Petal-like NiS-NiO/G-C3N4 Nanocomposite for High-Performance Symmetric Supercapacitor
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
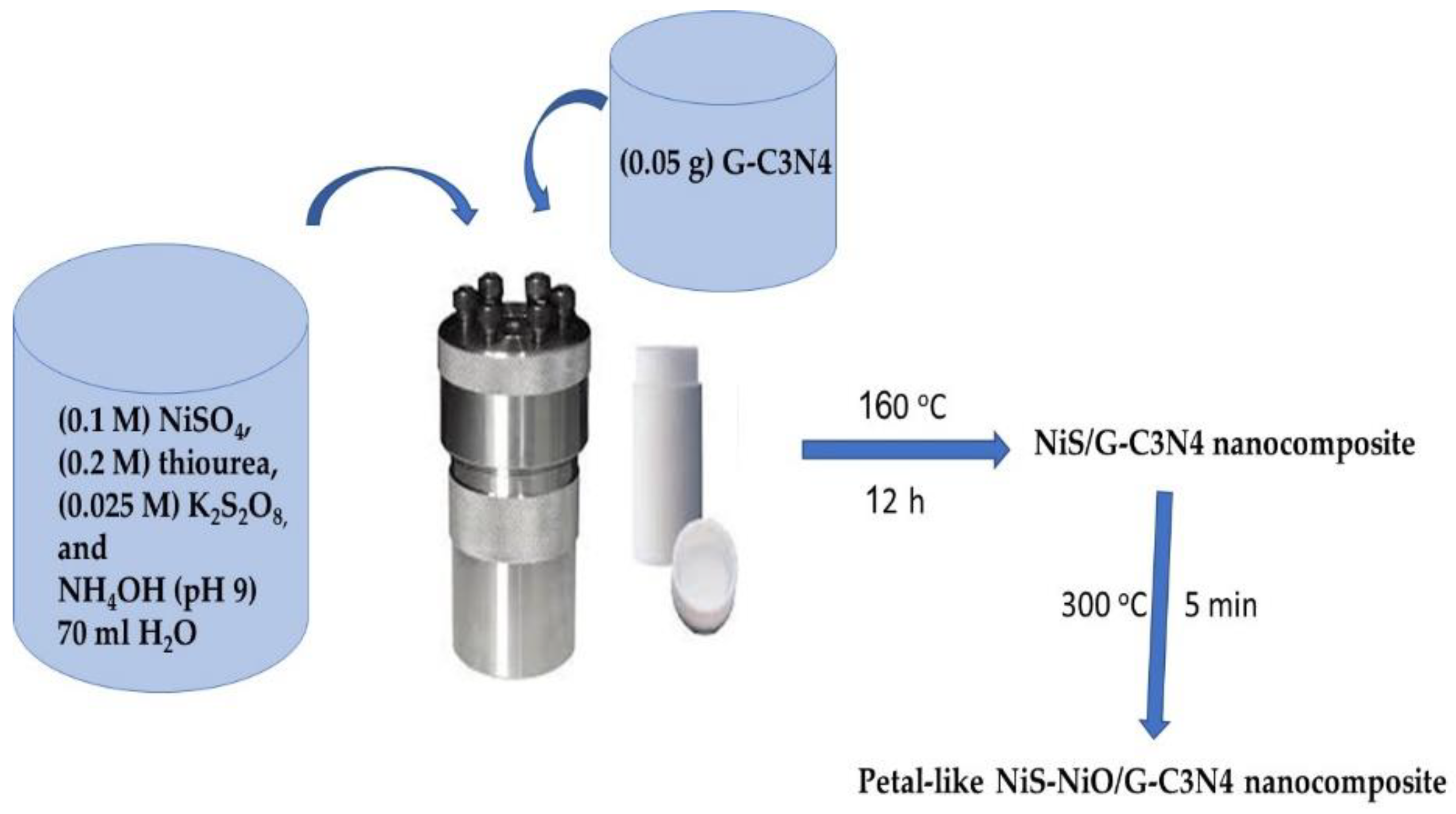
2.2. Preparation of NiS-NiO/G-C3N4 Nanocomposite
2.3. Synthesis and Testing of the Prepared Symmetric Supercapacitor
2.4. Characterization
3. Results and Discussion
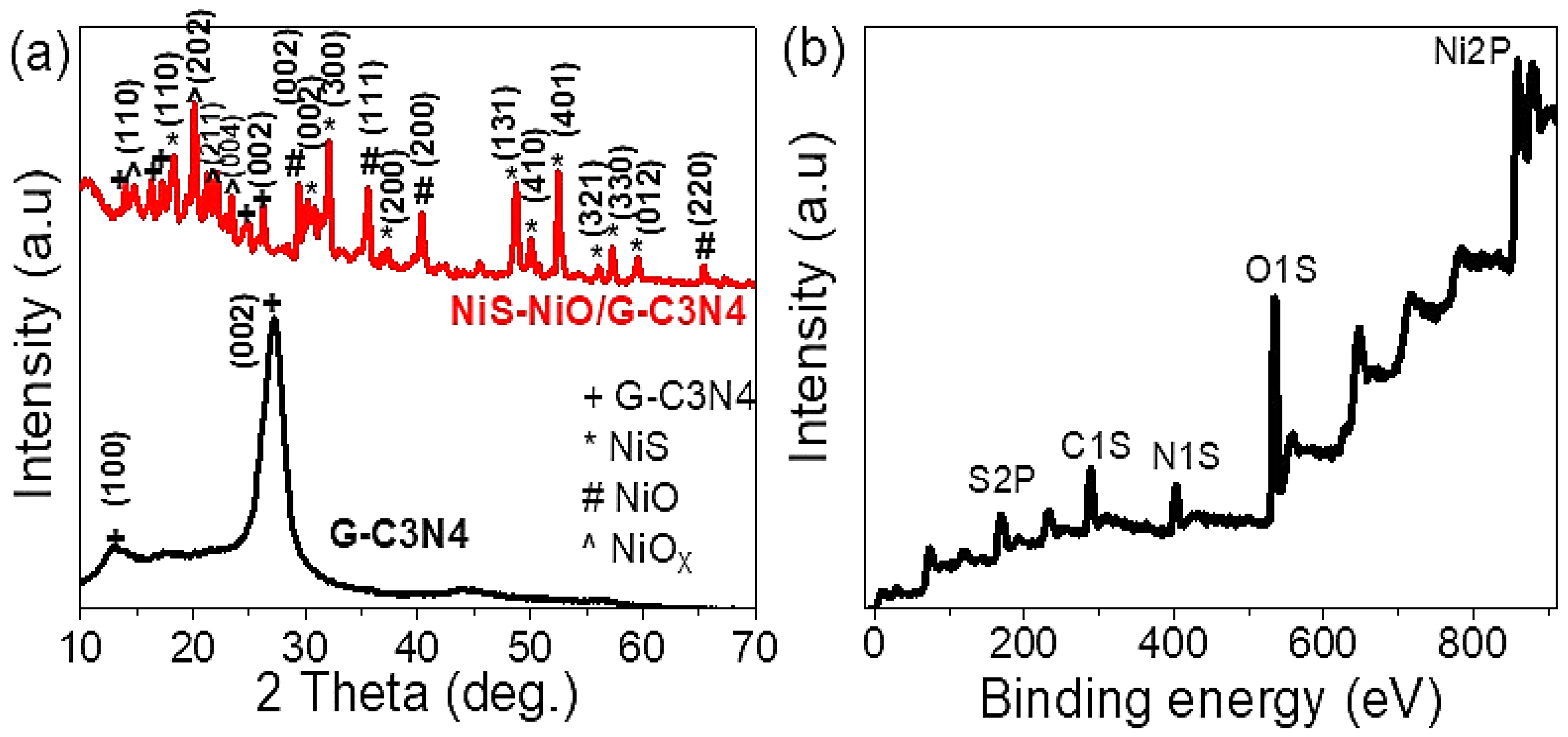
3.1. Analyses
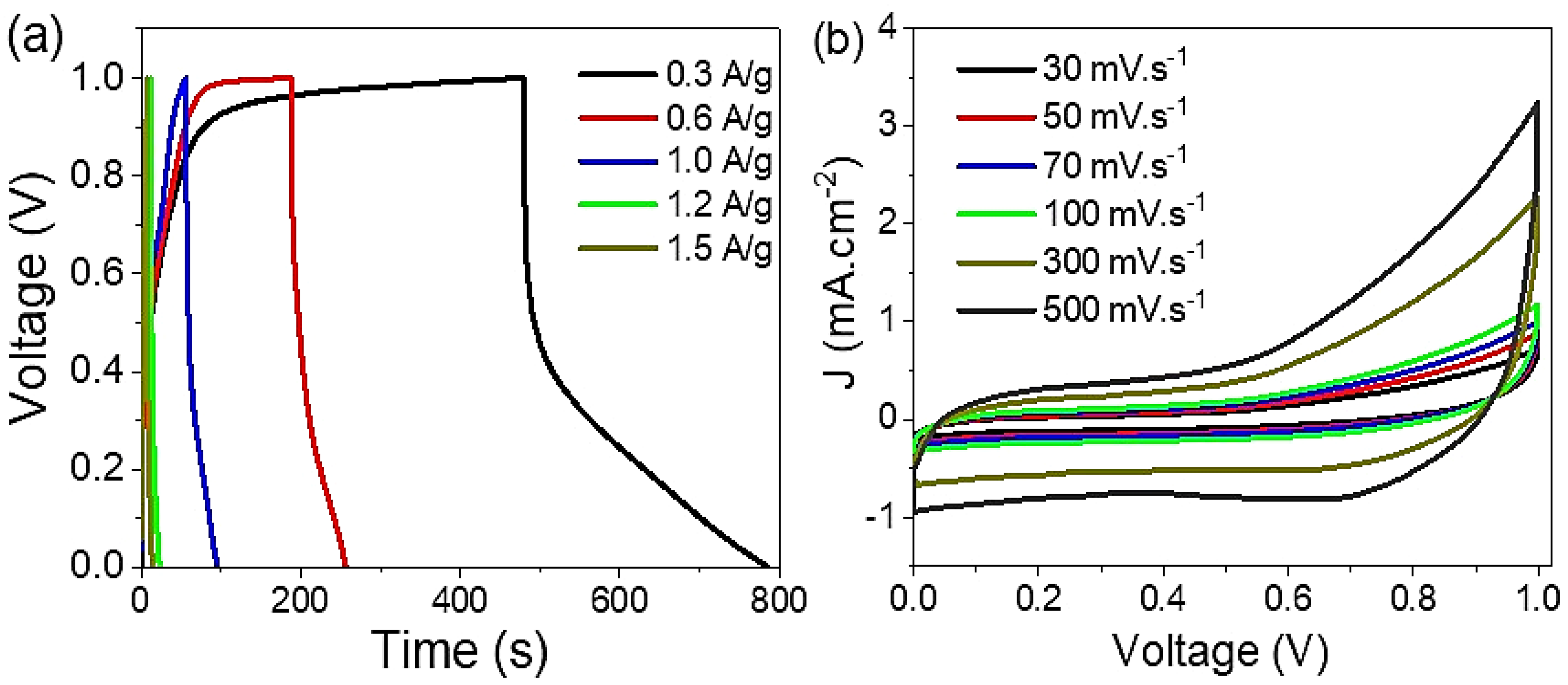
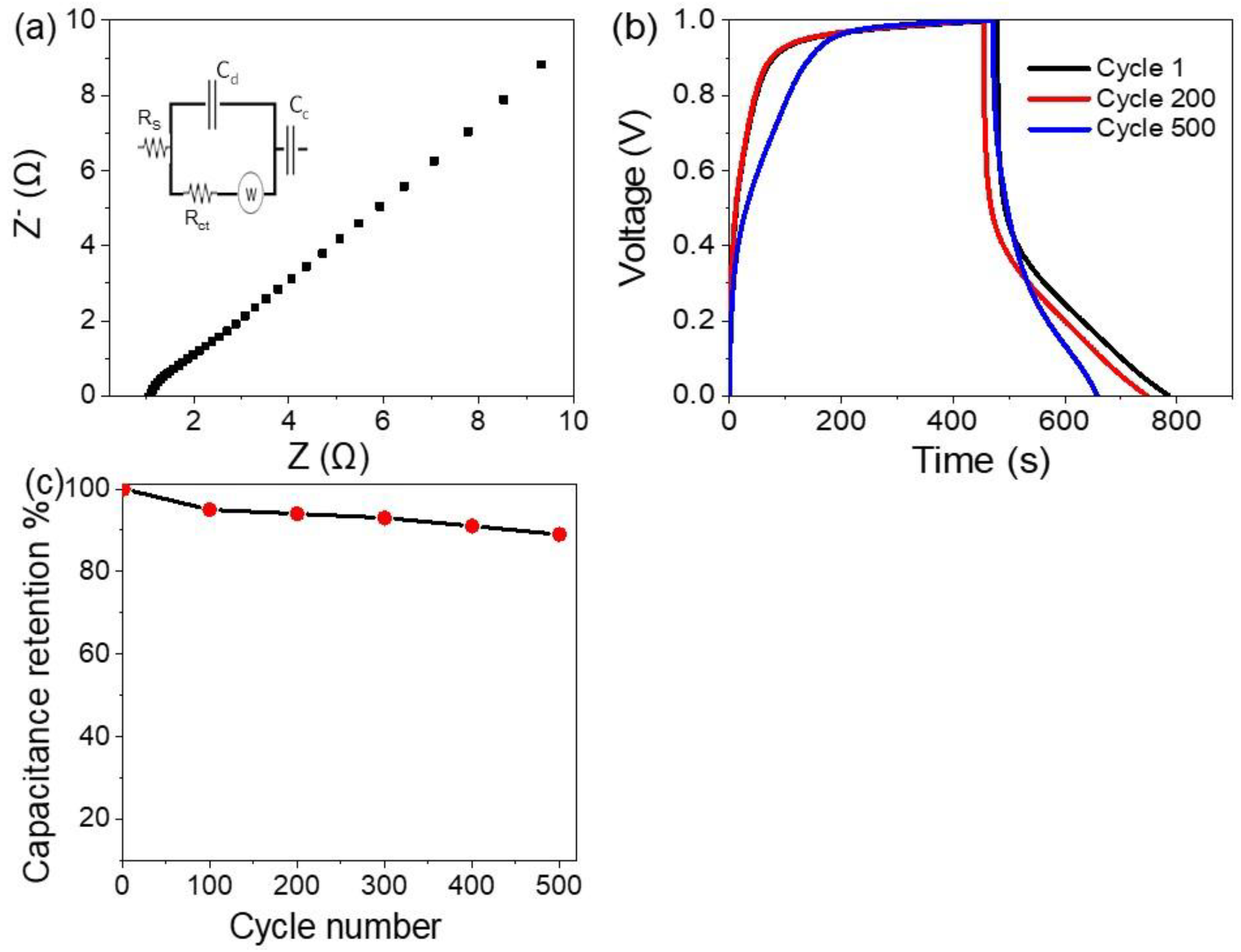
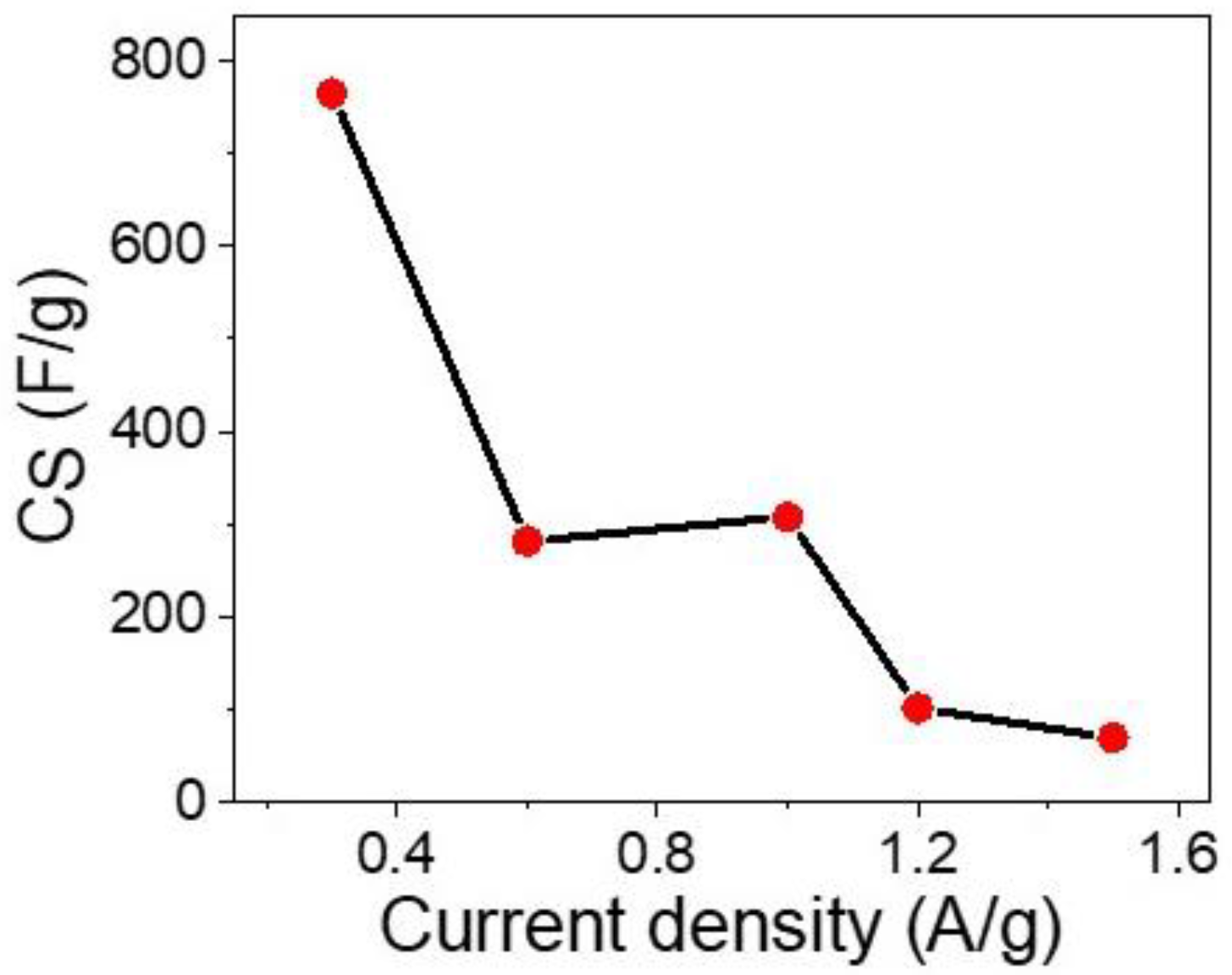
3.2. The Electrochemical Testing for the Prepared Supercapacitor
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdelazeez, A.A.A.; Trabelsi, A.B.G.; Alkallas, F.H.; Rabia, M. Successful 2D MoS2 Nanosheets Synthesis with SnSe Grid-like Nanoparticles: Photoelectrochemical Hydrogen Generation and Solar Cell Applications. Solar Energy 2022, 248, 251–259. [Google Scholar] [CrossRef]
- Shaban, M.; Ali, S.; Rabia, M. Design and application of nanoporous graphene oxide film for CO2, H2, and C2H2 gases sensing. J. Mater. Res. Technol. 2019, 8, 4510–4520. [Google Scholar] [CrossRef]
- Elsayed, A.M.; Rabia, M.; Shaban, M.; Aly, A.H.; Ahmed, A.M. Preparation of hexagonal nanoporous Al2O3/TiO2/TiN as a novel photodetector with high efficiency. Sci. Rep. 2021, 11, 17572. [Google Scholar] [CrossRef] [PubMed]
- Mai, L.Q.; Minhas-Khan, A.; Tian, X.; Hercule, K.M.; Zhao, Y.L.; Lin, X.; Xu, X. Synergistic interaction between redox-active electrolyte and binder-free functionalized carbon for ultrahigh supercapacitor performance. Nat. Commun. 2013, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, K.; Pazhamalai, P.; Mariappan, V.K.; Nardekar, S.S.; Sahoo, S.; Kim, S.J. Probing the energy conversion process in piezoelectric-driven electrochemical self-charging supercapacitor power cell using piezoelectrochemical spectroscopy. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mai, L.Q.; Yang, F.; Zhao, Y.L.; Xu, X.; Xu, L.; Luo, Y.Z. Hierarchical MnMoO4/CoMoO4 heterostructured nanowires with enhanced supercapacitor performance. Nat. Commun. 2011, 2, 1–5. [Google Scholar] [CrossRef]
- Chahal, P.; Madaswamy, S.L.; Lee, S.C.; Wabaidur, S.M.; Dhayalan, V.; Ponnusamy, V.K.; Dhanusuraman, R. Novel manganese oxide decorated polyaniline/graphitic carbon nitride nanohybrid material for efficient supercapacitor application. Fuel 2022, 330, 125531. [Google Scholar] [CrossRef]
- Javed, M.S.; Mateen, A.; Ali, S.; Zhang, X.; Hussain, I.; Imran, M.; Shah, S.S.A.; Han, W. The Emergence of 2D MXenes Based Zn-Ion Batteries: Recent Development and Prospects. Small 2022, 18, 2201989. [Google Scholar] [CrossRef]
- Javed, M.S.; Shah, S.S.A.; Najam, T.; Siyal, S.H.; Hussain, S.; Saleem, M.; Zhao, Z.; Mai, W. Achieving high-energy density and superior cyclic stability in flexible and lightweight pseudocapacitor through synergic effects of binder-free CoGa2O4 2D-hexagonal nanoplates. Nano Energy 2020, 77, 105276. [Google Scholar] [CrossRef]
- Javed, M.S.; Zhang, X.; Ali, S.; Mateen, A.; Idrees, M.; Sajjad, M.; Batool, S.; Ahmad, A.; Imran, M.; Najam, T.; et al. Heterostructured bimetallic–sulfide@layered Ti3C2Tx–MXene as a synergistic electrode to realize high-energy-density aqueous hybrid-supercapacitor. Nano Energy 2022, 101, 107624. [Google Scholar] [CrossRef]
- Javed, M.S.; Shaheen, N.; Hussain, S.; Li, J.; Shah, S.S.A.; Abbas, Y.; Ahmad, M.A.; Raza, R.; Mai, W. An ultra-high energy density flexible asymmetric supercapacitor based on hierarchical fabric decorated with 2D bimetallic oxide nanosheets and MOF-derived porous carbon polyhedra. J. Mater. Chem. A 2019, 7, 946–957. [Google Scholar] [CrossRef]
- Zheng, Z.X.; Wang, M.; Shi, X.Z.; Wang, C.M. Palladium Nanoparticles/Graphitic Carbon Nitride Nanosheets-Carbon Nanotubes as a Catalytic Amplification Platform for the Selective Determination of 17α-ethinylestradiol in Feedstuffs. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Santos, R.S.; Suresh Babu, R.; Devendiran, M.; Haddad, D.B.; de Barros, A.L.F. Facile synthesis of transition metal (M = Cu, Co) oxide grafted graphitic carbon nitride nanosheets for high performance asymmetric supercapacitors. Mater. Lett. 2022, 308, 131156. [Google Scholar] [CrossRef]
- Kumar Kuila, S.; Ghorai, A.; Midya, A.; Sekhar Tiwary, C.; Kumar Kundu, T. Chemisorption of gadolinium ions on 2D-graphitic carbon nitride nanosheet for enhanced solid-state supercapacitor performance. Chem. Phys. Lett. 2022, 796, 139572. [Google Scholar] [CrossRef]
- Rani, B.; Nayak, A.K.; Sahu, N.K. Electrochemical supercapacitor application of CoFe2O4 nanoparticles decorated over graphitic carbon nitride. Diam. Relat. Mater. 2021, 120, 108671. [Google Scholar] [CrossRef]
- Lin, R.; Li, Z.; Abou El Amaiem, D.I.; Zhang, B.; Brett, D.J.L.; He, G.; Parkin, I.P. A general method for boosting the supercapacitor performance of graphitic carbon nitride/graphene hybrids. J. Mater. Chem. A 2017, 5, 25545–25554. [Google Scholar] [CrossRef]
- Lu, C.; Chen, X. Carbon nanotubes/graphitic carbon nitride nanocomposites for all-solid-state supercapacitors. Sci. China Technol. Sci. 2020, 63, 1714–1720. [Google Scholar] [CrossRef]
- Pilathottathil, S.; Kavil, J.; Shahin Thayyil, M. Boosting ion dynamics by developing graphitic carbon Nitride/Carbon hybrid electrode materials for ionogel supercapacitor. Mater. Sci. Eng. B 2022, 276, 115573. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, J.; Liu, H.; Que, M.; Cai, X.; Tan, S.; Huang, L. Flower-like Ni(OH)2 hybridized g-C3N4 for high-performance supercapacitor electrode material. Mater. Lett. 2015, 145, 150–153. [Google Scholar] [CrossRef]
- Azizi-Toupkanloo, H.; Karimi-Nazarabad, M.; Shakeri, M.; Eftekhari, M. Photocatalytic mineralization of hard-degradable morphine by visible light-driven Ag@g-C3N4 nanostructures. Environ. Sci. Pollut. Res. 2019, 26, 30941–30953. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, P.; Joshi, C.; Manchanda, M.; Boukherroub, R.; Jain, S.L. Nickel Decorated on Phosphorous-Doped Carbon Nitride as an Efficient Photocatalyst for Reduction of Nitrobenzenes. Nanomaterials 2016, 6, 59. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Yuan, X.; Zhang, Z.; Mei, D.; Li, L.; Ma, Z.F.; Zhang, L.; Yang, J.; Zhang, J. Novel Flower-like Nickel Sulfide as an Efficient Electrocatalyst for Non-aqueous Lithium-Air Batteries. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Qiao, H.; Yang, H.; Zhang, C.; Yan, X. Characterization of NiO nanoparticles by anodic arc plasma method. J. Alloys Compd. 2009, 479, 855–858. [Google Scholar] [CrossRef]
- Rakshit, S.; Ghosh, S.; Chall, S.; Mati, S.S.; Moulik, S.P.; Bhattacharya, S.C. Controlled synthesis of spin glass nickel oxide nanoparticles and evaluation of their potential antimicrobial activity: A cost effective and eco friendly approach. RSC Adv. 2013, 3, 19348–19356. [Google Scholar] [CrossRef]
- Atta, A.; Abdelhamied, M.M.; Essam, D.; Shaban, M.; Alshammari, A.H.; Rabia, M. Structural and physical properties of polyaniline/silver oxide/silver nanocomposite electrode for supercapacitor applications. Int. J. Energy Res. 2021, 46, 6702–6710. [Google Scholar] [CrossRef]
- Gamal, A.; Shaban, M.; BinSabt, M.; Moussa, M.; Ahmed, A.M.; Rabia, M.; Hamdy, H. Facile Fabrication of Polyaniline/Pbs Nanocomposite for High-Performance Supercapacitor Application. Nanomaterials 2022, 12, 817. [Google Scholar] [CrossRef]
- Hao, J.; Huang, Y.; He, C.; Xu, W.; Yuan, L.; Shu, D.; Song, X.; Meng, T. Bio-templated fabrication of three-dimensional network activated carbons derived from mycelium pellets for supercapacitor applications. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Ramadan, M.; Abdellah, A.M.; Mohamed, S.G.; Allam, N.K. 3D Interconnected Binder-Free Electrospun MnO@C Nanofibers for Supercapacitor Devices. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Sayyah, E.S.M.; Shaban, M.; Rabia, M. A sensor of m-cresol nanopolymer/Pt-electrode film for detection of lead ions by potentiometric methods. Adv. Polym. Technol. 2018, 37, 1296–1304. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/adv.21788 (accessed on 1 November 2020). [CrossRef]
- Sayyah, S.M.; Shaban, M.; Rabia, M. Electropolymerization of m-Toluidin on Platinum Electrode from Aqueous Acidic Solution and Character of the Obtained Polymer. Adv. Polym. Technol. 2018, 37, 126–136. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/adv.21649 (accessed on 1 November 2020). [CrossRef]
- Mohamed, F.; Rabia, M.; Shaban, M. Synthesis and characterization of biogenic iron oxides of different nanomorphologies from pomegranate peels for efficient solar hydrogen production. J. Mater. Res. Technol. 2020, 9, 4255–4271. [Google Scholar] [CrossRef]
- Jones, P.K.; Stimming, U.; Lee, A.A. Impedance-based forecasting of lithium-ion battery performance amid uneven usage. Nat. Commun. 2022, 13, 1–9. [Google Scholar] [CrossRef]
- Qu, D.; Ji, W.; Qu, H. Probing process kinetics in batteries with electrochemical impedance spectroscopy. Commun. Mater. 2022, 3, 1–9. [Google Scholar] [CrossRef]
- Yu, J.; Fu, N.; Zhao, J.; Liu, R.; Li, F.; Du, Y.; Yang, Z. High Specific Capacitance Electrode Material for Supercapacitors Based on Resin-Derived Nitrogen-Doped Porous Carbons. ACS Omega 2019, 4, 15904–15911. [Google Scholar] [CrossRef]
- Naveed ur Rehman, M.; Munawar, T.; Nadeem, M.S.; Mukhtar, F.; Maqbool, A.; Riaz, M.; Manzoor, S.; Ashiq, M.N.; Iqbal, F. Facile synthesis and characterization of conducting polymer-metal oxide based core-shell PANI-Pr2O–NiO–Co3O4 nanocomposite: As electrode material for supercapacitor. Ceram. Int. 2021, 47, 18497–18509. [Google Scholar] [CrossRef]

| Element | Peak Location (eV) | Atomic % |
|---|---|---|
| O1s | 534.6 | 37.67 |
| Ni2p | 858.9 | 6.42 |
| N1s | 402.6 | 13.29 |
| C1s | 288.6 | 32.14 |
| S2p | 167.2 | 10.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trabelsi, A.B.G.; Essam, D.; H. Alkallas, F.; M. Ahmed, A.; Rabia, M. Petal-like NiS-NiO/G-C3N4 Nanocomposite for High-Performance Symmetric Supercapacitor. Micromachines 2022, 13, 2134. https://doi.org/10.3390/mi13122134
Trabelsi ABG, Essam D, H. Alkallas F, M. Ahmed A, Rabia M. Petal-like NiS-NiO/G-C3N4 Nanocomposite for High-Performance Symmetric Supercapacitor. Micromachines. 2022; 13(12):2134. https://doi.org/10.3390/mi13122134
Chicago/Turabian StyleTrabelsi, Amira Ben Gouider, Doaa Essam, Fatemah H. Alkallas, Ashour M. Ahmed, and Mohamed Rabia. 2022. "Petal-like NiS-NiO/G-C3N4 Nanocomposite for High-Performance Symmetric Supercapacitor" Micromachines 13, no. 12: 2134. https://doi.org/10.3390/mi13122134
APA StyleTrabelsi, A. B. G., Essam, D., H. Alkallas, F., M. Ahmed, A., & Rabia, M. (2022). Petal-like NiS-NiO/G-C3N4 Nanocomposite for High-Performance Symmetric Supercapacitor. Micromachines, 13(12), 2134. https://doi.org/10.3390/mi13122134






