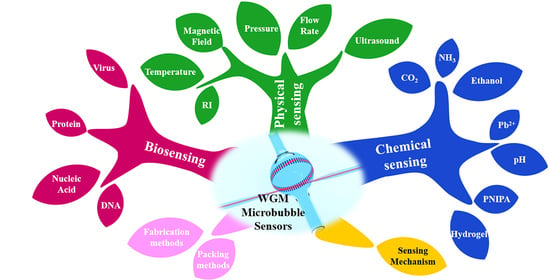
Optical Whispering-Gallery-Mode Microbubble Sensors
Abstract
:1. Introduction
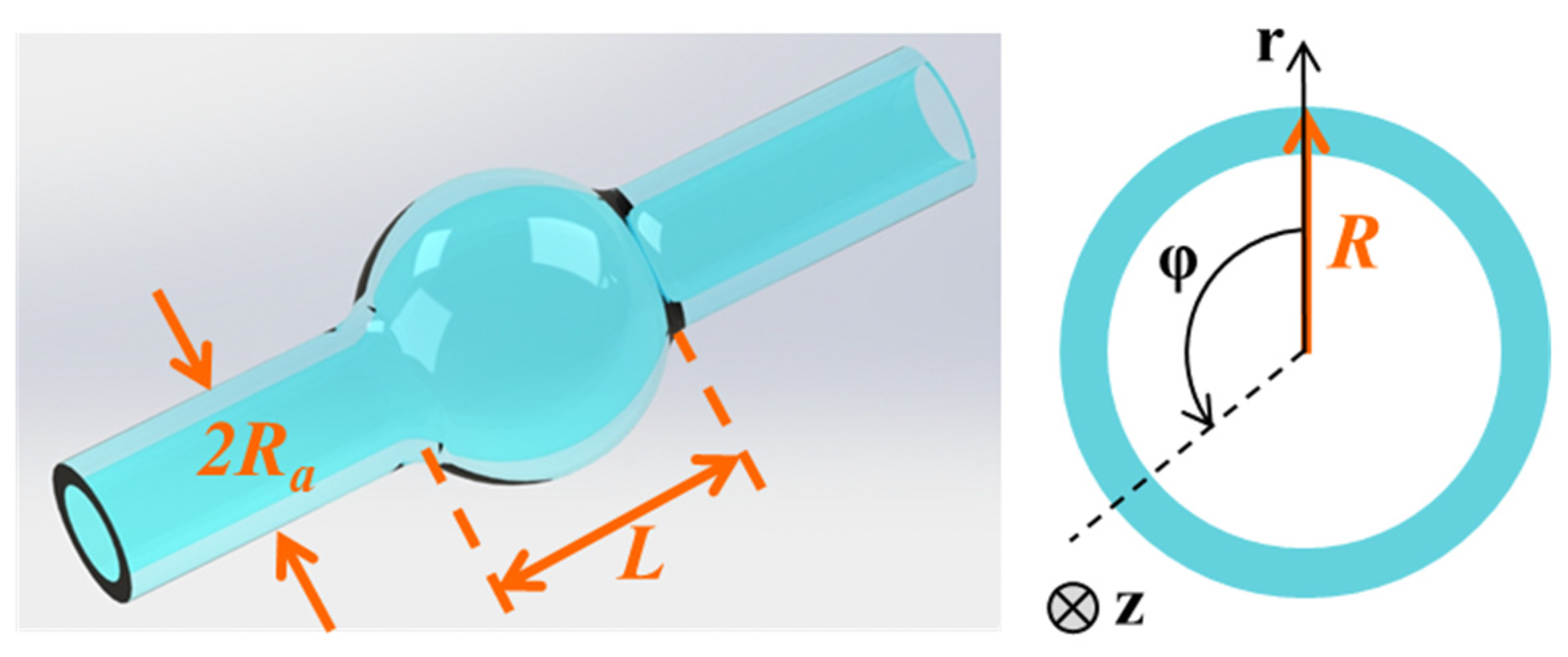
2. Optical Characteristics and Sensing Mechanism of a Microbubble Resonator
2.1. Optical Characteristics
2.2. Sensing Mechanism
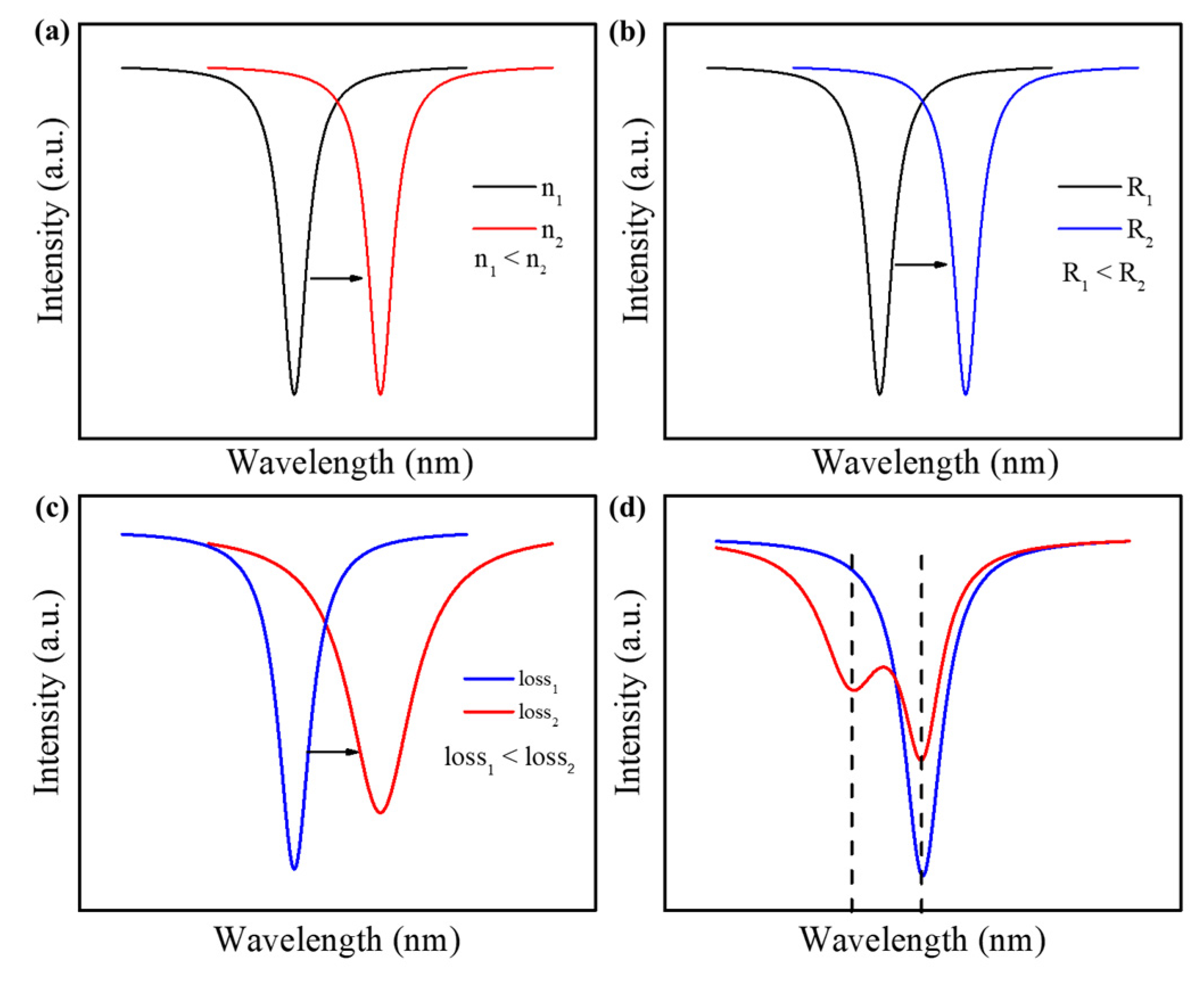
2.2.1. Wavelength Shift
2.2.2. Mode-Broadening
2.2.3. Mode-Splitting
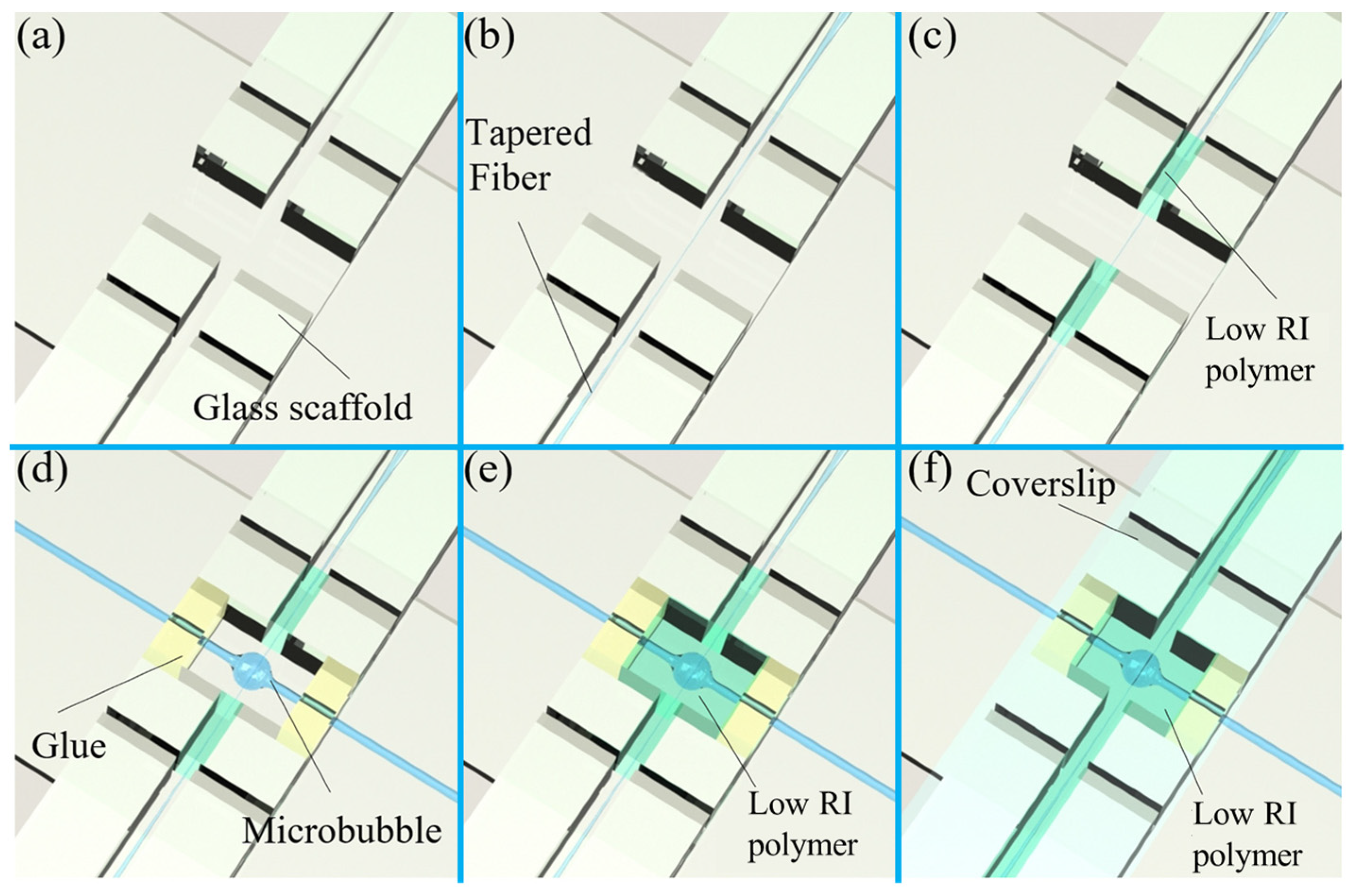
3. Fabrication and Packaging Methods of a Microbubble Resonator
3.1. Fabrication Methods of a Microbubble Resonator
3.2. Packaging Process of a Microbubble Resonator
4. Sensing Applications of Microbubble Resonators
4.1. Physical Sensing
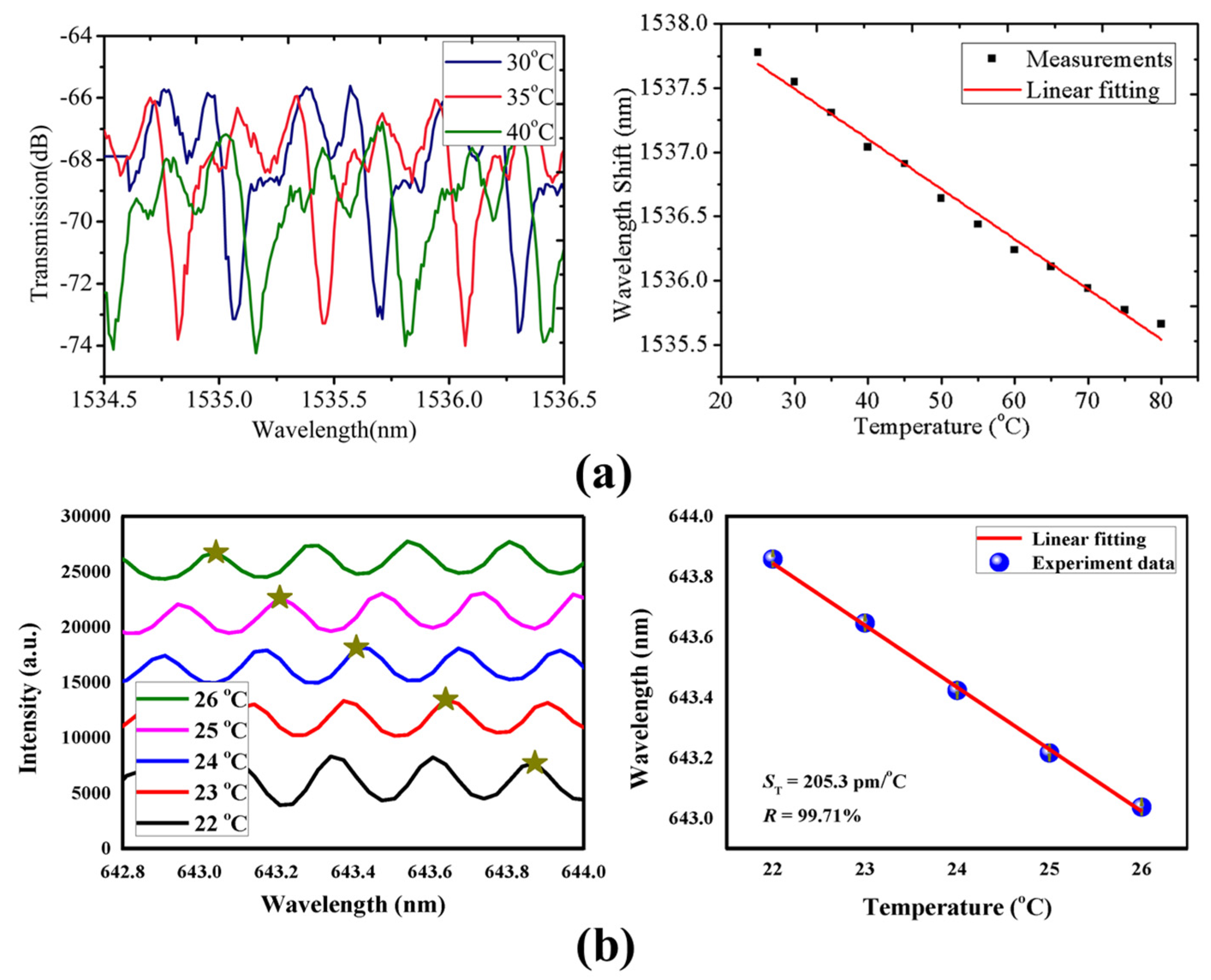
4.1.1. Temperature Sensing
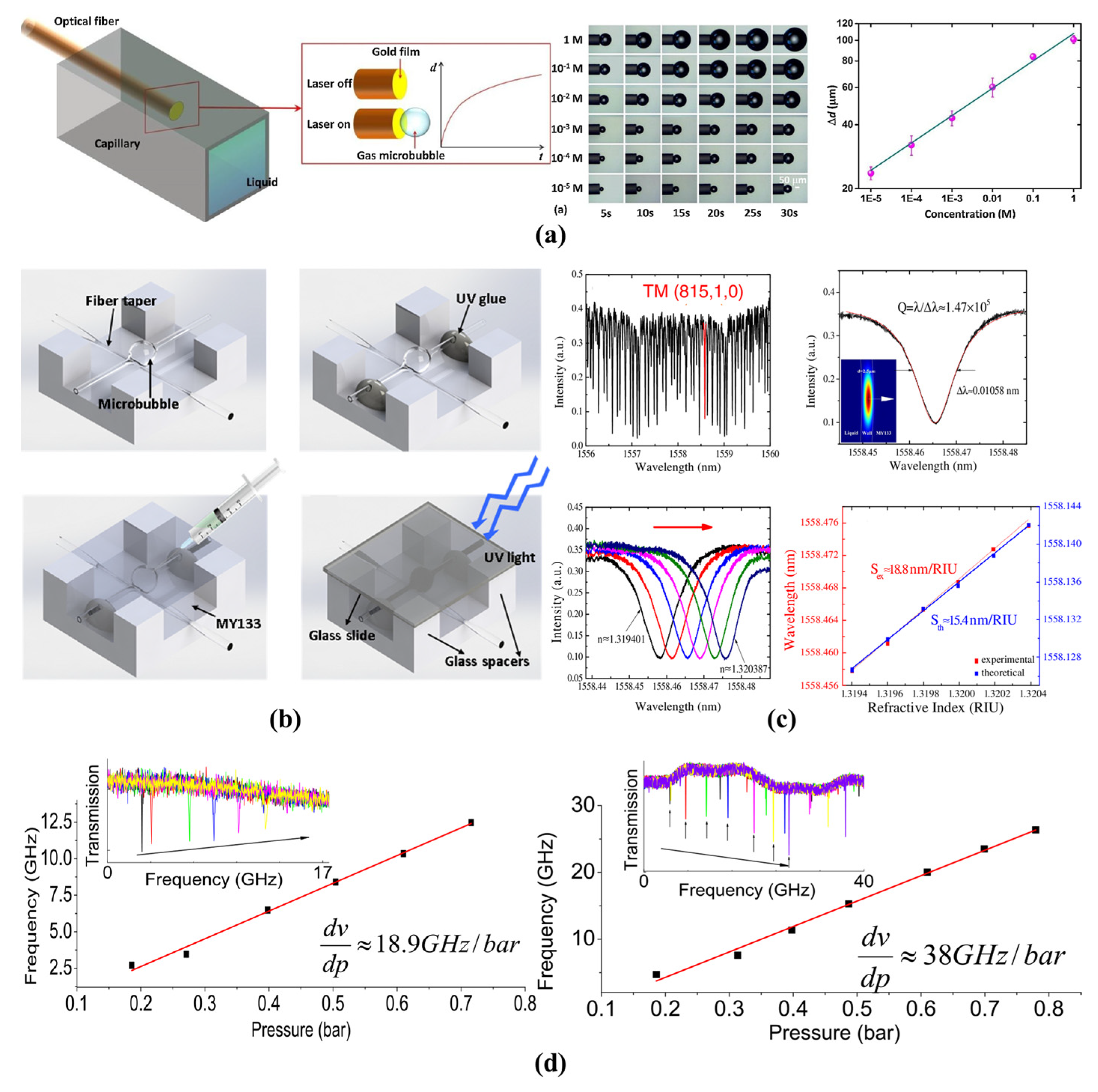
4.1.2. Bulk RI and Liquid-Concentration Sensing
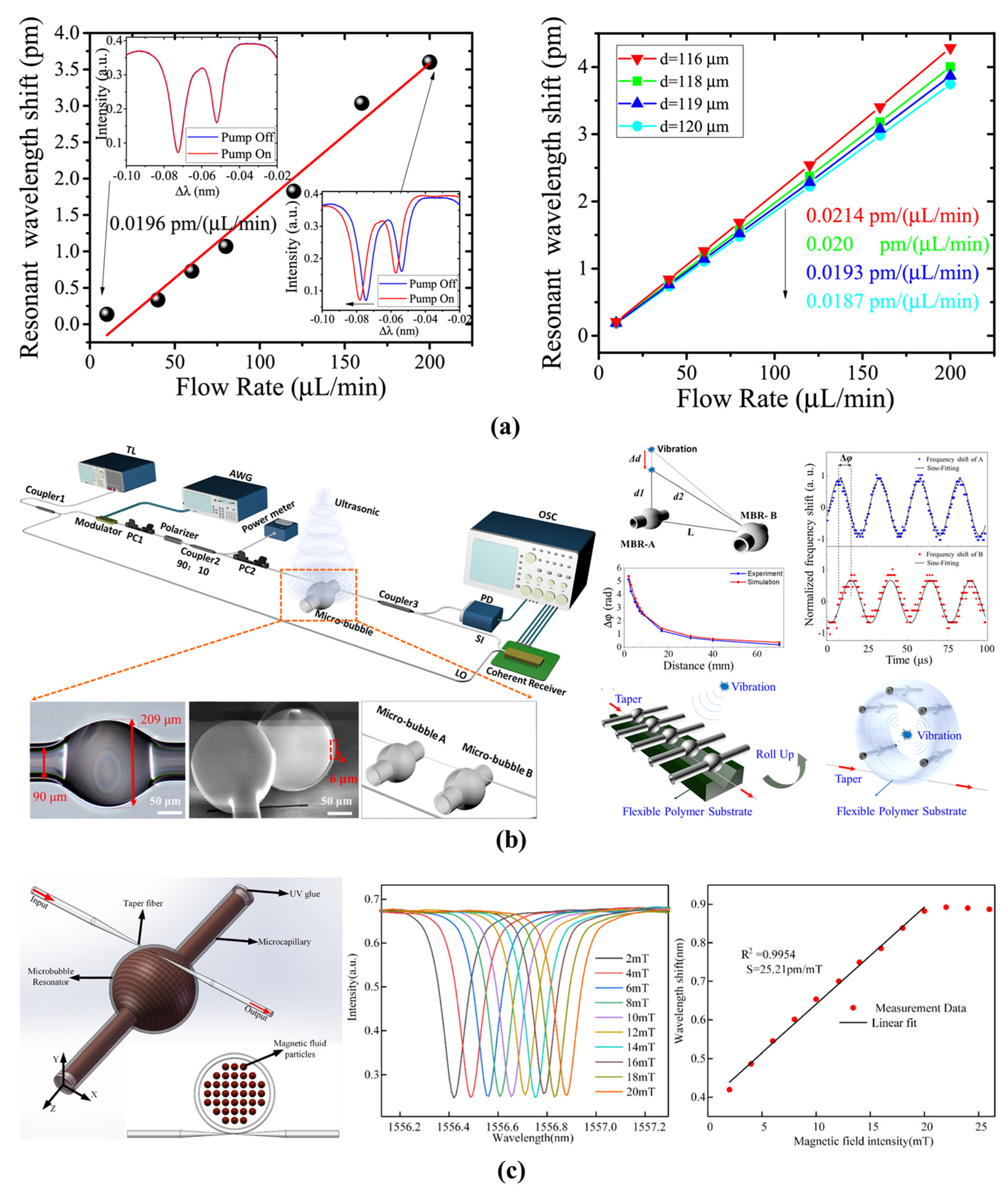
4.1.3. Pressure and Flow Rate Sensing
4.1.4. Magnetic-Field Sensing
4.2. Chemical Sensing
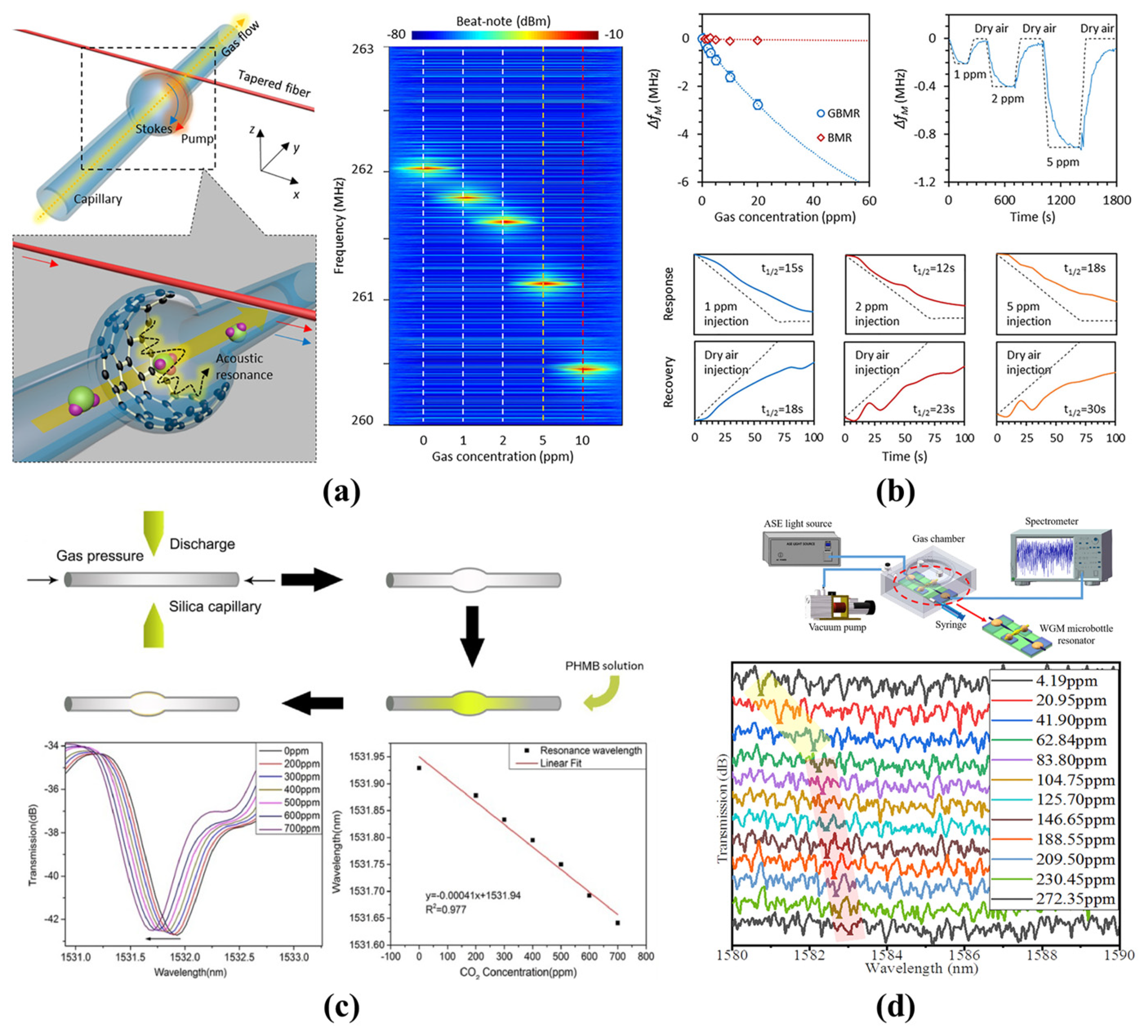
4.2.1. Gas Sensing
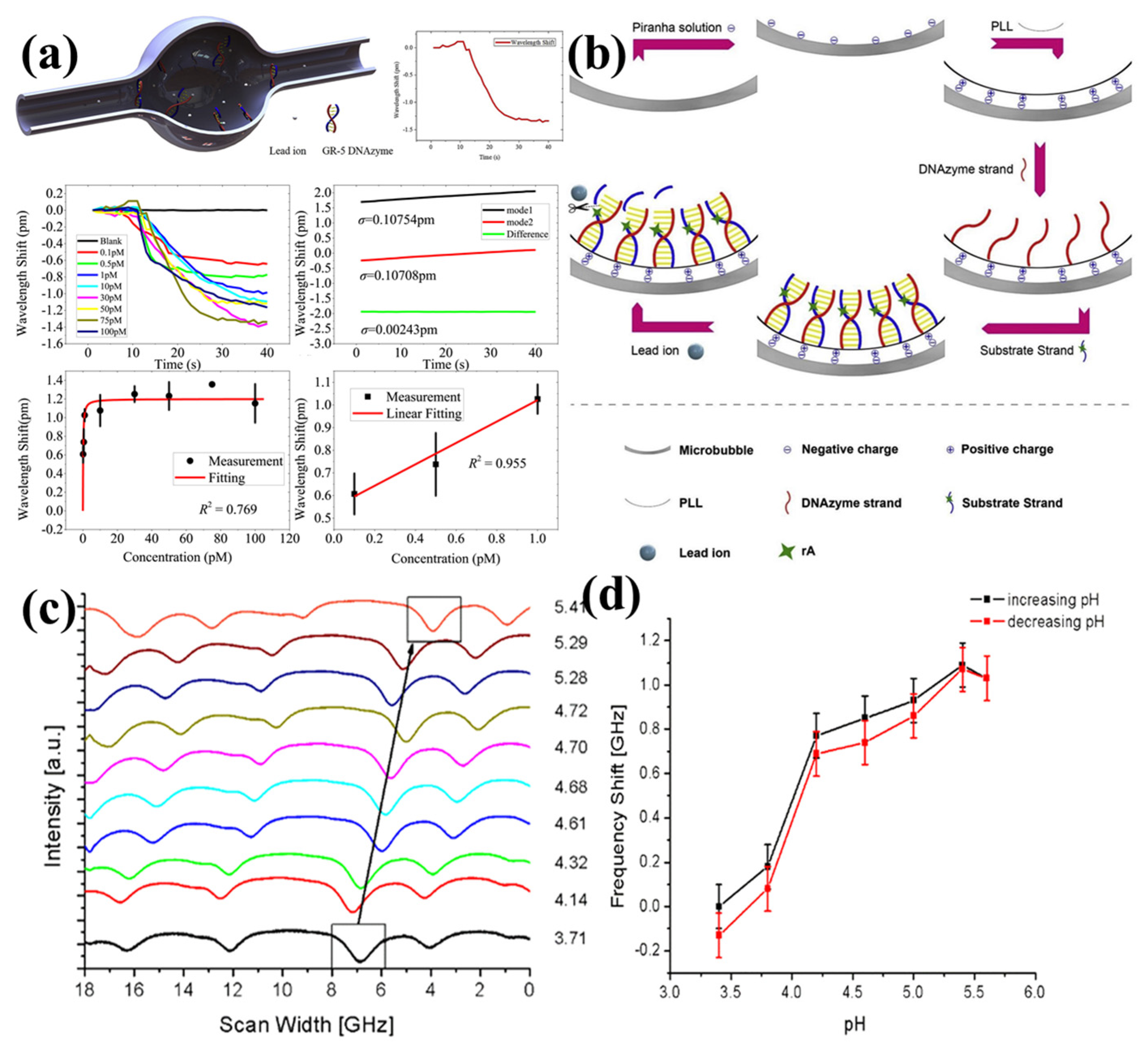
4.2.2. Ion and pH Sensing
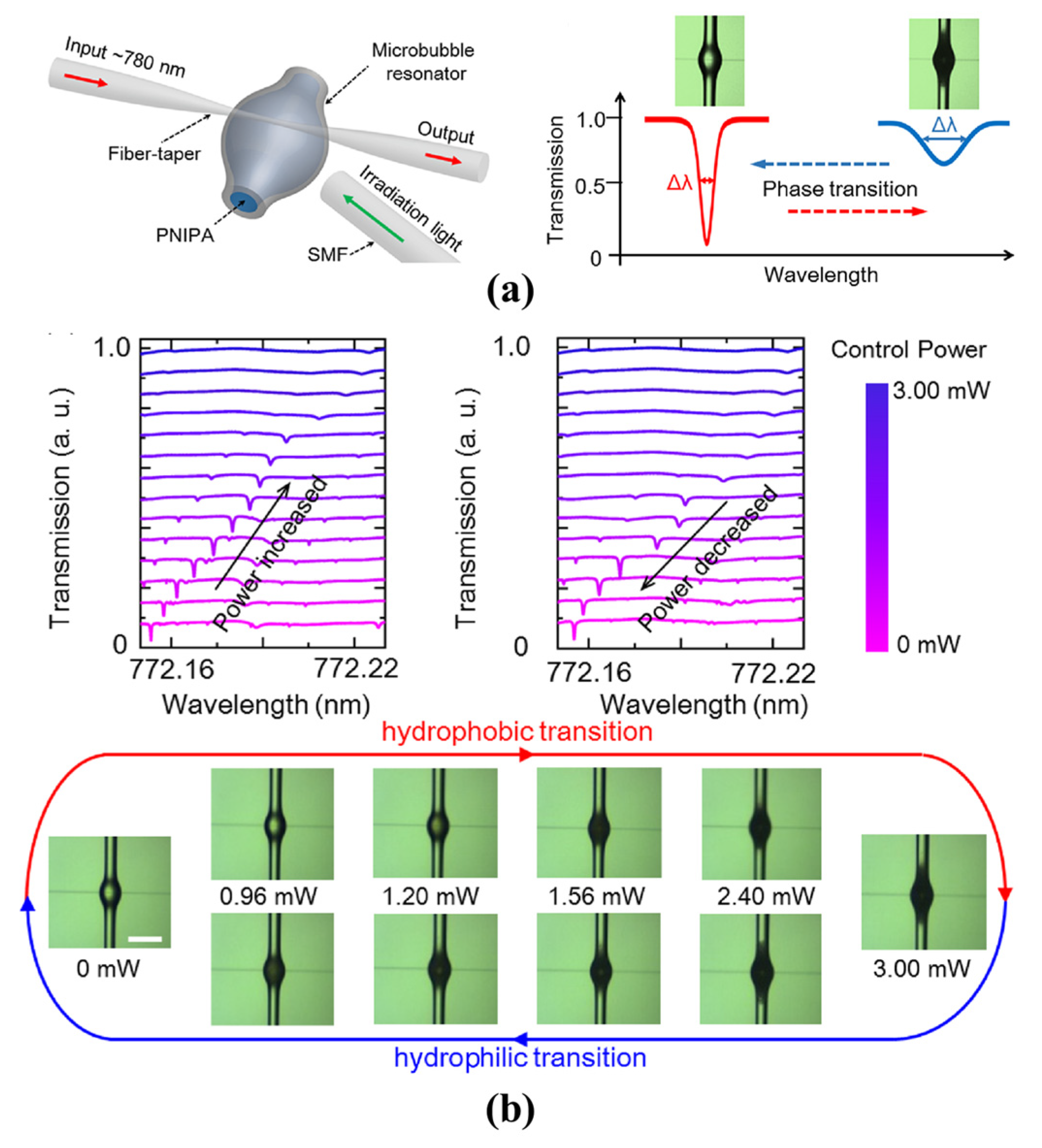
4.2.3. Hydrogel Phase Sensing
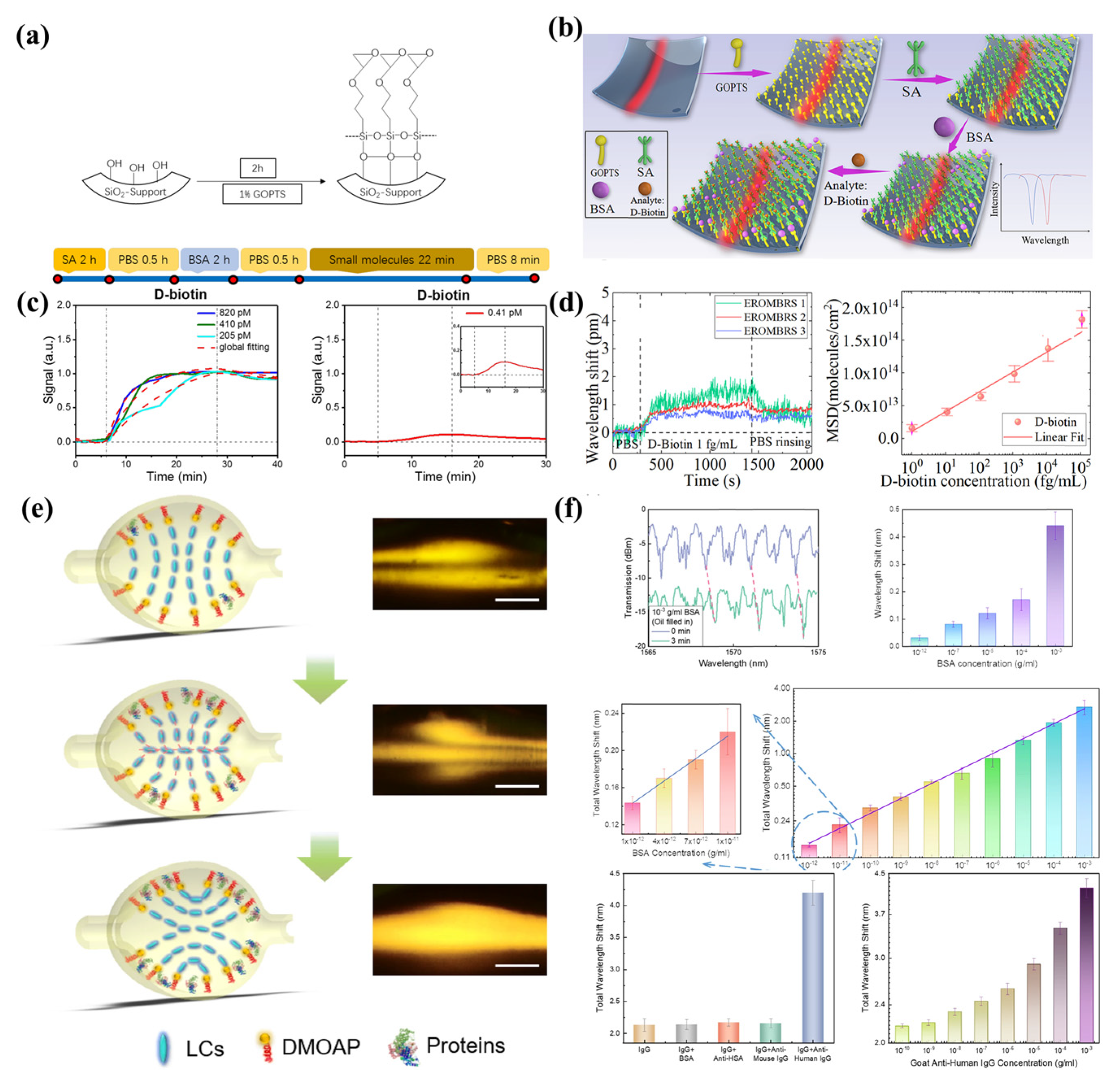
4.3. Biosensing
5. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Homola, J.; Yee, S.S.; Gauglitz, G. Surface plasmon resonance sensors. Sens. Actuators B Chem. 1999, 54, 3–15. [Google Scholar] [CrossRef]
- Hou, W.; Cronin, S.B. A review of surface plasmon resonance-enhanced photocatalysis. Adv. Funct. Mater. 2013, 23, 1612–1619. [Google Scholar] [CrossRef]
- Duveneck, G.L.; Abel, A.P.; Bopp, M.A.; Kresbach, G.M.; Ehrat, M. Planar waveguides for ultra-high sensitivity of the analysis of nucleic acids. Anal. Chim. Acta 2002, 469, 49–61. [Google Scholar] [CrossRef]
- Duveneck, G.L.; Pawlak, M.; Neuschäfer, D.; Bär, E.; Budach, W.; Pieles, U.; Ehrat, M. Novel bioaffinity sensors for trace analysis based on luminescence excitation by planar waveguides. Sens. Actuators B Chem. 1997, 38, 88–95. [Google Scholar] [CrossRef]
- Peters, K. Polymer optical fiber sensors—A review. Smart Mater. Struct. 2010, 20, 013002. [Google Scholar] [CrossRef]
- Zubia, J.; Arrue, J. Plastic optical fibers: An introduction to their technological processes and applications. Opt. Fiber Technol. 2001, 7, 101–140. [Google Scholar] [CrossRef]
- Vinet, J.Y.; Meers, B.; Man, C.N.; Brillet, A. Optimization of long-baseline optical interferometers for gravitational-wave detection. Phys. Rev. D 1988, 38, 433. [Google Scholar] [CrossRef]
- Kimble, H.J.; Lev, B.L.; Ye, J. Optical interferometers with reduced sensitivity to thermal noise. Phys. Rev. Lett. 2008, 101, 260602. [Google Scholar] [CrossRef]
- Vahala, K.J. Optical microcavities. Nature 2003, 424, 839–846. [Google Scholar] [CrossRef]
- Krioukov, E.; Klunder, D.J.W.; Driessen, A.; Greve, J.; Otto, C. Sensor based on an integrated optical microcavity. Opt. Lett. 2002, 27, 512–514. [Google Scholar] [CrossRef]
- Gather, M.C.; Yun, S.H. Single-cell biological lasers. Nat. Photonics 2011, 5, 406–410. [Google Scholar] [CrossRef]
- Wu, X.; Oo, M.K.K.; Reddy, K.; Chen, Q.; Sun, Y.; Fan, X. Optofluidic laser for dual-mode sensitive biomolecular detection with a large dynamic range. Nat. Commun. 2014, 5, 3779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Chen, Q.; Wang, Y.; Tan, X.; Fan, X. Stable High-Q Bouncing Ball Modes inside a Fabry–Pérot Cavity. ACS Photonics 2019, 6, 2470–2478. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, C.; Zhang, T.; Chen, J.; Liu, S.; Fan, X. Optofluidic laser array based on stable high-Q Fabry–Pérot microcavities. Lab Chip 2015, 15, 3862–3869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, E.; Grot, A.; Mirkarimi, L.W.; Sigalas, M.; Girolami, G. Ultracompact biochemical sensor built with two-dimensional photonic crystal microcavity. Opt. Lett. 2004, 29, 1093–1095. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K.; Barclay, P.E.; Painter, O.; Chen, J.; Cho, A.Y.; Gmachl, C. Experimental demonstration of a high quality factor photonic crystal microcavity. Appl. Phys. Lett. 2003, 83, 1915–1917. [Google Scholar] [CrossRef]
- Vučković, J.; Lončar, M.; Mabuchi, H.; Scherer, A. Design of photonic crystal microcavities for cavity QED. Phys. Rev. E 2001, 65, 016608. [Google Scholar] [CrossRef] [Green Version]
- Vuckovic, J.; Loncar, M.; Mabuchi, H.; Schere, A. Optimization of the Q factor in photonic crystal microcavities. IEEE J. Quantum Electron. 2002, 38, 850–856. [Google Scholar] [CrossRef] [Green Version]
- Eivazi, S.; Mozaffari, M.H. Numerical design and investigation of an optically pumped 1.55 μm single quantum dot photonic crystal-based laser. Photonic Nanostruct. 2018, 32, 42–46. [Google Scholar] [CrossRef]
- Mozaffari, M.H.; Ebnali-Heidari, M.; Moravvej-Farshi, M.K. A proposal for ultra-sensitive intensity-based biosensing via photonic crystal optofluidic biolaser. Laser Phys. 2019, 29, 035803. [Google Scholar] [CrossRef]
- Vollmer, F.; Arnold, S.; Keng, D. Single virus detection from the reactive shift of a whispering-gallery mode. Proc. Natl. Acad. Sci. USA 2008, 105, 20701–20704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vollmer, F.; Arnold, S. Whispering-gallery-mode biosensing: Label-free detection down to single molecules. Nat. Methods 2008, 5, 591–596. [Google Scholar] [CrossRef]
- Foreman, M.R.; Swaim, J.D.; Vollmer, F. Whispering gallery mode sensors. Adv. Opt. Photonics 2015, 7, 168–240. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Ozdemir, S.K.; Xiao, Y.F.; Li, L.; He, L.; Chen, D.R.; Yang, L. On-chip single nanoparticle detection and sizing by mode splitting in an ultrahigh-Q microresonator. Nat. Photonics 2010, 4, 46–49. [Google Scholar] [CrossRef]
- Righini, G.C.; Soria, S. Biosensing by WGM microspherical resonators. Sensors 2016, 16, 905. [Google Scholar] [CrossRef] [Green Version]
- Bianucci, P. Optical microbottle resonators for sensing. Sensors 2016, 16, 1841. [Google Scholar] [CrossRef] [Green Version]
- Bozzola, A.; Perotto, S.; Angelis, F.D. Hybrid plasmonic–photonic whispering gallery mode resonators for sensing: A critical review. Analyst 2017, 142, 883–898. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.; Baaske, M.D.; Vollmer, F. Towards next-generation label-free biosensors: Recent advances in whispering gallery mode sensors. Lab Chip 2017, 17, 1190–1205. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, S.; Humbert, G.; Ho, H.P. Microfluidic whispering gallery mode optical sensors for biological applications. Laser Photonics Rev. 2020, 14, 2000135. [Google Scholar] [CrossRef]
- Jiang, X.; Qavi, A.J.; Huang, S.H.; Yang, L. Whispering-gallery sensors. Matter 2020, 3, 371–392. [Google Scholar] [CrossRef]
- Toropov, N.; Cabello, G.; Serrano, M.P.; Gutha, R.R.; Rafti, M.; Vollmer, F. Review of biosensing with whispering-gallery mode lasers. Light Sci. Appl. 2021, 10, 42. [Google Scholar] [CrossRef]
- Feng, Z.; Bai, L. Advances of optofluidic microcavities for microlasers and biosensors. Micromachines 2018, 9, 122. [Google Scholar] [CrossRef] [Green Version]
- Mozaffari, M.H.; Farmani, A. On-chip single-mode optofluidic microresonator dye laser sensor. IEEE Sens. J. 2019, 20, 3556–3563. [Google Scholar] [CrossRef]
- Savchenkov, A.A.; Matsko, A.B.; Ilchenko, V.S.; Maleki, L. Optical resonators with ten million finesse. Opt. Express 2007, 15, 6768–6773. [Google Scholar] [CrossRef] [PubMed]
- Rayleigh, L. The problem of the whispering gallery. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1910, 20, 1001–1004. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.; Li, H.; Suter, J.D.; Reddy, K.; Sun, Y.; Fan, X. Tunable single mode lasing from an on-chip optofluidic ring resonator laser. Appl. Phys. Lett. 2011, 98, 061103. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Li, H.; Liu, L.; Xu, L. Unidirectional single-frequency lasing from a ring-spiral coupled microcavity laser. Appl. Phys. Lett. 2008, 93, 081105. [Google Scholar] [CrossRef]
- Ren, L.; Wu, X.; Li, M.; Zhang, X.; Liu, L.; Xu, L. Ultrasensitive label-free coupled optofluidic ring laser sensor. Opt. Lett. 2012, 37, 3873–3875. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, L.; Wu, X.; Li, H.; Liu, L.; Xu, L. Coupled optofluidic ring laser for ultrahigh-sensitive sensing. Opt. Express 2011, 19, 22242–22247. [Google Scholar] [CrossRef]
- Tu, X.; Wu, X.; Li, M.; Liu, L.; Xu, L. Ultraviolet single-frequency coupled optofluidic ring resonator dye laser. Opt. Express 2012, 20, 19996–20001. [Google Scholar] [CrossRef]
- Armani, D.; Min, B.; Martin, A.; Vahala, K.J. Electrical thermo-optic tuning of ultrahigh-Q microtoroid resonators. Appl. Phys. Lett. 2004, 85, 5439–5441. [Google Scholar] [CrossRef] [Green Version]
- Hossein-Zadeh, M.; Vahala, K.J. Free ultra-high-Q microtoroid: A tool for designing photonic devices. Opt. Express 2007, 15, 166–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, J. Label-free single exosome detection using frequency-locked microtoroid optical resonators. ACS Photonics 2015, 2, 1241–1245. [Google Scholar] [CrossRef]
- Wang, T.J.; Chen, P.T.; Hsiao, W.C.; Chen, C.H. High-Q LiNbO3 microtoroid resonators. J. Light Technol. 2016, 34, 3306–3311. [Google Scholar] [CrossRef]
- Xiao, Y.F.; He, L.; Zhu, J.; Yang, L. Electromagnetically induced transparency-like effect in a single polydimethylsiloxane-coated silica microtoroid. Appl. Phys. Lett. 2009, 94, 231115. [Google Scholar] [CrossRef]
- Zhu, J.; Özdemir, Ş.K.; He, L.; Chen, D.R.; Yang, L. Single virus and nanoparticle size spectrometry by whispering-gallery-mode microcavities. Opt. Express 2011, 19, 16195–16206. [Google Scholar] [CrossRef]
- Kippenberg, T.J.; Kalkman, J.; Polman, A.; Vahala, K.J. Demonstration of an erbium-doped microdisk laser on a silicon chip. Phys. Rev. A 2006, 74, 051802. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Li, H.; Liu, L.; Xu, L. High quality Direct Photo-Patterned Microdisk Lasers with Organic–Inorganic Hybrid Materials. IEEE J. Quantum Electron. 2007, 44, 75–80. [Google Scholar] [CrossRef]
- Guo, Z.; Qin, Y.; Chen, P.; Hu, J.; Zhou, Y.; Zhao, X.; Liu, Z.; Fei, Y.; Jiang, X.; Wu, X. Hyperboloid-Drum Microdisk Laser Biosensors for Ultrasensitive Detection of Human IgG. Small 2020, 16, 2000239. [Google Scholar] [CrossRef]
- Li, Y.; Feng, L.; Li, F.; Hu, P.; Du, M.; Su, X.; Sun, D.; Tang, H.; Li, Q.; Yun, F. Three-dimensional anisotropic microlaser from GaN-based self-bent-up microdisk. ACS Photonics 2018, 5, 4259–4264. [Google Scholar] [CrossRef]
- Lu, Q.; Chen, X.; Fu, L.; Xie, S.; Wu, X. On-Chip Real-Time chemical sensors based on water-immersion-objective pumped whispering-gallery-mode microdisk laser. Nanomaterials 2019, 9, 479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, R.; Ling, B.; Sun, X.W.; Sun, H.D. Room temperature excitonic whispering gallery mode lasing from high-quality hexagonal ZnO microdisks. Adv. Mater. 2011, 23, 2199–2204. [Google Scholar] [CrossRef] [PubMed]
- Chantada, L.; Nikolaev, N.I.; Ivanov, A.L.; Borri, P.; Langbein, W. Optical resonances in microcylinders: Response to perturbations for biosensing. J. Opt. Soc. Am. B 2008, 25, 1312–1321. [Google Scholar] [CrossRef]
- Lee, S.B.; Lee, J.H.; Chang, J.S.; Moon, H.J.; Kim, S.W.; An, K. Observation of scarred modes in asymmetrically deformed microcylinder lasers. Phys. Rev. Lett. 2002, 88, 033903. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Shang, L.; Tu, X.; Liu, L.; Xu, L. Coupling variation induced ultrasensitive label-free biosensing by using single mode coupled microcavity laser. J. Am. Chem. Soc. 2009, 131, 16612–16613. [Google Scholar] [CrossRef]
- Wan, H.; Chen, J.; Wan, C.; Zhou, Q.; Wang, J.; Zhang, Z. Optofluidic microcapillary biosensor for label-free, low glucose concentration detection. Biomed. Opt. Express 2019, 10, 3929–3937. [Google Scholar] [CrossRef]
- Kurahashi, N.; Nguyen, V.C.; Sasaki, F.; Yanagi, H. Whispering gallery mode lasing in lead halide perovskite crystals grown in microcapillary. Appl. Phys. Lett. 2018, 113, 011107. [Google Scholar] [CrossRef]
- Zhu, X.; Zhan, Z.; Li, J.; Li, M.; Song, Y. High-sensitivity temperature sensor based on Fano resonance in an optofluidic microcapillary resonator. Appl. Opt. 2020, 59, 1259–1262. [Google Scholar] [CrossRef]
- Ward, J.; Benson, O. WGM microresonators: Sensing, lasing and fundamental optics with microspheres. Laser Photonics Rev. 2011, 5, 553–570. [Google Scholar] [CrossRef]
- Braunfelds, J.; Murnieks, R.; Salgals, T.; Brice, I.; Sharashidze, T.; Lyashuk, I.; Ostrovskis, A.; Spolitis, S.; Alnis, J.; Porins, J. Frequency comb generation in WGM microsphere based generators for telecommunication applications. Quantum Electron. 2020, 50, 1043. [Google Scholar] [CrossRef]
- Hanumegowda, N.M.; Stica, C.J.; Patel, B.C.; White, I.; Fan, X. Refractometric sensors based on microsphere resonators. Appl. Phys. Lett. 2005, 87, 201107. [Google Scholar] [CrossRef]
- Awerkamp, P.A.; Fish, D.; King, M.; Hill, D.; Nordin, G.P.; Camacho, R.M. 3D printed mounts for microdroplet resonators. Opt. Express 2022, 30, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Wu, X.; Liu, L.; Xu, L. Mode-selective lasing in high-Q polymer micro bottle resonators. Opt. Express 2015, 23, 22740–22745. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wu, X.; Liu, L.; Fan, X.; Xu, L. Self-referencing optofluidic ring resonator sensor for highly sensitive biomolecular detection. Anal. Chem. 2013, 85, 9328–9332. [Google Scholar] [CrossRef] [PubMed]
- Riesen, N.; Zhang, W.Q.; Monro, T.M. Dispersion analysis of whispering gallery mode microbubble resonators. Opt. Express 2016, 24, 8832–8847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riesen, N.; Zhang, W.Q.; Monro, T.M. Dispersion in silica microbubble resonators. Opt. Lett. 2016, 41, 1257–1260. [Google Scholar] [CrossRef]
- Frigenti, G.; Cavigli, L.; Ratto, F.; Centi, S.; Murzina, T.V.; Farnesi, D.; Pelli, S.; Soria, S.; Conti, G.N. Microbubble resonators for scattering-free absorption spectroscopy of nanoparticles. Opt. Express 2021, 29, 31130–31136. [Google Scholar] [CrossRef]
- Farnesi, D.; Barucci, A.; Righini, G.C.; Conti, G.N.; Soria, S. Generation of hyper-parametric oscillations in silica microbubbles. Opt. Lett. 2015, 40, 4508–4511. [Google Scholar] [CrossRef]
- Farnesi, D.; Righini, G.; Conti, G.N.; Soria, S. Efficient frequency generation in phoxonic cavities based on hollow whispering gallery mode resonators. Sci. Rep. 2017, 7, 44198. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Ward, J.; Chormaic, S.N. Quasi-droplet microbubbles for high resolution sensing applications. Opt. Express 2014, 22, 6881–6898. [Google Scholar] [CrossRef] [Green Version]
- Lu, Q.; Chen, X.; Liu, X.; Fu, L.; Zou, C.L.; Xie, S. Tunable optofluidic liquid metal core microbubble resonator. Opt. Express 2020, 28, 2201–2209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, L.; Xu, L. Ultralow sensing limit in optofluidic micro-bottle resonator biosensor by self-referenced differential-mode detection scheme. Appl. Phys. Lett. 2014, 104, 033703. [Google Scholar] [CrossRef]
- Watkins, A.; Ward, J.; Wu, Y.; Chormaic, S.N. Single-input spherical microbubble resonator. Opt. Lett. 2011, 36, 2113–2115. [Google Scholar] [CrossRef] [PubMed]
- Frigenti, G.; Cavigli, L.; Fernández-Bienes, A.; Ratto, F.; Centi, S.; García-Fernández, T.; Conti, G.N.; Soria, S. Resonant microbubble as a microfluidic stage for all-optical photoacoustic sensing. Phys. Rev. Appl. 2019, 12, 014062. [Google Scholar] [CrossRef]
- Frigenti, G.; Cavigli, L.; Fernández-Bienes, A.; Ratto, F.; Centi, S.; García-Fernández, T.; Nunzi Conti, G.; Soria, S. Microbubble resonators for all-optical photoacoustics of flowing contrast agents. Sensors 2020, 20, 1696. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Li, M.; Wu, X.; Liu, L.; Xu, L. 2-D optical/opto-mechanical microfluidic sensing with micro-bubble resonators. Opt. Express 2015, 23, 17659–17664. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, X.; Liu, L.; Xu, L. Optical spring effect in micro-bubble resonators and its application for the effective mass measurement of optomechanical resonant mode. Sensors 2017, 17, 2256. [Google Scholar] [CrossRef] [Green Version]
- Cosci, A.; Berneschi, S.; Giannetti, A.; Farnesi, D.; Cosi, F.; Baldini, F.; Conti, G.N.; Soria, S.; Barucci, A.; Righini, G.; et al. Resonance frequency of optical microbubble resonators: Direct measurements and mitigation of fluctuations. Sensors 2016, 16, 1405. [Google Scholar] [CrossRef] [Green Version]
- Lu, Q.; Li, M.; Liao, J.; Liu, S.; Wu, X.; Liu, L.; Xu, L. Strong coupling of hybrid and plasmonic resonances in liquid core plasmonic micro-bubble cavities. Opt. Lett. 2015, 40, 5842–5845. [Google Scholar] [CrossRef]
- Lu, Q.; Liu, S.; Wu, X.; Liu, L.; Xu, L. Stimulated Brillouin laser and frequency comb generation in high-Q microbubble resonators. Opt. Lett. 2016, 41, 1736–1739. [Google Scholar] [CrossRef]
- Liao, J.; Wu, X.; Liu, L.; Xu, L. Fano resonance and improved sensing performance in a spectral-simplified optofluidic micro-bubble resonator by introducing selective modal losses. Opt. Express 2016, 24, 8574–8580. [Google Scholar] [CrossRef]
- Hogan, L.T.; Horak, E.H.; Ward, J.M.; Knapper, K.A.; Chormaic, S.N.; Goldsmith, R.H. Toward real-time monitoring and control of single nanoparticle properties with a microbubble resonator spectrometer. ACS Nano 2019, 13, 12743–12757. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fan, X.; Li, Z.; Tang, T.; Wu, F.; Shen, D.; Wu, X. Stabilizing and Tuning the Laser Frequencies in Er-Doped Fiber Ring Lasers Based on Microbubble Resonators. IEEE Photonics J. 2017, 9, 1502509. [Google Scholar] [CrossRef]
- Louyer, Y.; Meschede, D.; Rauschenbeutel, A. Tunable whispering-gallery-mode resonators for cavity quantum electrodynamics. Phys. Rev. A 2005, 72, 031801. [Google Scholar] [CrossRef] [Green Version]
- Pöllinger, M.; O’Shea, D.; Warken, F.; Rauschenbeutel, A. Ultrahigh-Q tunable whispering-gallery-mode microresonator. Phys. Rev. lett. 2009, 103, 053901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sumetsky, M.; Dulashko, Y.; Windeler, R.S. Optical microbubble resonator. Opt. Lett. 2010, 35, 898–900. [Google Scholar] [CrossRef] [PubMed]
- Sumetsky, M.; Dulashko, Y.; Windeler, R.S. Super free spectral range tunable optical microbubble resonator. Opt. Lett. 2010, 35, 1866–1868. [Google Scholar] [CrossRef]
- Vollmer, F.; Yu, D. Optical Whispering Gallery Modes for Biosensing; Springer: Berlin/Heidelberg, Germany, 2020; pp. 145–149. [Google Scholar]
- Tang, T.; Wu, X.; Liu, L.; Xu, L. Packaged optofluidic microbubble resonators for optical sensing. Appl. Opt. 2016, 55, 395–399. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, C.; Guo, Z.; Wang, B.; Wu, X.; Fei, Y. Highly sensitive label-free detection of small molecules with an optofluidic microbubble resonator. Micromachines 2018, 9, 274. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Duan, B.; Wang, A.; Pan, Y.; Wang, C.; Ji, Y.; Chen, J.H. Packaged microbubble resonator for versatile optical sensing. J. Light Technol. 2020, 38, 4555–4559. [Google Scholar] [CrossRef]
- Yang, D.; Wang, A.; Chen, J.H.; Yu, X.C.; Lan, C.; Ji, Y.; Xiao, Y.F. Real-time monitoring of hydrogel phase transition in an ultrahigh Q microbubble resonator. Photonics Res. 2020, 8, 497–502. [Google Scholar] [CrossRef]
- Guo, Z.; Lu, Q.; Zhu, C.; Wang, B.; Zhou, Y.; Wu, X. Ultra-sensitive biomolecular detection by external referencing optofluidic microbubble resonators. Opt. Express 2019, 27, 12424–12435. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Liu, J.; Zheng, Z. Self-expanding fabricated optical millibubble resonators with strong thermo-optical effect. Opt. Commun. 2020, 472, 125821. [Google Scholar] [CrossRef]
- Ward, J.M.; Yang, Y.; Chormaic, S.N. Highly sensitive temperature measurements with liquid-core microbubble resonators. IEEE Photonics Technol. Lett. 2013, 25, 2350–2353. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Su, H.; Zhu, F.; Yi, G.; Wang, J. Magnetic-field tuning whispering gallery mode based on hollow microbubble resonator with Terfenol-D-fixed. Appl. Opt. 2019, 58, 8889–8893. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, W.; Wang, R.; Xing, E.; Jing, N.; Zhou, Y.; Tang, J.; Liu, J. Magnetic sensor based on WGM hollow microbubble resonator filled with magnetic fluid. Opt. Commun. 2021, 497, 127148. [Google Scholar] [CrossRef]
- Henze, R.; Seifert, T.; Ward, J.; Benson, O. Tuning whispering gallery modes using internal aerostatic pressure. Opt. Lett. 2011, 36, 4536–4538. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Liao, J.; Liu, S.; Wu, X.; Liu, L.; Xu, L. Precise measurement of micro bubble resonator thickness by internal aerostatic pressure sensing. Opt. Express 2016, 24, 20855–20861. [Google Scholar] [CrossRef]
- Chen, Z.; Guo, Z.; Mu, X.; Li, Q.; Wu, X.; Fu, H.Y. Packaged microbubble resonator optofluidic flow rate sensor based on Bernoulli Effect. Opt. Express 2019, 27, 36932–36940. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Zhao, S.; Yu, Y.; Sun, H.; Yang, Y.; Dong, Y.; Huang, Y.; Wang, T. High-Sensitivity Flow Rate Sensor Enabled by Higher Order Modes of Packaged Microbottle Resonator. IEEE Photonics Technol. Lett. 2021, 33, 599–602. [Google Scholar] [CrossRef]
- Berneschi, S.; Farnesi, D.; Cosi, F.; Conti, G.N.; Pelli, S.; Righini, G.C.; Soria, S. High Q silica microbubble resonators fabricated by arc discharge. Opt. Lett. 2011, 36, 3521–3523. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wu, X.; Liu, L.; Xu, L. Kerr parametric oscillations and frequency comb generation from dispersion compensated silica micro-bubble resonators. Opt. Express 2013, 21, 16908–16913. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Liu, T.; Jiang, J.; Liu, K.; Chen, W.; Zhang, X.; Lin, X.; Liu, W. High Q silica microbubble resonators fabricated by heating a pressurized glass capillary. In Advanced Sensor Systems and Applications VI; International Society for Optics and Photonics: Beijing, China, 2014. [Google Scholar]
- Jiang, J.; Liu, Y.; Liu, K.; Wang, S.; Ma, Z.; Zhang, Y.; Niu, P.; Shen, L.; Liu, T. Wall-thickness-controlled microbubble fabrication for WGM-based application. Appl. Opt. 2020, 59, 5052–5057. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Saurabh, S.; Ward, J.M.; Chormaic, S.N. High-Q, ultrathin-walled microbubble resonator for aerostatic pressure sensing. Opt. Express 2016, 24, 294–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.L.; Gong, Y.; Wu, Y.; Rao, Y.J.; Peng, G.D.; Fan, X. Lab-on-tip based on photothermal microbubble generation for concentration detection. Sens. Actuators B Chem. 2018, 255, 2504–2509. [Google Scholar] [CrossRef]
- He, C.; Sun, H.; Mo, J.; Yang, C.; Feng, G.; Zhou, H.; Zhou, S. Temperature sensor based on high-Q polymethylmethacrylate optical microbubble. Laser Phys. 2018, 28, 076202. [Google Scholar] [CrossRef]
- Wang, P.; Ward, J.; Yang, Y.; Feng, X.; Brambilla, G.; Farrell, G.; Chormaic, S.N. Lead-silicate glass optical microbubble resonator. Appl. Phys. Lett. 2015, 106, 061101. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Zhang, J.; Wang, R.; Li, A.; Zhang, M.; Wang, S.; Wang, P.; Ward, J.M.; Chormaic, S.N. A tellurite glass optical microbubble resonator. Opt. Express 2020, 28, 32858–32868. [Google Scholar] [CrossRef]
- Zhu, N.; Shi, B.; Guo, Y.; Han, B.; Zhang, Y. Polydimethylsiloxane self-assembled whispering gallery mode microbottle resonator for ethanol sensing. Opt. Mater. 2020, 107, 110024. [Google Scholar] [CrossRef]
- Yang, Y.; Lei, F.; Kasumie, S.; Xu, L.; Ward, J.M.; Yang, L. Tunable erbium-doped microbubble laser fabricated by sol-gel coating. Opt. Express 2017, 25, 1308–1313. [Google Scholar] [CrossRef] [Green Version]
- Cai, L.; Pan, J.; Hu, S. Overview of the coupling methods used in whispering gallery mode resonator systems for sensing. Opt. Lasers Eng. 2020, 127, 105968. [Google Scholar] [CrossRef]
- Cai, M.; Painter, O.; Vahala, K.J. Observation of critical coupling in a fiber taper to a silica-microsphere whispering-gallery mode system. Phys. Rev. Lett. 2000, 85, 74–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Liu, B.; Zhang, H.; Lin, W.; Li, Y.; Liu, H.; Song, B. Excitation of whispering gallery modes in silica capillary using SMF-MMF joint with lateral offset. IEEE Photonics Technol. Lett. 2017, 29, 1027–1030. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Y.; Sun, H. Advances and prospects for whispering gallery mode microcavities. Adv. Opt. Mater. 2015, 3, 1136–1162. [Google Scholar] [CrossRef]
- Chen, X.; Fu, L.; Lu, Q.; Wu, X.; Xie, S. Packaged droplet microresonator for thermal sensing with high sensitivity. Sensors 2018, 18, 3881. [Google Scholar] [CrossRef] [Green Version]
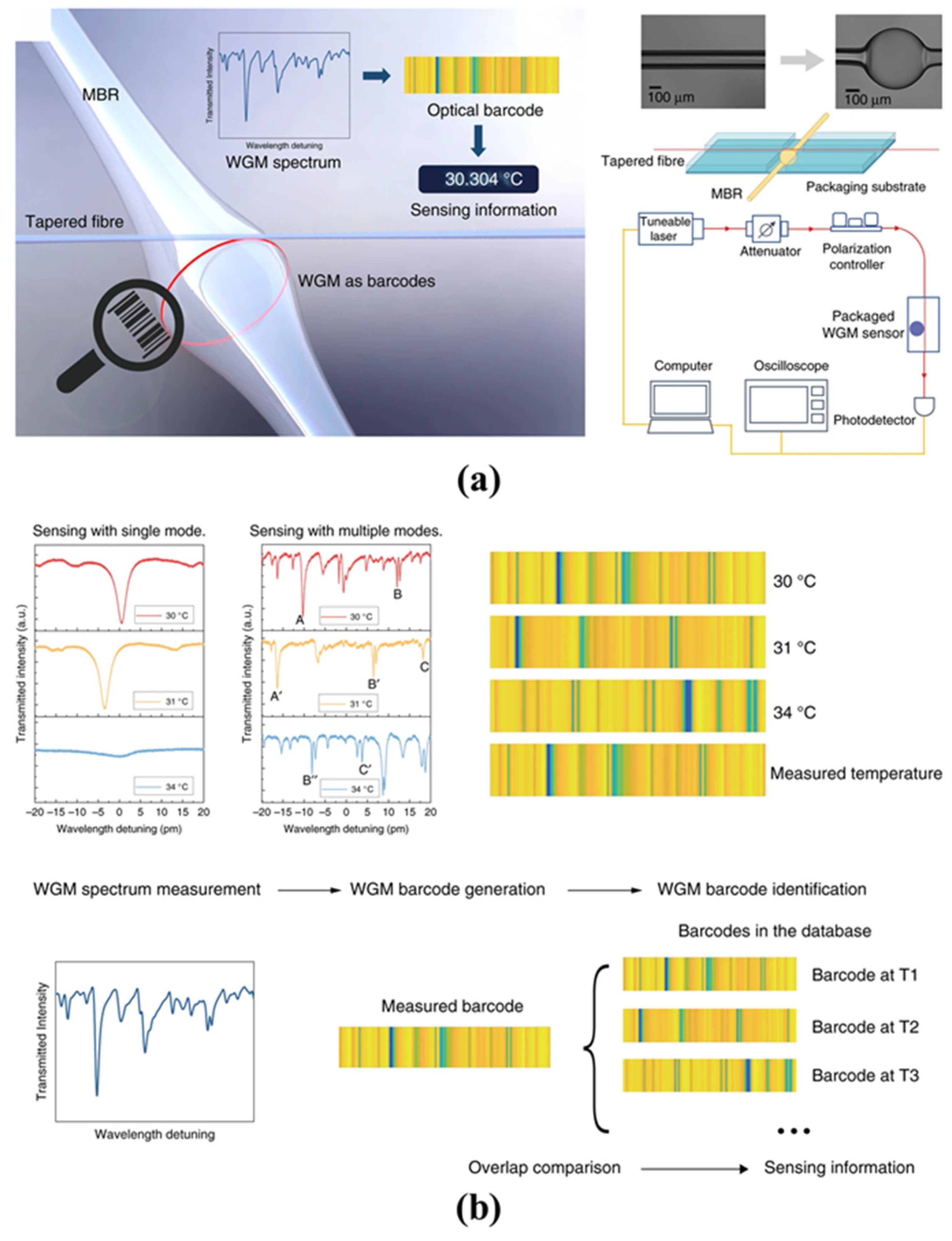
- Liao, J.; Yang, L. Optical whispering-gallery mode barcodes for high-precision and wide-range temperature measurements. Light Sci. Appl. 2021, 10, 32. [Google Scholar] [CrossRef]
- Liao, J.; Qavi, A.J.; Dong, R.; Yang, L. Packaging of optofluidic microbubble resonator sensors. In Proceedings of the Chemical, Biological, Radiological, Nuclear, and Explosives (CBRNE) Sensing XX, Baltimore, MD, USA, 17 May 2019. [Google Scholar]
- Pan, J.; Zhang, B.; Liu, Z.; Zhao, J.; Feng, Y.; Wan, L.; Li, Z. Microbubble resonators combined with a digital optical frequency comb for high-precision air-coupled ultrasound detectors. Photonics Res. 2020, 8, 303–310. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Liu, B.; Li, Y.; Song, B. Whispering-gallery-mode tuning in a magnetic-fluid-infiltrated microbubble resonator based on laser-induced photo-thermal effect. In Proceedings of the Asia-Pacific Optical Sensors Conference, Shanghai, China, 11–14 October 2016. [Google Scholar]
- Yao, B.; Yu, C.; Wu, Y.; Huang, S.W.; Wu, H.; Gong, Y.; Chen, Y.; Li, Y.; Wong, C.W.; Fan, X.; et al. Graphene-enhanced Brillouin optomechanical microresonator for ultrasensitive gas detection. Nano Lett. 2017, 17, 4996–5002. [Google Scholar] [CrossRef]
- Li, H.; Sun, B.; Yuan, Y.; Yang, J. Guanidine derivative polymer coated microbubble resonator for high sensitivity detection of CO2 gas concentration. Opt. Express 2019, 27, 1991–2000. [Google Scholar] [CrossRef]
- Peng, Z.D.; Yu, C.Q.; Ren, H.L.; Zou, C.L.; Guo, G.C.; Dong, C.H. Gas identification in high-Q microbubble resonators. Opt. Lett. 2020, 45, 4440–4443. [Google Scholar] [CrossRef]
- Fu, L.; Lu, Q.; Liu, X.; Chen, X.; Wu, X.; Xie, S. Combining whispering gallery mode optofluidic microbubble resonator sensor with GR-5 DNAzyme for ultra-sensitive lead ion detection. Talanta 2020, 213, 120815. [Google Scholar] [CrossRef] [PubMed]
- Stoian, R.I.; Lavine, B.K.; Rosenberger, A.T. pH sensing using whispering gallery modes of a silica hollow bottle resonator. Talanta 2019, 194, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.Q.; Chen, J.; Cao, Q.T.; Duan, B.; Chen, H.J.; Yu, X.C.; Xiao, Y.F. Operando monitoring transition dynamics of responsive polymer using optofluidic microcavities. Light Sci. Appl. 2021, 10, 128. [Google Scholar] [CrossRef] [PubMed]
- Barucci, A.; Berneschi, S.; Giannetti, A.; Baldini, F.; Cosci, A.; Pelli, S.; Farnesi, D.; Righini, G.C.; Soria, S.; Conti, G.N. Optical microbubble resonators with high refractive index inner coating for bio-sensing applications: An analytical approach. Sensors 2016, 16, 1992. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Liu, Y.; Gong, C.; Yuan, Z.; Shen, L.; Chang, P.; Liu, K.; Xu, T.; Jiang, Y.; Cheng, Y.C. Liquid crystal-amplified optofluidic biosensor for ultra-highly sensitive and stable protein assay. PhotoniX 2021, 2, 18. [Google Scholar] [CrossRef]
- Yu, X.C.; Tang, S.J.; Liu, W.; Xu, Y.; Gong, Q.; Chen, Y.L.; Xiao, Y.F. Single-molecule optofluidic microsensor with interface whispering gallery modes. Proc. Natl. Acad. Sci. USA 2022, 119, e2108678119. [Google Scholar] [CrossRef]
- Berneschi, S.; Baldini, F.; Cosci, A.; Farnesi, D.; Conti, G.N.; Tombelli, S.; Trono, C.; Pelli, S.; Giannetti, A. Fluorescence biosensing in selectively photo–activated microbubble resonators. Sens. Actuators B Chem. 2017, 242, 1057–1064. [Google Scholar] [CrossRef]



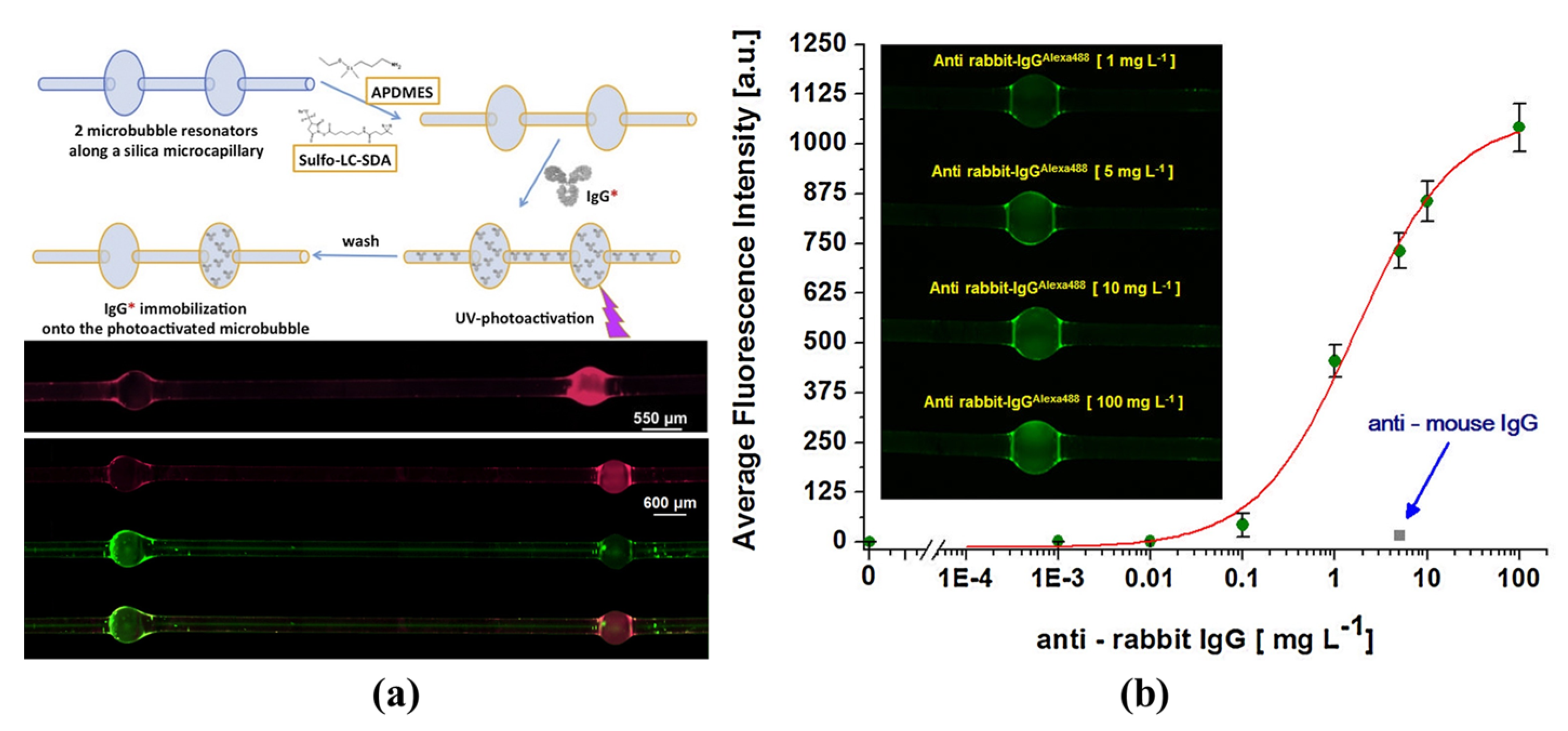
| Application | Q-Factor | Fabrication Material | Sensitivity | Dynamic Detection Ranges | Detection Limit | |
|---|---|---|---|---|---|---|
| Temperature Sensing | [95] | 106 | Glass capillary | 100 GHz/K | - | 8.5 mK |
| [108] | 1.6 × 104 | PMMA | 39 pm/°C | 25 °C–80 °C | - | |
| [117] | 5.8 × 103 | Silica capillary | 205 pm/°C | 22 °C–26 °C | - | |
| [118] | 106–107 | Silica capillary | 205 pm/°C | ~65 ℃ | 0.002 ℃ | |
| Liquid-concentration Sensing | [89] | 5.8 × 106 | Silica capillary | 18.8 nm/RIU | - | 5.4 × 10−5 RIU |
| [93] | 1.8 × 106 | Silica capillary | 11.4 nm/RIU | - | 5.5 × 10−5 RIU | |
| Pressure Sensing | [98] | 103–107 | Glass capillary | −1.1 GHz/bar | - | - |
| [99] | - | Silica microcapillary | 6.21 GHz/bar | - | 0.02 μm | |
| [106] | 5 × 107 | Silica Capillary | 38 GHz/bar | - | 0.17 mbar | |
| [119] | 1.5 × 106 | Silica capillary | 51.2 pm/bar | - | - | |
| Flow Rate Sensing | [100] | 1.8 × 105 | Silica capillary | 0.0196 pm/(μL/min) | 10 μL/min–200 μL/min | - |
| [101] | 1.7 × 105 | Silica capillary | 0.079 pm/(μL/min) | 0 μL/min–200 μL/min | - | |
| Magnetic Sensing | [96] | 2.1 × 104 | Hollow Fiber | 0.08 pm/mT | 0.41 mT–21.8 mT | - |
| [97] | 4.0 × 106 | Microcapillary (Magnetic Fluid) | 25.2 pm/mT | 2 mT–20 mT | - | |
| Gas Sensing | [111] | 2.2 × 104 | PDMS | 36.24 pm/ppm | 4.2 ppm–272.4 ppm | - |
| [122] | 4.4 × 104 | Silica capillary (Graphene) | 200 kHz/ppm | 1 ppm–400 ppm | 1 ppb | |
| [123] | 1.1 × 105 | Silica capillary (PHMB) | 0.46 pm/ppm | 200 ppm–700 ppm | 50 ppm | |
| Ion Sensing | [125] | 4.7 × 104 | Silica capillary | 265.2 nm/RIU | 0.1 pM–100 pM | 15 fM |
| pH Sensing | [126] | - | Silica capillary (polyNIPA) | 33 nm/RIU | pH 3.4–pH 5.6 | pH 0.06 |
| Hydrogel Sensing | [92] | 9.1 × 107 | Silica capillary | - | Hydrophobic -hydrophilic | - |
| Biosensing | [64] | 3.0 × 105 | Silica capillary | - | 0 ng/mL–20 ng/mL | 2 pg/mL |
| [90] | 3.7 × 105 | Silica capillary | - | - | 0.41 pM | |
| [93] | 1.8 × 106 | Silica capillary | 11.4 nm/RIU | 1 fg/mL–100 pg/mL | 1 fg/mL | |
| [129] | 1.4 × 104 | Silica capillary (5CB liquid crystal) | - | 10−12 g/Ml–10−3 g/mL | 1.92 fM | |
| [130] | 8.6 × 108 | Microcapillary | ~0.9 fm/(pg.cm−2) | - | 0.3 pg/cm2 | |
| [131] | 3 × 107 | Silica microcapillary | - | 10−3 mg/L–102 mg/L | 0.015 mg/L | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Guo, Z.; Zhou, Y.; Guo, J.; Liu, Z.; Li, Y.; Luo, M.; Wu, X. Optical Whispering-Gallery-Mode Microbubble Sensors. Micromachines 2022, 13, 592. https://doi.org/10.3390/mi13040592
Zhao X, Guo Z, Zhou Y, Guo J, Liu Z, Li Y, Luo M, Wu X. Optical Whispering-Gallery-Mode Microbubble Sensors. Micromachines. 2022; 13(4):592. https://doi.org/10.3390/mi13040592
Chicago/Turabian StyleZhao, Xuyang, Zhihe Guo, Yi Zhou, Junhong Guo, Zhiran Liu, Yuxiang Li, Man Luo, and Xiang Wu. 2022. "Optical Whispering-Gallery-Mode Microbubble Sensors" Micromachines 13, no. 4: 592. https://doi.org/10.3390/mi13040592
APA StyleZhao, X., Guo, Z., Zhou, Y., Guo, J., Liu, Z., Li, Y., Luo, M., & Wu, X. (2022). Optical Whispering-Gallery-Mode Microbubble Sensors. Micromachines, 13(4), 592. https://doi.org/10.3390/mi13040592