Oligoimide-Mediated Graphene Oxide-Epoxy Nanocomposites with Enhanced Thermal Conductivity and Mechanical Properties
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Measurements
2.2. Instrumentation
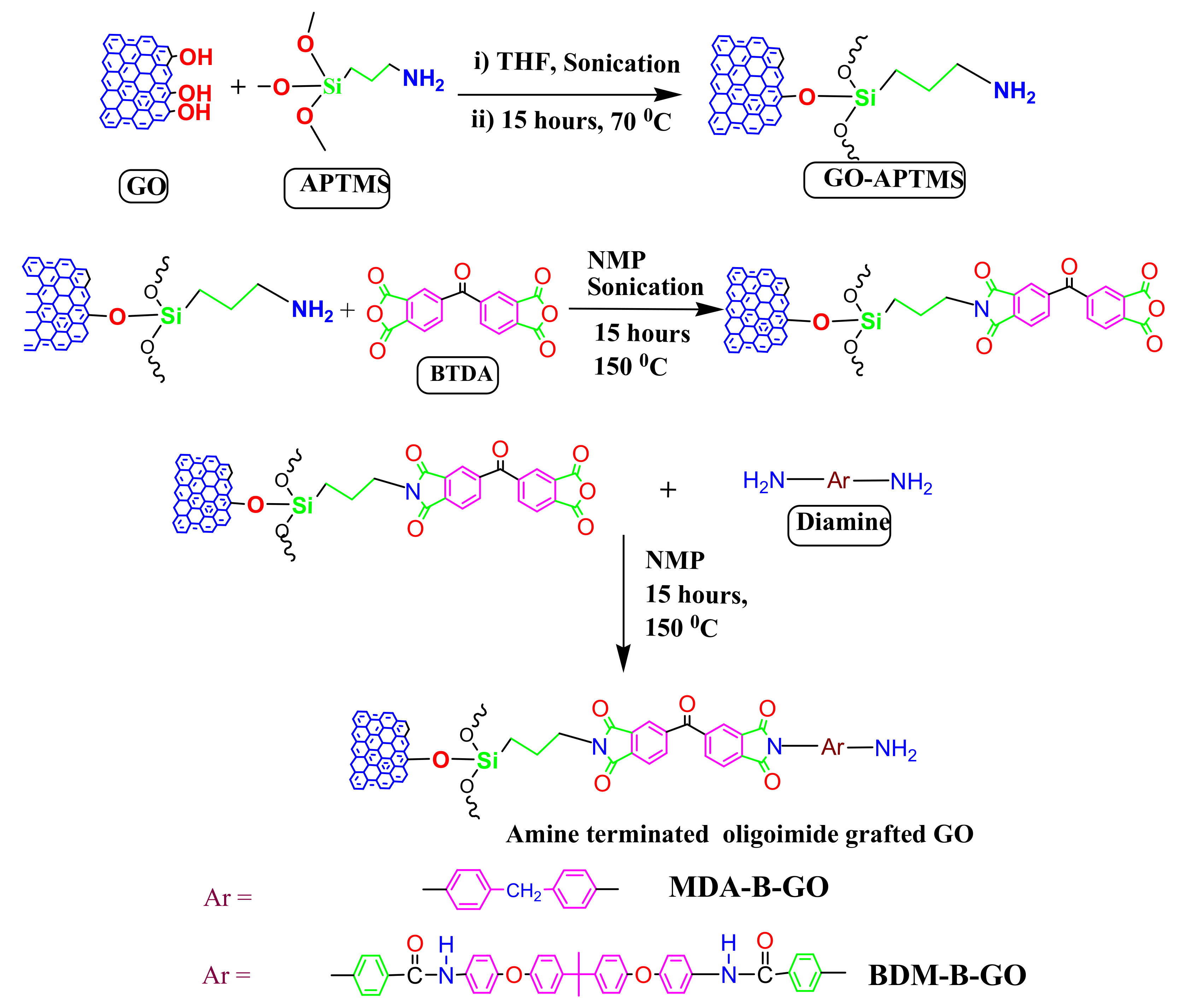
2.3. Surface Modification of Graphene Oxide
2.4. Preparation of MDA-B-GO and BDM-B-GO-Epoxy Nanocomposites
3. Results and Discussion
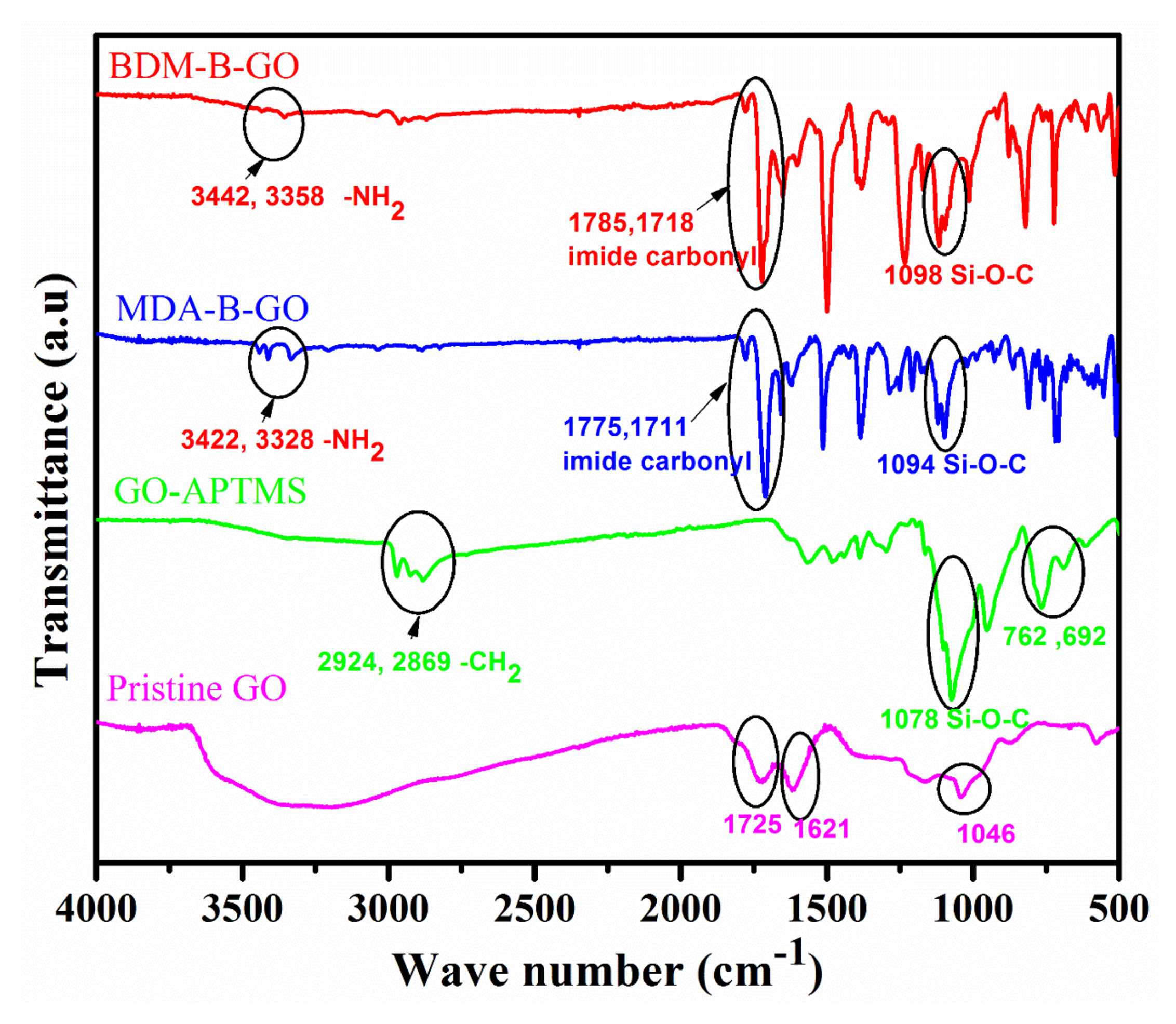
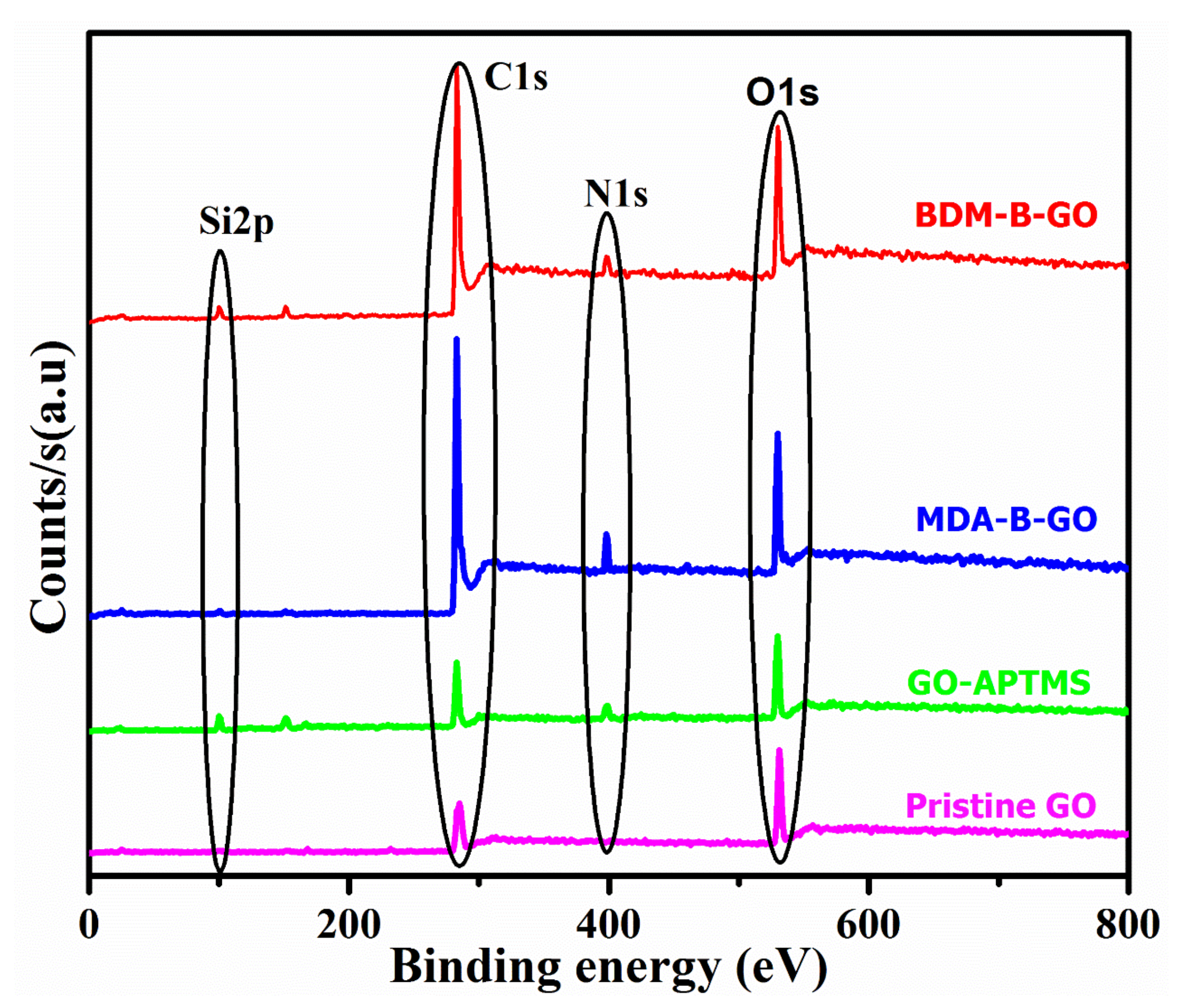
3.1. Surface Modification of GO
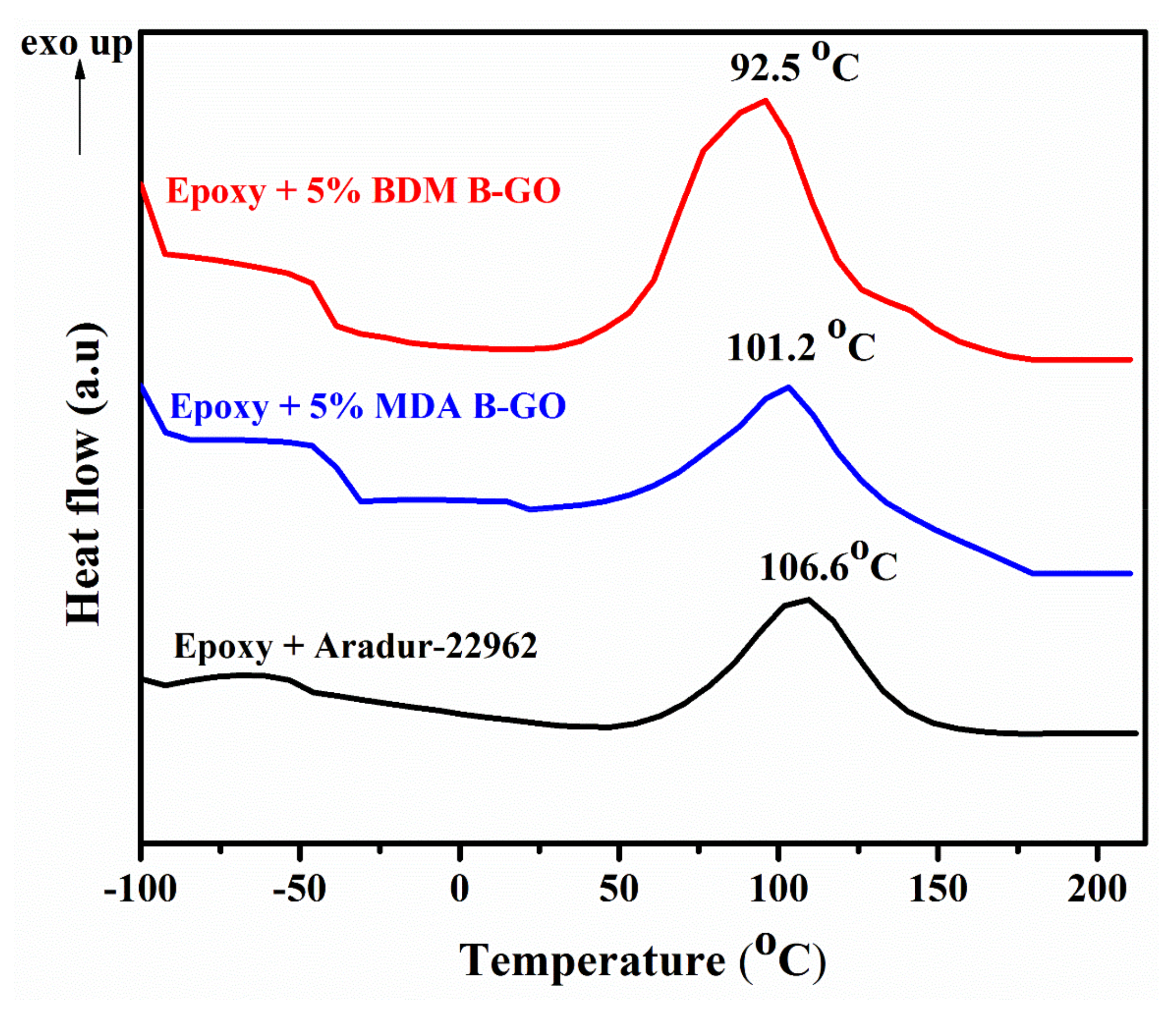
3.2. Curing Potential of MDA-B-GO and BDM-B-GO
4. Characterization of Epoxy Nanocomposites
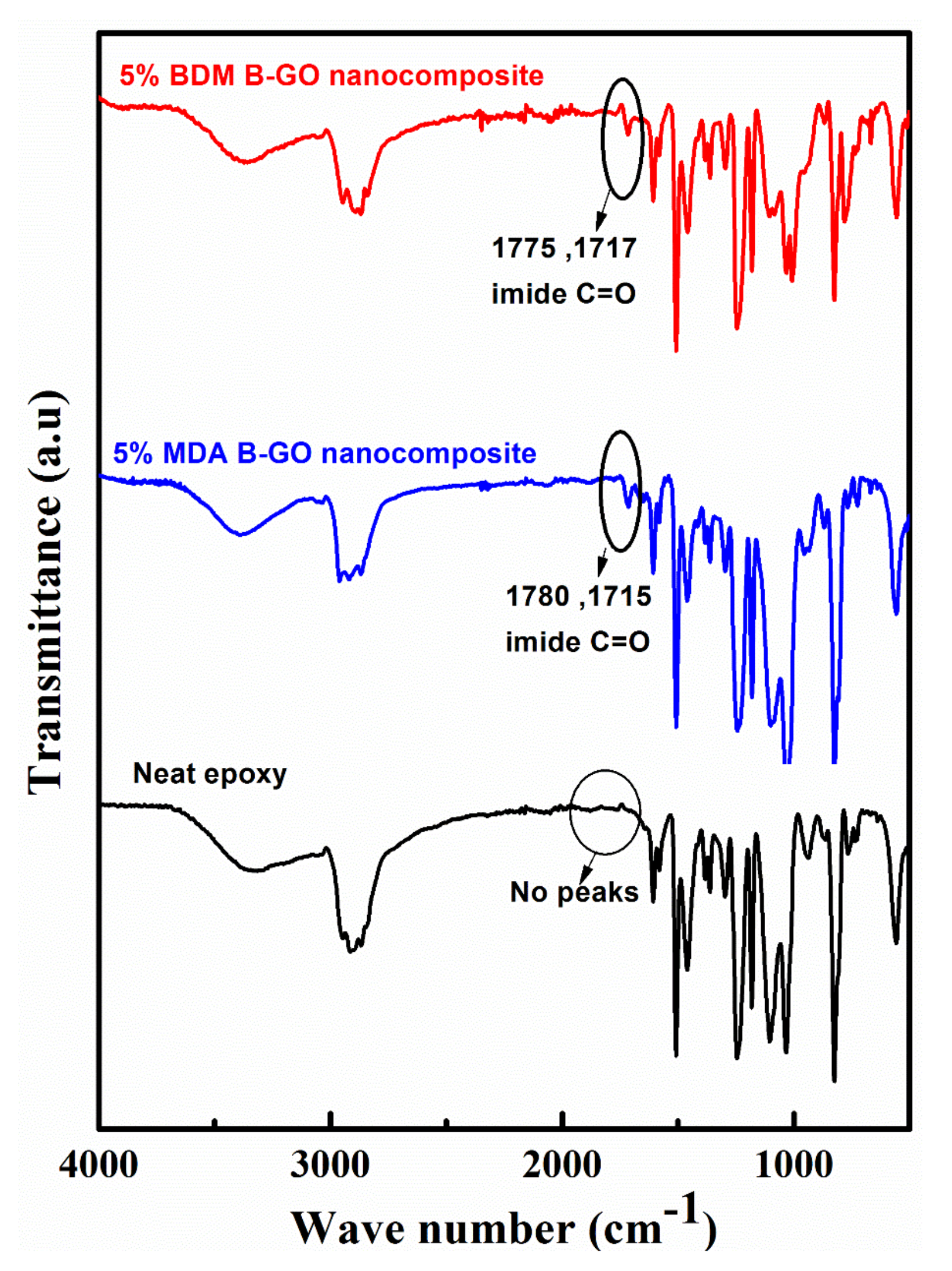
4.1. FTIR of Neat Epoxy and Nanocomposites
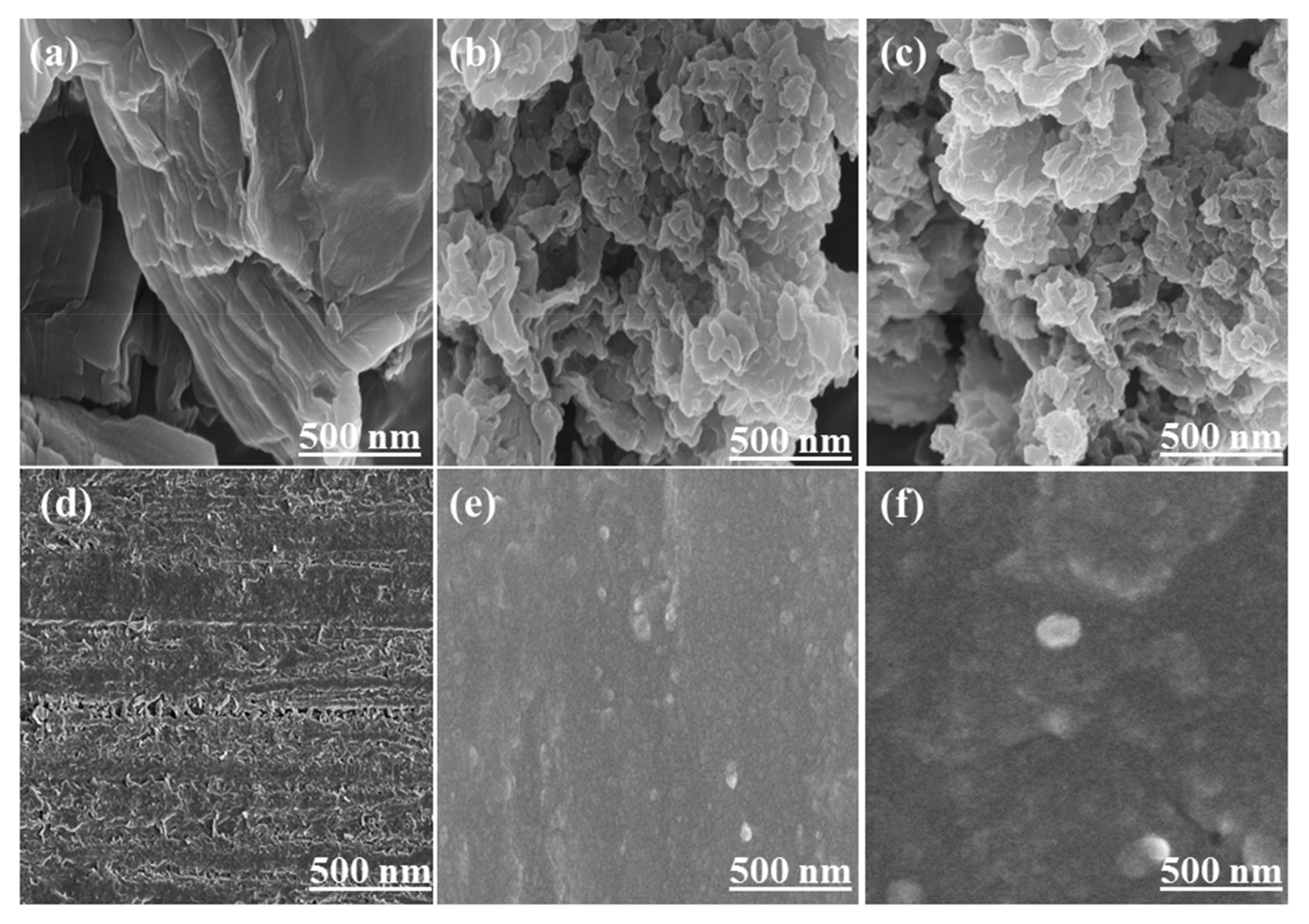
4.2. Morphological Studies
5. Thermal Properties
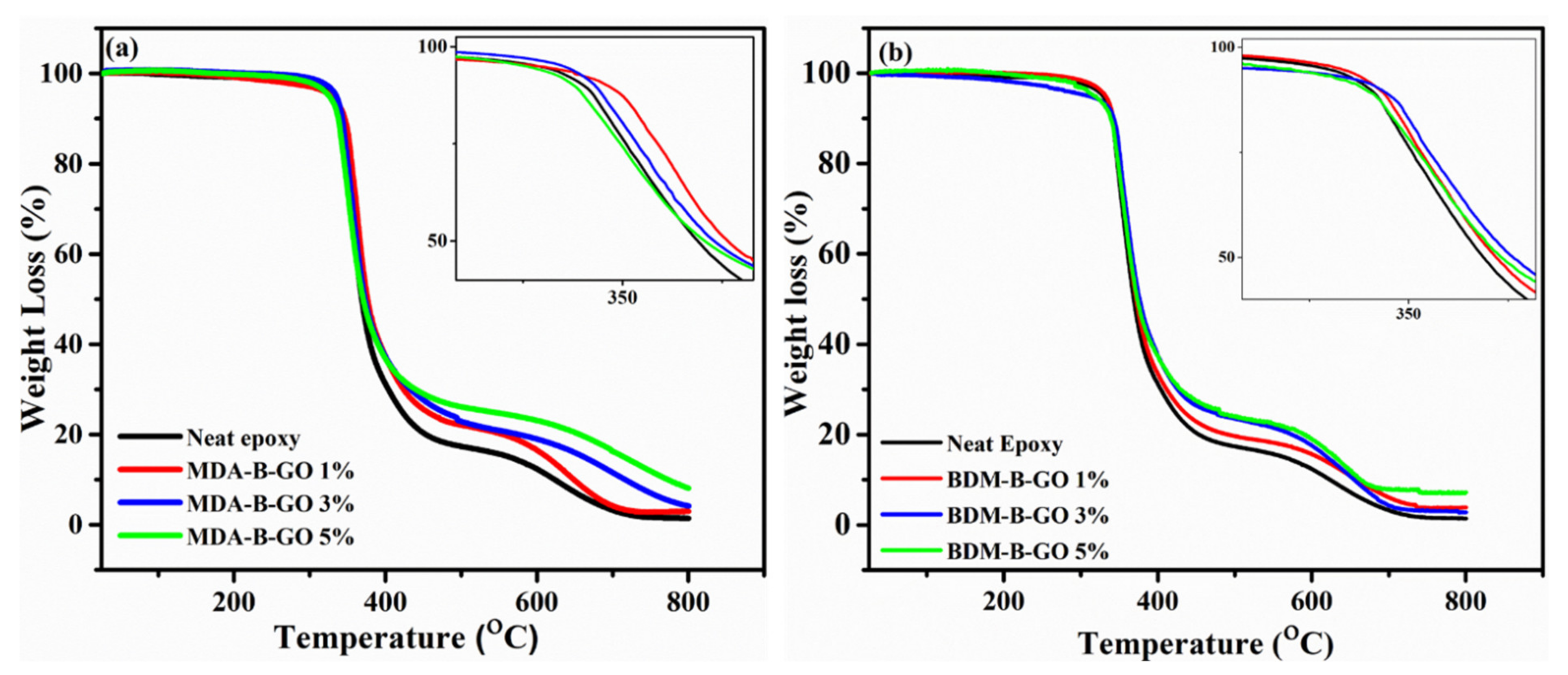
5.1. TGA Analysis
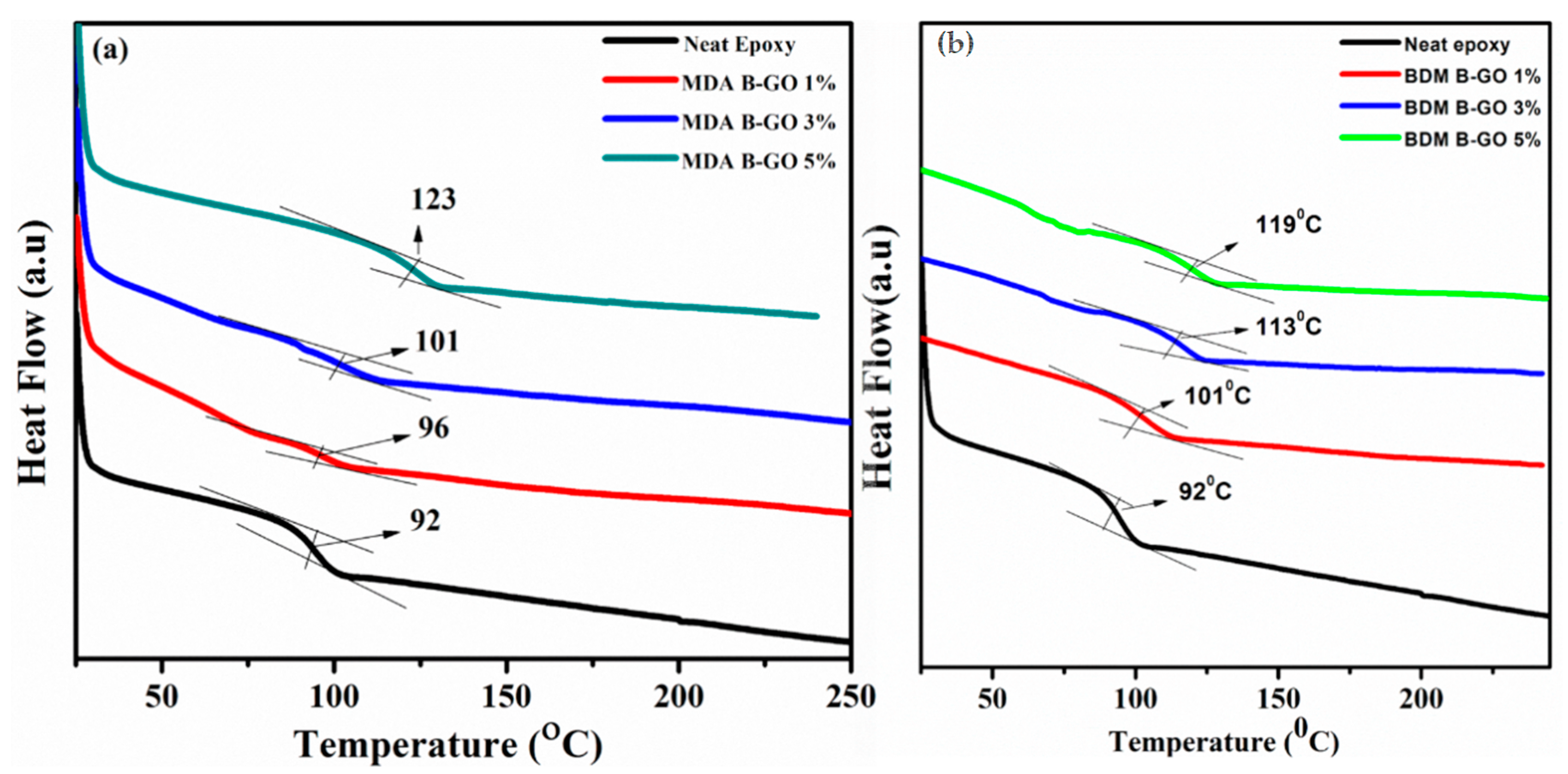
5.2. DCS Analysis
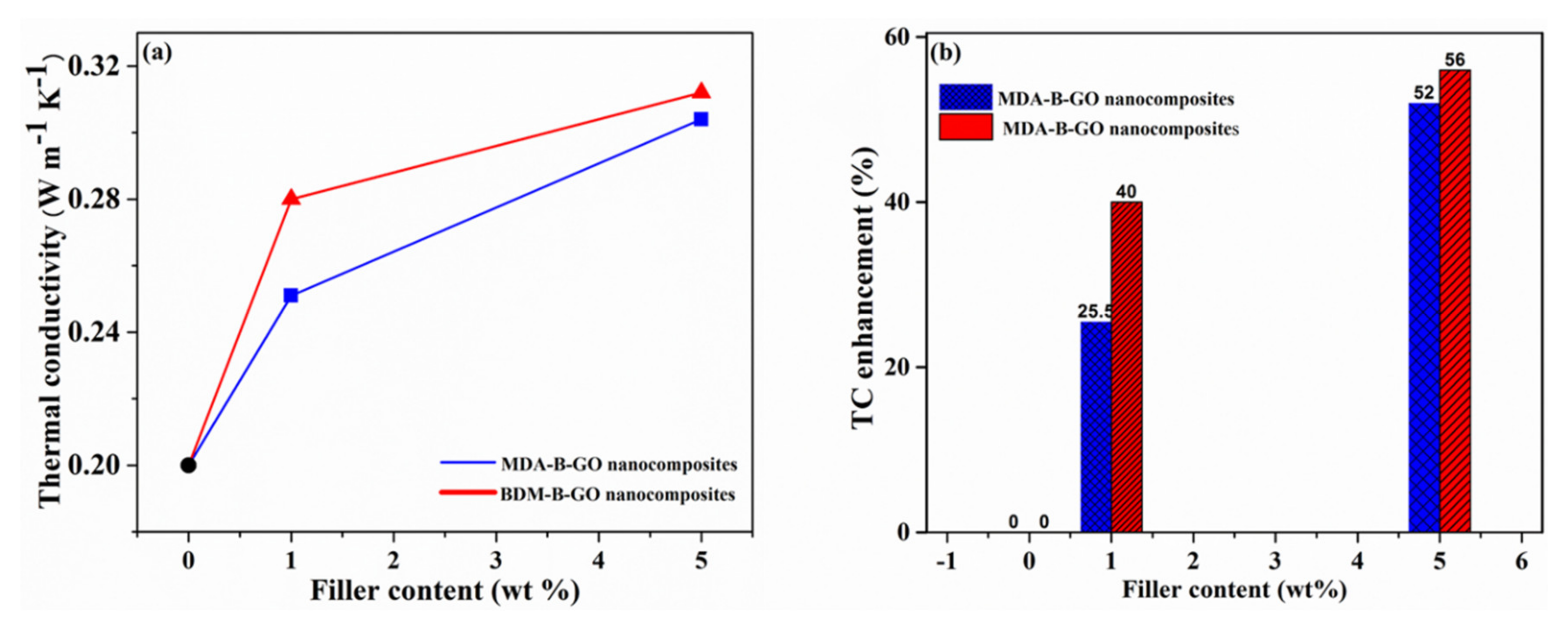
5.3. Thermal Conductivity
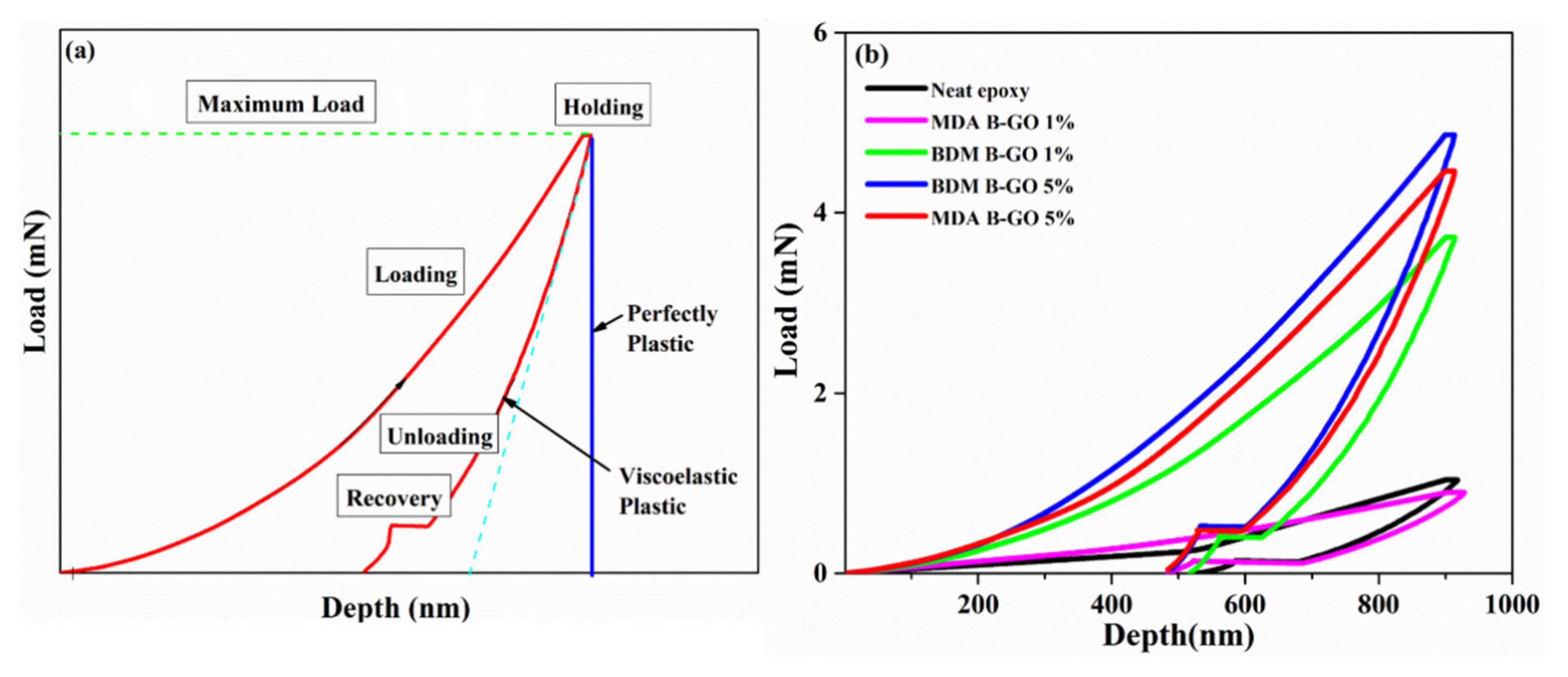
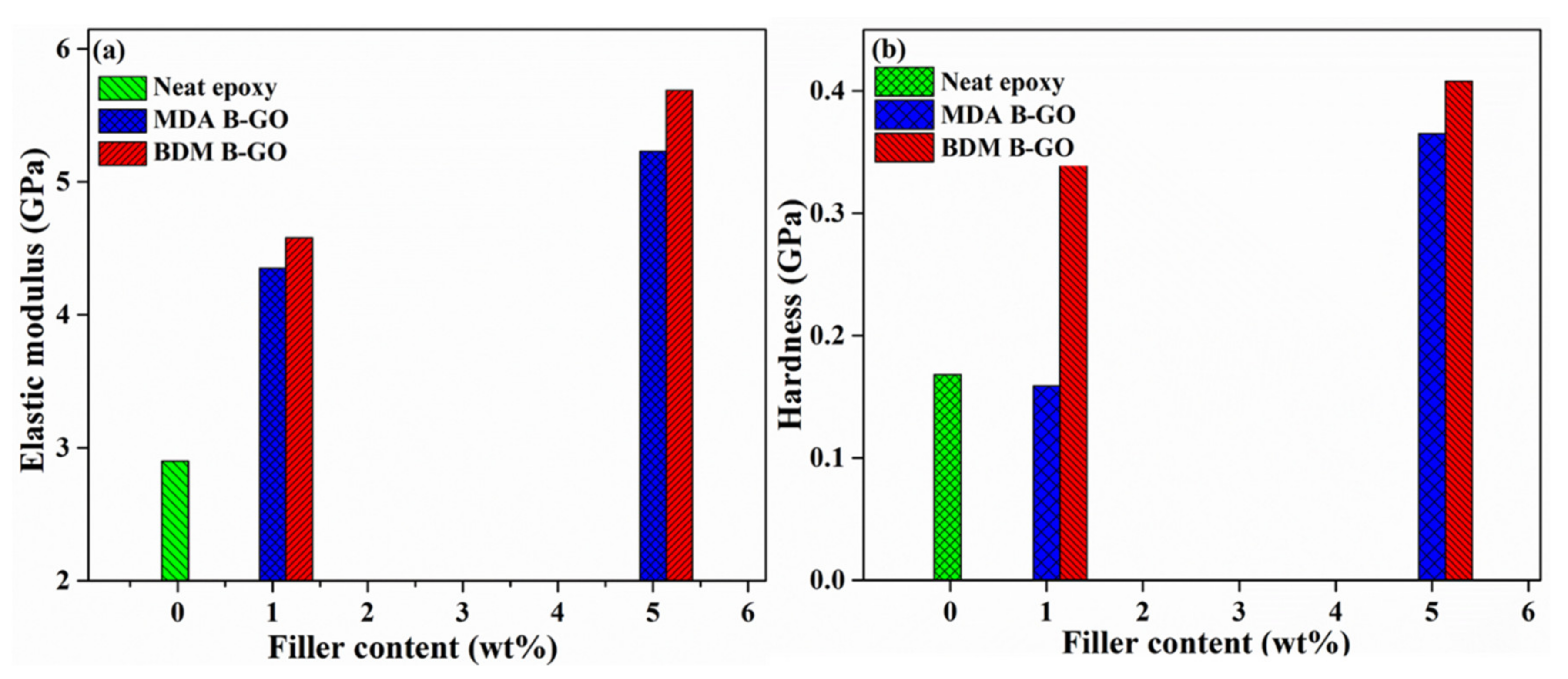
5.4. Mechanical Properties
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, L.; Cui, L.; Li, Z.; Lu, H.; Qi, X.; Wang, W.; Jin, X.; Dong, Y.; Fu, Y.; Jiang, W.; et al. Thermodynamic coupling behavior and energy harvesting of vapor grown carbon fiber/graphene oxide/epoxy shape memory composites. Compos. Sci. Technol. 2021, 203, 108583. [Google Scholar] [CrossRef]
- Chi, Z.; Wang, C.; Dong, Y.; Zhou, Y.; Xu, H.; Islam, Z.; Qian, C.; Fu, Y. MXene/epoxy-based shape memory nanocomposites with highly stable thermal-mechanical coupling effect for constructing an effective information transmission medium. Compos. Sci. Technol. 2022, 225, 109505. [Google Scholar] [CrossRef]
- Wang, Q.; Wen, G.; Liu, L.; Zhu, B.; Su, D. Promising commercial reinforcement to the nanodiamond/epoxy composite by grafting ammonium ions. J. Mater. Sci. Technol. 2018, 34, 990–994. [Google Scholar] [CrossRef]
- Wang, Q.; Su, D.S.; Wang, D.-Y. Carbon Nanotube/Epoxy Composites for Improved Fire Safety. ACS Appl. Nano Mater. 2020, 3, 4253–4264. [Google Scholar] [CrossRef]
- Qin, J.; Zhang, W.; Yang, R. Synthesis of novel phosphonium bromide-montmorillonite nanocompound and its performance in flame retardancy and dielectric properties of epoxy resins. Polym. Compos. 2021, 42, 362–374. [Google Scholar] [CrossRef]
- Zheng, N.; He, J.; Zhao, D.; Huang, Y.; Gao, J.; Mai, Y.-W. Improvement of atomic oxygen erosion resistance of carbon fiber and carbon fiber/epoxy composite interface with a silane coupling agent. Mater. Des. 2016, 109, 171–178. [Google Scholar] [CrossRef]
- Mostovoy, A.S.; Yakovlev, A.V. Reinforcement of Epoxy Composites with Graphite-Graphene Structures. Sci. Rep. 2019, 9, 16246. [Google Scholar] [CrossRef] [Green Version]
- Amirbeygi, H.; Khosravi, H.; Tohidlou, E. Reinforcing effects of aminosilane-functionalized graphene on the tribological and mechanical behaviors of epoxy nanocomposites. J. Appl. Polym. Sci. 2019, 136, 47410. [Google Scholar] [CrossRef]
- Sun, A.; Mu, L.; Hu, X. Graphene Oxide Quantum Dots as Novel Nanozymes for Alcohol Intoxication. ACS Appl. Mater. Interfaces 2017, 9, 12241–12252. [Google Scholar] [CrossRef]
- Chhetri, S.; Adak, N.C.; Samanta, P.; Mallisetty, P.K.; Murmu, N.C.; Kuila, T. Interface engineering for the improvement of mechanical and thermal properties of covalent functionalized graphene/epoxy composites. J. Appl. Polym. Sci. 2018, 135, 46124. [Google Scholar] [CrossRef]
- Chatterjee, S.; Wang, J.W.; Kuo, W.S.; Tai, N.H.; Salzmann, C.; Li, W.L.; Hollertz, R.; Nüesch, F.A.; Chu, B.T.T. Mechanical reinforcement and thermal conductivity in expanded graphene nanoplatelets reinforced epoxy composites. Chem. Phys. Lett. 2012, 531, 6–10. [Google Scholar] [CrossRef]
- Yu, J.W.; Jung, J.; Choi, Y.-M.; Choi, J.H.; Yu, J.; Lee, J.K.; You, N.-H.; Goh, M. Enhancement of the crosslink density, glass transition temperature, and strength of epoxy resin by using functionalized graphene oxide co-curing agents. Polym. Chem. 2016, 7, 36–43. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, Z.; Tjiu, W.W.; Lv, J.; Wei, C. Preparation and characterization of epoxy nanocomposites containing surface-modified graphene oxide. J. Appl. Polym. Sci. 2014, 131, 40236. [Google Scholar] [CrossRef]
- Mostovoy, A.S.; Kadykova, Y.A.; Bekeshev, A.Z.; Tastanova, L.K. Epoxy composites modified with microfibers of potassium polytitanates. J. Appl. Polym. Sci. 2018, 135, 46651. [Google Scholar] [CrossRef]
- Khan, M.I.; Siddiqi, H.M.; Park, C.H.; Han, J.; Park, H.; Kim, B.; Hassan, M.; Akhter, T. High performance epoxy nanocomposites with enhanced thermal and mechanical properties by incorporating amine-terminated oligoimide-grafted graphene oxide. High Perform. Polym. 2020, 32, 569–587. [Google Scholar] [CrossRef]
- Riddick, J.A.; Toops, E.C.; Weissberger, A. Organic Solvents: Physical Properties and Methods of Purification; Wiley-Interscience: Hoboken, NJ, USA, 1970. [Google Scholar]
- Gholipour-Mahmoudalilou, M.; Roghani-Mamaqani, H.; Azimi, R.; Abdollahi, A. Preparation of hyperbranched poly (amidoamine)-grafted graphene nanolayers as a composite and curing agent for epoxy resin. Appl. Surf. Sci. 2018, 428, 1061–1069. [Google Scholar] [CrossRef]
- Chen, W.-Q.; Li, Q.-T.; Li, P.-H.; Zhang, Q.-Y.; Xu, Z.-S.; Chu, P.K.; Wang, X.-B.; Yi, C.-F. In situ random co-polycondensation for preparation of reduced graphene oxide/polyimide nanocomposites with amino-modified and chemically reduced graphene oxide. J. Mater. Sci. 2015, 50, 3860–3874. [Google Scholar] [CrossRef]
- Wang, F.; Drzal, L.T.; Qin, Y.; Huang, Z. Mechanical properties and thermal conductivity of graphene nanoplatelet/epoxy composites. J. Mater. Sci. 2015, 50, 1082–1093. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Sato, N.; Tölle, F.; Mülhaupt, R.; Fiedler, B.; Schulte, K. Fracture toughness and failure mechanism of graphene based epoxy composites. Compos. Sci. Technol. 2014, 97, 90–99. [Google Scholar] [CrossRef]
- Teng, C.-C.; Ma, C.-C.M.; Lu, C.-H.; Yang, S.-Y.; Lee, S.-H.; Hsiao, M.-C.; Yen, M.-Y.; Chiou, K.-C.; Lee, T.-M. Thermal conductivity and structure of non-covalent functionalized graphene/epoxy composites. Carbon 2011, 49, 5107–5116. [Google Scholar] [CrossRef]
- Yu, Z.; Di, H.; Ma, Y.; He, Y.; Liang, L.; Lv, L.; Ran, X.; Pan, Y.; Luo, Z. Preparation of graphene oxide modified by titanium dioxide to enhance the anti-corrosion performance of epoxy coatings. Surf. Coat. Technol. 2015, 276, 471–478. [Google Scholar] [CrossRef]
- Chiang, C.-L.; Hsu, S.-W. Synthesis, characterization and thermal properties of novel epoxy/expandable graphite composites. Polym. Int. 2010, 59, 119–126. [Google Scholar] [CrossRef]
- Yadav, A.; Kumar, A.; Sharma, K.; Shukla, M.K. Investigating the Effects of Amine Functionalized Graphene on the Mechanical Properties of Epoxy Nanocomposites. Mater. Today Proc. 2019, 11, 837–842. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Brito, F.S.; Franceschi, W.; Simonetti, E.A.N.; Cividanes, L.S.; Chipara, M.; Lozano, K. Functionalized graphene oxide as reinforcement in epoxy based nanocomposites. Surf. Interfaces 2018, 10, 100–109. [Google Scholar] [CrossRef]
- Ribeiro, H.; da Silva, W.M.; Neves, J.C.; Calado, H.D.R.; Paniago, R.; Seara, L.M.; Mercês Camarano, D.; Silva, G.G. Multifunctional nanocomposites based on tetraethylenepentamine-modified graphene oxide/epoxy. Polym. Test. 2015, 43, 182–192. [Google Scholar] [CrossRef]
- Xu, X.; Chen, J.; Zhou, J.; Li, B. Thermal Conductivity of Polymers and Their Nanocomposites. Adv. Mater. 2018, 30, 1705544. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Qian, X.; Yang, R. Thermal conductivity of polymers and polymer nanocomposites. Mater. Sci. Eng. R Rep. 2018, 132, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Zong, P.; Fu, J.; Chen, L.; Yin, J.; Dong, X.; Yuan, S.; Shi, L.; Deng, W. Effect of aminopropylisobutyl polyhedral oligomeric silsesquioxane functionalized graphene on the thermal conductivity and electrical insulation properties of epoxy composites. RSC Adv. 2016, 6, 10498–10506. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 2011, 7, 1564–1583. [Google Scholar] [CrossRef]
- Sánchez, M.; Rams, J.; Campo, M.; Jiménez-Suárez, A.; Ureña, A. Characterization of carbon nanofiber/epoxy nanocomposites by the nanoindentation technique. Compos. Part B Eng. 2011, 42, 638–644. [Google Scholar] [CrossRef]
- Li, X.; Gao, H.; Scrivens, W.A.; Fei, D.; Xu, X.; Sutton, M.A.; Reynolds, A.P.; Myrick, M.L. Nanomechanical characterization of single-walled carbon nanotube reinforced epoxy composites. Nanotechnology 2004, 15, 1416–1423. [Google Scholar] [CrossRef]
- Ferencz, R.; Sanchez, J.; Blümich, B.; Herrmann, W. AFM nanoindentation to determine Young’s modulus for different EPDM elastomers. Polym. Test. 2012, 31, 425–432. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, W.; Li, D.; Sun, Y.; Wang, Z.; Hou, C.; Chen, L.; Cao, Y.; Liu, Y. Mechanical and anticorrosive properties of graphene/epoxy resin composites coating prepared by in-situ method. Int. J. Mol. Sci. 2015, 16, 2239–2251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, M.C.; de Castro, V.G.; Seara, L.M.; Diniz, V.P.A.; Lavall, R.L.; Silva, G.G. Thermosetting polyurethane-multiwalled carbon nanotube composites: Thermomechanical properties and nanoindentation. J. Appl. Polym. Sci. 2014, 131, 41207. [Google Scholar] [CrossRef]

| Nanocomposites Type | Codes | Filler % | Amount of Total Mixture (g) | Amount of Epoxy (g) | Amount of Hardener (g) | Amount of Filler (g) |
|---|---|---|---|---|---|---|
| Neat epoxy | 0 | 3.75 | 3.0000 | 0.7500 | 0 | |
| MDA-B-GO-epoxy nanocomposites | MDA-B-GO 1 | 1 | 3.75 | 2.9700 | 0.7425 | 0.0375 |
| MDA-B-GO 3 | 3 | 3.75 | 2.9100 | 0.7275 | 0.1125 | |
| MDA-B-GO 5 | 5 | 3.75 | 2.8500 | 0.7125 | 0.1875 | |
| BDM-B-GO-epoxy nanocomposites | BDM-B-GO 1 | 1 | 3.75 | 2.9700 | 0.7425 | 0.0375 |
| BDM-B-GO 3 | 3 | 3.75 | 2.9100 | 0.7275 | 0.1125 | |
| BDM-B-GO 5 | 5 | 3.75 | 2.8500 | 0.7125 | 0.1875 |
| Graphene Type | % C | % O | % N | % Si | C:O | C:Si |
|---|---|---|---|---|---|---|
| GO | 67.65 | 32.35 | - | - | 2.09 | - |
| GO-APTMS | 54.04 | 23.29 | 10.18 | 12.49 | 2.33 | 4.32 |
| MDA-B-GO | 78.08 | 14.37 | 5.74 | 1.81 | 5.43 | 43.13 |
| BDM-B-GO | 74.98 | 16.85 | 4.01 | 4.16 | 4.44 | 18.02 |
| Sample | Tmax (°C) | ∆H (mJ·g−1) | % Reduction in ∆H |
|---|---|---|---|
| Epoxy cured with hardener Aradur-22962 | 106.6 | 166.79 | - |
| Epoxy cured with 5 % MDA-B-GO | 101.2 | 39.01 | 76.6 |
| Epoxy cured with 5 % BDM-B-GO | 92.5 | 90.53 | 45.7 |
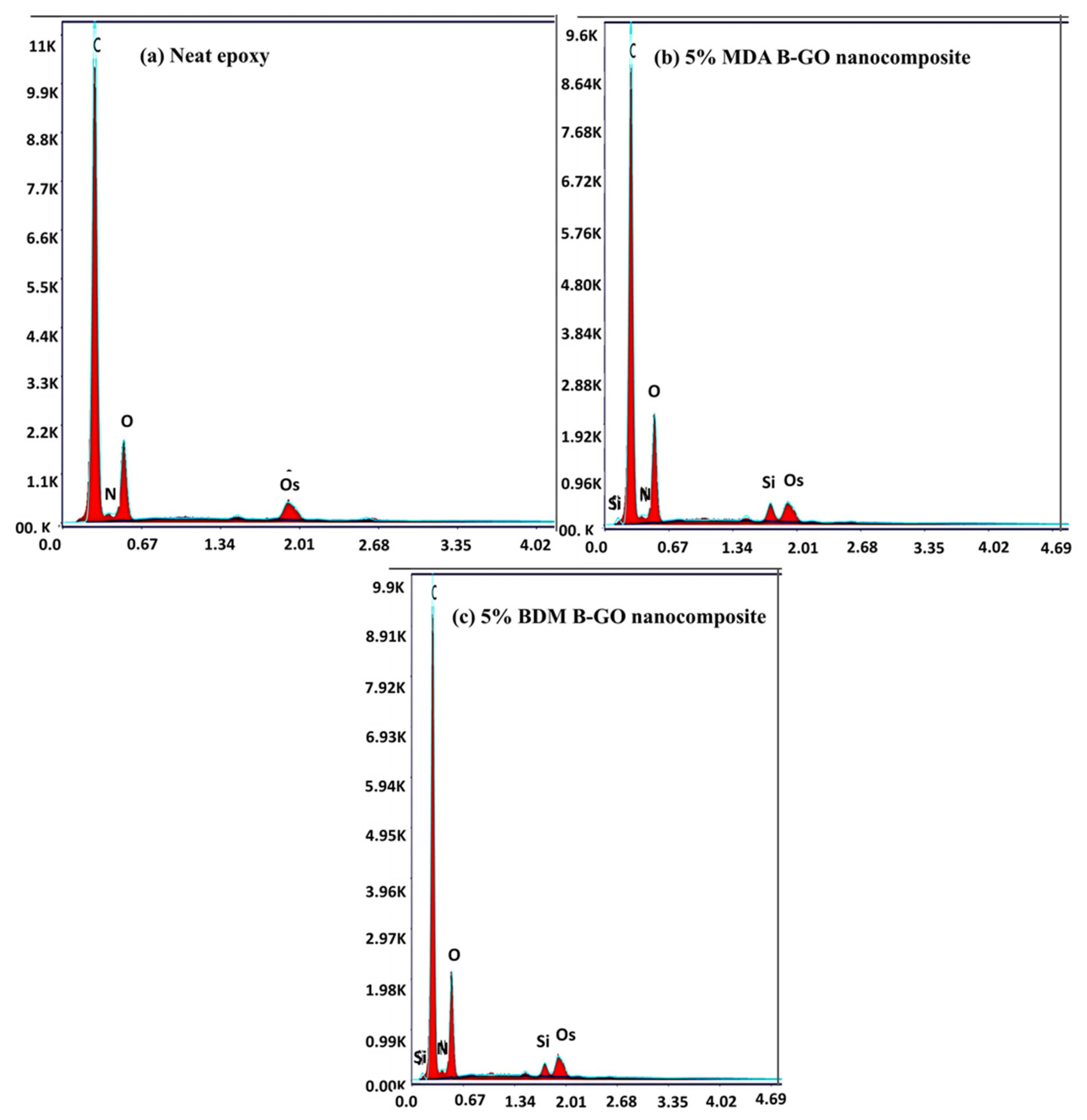
| Element | Neat Epoxy | MDA-B-GO-Epoxy Nanocomposite | BDM-B-GO-Epoxy Nanocomposite | |||
|---|---|---|---|---|---|---|
| Weight % | Atomic % | Weight % | Atomic % | Weight % | Atomic % | |
| C | 77.99 | 82.36 | 73.17 | 78.98 | 74.37 | 79.88 |
| N | 1.72 | 1.56 | 1.05 | 0.98 | 0.97 | 0.90 |
| O | 20.29 | 16.08 | 23.28 | 18.88 | 22.77 | 18.36 |
| Si | - | - | 2.53 | 1.17 | 1.89 | 0.87 |
| Type of GO-Epoxy Nanocomposites | GO Filler Content (%) | IDT (°C) | T10 (°C) | T50 (°C) | Tmax (°C) | R800 (%) |
|---|---|---|---|---|---|---|
| 0 (Neat Epoxy) | 294 | 340 | 368 | 626 | 1.34 | |
| 1 | 263 | 346 | 377 | 653 | 3.0 | |
| MDA-B-GO-epoxy nanocomposites | 3 | 316 | 343 | 373 | 720 | 4.1 |
| 5 | 300 | 337 | 371 | 774 | 7.70 | |
| 1 | 308 | 342 | 373 | 657 | 2.89 | |
| BDM-B-GO-epoxy nanocomposites | 3 | 287 | 343 | 376 | 655 | 3.81 |
| 5 | 218 | 338 | 372 | 663 | 7.20 |
| Type of GO-Epoxy Nanocomposites | Filler Content (Wt %) | Hardness (GPa)′ | Elastic Modulus (GPa) | Plasticity Index |
|---|---|---|---|---|
| Neat Epoxy | 0 | 0.168 | 2.90 | 0.60 |
| MDA-B-GO-epoxy nanocomposites | 1 | 0.159 | 4.35 | 0.57 |
| 5 | 0.364 | 5.23 | 0.53 | |
| BDM-B-GO-epoxy nanocomposites | 1 | 0.36 | 4.58 | 0.57 |
| 5 | 0.408 | 5.69 | 0.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.I.; Akhter, T.; Siddiqi, H.M.; Lee, Y.J.; Park, H.; Hassan, M.u.; Park, C.H. Oligoimide-Mediated Graphene Oxide-Epoxy Nanocomposites with Enhanced Thermal Conductivity and Mechanical Properties. Micromachines 2022, 13, 1379. https://doi.org/10.3390/mi13091379
Khan MI, Akhter T, Siddiqi HM, Lee YJ, Park H, Hassan Mu, Park CH. Oligoimide-Mediated Graphene Oxide-Epoxy Nanocomposites with Enhanced Thermal Conductivity and Mechanical Properties. Micromachines. 2022; 13(9):1379. https://doi.org/10.3390/mi13091379
Chicago/Turabian StyleKhan, Muhammad Inshad, Toheed Akhter, Humaira Masood Siddiqi, Young Jun Lee, Hyeonjung Park, Muhmood ul Hassan, and Chan Ho Park. 2022. "Oligoimide-Mediated Graphene Oxide-Epoxy Nanocomposites with Enhanced Thermal Conductivity and Mechanical Properties" Micromachines 13, no. 9: 1379. https://doi.org/10.3390/mi13091379
APA StyleKhan, M. I., Akhter, T., Siddiqi, H. M., Lee, Y. J., Park, H., Hassan, M. u., & Park, C. H. (2022). Oligoimide-Mediated Graphene Oxide-Epoxy Nanocomposites with Enhanced Thermal Conductivity and Mechanical Properties. Micromachines, 13(9), 1379. https://doi.org/10.3390/mi13091379






