Tetracycline Adsorption on Magnetic Sludge Biochar: Effects of pH, Humic Acid (HA), and Fulvic Acid (FA)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Magnetic Sludge Biochar
2.3. Characterization of Magnetic Sludge Biochar
2.4. Batch Sorption Experiment
3. Results and Discussion
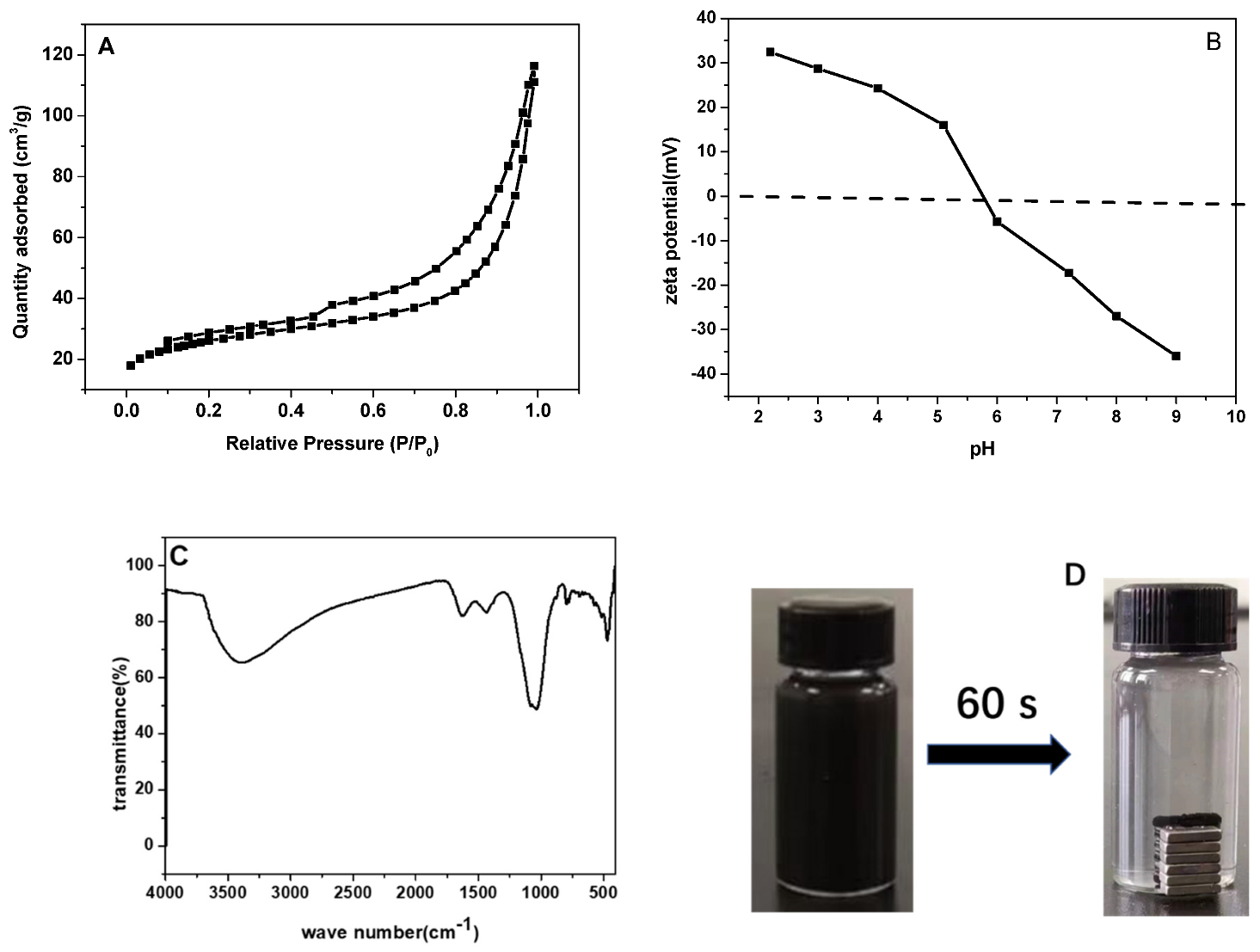
3.1. Characterization of Magnetic Sludge Biochar
3.2. Adsorption of TC on Magnetic Sludge Biochar in the Absence of NOM
3.2.1. Effect of Initial pH of TC
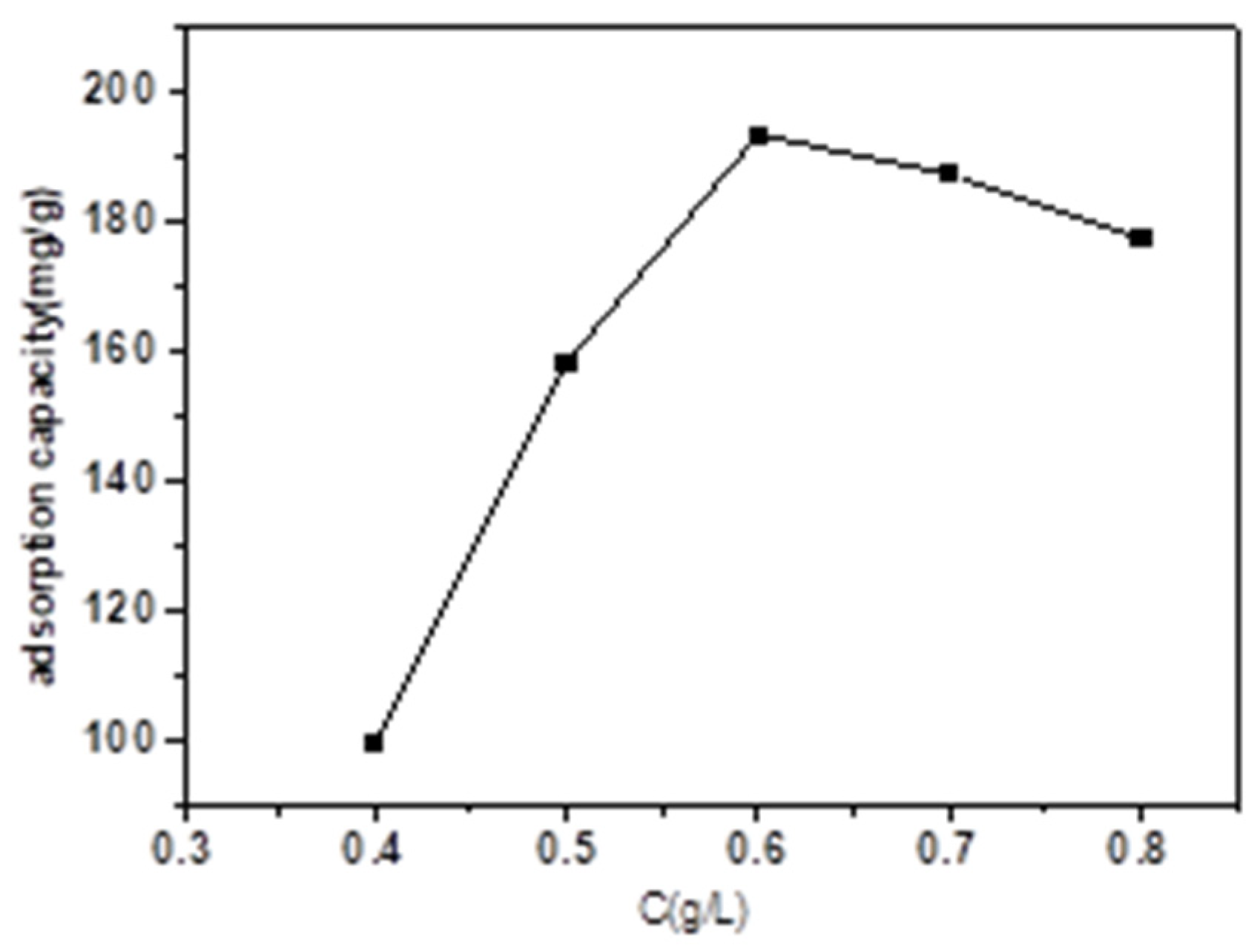
3.2.2. Effect of Magnetic Biochar Dose
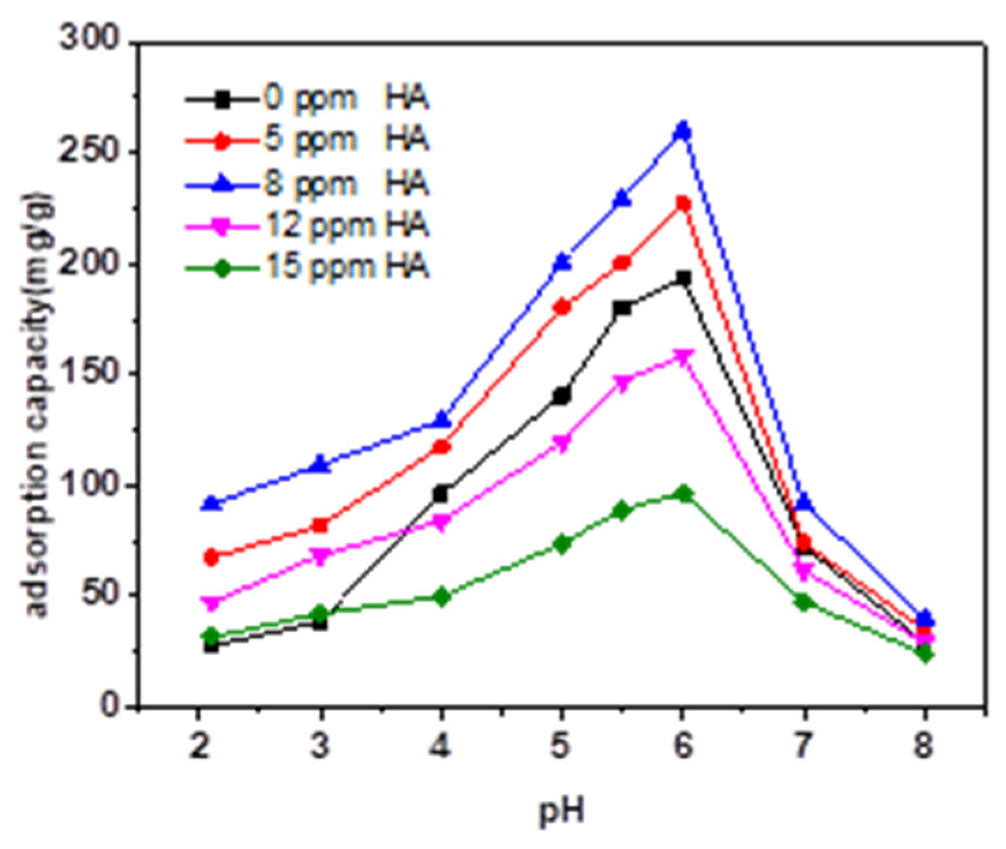
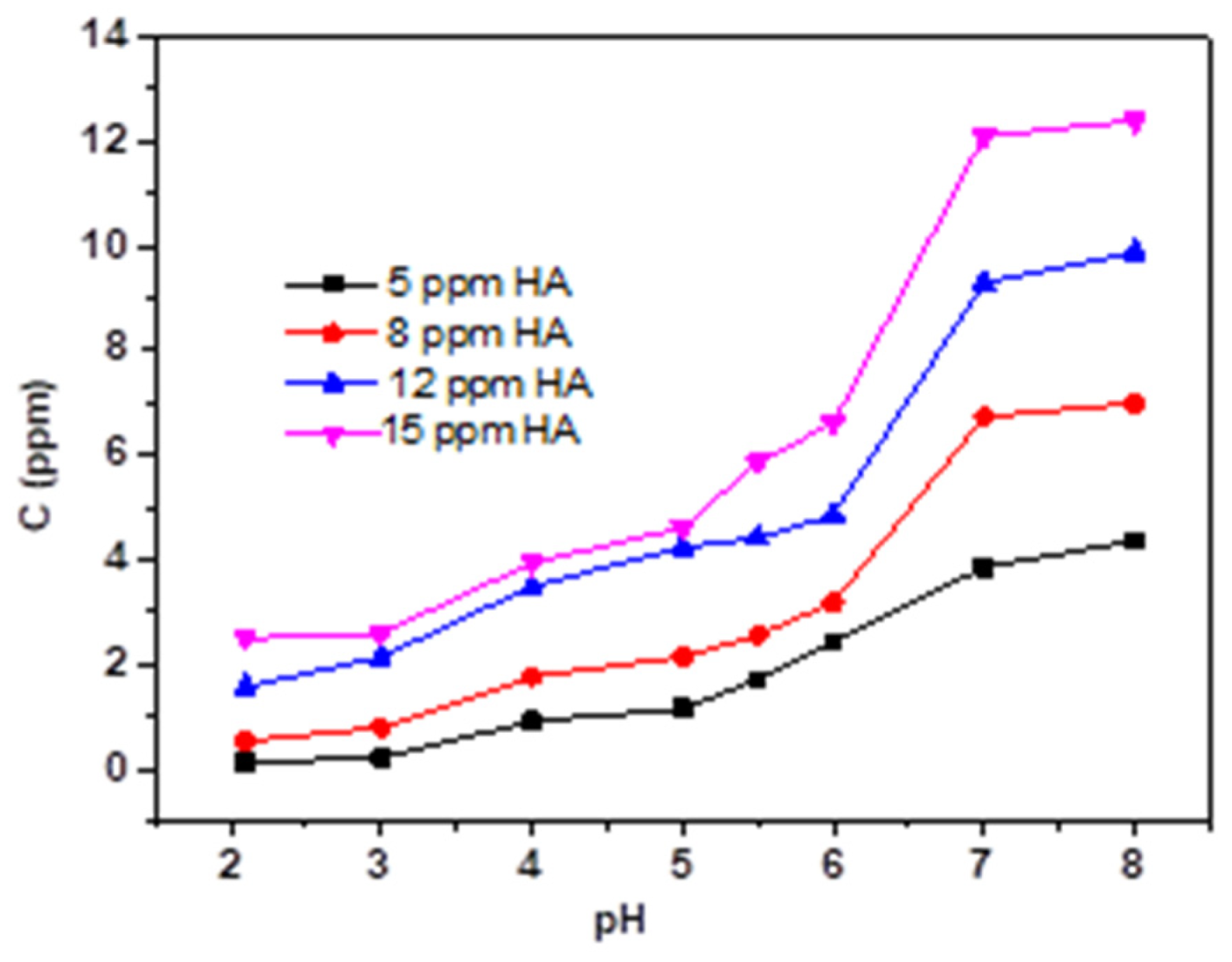
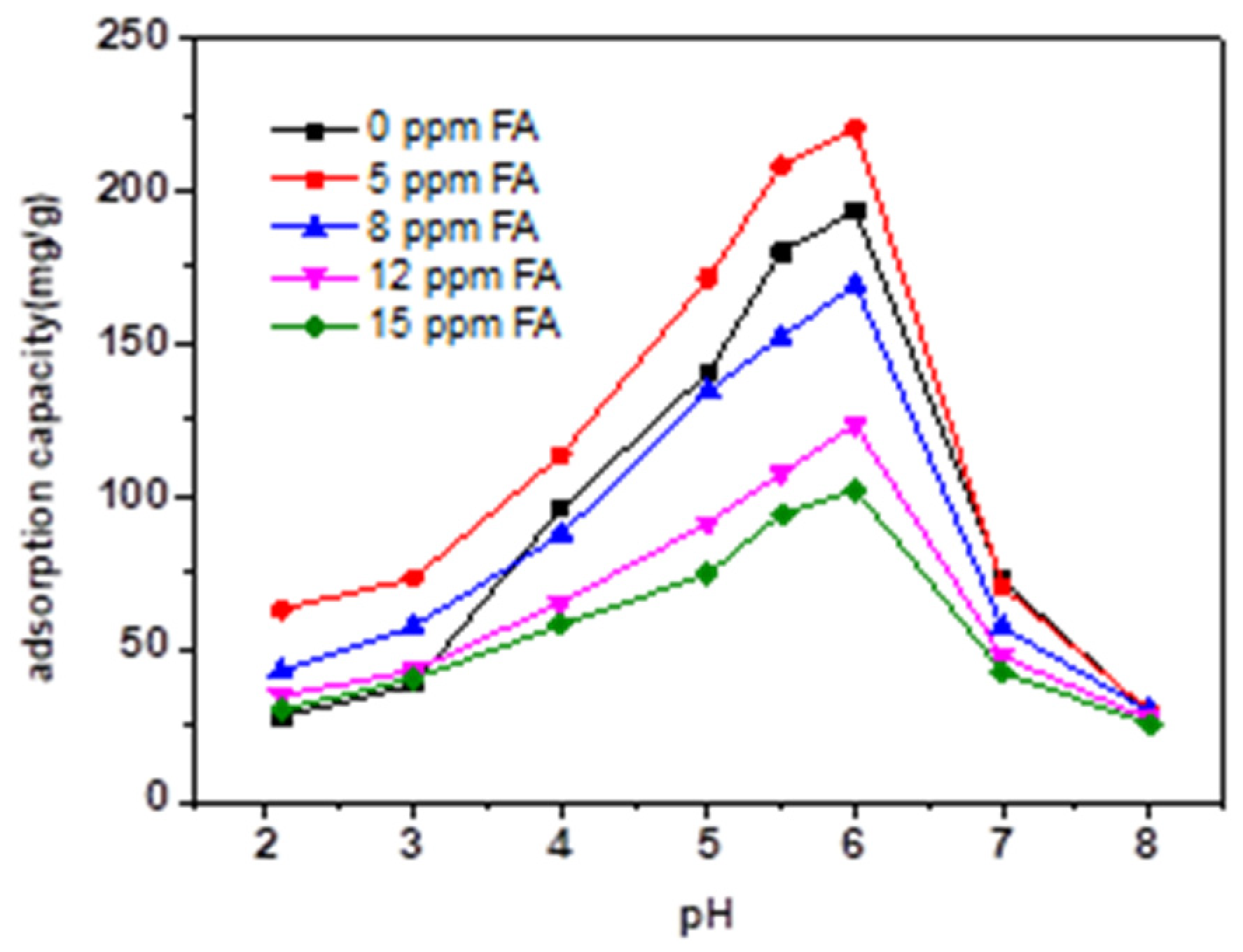
3.3. Effect of HA and FA on the Adsorption Behavior of TC
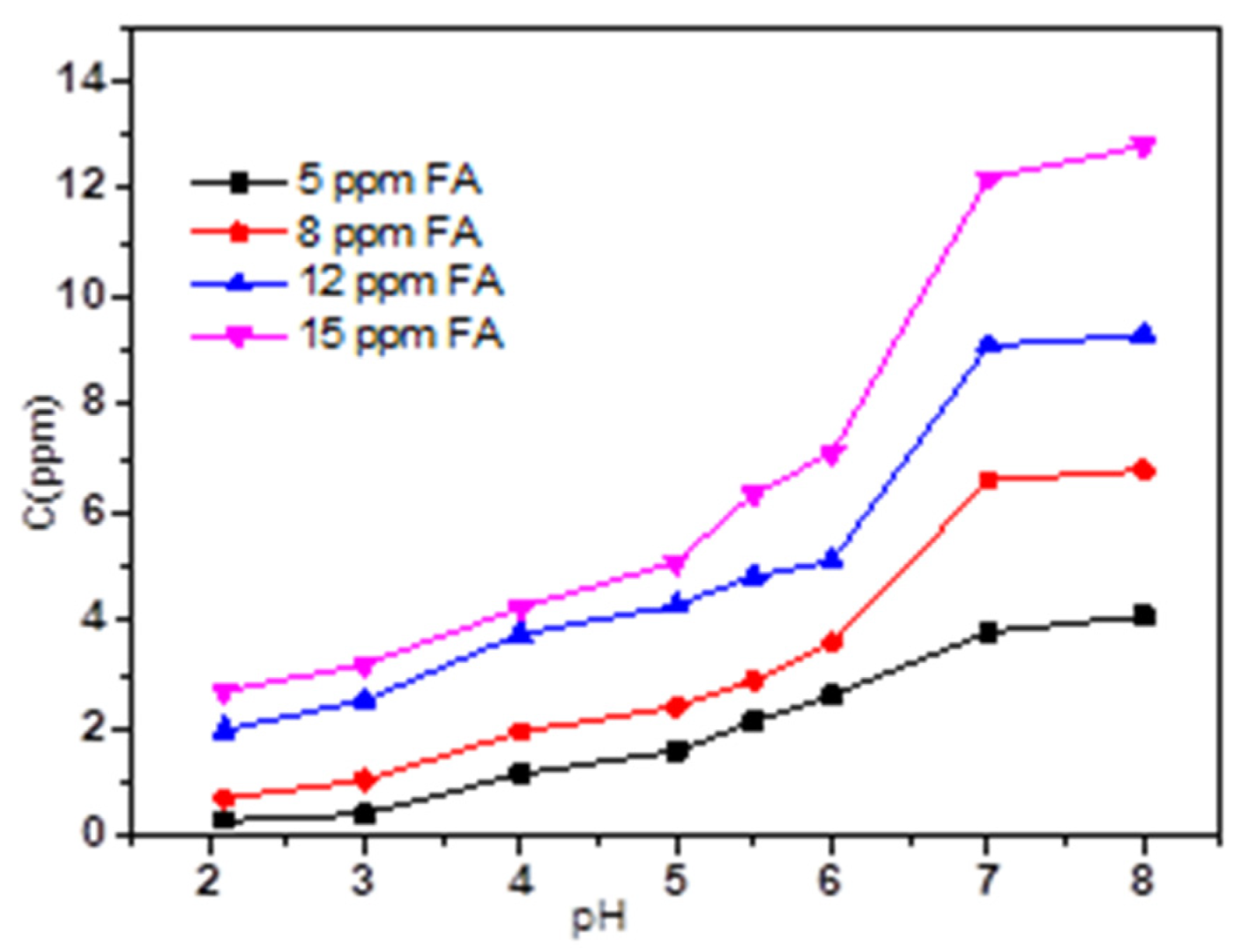
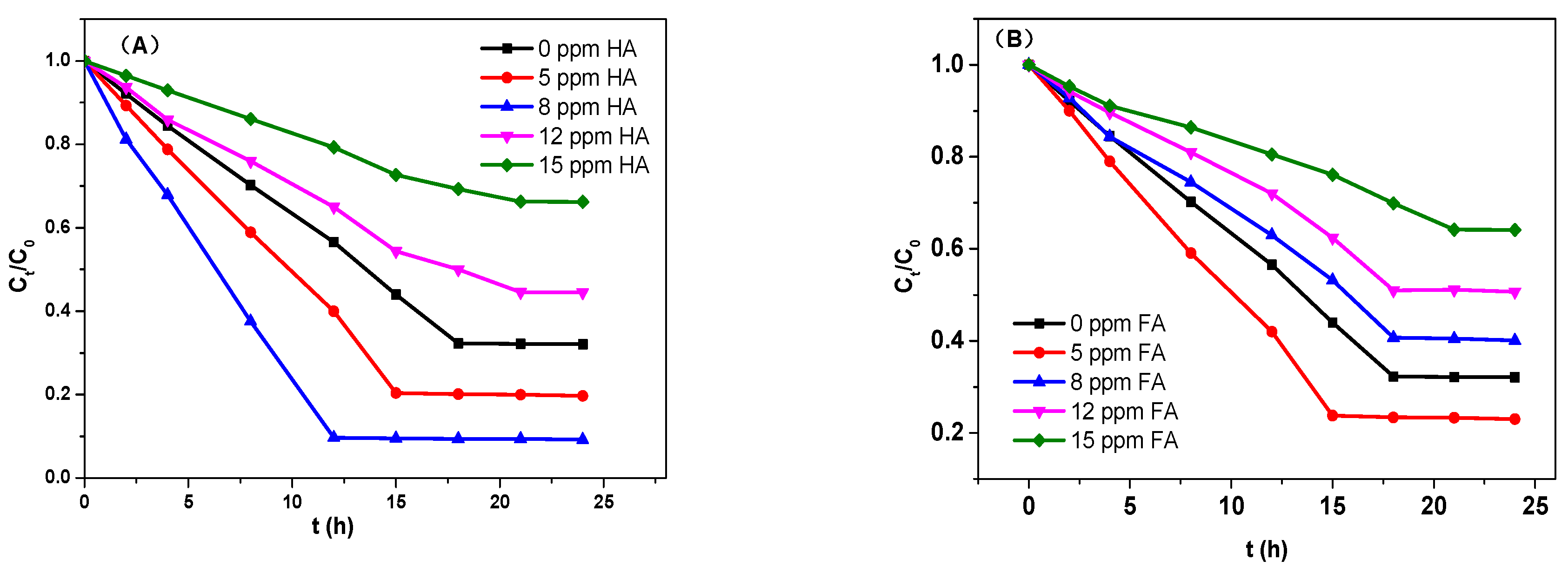
3.4. Adsorption Performance of TC on Magnetic Sludge Biochar in Coexistence of HA and FA System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Feng, Z.; Yuan, R.; Wang, F.; Chen, Z.; Chen, H. Preparation of magnetic biochar and its application in catalytic degradation of organic pollutants: A review. Sci. Total Environ. 2020, 765, 142673. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Huang, F.; Zeng, G.; Huang, D.; Qin, L.; Cheng, M.; Zhang, C.; Li, B.; Yi, H.; Liu, S. Fabrication of novel magnetic MnFe2O4/bio-char composite and heterogeneous photo-Fenton degradation of tetracycline in near neutral pH. Chemosphere 2019, 224, 910–921. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Sun, Y.; Tsang, D.; Lin, D. Environmental transformations and ecological effects of iron-based nanoparticles. Environ. Pollut. 2017, 232, 10–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, W.; Wang, G.; Zhang, Y.; Gao, Y.; Boyd, S.A.; Teppen, B.J.; Tiedje, J.M.; Zhu, D.; Li, H. Bioavailability of Soil-Sorbed Tetracycline to Escherichia coli under Unsaturated Conditions. Environ. Sci. Technol. 2017, 51, 6165–6173. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Yu, J.; Pang, Y.; Zeng, G.; Deng, Y.; Wang, J.; Ren, X.; Ye, S.; Peng, B.; Feng, H. Sustainable efficient adsorbent: Alkali-acid modified magnetic biochar derived from sewage sludge for aqueous organic contaminant removal. Chem. Eng. J. 2018, 336, 160–169. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.M.; Zhang, R.; Liu, X.G.; Song, G.X.; Chen, X.F.; Wang, Y.; Kong, J.L. Highly efficient As(V)/Sb(V) removal by magnetic sludge composite: Synthesis, characterization, equilibrium, and mechanism studies. RSC Adv. 2016, 6, 42876–42884. [Google Scholar] [CrossRef]
- Bian, R.; Zhu, J.; Chen, Y.; Yu, Y.; Zhu, S.; Zhang, L.; Huo, M. Resource recovery of wastewater treatment sludge: Synthesis of a magnetic cancrinite adsorbent. RSC Adv. 2019, 9, 36248–36255. [Google Scholar] [CrossRef]
- Yuan, S.J.; Dai, X.H. Sewage sludge-based functional nanomaterials: Development and applications. Environ. Sci. Nano 2017, 14, 17–26. [Google Scholar] [CrossRef]
- Nebbioso, A.; Piccolo, A. Molecular characterization of dissolved organic matter (DOM): A critical review. Anal. Bioanal. Chem. 2013, 405, 109–124. [Google Scholar] [CrossRef]
- Philippe, A.; Schaumann, G.E. Interactions of Dissolved Organic Matter with Natural and Engineered Inorganic Colloids: A Review. Environ. Sci. Technol. 2014, 48, 8946–8962. [Google Scholar] [CrossRef]
- Cornelissen, G.; Gustafsson, O. Effects of added PAHs and precipitated humic acid coatings on phenanthrene sorption to environmental Black carbon. Environ. Pollut. 2006, 141, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Pang, Y.; Yang, Q.; Wang, D.; Li, X.; Wang, L.; Lei, M.; Liu, J. Enhanced ciprofloxacin removal by sludge-derived biochar: Effect of humic acid. Chemosphere 2019, 231, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Lian, F.; Sun, B.; Chen, X.; Zhu, L.; Liu, Z.; Xing, B. Effect of humic acid (HA) on sulfonamide sorption by biochars. Environ. Pollut. 2015, 204, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Park, C.M.; Han, J.; Chu, K.H.; Al-Hamadani, Y.; Her, N.; Heo, J.; Yoon, Y. Influence of solution pH, ionic strength, and humic acid on cadmium adsorption onto activated biochar: Experiment and modeling. J. Ind. Eng. Chem. 2017, 48, 186–193. [Google Scholar] [CrossRef]
- Sharma, V.K.; Johnson, N.; Cizmas, L.; Mcdonald, T.J.; Kim, H. A review of the influence of treatment strategies on antibiotic resistant bacteria and antibiotic resistance genes. Chemosphere 2016, 150, 702–714. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, F.; Duan, L.; Yang, H.; Gao, J. Tetracycline adsorption onto rice husk ash, an agricultural waste: Its kinetic and thermodynamic studies. J. Mol. Liq. 2016, 222, 487–494. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, C.; Deng, D.; Li, Y.; Luo, L. Factors affecting sorption behaviors of tetracycline to soils: Importance of soil organic carbon, pH and Cd contamination. Ecotoxicol. Environ. Saf. 2020, 197, 110572. [Google Scholar] [CrossRef]
- Acosta, R.; Fierro, V.; Martinez, D.; Nabarlatz, D.; Celzard, A. Tetracycline adsorption onto activated carbons produced by KOH activation of tyre pyrolysis char. Chemosphere 2016, 149, 168–176. [Google Scholar] [CrossRef]
- Luo, Y.; Xu, L.; Rysz, M.; Wang, Y.; Zhang, H.; Alvarez, P. Occurrence and Transport of Tetracycline, Sulfonamide, Quinolone, and Macrolide Antibiotics in the Haihe River Basin, China. Environ. Sci. Technol. 2011, 45, 1827–1833. [Google Scholar] [CrossRef]
- Wang, J.; Liao, Z.; Ifthikar, J.; Shi, L.; Chen, Z.; Chen, Z. One-step preparation and application of magnetic sludge-derived biochar on acid orange 7 removal via both adsorption and persulfate based oxidation. RSC Adv. 2017, 7, 18696–18706. [Google Scholar] [CrossRef] [Green Version]
- Ferreiro, E.A.; Bussetti, S. Thermodynamic parameters of adsorption of 1,10-phenanthroline and 2,2′-bipyridyl on hematite, kaolinite and montmorillonites. Colloids Surf. A Physicochem. Eng. Asp. 2007, 301, 117–128. [Google Scholar] [CrossRef]
- Kong, S.; Wang, Y.; Zhan, H.; Liu, M.; Liang, L.; Hu, Q. Competitive adsorption of humic acid and arsenate on nanoscale iron–manganese binary oxide-loaded zeolite in groundwater. J. Geochem. Explor. 2014, 144, 220–225. [Google Scholar] [CrossRef]
- Chen, T.; Zhou, Z.; Xu, S.; Wang, H.; Lu, W. Adsorption behavior comparison of trivalent and hexavalent chromium on biochar derived from municipal sludge. Bioresour. Technol. 2015, 190, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, Q.; Feng, R.; Xu, W.; Wei, D. Magnetic activated sludge for Cu(II) and Cd(II) removal: Adsorption performance and mechanism studies. New J. Chem. 2019, 43, 18062–18071. [Google Scholar] [CrossRef]
- Wei, J.; Liu, Y.; Li, J.; Zhu, Y.; Yu, H.; Peng, Y. Adsorption and co-adsorption of tetracycline and doxycycline by one-step synthesized iron loaded sludge biochar. Chemosphere 2019, 236, 124254. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dong, Y.-n.; Zhu, M.; Li, X.; Keller, A.A.; Wang, T.; Li, F. Heteroaggregation of engineered nanoparticles and kaolin clays in aqueous environments. Water Res. 2015, 80, 130–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, S.; Liu, J.; Yin, Y.; Shen, M. Interactions between engineered nanoparticles and dissolved organic matter: A review on mechanisms and environmental effects. J. Environ. Sci. 2018, 63, 198–217. [Google Scholar] [CrossRef]
- Yu, W.; Wang, L.; Fang, G.; Herath, H.; Zhou, D. Enhanced PCBs sorption on biochars as affected by environmental factors: Humic acid and metal cations. Environ. Pollut. 2013, 172, 86–93. [Google Scholar]


| Raw Materials | Preparation Method | NOM | Adsorbate | Effect | Reference |
|---|---|---|---|---|---|
| crop straws | pyrolysis | HA | Sulfamethoxazole sulfonamide | Declined (2.5–30 ppm) Improved (>30 ppm) Improved (2.5–30 ppm) | [13] |
| declined | |||||
| (>30 ppm) | |||||
| pine needles wheat straw sludge | pyrolysis | HA HA | polychlorinated biphenyls ciprofloxacin | Enhanced (10 ppm) Promoted (10 ppm) | [28] [12] |
| sludge | pyrolysis | HA FA | tetracycline | Improved (≤8 ppm) Improved (≤5 ppm) | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Yang, M.; Long, D.; Yang, F.; Luo, S. Tetracycline Adsorption on Magnetic Sludge Biochar: Effects of pH, Humic Acid (HA), and Fulvic Acid (FA). Micromachines 2022, 13, 1057. https://doi.org/10.3390/mi13071057
Wu Y, Yang M, Long D, Yang F, Luo S. Tetracycline Adsorption on Magnetic Sludge Biochar: Effects of pH, Humic Acid (HA), and Fulvic Acid (FA). Micromachines. 2022; 13(7):1057. https://doi.org/10.3390/mi13071057
Chicago/Turabian StyleWu, Yuanhui, Meizhi Yang, Dan Long, Fanian Yang, and Suxing Luo. 2022. "Tetracycline Adsorption on Magnetic Sludge Biochar: Effects of pH, Humic Acid (HA), and Fulvic Acid (FA)" Micromachines 13, no. 7: 1057. https://doi.org/10.3390/mi13071057
APA StyleWu, Y., Yang, M., Long, D., Yang, F., & Luo, S. (2022). Tetracycline Adsorption on Magnetic Sludge Biochar: Effects of pH, Humic Acid (HA), and Fulvic Acid (FA). Micromachines, 13(7), 1057. https://doi.org/10.3390/mi13071057






