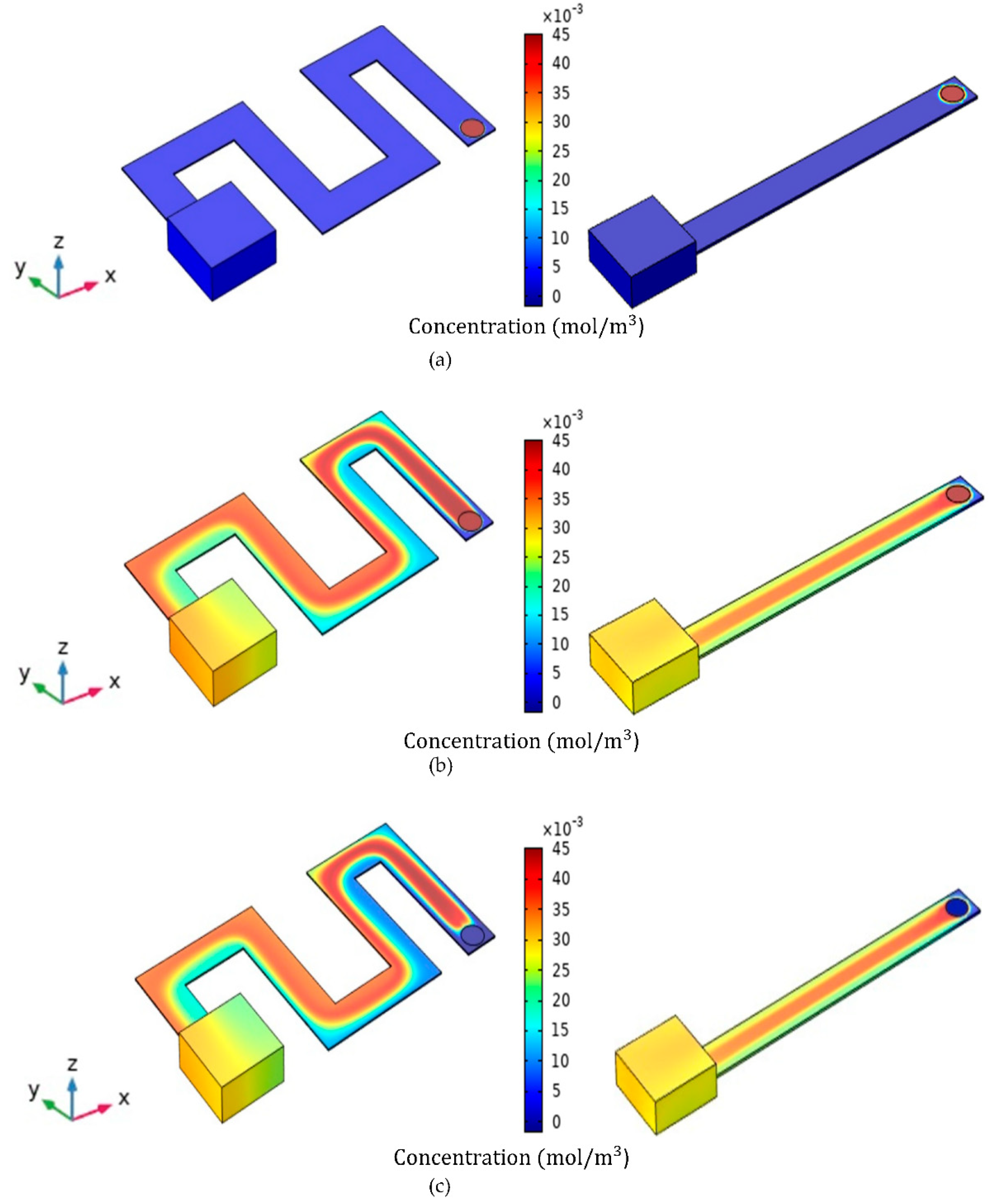
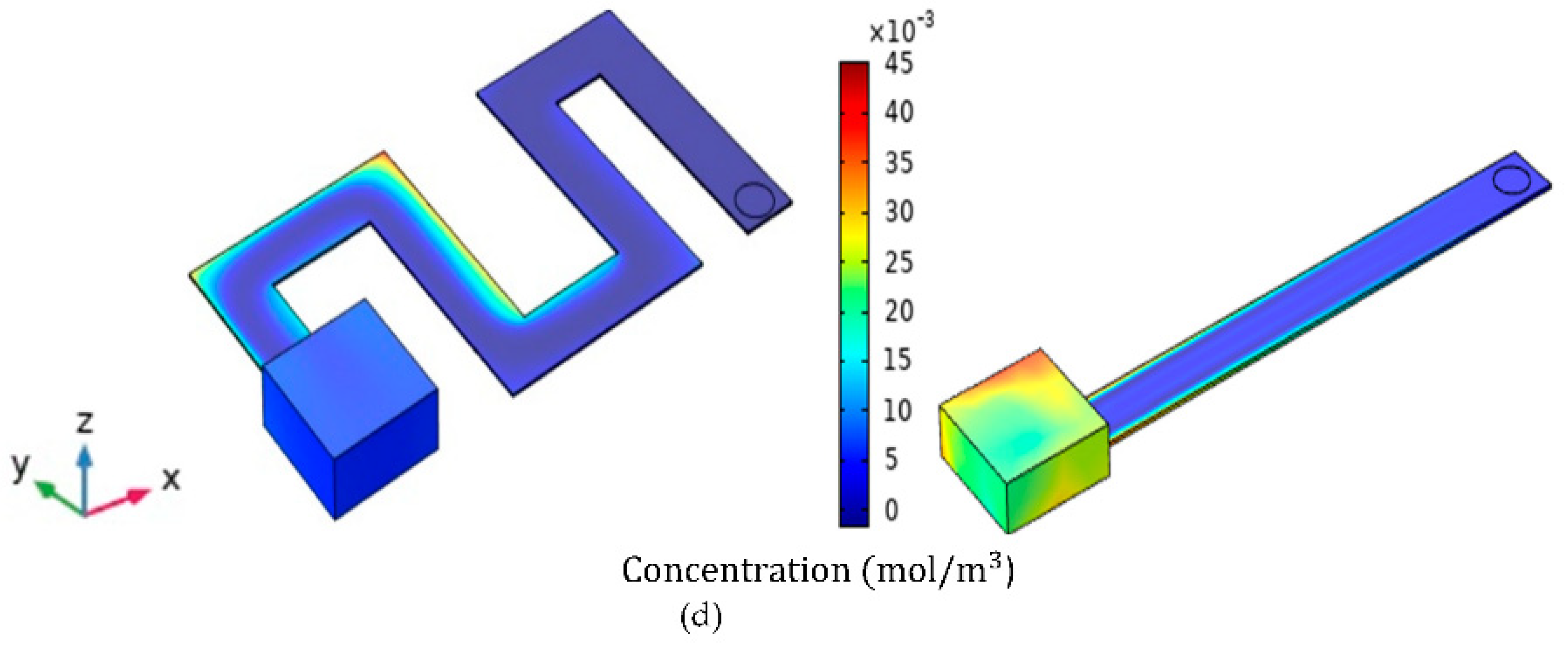

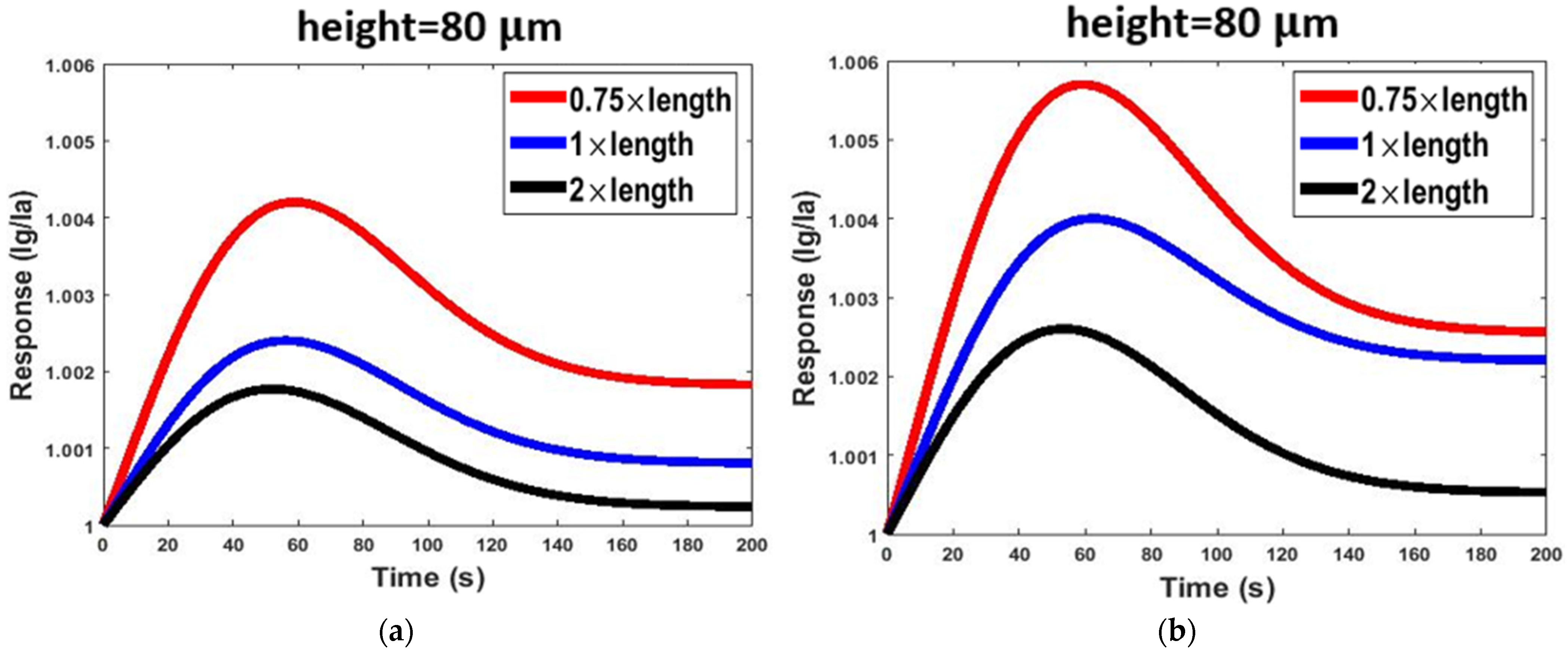

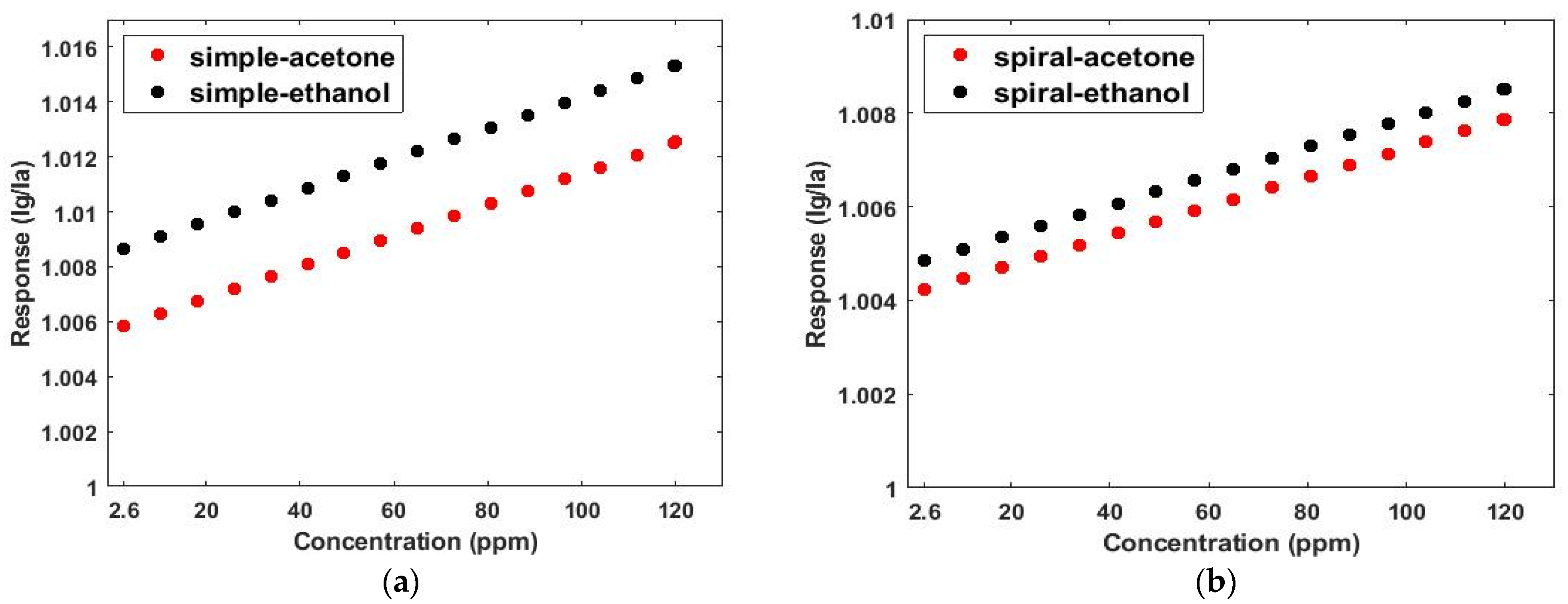

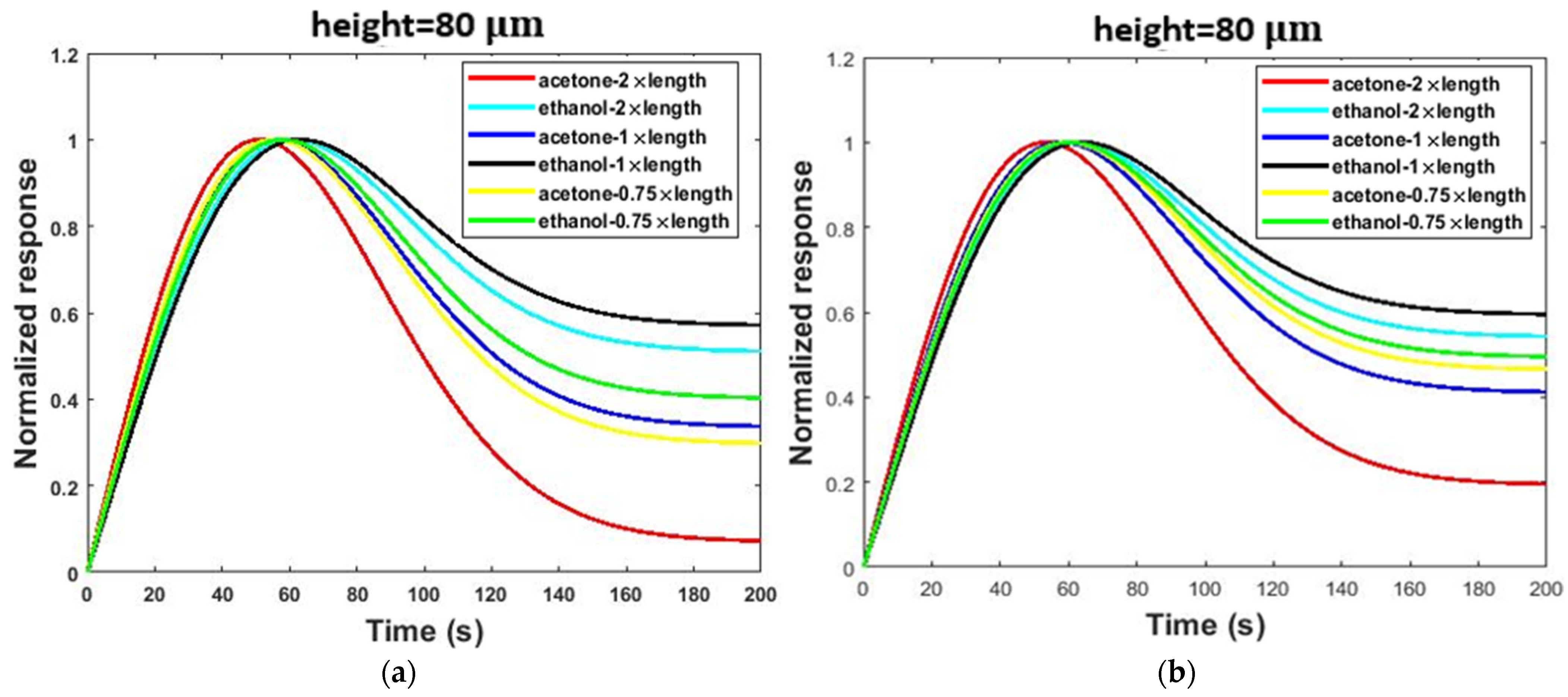


| Simulation Round | Channel Width | Channel Height | Channel Length |
|---|---|---|---|
| 1 | W | 1 × H | 0.75 × L |
| 2 1 | W | 1 × H | 1 × L |
| 3 | W | 1 × H | 2 × L |
| 4 | W | 2 × H | 0.75 × L |
| 5 | W | 2 × H | 1 × L |
| 6 | W | 2 × H | 2 × L |
| 7 | W | 3 × H | 0.75 × L |
| 8 | W | 3 × H | 1 × L |
| 9 | W | 3 × H | 2 × L |
| Dimensions | Simple | Serpentine | ||
|---|---|---|---|---|
| W | H | L | SAB | SAB |
| 3 mm | 80 µm | 60 mm | 337 | 448 |
| 3 mm | 80 µm | 30 mm | 141 | 221 |
| 3 mm | 80 µm | 22.5 mm | 28 | 103 |
| Dimensions | Simple | Serpentine | ||
|---|---|---|---|---|
| W | L | H | SAB | SAB |
| 3 mm | 30 mm | 80 µm | 141 | 221 |
| 3 mm | 30 mm | 160 µm | 24 | 114 |
| 3 mm | 30 mm | 240 µm | 16 | 72 |
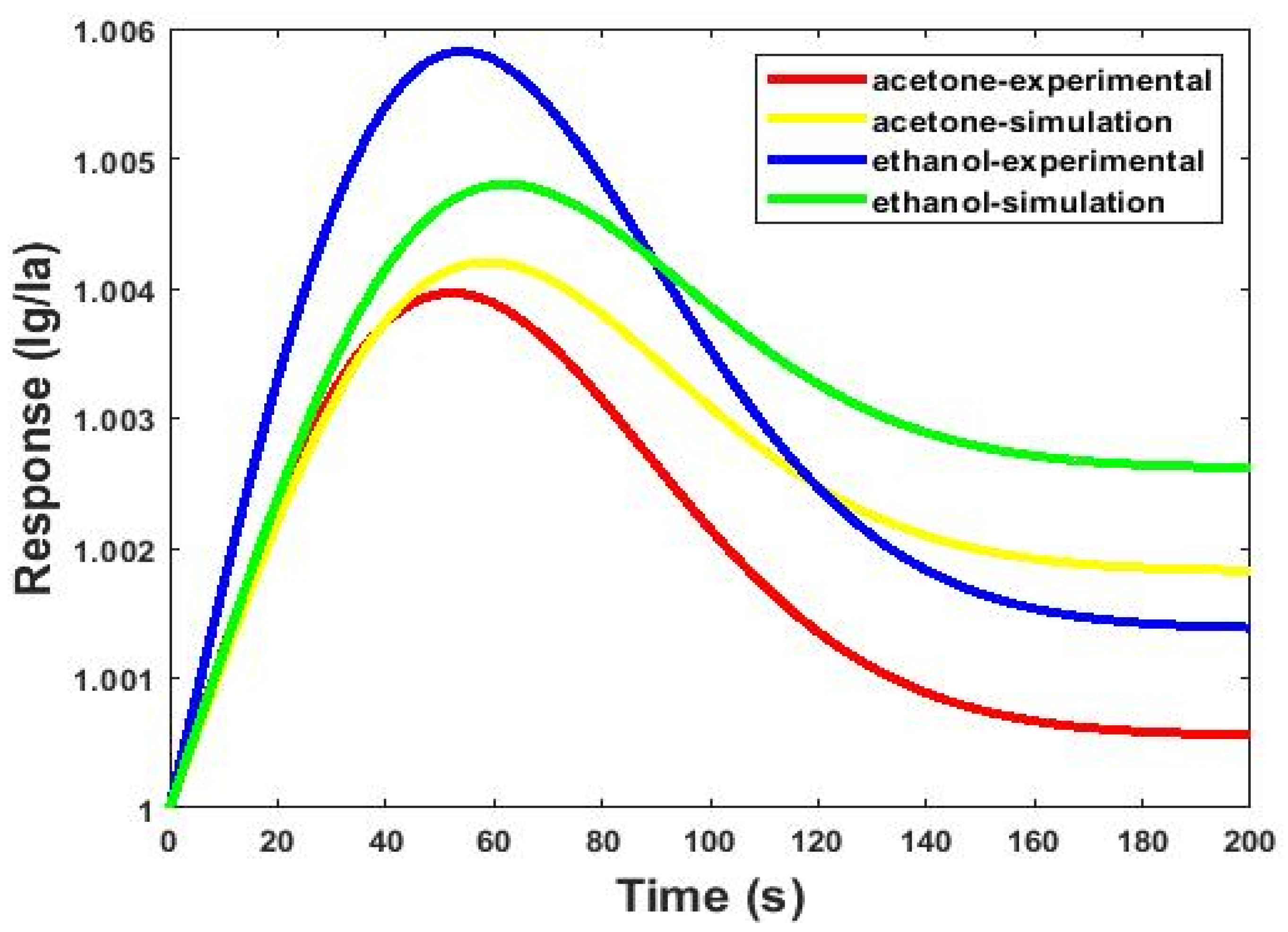
| Responses and Selectivity Indicators of the Microfluidic-Based Gas Sensor | |||
|---|---|---|---|
| Response to Acetone | Response to Ethanol | SAB | |
| Simulation | 1.0042 | 1.0048 | 103 |
| Experimental | 1.0039 | 1.0058 | 98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aghaseyedi, M.; Salehi, A.; Valijam, S.; Shooshtari, M. Gas Selectivity Enhancement Using Serpentine Microchannel Shaped with Optimum Dimensions in Microfluidic-Based Gas Sensor. Micromachines 2022, 13, 1504. https://doi.org/10.3390/mi13091504
Aghaseyedi M, Salehi A, Valijam S, Shooshtari M. Gas Selectivity Enhancement Using Serpentine Microchannel Shaped with Optimum Dimensions in Microfluidic-Based Gas Sensor. Micromachines. 2022; 13(9):1504. https://doi.org/10.3390/mi13091504
Chicago/Turabian StyleAghaseyedi, Maryam, Alireza Salehi, Shayan Valijam, and Mostafa Shooshtari. 2022. "Gas Selectivity Enhancement Using Serpentine Microchannel Shaped with Optimum Dimensions in Microfluidic-Based Gas Sensor" Micromachines 13, no. 9: 1504. https://doi.org/10.3390/mi13091504
APA StyleAghaseyedi, M., Salehi, A., Valijam, S., & Shooshtari, M. (2022). Gas Selectivity Enhancement Using Serpentine Microchannel Shaped with Optimum Dimensions in Microfluidic-Based Gas Sensor. Micromachines, 13(9), 1504. https://doi.org/10.3390/mi13091504








