Cu2S Nanoflakes Decorated with NiS Nanoneedles for Enhanced Oxygen Evolution Activity
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kuo, D.Y.; Kawasaki, J.K.; Nelson, J.N.; Kloppenburg, J.; Hautier, G.; Shen, K.M.; Schlom, D.G.; Suntivich, J. Influence of surface adsorption on the oxygen evolution reaction on IrO2(110). J. Am. Chem. Soc. 2017, 139, 3473–3479. [Google Scholar] [CrossRef] [PubMed]
- Stoerzinger, K.A.; Diaz-Morales, O.; Kolb, M.; Rao, R.R.; Frydendal, R.; Qiao, L.; Wang, X.R.; Halck, N.B.; Rossmeisl, J.; Hansen, H.A.; et al. Orientation-dependent oxygen evolution on RuO2 without lattice exchange. ACS Energy Lett. 2017, 2, 876–881. [Google Scholar] [CrossRef] [Green Version]
- Cai, P.; Huang, J.; Chen, J.; Wen, Z. Oxygen-containing amorphous cobalt sulfide porous nanocubes as high-activity electrocatalysts for the oxygen evolution reaction in an alkaline/neutral medium. Angew. Chem. 2017, 56, 4858–4861. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.P.; Lu, X.F.; Zang, S.Q.; Lou, X.W. Non-noble-metal-based electrocatalysts toward the oxygen evolution reaction. Adv. Funct. Mater. 2020, 30, 1910274. [Google Scholar] [CrossRef]
- Peng, L.; Shah, S.S.A.; Wei, Z. Recent developments in metal phosphide and sulfide electrocatalysts for oxygen evolution reaction. Chin. J. Catal. 2018, 39, 1575–1593. [Google Scholar] [CrossRef]
- Cui, M.; Yang, C.; Li, B.; Dong, Q.; Wu, M.; Hwang, S.; Xie, H.; Wang, X.; Wang, G.; Hu, L. High-entropy metal sulfide nanoparticles promise high-performance oxygen evolution reaction. Adv. Energy Mater. 2020, 11, 2002887. [Google Scholar] [CrossRef]
- Thangasamy, P.; Oh, S.; Nam, S.; Randriamahazaka, H.; Oh, I.K. Ferrocene-incorporated cobalt sulfide nanoarchitecture for superior oxygen evolution reaction. Small 2020, 16, 2001665. [Google Scholar] [CrossRef]
- Pujari, R.B.; Gund, G.S.; Patil, S.J.; Park, H.S.; Lee, D.W. Anion-exchange phase control of manganese sulfide for oxygen evolution reaction. J. Mater. Chem. A 2020, 8, 3901–3909. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Zhuo, S.; Liu, S.; Han, A.; Li, L.; Tian, Y. Flower-like CoO@Cu2S nanocomposite for enhanced oxygen evolution reaction. Appl. Surf. Sci. 2021, 555, 149441. [Google Scholar] [CrossRef]
- He, L.; Zhou, D.; Lin, Y.; Ge, R.; Hou, X.; Sun, X.; Zheng, C. Ultrarapid in situ synthesis of Cu2S nanosheet arrays on copper foam with room-temperature-active iodine plasma for efficient and cost-effective oxygen evolution. ACS Catal. 2018, 8, 3859–3864. [Google Scholar] [CrossRef]
- Deng, S.; Shen, Y.; Xie, D.; Lu, Y.; Yu, X.; Yang, L.; Wang, X.; Xia, X.; Tu, J. Directional construction of Cu2S branch arrays for advanced oxygen evolution reaction. J. Energy Chem. 2019, 39, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Qian, Y.; Zhang, H.; Zhang, L.; Chai, Q.; Wang, Q.; Du, J.; Han, Y.; Wang, W.; Kang, D.J. Highly efficient oxygen evolution electrocatalysts based on nanosheet-shaped CuS in situ grown on carbon cloth. Ceram. Int. 2019, 45, 10664–10671. [Google Scholar] [CrossRef]
- Yang, D.; Gao, L.; Yang, J.H. Facile synthesis of ultrathin Ni(OH)2-Cu2S hexagonal nanosheets hybrid for oxygen evolution reaction. J. Power Sources 2017, 359, 52–56. [Google Scholar] [CrossRef]
- Guan, X.; Sun, X.; Feng, H.; Zhang, J.; Wen, H.; Tian, W.; Zheng, D.; Yao, Y. Rational interface engineering of Cu2S-CoOx/CF enhances oxygen evolution reaction activity. Chem. Commun. 2020, 56, 13571–13574. [Google Scholar] [CrossRef]
- Zuo, Y.; Liu, Y.; Li, J.; Du, R.; Han, X.; Zhang, T.; Arbiol, J.; Divins, N.J.; Llorca, J.; Guijarro, N.; et al. In situ electrochemical oxidation of Cu2S into CuO nanowires as a durable and efficient electrocatalyst for oxygen evolution reaction. Chem. Mater. 2019, 31, 7732–7743. [Google Scholar] [CrossRef] [Green Version]
- Ding, X.; Li, W.; Kuang, H.; Qu, M.; Cui, M.; Zhao, C.; Qi, D.C.; Oropeza, F.E.; Zhang, K.H.L. An Fe stabilized metallic phase of NiS2 for the highly efficient oxygen evolution reaction. Nanoscale 2019, 11, 23217–23225. [Google Scholar] [CrossRef]
- Khan, N.A.; Rashid, N.; Junaid, M.; Zafar, M.N.; Faheem, M.; Ahmad, I. NiO/NiS heterostructures: An efficient and stable electrocatalyst for oxygen evolution reaction. ACS Appl. Energy Mater. 2019, 2, 3587–3594. [Google Scholar] [CrossRef]
- Xue, Z.; Li, X.; Liu, Q.; Cai, M.; Liu, K.; Liu, M.; Ke, Z.; Liu, X.; Li, G. Interfacial electronic structure modulation of NiTe nanoarrays with NiS nanodots facilitates electrocatalytic oxygen evolution. Adv. Mater. 2019, 31, 1900430. [Google Scholar] [CrossRef]
- Wang, L.; Qian, Y.; Du, J.; Wu, H.; Wang, Z.; Li, G.; Li, K.; Wang, W.; Kang, D.J. Facile synthesis of cactus-shaped CdS-Cu9S5 heterostructure on copper foam with enhanced photoelectrochemical performance. Appl. Surf. Sci. 2019, 492, 849–855. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, H.; Hao, J.; Lu, S.; Duan, F.; Xu, F.; Du, M. One-dimensional, space-confined, solid-phase growth of the Cu9S5@MoS2 core-shell heterostructure for electrocatalytic hydrogen evolution. J. Colloid Interface Sci. 2021, 595, 88–97. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.; Zhu, H.; Lu, S.; Ma, P.; Dong, W.; Duan, F.; Chen, M.; Du, M. Understanding the role of nanoscale heterointerfaces in core/shell structures for water splitting: Covalent bonding interaction boosts the activity of binary transition-metal sulfides. ACS Appl. Mater. Interfaces 2020, 12, 6250–6261. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, X.; Zhang, B.; Hou, W.; Lv, S.; Shi, Y. Controlled synthesis and fine-tuned interface of NiS nanoparticles/Bi2WO6 nanosheets heterogeneous as electrocatalyst for oxygen evolution reaction. Appl. Surf. Sci. 2020, 526, 146718. [Google Scholar] [CrossRef]
- Luo, Y.; Qin, J.; Yang, G.; Luo, S.; Zhao, Z.; Chen, M.; Ma, J. N-Ni-S coordination sites of NiS/C3N4 formed by an electrochemical-pyrolysis strategy for boosting oxygen evolution reaction. Chem. Eng. J. 2021, 410, 128394. [Google Scholar] [CrossRef]
- Jiang, S.; Shao, H.; Cao, G.; Li, H.; Xu, W.; Li, J.; Fang, J.; Wang, X. Waste cotton fabric derived porous carbon containing Fe3O4/NiS nanoparticles for electrocatalytic oxygen evolution. J. Mater. Sci. Technol. 2020, 59, 92–99. [Google Scholar] [CrossRef]
- Jia, Q.; Wang, X.; Wei, S.; Zhou, C.; Wang, J.; Liu, J. Porous flower-like Mo-doped NiS heterostructure as highly efficient and robust electrocatalyst for overall water splitting. Appl. Surf. Sci. 2019, 484, 1052–1060. [Google Scholar] [CrossRef]
- Li, M.; Qian, Y.; Du, J.; Wu, H.; Zhang, L.; Li, G.; Li, K.; Wang, W.; Kang, D.J. CuS nanosheets decorated with CoS2 nanoparticles as an efficient electrocatalyst for enhanced hydrogen evolution at all pH values. ACS Sustain. Chem. Eng. 2019, 7, 14016–14022. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, L.; Park, M.; Song, K.; Choi, H.; Shi, H.; Lee, H.J.; Pang, H. Nickel sulfide nanorods decorated on graphene as advanced hydrogen evolution electrocatalysts in acidic and alkaline media. J. Colloid Interface Sci. 2022, 608, 2633–2640. [Google Scholar] [CrossRef]
- Ni, S.; Lv, X.; Li, T.; Yang, X. Fabrication of Cu2S cathode for Li-ion battery via a low temperature dry thermal sulfuration method. Mater. Chem. Phys. 2013, 143, 349–354. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, R.; Jiang, P.; Wang, G.; Chen, Y.; Wu, X.; Zhang, C. Efficient photoelectrochemical hydrogen generation from water using a robust photocathode formed by CdTe QDs and nickel ion. ACS Sustain. Chem. Eng. 2015, 3, 2429–2434. [Google Scholar] [CrossRef]
- Zhang, J.; Li, W.; Li, Y.; Zhong, L.; Xu, C. Self-optimizing bifunctional CdS/Cu2S with coexistence of light-reduced CuO for highly efficient photocatalytic H2 generation under visible-light irradiation. Appl. Catal. B Environ. 2017, 217, 30–36. [Google Scholar] [CrossRef]
- Guan, B.; Li, Y.; Yin, B.; Liu, K.; Wang, D.; Zhang, H.; Cheng, C. Synthesis of hierarchical NiS microflowers for high performance asymmetric supercapacitor. Chem. Eng. J. 2017, 308, 1165–1173. [Google Scholar] [CrossRef]
- Liu, P.; Yan, J.; Mao, J.; Li, J.; Liang, D.; Song, W. In-plane intergrowth CoS2/MoS2 nanosheets: Binary metal–organic framework evolution and efficient alkaline HER electrocatalysis. J. Mater. Chem. A 2020, 8, 11435–11441. [Google Scholar] [CrossRef]
- Qian, Y.; Yu, J.; Zhang, Y.; Zhang, F.; Su, C.; Shi, H.; Kang, D.J.; Pang, H. Interfacial microenvironment modulation enhancing catalytic kinetics of binary metal sulfides heterostructures for advanced water splitting electrocatalysts. Small Methods 2022, 6, 2101186. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yang, S.; Tian, Z.; Zhu, B. NiS and graphene as dual cocatalysts for the enhanced photocatalytic H2 production activity of g-C3N4. Appl. Surf. Sci. 2019, 469, 657–665. [Google Scholar] [CrossRef]
- Qian, Y.; Yu, J.; Zhang, F.; Kang, Y.; Su, C.; Pang, H. Facile synthesis of sub-10 nm ZnS/ZnO nanoflakes for high-performance flexible triboelectric nanogenerators. Nano Energy 2021, 88, 106256. [Google Scholar] [CrossRef]
- Hao, Z.; Wei, P.; Kang, H.; Yang, Y.; Li, J.; Chen, X.; Guo, D.; Liu, L. Three-dimensional Fe3S4@NiS hollow nanospheres as efficient electrocatalysts for oxygen evolution reaction. J. Electroanal. Chem. 2019, 850, 113436. [Google Scholar] [CrossRef]
- Zhu, G.; Xie, X.; Liu, Y.; Li, X.; Xu, K.; Shen, X.; Yao, Y.; Shah, S.A. Fe3O4@NiSx/rGO composites with amounts of heterointerfaces and enhanced electrocatalytic properties for oxygen evolution. Appl. Surf. Sci. 2018, 442, 256–263. [Google Scholar] [CrossRef]
- Chakraborty, B.; Kalra, S.; Beltran-Suito, R.; Das, C.; Hellmann, T.; Menezes, P.W.; Driess, M. A Low-temperature molecular precursor approach to copper-based nano-sized digenite mineral for efficient electrocatalytic oxygen evolution reaction. Chem. Asian J. 2020, 15, 852–859. [Google Scholar] [CrossRef] [Green Version]
- Kundu, J.; Khilari, S.; Bhunia, K.; Pradhan, D. Ni-doped CuS as an efficient electrocatalyst for the oxygen evolution reaction. Catal. Sci. Technol. 2019, 9, 406–417. [Google Scholar] [CrossRef]
- Zhao, G.; Li, P.; Cheng, N.; Dou, S.X.; Sun, W. An Ir/Ni(OH)2 heterostructured electrocatalyst for the oxygen evolution reaction: Breaking the scaling relation, stabilizing iridium(V), and beyond. Adv. Mater. 2020, 32, 2000872. [Google Scholar] [CrossRef]
- Zhang, S.; Xue, H.; Li, W.L.; Sun, J.; Guo, N.; Song, T.; Dong, H.; Zhang, J.; Ge, X.; Zhang, W.; et al. Constructing precise coordination of nickel active sites on hierarchical porous carbon framework for superior oxygen reduction. Small 2021, 17, 2102125. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.J.; Lu, X.Y.; Ju, R.; Chen, H.; Shao, L.; Zhai, X.; Li, Y.N.; Fan, F.Q.; Fu, Y.; Qi, W. Preparation of MOF film/aerogel composite catalysts via substrate-seeding secondary-growth for the oxygen evolution reaction and CO2 cycloaddition. Angew. Chem. 2021, 60, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Eom, T.; Cho, M.; Lee, H.J.; Pang, H. Fabrication of defect-rich bifunctional hollow NiTe2 nanotubes for high performance hydrogen evolution electrocatalysts and supercapacitors. J. Energy Storage 2021, 42, 103098. [Google Scholar] [CrossRef]
- Qian, Y.; Du, J.; Kang, D.J. Enhanced electrochemical performance of porous Co-doped TiO2 nanomaterials prepared by a solvothermal method. Microporous Mesoporous Mater. 2019, 273, 148–155. [Google Scholar] [CrossRef]
- Zheng, F.; Zhang, W.; Zhang, X.; Zhang, Y.; Chen, W. Sub-2 nm ultrathin and robust 2D FeNi layered double hydroxide nanosheets packed with 1D FeNi-MOFs for enhanced oxygen evolution electrocatalysis. Adv. Funct. Mater. 2021, 31, 2103318. [Google Scholar] [CrossRef]

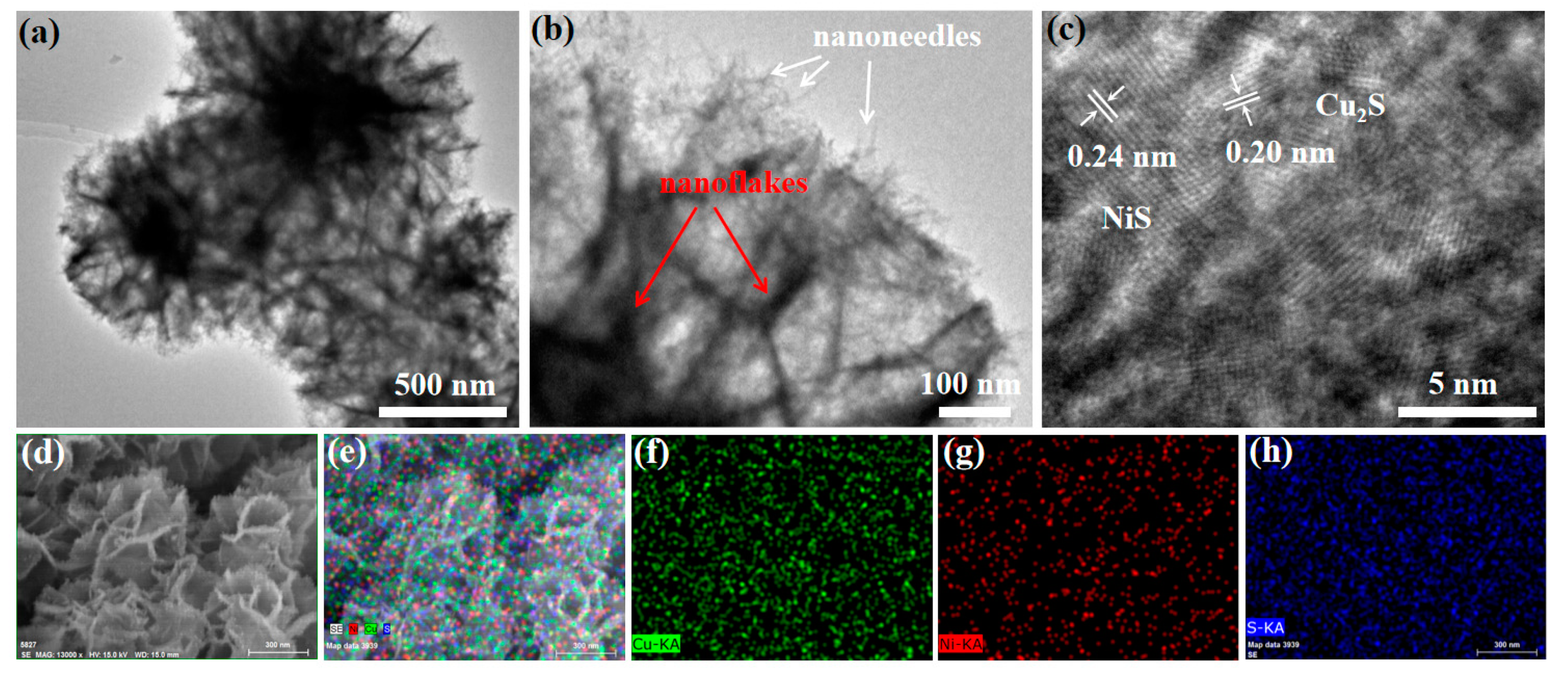
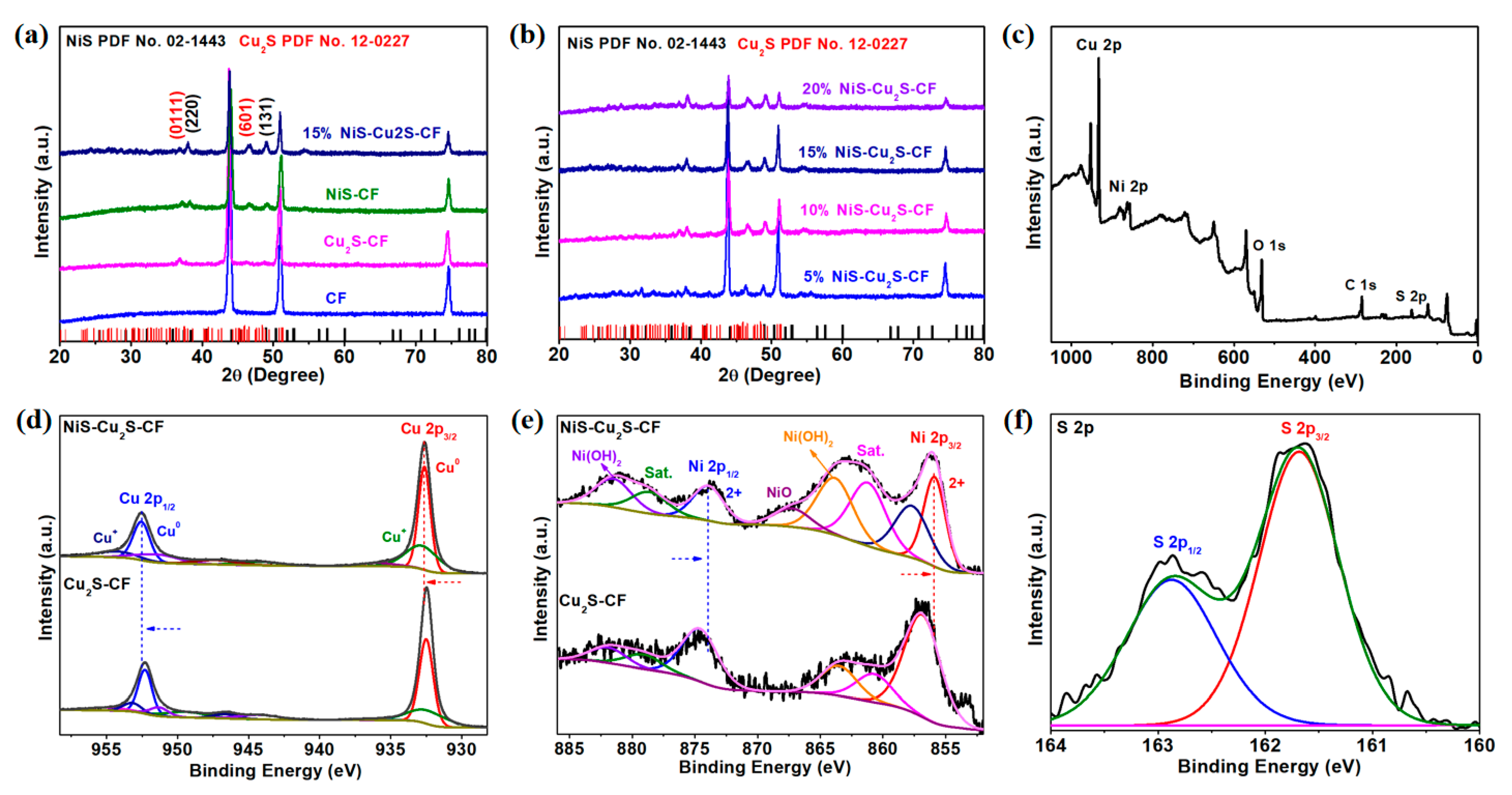
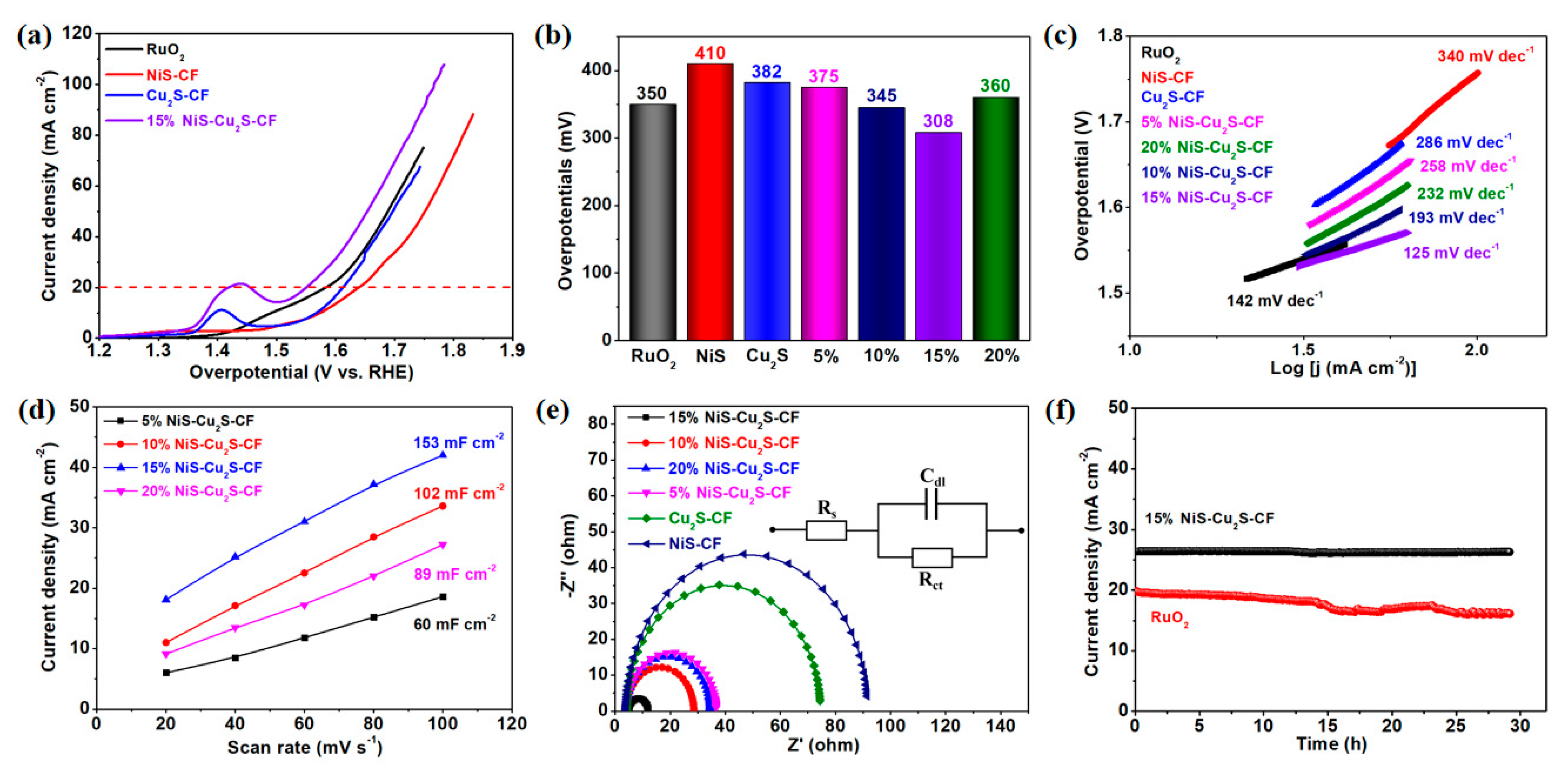
| Electrocatalysts | Overpotential (mV) | Current Density (mA cm−2) | Ref. |
|---|---|---|---|
| 15% NiS-Cu2S-CF | 308 | 20 | This work |
| Cu2S-CF | 336 | 20 | [10] |
| CuS-CC | 358 | 10 | [12] |
| Cu2S-Ni(OH)2 | 500 | 10 | [13] |
| Cu2S-CuO | 380 | 10 | [15] |
| NiS-Bi2WO6 | 527 | 10 | [22] |
| NiS-C3N4 | 334 | 10 | [23] |
| NiS-Fe3O4 | 310 | 10 | [24] |
| NiS-Fe3S4 | 338 | 10 | [36] |
| NiSx-Fe3O4-rGO | 330 | 10 | [37] |
| Cu9S5-Ni foam | 301 | 10 | [38] |
| 3% Ni-doped CuS | 390 | 10 | [39] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Li, M.; Lyu, Y.; Liu, J.; Du, J.; Kang, D.J. Cu2S Nanoflakes Decorated with NiS Nanoneedles for Enhanced Oxygen Evolution Activity. Micromachines 2022, 13, 278. https://doi.org/10.3390/mi13020278
Wang L, Li M, Lyu Y, Liu J, Du J, Kang DJ. Cu2S Nanoflakes Decorated with NiS Nanoneedles for Enhanced Oxygen Evolution Activity. Micromachines. 2022; 13(2):278. https://doi.org/10.3390/mi13020278
Chicago/Turabian StyleWang, Le, Mancong Li, Yingxin Lyu, Jiawen Liu, Jimin Du, and Dae Joon Kang. 2022. "Cu2S Nanoflakes Decorated with NiS Nanoneedles for Enhanced Oxygen Evolution Activity" Micromachines 13, no. 2: 278. https://doi.org/10.3390/mi13020278
APA StyleWang, L., Li, M., Lyu, Y., Liu, J., Du, J., & Kang, D. J. (2022). Cu2S Nanoflakes Decorated with NiS Nanoneedles for Enhanced Oxygen Evolution Activity. Micromachines, 13(2), 278. https://doi.org/10.3390/mi13020278







