Abstract
Graphitic carbon nitride (G-C3N4) was synthesized through the direct combustion of urea in the air. The CoS-Co2O3/G-C3N4 composite was synthesized via the hydrothermal method of G-C3N4 using cobalt salts. The morphological and chemical structures were determined through XRD, XPS, SEM, and TEM. XRD and XPS analyses confirmed the chemical structure, function groups, and elements percentage of the prepared nanocomposite. SEM measurements illustrated the formation of G-C3N4 sheets, as well as the flower shape of the CoS-Co2O3/G-C3N4 composite, evidenced through the formation of nano appendages over G-C3N4 sheets. TEM confirmed the 2D nanosheets of G-C3N4 with an average width and length of 80 nm and 170 nm, respectively. Two symmetric electrodes for the supercapacitor from the CoS-Co2O3/G-C3N4 composite. Electrochemical measurements were carried out to determine the charge/discharge, cyclic voltammetry, stability, and impedance of the prepared supercapacitor. The measurements were carried out under acid (0.5 M HCL) and basic (6.0 M NaOH) mediums. The charge and discharge lifetime values in the acid and base medium were 85 and 456 s, respectively. The cyclic voltammetry behavior was rectangular in a base medium for the pseudocapacitance feature. The supercapacitor had 100% stability retention up to 600 cycles; then, the stability decreased to 98.5% after 1000 cycles. The supercapacitor displayed a specific capacitance (CS) of 361 and 92 F/g, and an energy density equal to 28.7 and 30.2 W h kg−1 in the basic and acidic mediums, respectively. Our findings demonstrate the capabilities of supercapacitors to become an alternative solution to batteries, owing to their easy and low-cost manufacturing technique.
1. Introduction
Sustainable energy has become a promising field owing to cost-effective energy sources. Supercapacitors have been considered replacements for batteries. This is due to their simple charging, lasting a long time before being completely discharged [1,2]. Three types of supercapacitors, i.e., double-layer, hybrid, and pseudo-capacitors, have been developed so far. However, commercial supercapacitors are based on carbon materials (double-layer supercapacitors). Nevertheless, such capacitors have a large drawback related to the limited energy produced [3]. This has motivated researchers to develop advanced supercapacitors based on two composite carbon materials (hybrid supercapacitors). Indeed, the unique combination of composite materials ensures a better efficiency of supercapacitors that remains related to the redox reaction of the used material [4]. Particularly, the combination of carbon materials with redox materials in developing a supercapacitor ensures a significant enhancement of the capacitance through the charge storage inside the layers.
On the other hand, the electrochemistry of supercapacitors highly depends on the morphology of the used materials. For instance, using carbon layer materials decorated with additional metal oxides or sulfides increases the surface area that consequently originates extra active sites [5,6,7,8]. Consequently, this raises the charge storage inside the composite materials [3].
Graphitic carbon nitride (G-C3N4) is considered a promising material due to its simple preparation in a high-mass product, through the combustion of organic nitrogen materials such as urea and thiourea. G-C3N4 has great properties related to charge storage, represented by high surface area and charge chemical durability. This is assigned to the chemical structure of G-C3N4 of tris-triazine (C6N7) in the ternary amino group [9]. Santos et al. fabricated CoO and CuO decorated on G-C3N4; a specific capacitance (CS) of 124.75 and 84.28 at 0.5 A g were found, respectively [10]. Kuila et al. synthesized Gd decorated on G-C3N4 for supercapacitor applications; a capacitance equal to 2.59 mF cm−2 at 10 mV s−1 was obtained [11]. Furthermore, Rani et al. investigated the CoFe2O4/G-C3N4 composite, they demonstrated the impact of G-C3N4 on enhancing the electrical properties of the supercapacitor [12]. On the other hand, additional studies have been carried out on graphitic materials, such as mesoporous carbon nitride/graphene from 0.5 M H2SO4 as an electrolyte; a CS of 240 F/g was produced [13]. Additionally, the Cs of G-C3N4/graphene electrode from 1 M KOH were studied and its value was equal to 265 F/g [14]. Similarly, a supercapacitor from 0.4 M LiClO4 demonstrated a CS of 264 F/g [15]. Additionally, G-C3N4/carbon nanotube was used as the supercapacitor from a polyvinyl alcohol/H2SO4 electrolyte; the produced CS value was 148 F/g [16]. G-C3N4/activated carbon from 1 M Na2SO4; the produced CS value was 266 F/g [17].
Herein, a flower-shaped CoS-Co2O3/G-C3N4 nanocomposite for a two-symmetric-electrodes supercapacitor is prepared and tested in base and acid mediums. All the chemical analyses for confirming the chemical structure and morphology are carried out. The electrochemical properties are tested by measuring the charge/discharge, cyclic voltammetry, impedance, and stability of the prepared supercapacitor. The supercapacitor demonstrates a promising electrical property in the basic medium in comparison to the acid medium. The supercapacitor has a great performance in comparison to instances in the literature.
2. Experimental Section
2.1. Materials
Urea, thiourea, HCl, NaCl, and NaOH were purchased from Piochem Co., Cairo, Egypt. While cobalt acetate (Co(CH3COOH)2) and ammonia (NH4OH) were purchased from El Naser chemical Co., Cairo, Egypt. Nafion (5 wt.% dissolved in methanol) was obtained through Sigma–Aldrich, St. Louis, MO, USA.
2.2. Preparation of CoS-Co2O3/G-C3N4 Nanocomposite
First, 10 g of urea was well annealed in a tube furnace at 550 °C for 2 h under an N2 atmosphere. This led to the combustion and conversion of urea to G-C3N4.
For the preparation of the composite, (0.1 M) Co(CH3COOH)2, (0.2 M) thiourea, and (0.5 M) urea were dissolved in 40 mL H2O using an ultrasonic stirrer. Then, the pH solution was raised to 9 using an aqueous ammonia solution. Meanwhile, 0.05 g C3N4 was suspended in 30 mL H2O. Then, both solutions were added to each other inside the autoclave, in which the hydrothermal reaction was carried out at 160 °C for 12 h. After that, the produced powder was collected, washed, and dried at 60 °C for 5 h. Finally, the powder was annealed at 300 °C for 10 min. This process caused the formation of the CoS-Co2O3/G-C3N4 nanocomposite.
2.3. Synthesis and Electrochemical Testing of the Supercapacitor
Synthesis of the symmetric supercapacitor was carried out using the main prepared materials CoS-Co2O3/G-C3N4 nanocomposite. This material was used as a paste by mixing with (0.045 g) of nafion and 0.75 mL ethanol. Then, 0.003 g of this paste was loaded over an Au sheet (1 cm2). The supercapacitor was constructed by combining these two electrodes via a filter paper wetted with the electrolyte as a separator. Measurements were carried out under different electrolytes, such as HCl, NaOH, and NaCl, using an electrochemical workstation (CHI660E, USA) at 25 °C.
2.4. Characterization Process
The prepared materials were characterized using different analytical tools for confirming their chemical and morphological properties. X-ray diffraction (X’Pert Pro, Almelo Holland) and X-ray photoelectron spectroscopy were used to confirm the chemical structure of the prepared materials. The morphologies were determined using a scanning electron microscope, SEM, (ZEISS SUPRA 55 VP, Oberkochen, Germany) and a transmitted electron microscope (TEM), (JEOL JEM-2100).
3. Results and Discussion
The chemical and structural composition of the graphitic-like layer structures of G-C3N4 and CoS-Co2O3/G-C3N4 were confirmed using the XRD pattern (see Figure 1a). The G-C3N4 displayed two distinct diffraction peaks: a weak (100) diffraction peak at 13.03°, attributed to the in-planar structural packing motif with a separation of 0.679 nm, and a strong other peak located at 27.20° corresponding to the (002) peak of the interlayer d-spacing of 0.327 nm. The two peaks at 13.03° and 27.20° were matched with previous studies (JCPDS 87–1526 card) [18] and (JCPDS) 87–1526 for g-C3N4 [19]. The composite exhibited diffraction peaks corresponding to both g-C3N4 and CoS-Co2O3, reflecting the presence of two phases. The CoS nanomaterial had three peaks for the growth directions (101), (102), and (110), located at 32.45, 42.73, and 51.22°, respectively [20]. The Co2O3 nanomaterial had four peaks for the growth directions (311), (400), (422), and (440) for the positions 30.56, 38.51, 46.51, and 54.64°, respectively [21].
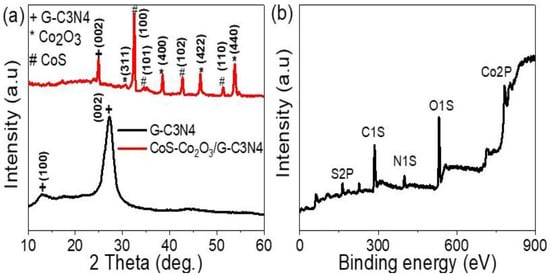
Figure 1.
(a) XRD of G-C3N4 and CoS-Co2O3/G-C3N4 composite and (b) XPS of CoS-Co2O3/G-C3N4 composite.
The chemical and structural composition was investigated via X-ray photoelectron spectroscopy (XPS) (see Figure 1b). Numerous elements were located in the sample, such as O, C, Co, N, and S (see Table 1). The O element was located at 532.5 eV and resulted from Co2O3 or oxygen inside the G-C3N4. Although the C1s spectrum was located at about 285.9 eV which can from the utilized pure G-C3N4, several bonds such as C-C, C-NH2, and N-C=N bonding at about 284, 286, and 288, respectively, were also distinguished. A small shift of the C-C XPS peak to higher binding energy was located, representing the chemical bonding between G-C3N4 and CoS-Co2O3. The N 1s strong peak centering at 399.7 eV was due to the nitrogen atom inside the G-C3N4. The S2p peak was located at 163.1 eV, which could be assigned to S-Co bonding. Furthermore, a Co2p spectrum peak was located at 782.25 eV, which could be assigned to the high binding between CoS-Co2O3 and G-C3N4.

Table 1.
The binding energy and percent of elements in the composite CoS-Co2O3/G-C3N4 were calculated from the XPS analysis.
The morphological and structural analysis of the samples were based on the correlation of SEM, TEM, and theoretical modeling images. Figure 2 represents SEM images of the (a) G-C3N4 and (b) CoS-Co2O3/G-C3N4 composites. The G-C3N4 has 2D nanosheet properties; the formed sheets enfold and crumple in some areas. The 2D nanosheets have an average width and length of 80 nm and 170 nm. It can be seen that both images reveal a thin sheet structure. Indeed, this was clearly distinguished through the TEM image (Figure 2c) of the G-C3N4, illustrating flat irregular shaped sheets with wrinkles. The dark area covering the sheets confirms CoS-Co2O3 incorporation in G-C3N4 sheets.
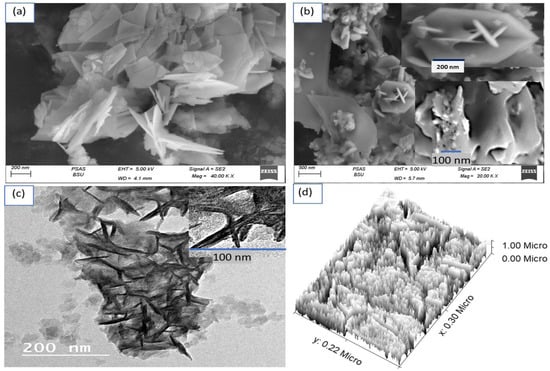
Figure 2.
SEM of (a) G-C3N4 and (b) CoS-Co2O3/G-C3N4 composite. (c) TEM and (d) theoretical image of CoS-Co2O3/G-C3N4 composite.
The theoretical modeling is shown in Figure 2d; the roughness and cross-section are clearly displayed. The formed CoS-Co2O3/G C3N4 composite is highly homogeneous roughness, in which the small particles coat the sheets represented by the G-C3N4.
The Electrochemical Study of the Prepared Supercapacitor
The charge and discharge behavior of CoS-Co2O3/G-C3N4 composite nanomaterials were studied under different electrolytes; 6 M NaOH and 0.5 M HCL (see Figure 3a,b). Measurements were carried out in a potential window (0 to 1.0 V) at 25 °C, through a current density of 0.4 to 1.0 A/g. Moreover, the supercapacitor based on two symmetric structured electrodes was used, in which 0.0003 g paste was loaded over each electrode; then, a filter paper saturated with the selected electrolyte was used as a separator between these two electrodes. From the curve behavior, the capacitive properties affected the current density, which decreased with an increase in the inserted current density. Furthermore, the capacitive properties had a greater value in the basic medium in comparison with the acid medium. This is related to the effect of the HCl electrolyte on Co2O3 under the reaction of an acid with a base. The charge and discharge lifetime values in the acid and base mediums were 85 and 456 s, respectively. This confirmed the non-linear curves and change in behavior in the base medium [22,23,24]. The formation of the OH- group caused increasing negative charge on the supercapacitor electrode that enhanced the pseudocapacitance properties of the prepared supercapacitor. On the other hand, the H+ ions caused the same effect, but the acid-base reaction under the presence of Co2O3 decreased the total capacitance.
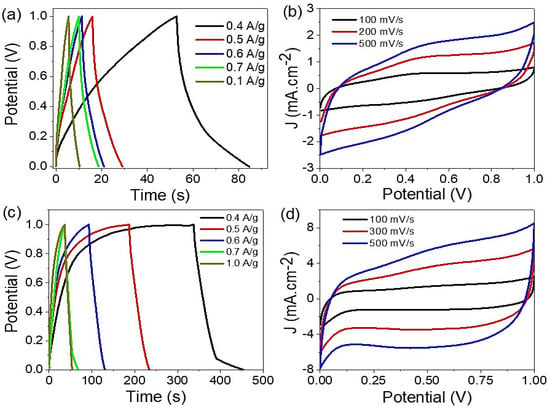
Figure 3.
(a,c) Charge and discharge and (b,d) cyclic voltammetry curves for CoS-Co2O3/G-C3N4 nanocomposite supercapacitor under HCl and NaOH electrolytes, respectively.
The cyclic voltammetry behavior confirmed the charge and discharge behavior [25,26], where the produced current density values had a greater value in the base medium than in the acid medium (see Figure 3c,d). The rectangular shape appeared in the base medium, which confirmed the pseudo-capacitance feature [27,28], in which the surface area increased by increasing the scan rate from 100 to 500 mV.s−1. This confirmed the diffusion time for the electrolyte to diffuse inside the supercapacitor paste. The oxidation and reduction behavior increased on both sides of the cyclic voltammetry curve under the rectangular formed shape. The cyclic voltammetry behavior depends on the electrolyte used, in which the rectangular shape is confirmed from a base medium for the pseudo-capacitance feature.
The stability of the supercapacitor electrodes is determined through the charge and discharge behavior at a current density of 0.4 A/g for 1000 cycles using two different electrolytes, 0.5 M HCl and 6 M NaOH (see, Figure 4a,b). From these figures, the two supercapacitors in both the acid and base mediums have greater stability up to 1000 cycles, but the supercapacitor in the base medium obtains better behavior. Figure 4c gives the relation of cycle numbers and the capacitance retention in both acid and base mediums. From this figure, both supercapacitors have great stability up to 400 cycles, but then the supercapacitor in the acid medium faces a small stability loss, which may be due to the reaction of HCl with the Co2O3 inside the paste of the electrode. The supercapacitor in the base medium has 100% stability retention up to 600 cycles; then, the stability decreases to 98.5% up to 1000 cycles.
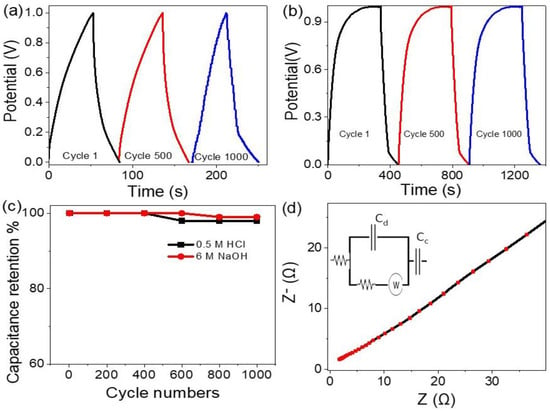
Figure 4.
The cycle lifetime of the supercapacitor using the electrolytes (a) 0.5 M HCl and (b) M NaOH. (c) Capacitance retention using different electrolytes and (d) the impedance of the supercapacitor in basic medium 6 M NaOH.
The relation between the real (Z) and imaginary (Z−) impedance (Nyquist plot) is shown in Figure 4d for the supercapacitor in 6 M NaOH. Moreover, the impedance circuit represents the electrochemical contact of the prepared supercapacitor (see insert Figure 4d), in which the series and charge transfer resistance represent the electrolyte and electrolyte/electrode resistances, respectively, while the Warburg impedance represents the resistance of the electrode material [29,30]. Moreover, the capacitance appears in the circuit that represents the contact. The supercapacitor displays a very small Z− value, confirming the greater behavior of NaOH as an electrolyte for charge transfer (Rct) between the electrolyte and the electrode (see Figure 4d). RCT characterizes the rate of redox reactions at the electrode/electrolyte interface, while Cdl occurs at the interfaces between solids and ionic solutions due to separations of ionic and electronic charges. The values of RS and RCT are 1.54 and 6.4 Ω, respectively, while the Cdl value is 18 µF.
For calculation of the performance of the prepared supercapacitor, the specific capacitance (CS) was determined in the acid and base mediums (see, Figure 5a). Such a calculation is based on Equation (1) [31,32], in which I, , and m represent current (A), discharge time (s), potential window (V), and the paste mass (g), respectively. The supercapacitor has CS values of 361 and 92 F/g in the basic and acidic mediums, respectively. In addition, the energy density (E) represents the capacitance of the prepared supercapacitor. These E values were calculated for the supercapacitor from 6 M NaOH and 0.5 M HCl, as shown in Figure 5b and Equation (2). This E value depends on potential windows Vmax and Vmin, in which the produced E values in the acid and base mediums are 28.7 and 30.2 W h kg−1, respectively.
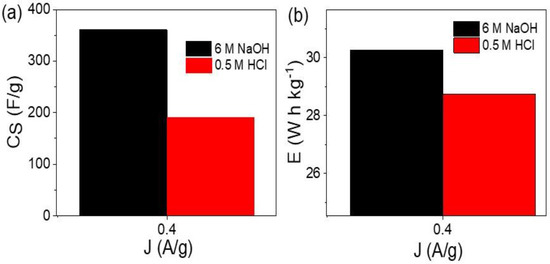
Figure 5.
(a) The specific capacitance and (b) energy density for the prepared two symmetric electrodes supercapacitor in acid and base mediums.
4. Conclusions
Flower-shaped CoS-Co2O3/G-C3N4 composite was prepared using the hydrothermal method for two-symmetric-electrodes supercapacitor application. Such a composite was based on G-C3N4, first prepared using the combustion process in ambient air. The morphological properties were confirmed through SEM and TEM analyses. The chemical structures were confirmed using XRD and XPS analyses, in which all the functional groups and elements were confirmed. The electrochemical studies were carried out by testing the prepared supercapacitor from the HCl and NaOH mediums. The supercapacitor parameters, charge/discharge, cyclic voltammetry, stability, and impedance of the prepared supercapacitor, were studied. The supercapacitor had a significant response in the basic medium in comparison to the acid medium. The supercapacitor had 100 and 98.5% stability retention up to 600 and 1000 cycles, respectively. The supercapacitor had CS values of 361 and 92 F/g in the basic and acidic mediums, respectively. Moreover, the E values were 28.7 and 30.2 W h kg−1, respectively. This promising prepared supercapacitor device has great electrochemical properties that qualifies it for industrial applications.
Author Contributions
Conceptualization, M.R. and A.B.G.T.; Data curation, M.R., D.E., F.H.A., A.B.G.T., S.E. and M.S.; Formal analysis M.R., D.E., F.H.A., A.B.G.T., S.E. and M.S.; Investigation, F.H.A., A.B.G.T. and M.R.; Methodology M.R., A.B.G.T. and D.E.; Supervision, M.R., D.E., F.H.A. and A.B.G.T.; Writing—original draft, M.R., D.E., F.H.A., A.B.G.T., S.E. and M.S.; Writing—review and editing, M.R. and A.B.G.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R38), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Data Availability Statement
Not applicable.
Acknowledgments
The authors express their gratitude to Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R38), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mai, L.Q.; Minhas-Khan, A.; Tian, X.; Hercule, K.M.; Zhao, Y.L.; Lin, X.; Xu, X. Synergistic Interaction between Redox-Active Electrolyte and Binder-Free Functionalized Carbon for Ultrahigh Supercapacitor Performance. Nat. Commun. 2013, 4, 2923. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, K.; Pazhamalai, P.; Mariappan, V.K.; Nardekar, S.S.; Sahoo, S.; Kim, S.J. Probing the Energy Conversion Process in Piezoelectric-Driven Electrochemical Self-Charging Supercapacitor Power Cell Using Piezoelectrochemical Spectroscopy. Nat. Commun. 2020, 11, 2351. [Google Scholar] [CrossRef] [PubMed]
- Mai, L.Q.; Yang, F.; Zhao, Y.L.; Xu, X.; Xu, L.; Luo, Y.Z. Hierarchical MnMoO4/CoMoO4 Heterostructured Nanowires with Enhanced Supercapacitor Performance. Nat. Commun. 2011, 2, 381. [Google Scholar] [CrossRef] [PubMed]
- Chahal, P.; Madaswamy, S.L.; Lee, S.C.; Wabaidur, S.M.; Dhayalan, V.; Ponnusamy, V.K.; Dhanusuraman, R. Novel Manganese Oxide Decorated Polyaniline/Graphitic Carbon Nitride Nanohybrid Material for Efficient Supercapacitor Application. Fuel 2022, 330, 125531. [Google Scholar] [CrossRef]
- Rabia, M.; Mohamed, H.S.H.; Shaban, M.; Taha, S. Preparation of Polyaniline/PbS Core-Shell Nano/Microcomposite and Its Application for Photocatalytic H2 Electrogeneration from H2O. Sci. Rep. 2018, 8, 1107. [Google Scholar] [CrossRef]
- Mohamed, H.S.H.; Rabia, M.; Zhou, X.G.; Qin, X.S.; Khabiri, G.; Shaban, M.; Younus, H.A.; Taha, S.; Hu, Z.Y.; Liu, J.; et al. Phase-Junction Ag/TiO2 Nanocomposite as Photocathode for H2 Generation. J. Mater. Sci. Technol. 2021, 83, 179–187. [Google Scholar] [CrossRef]
- Mohamed, F.; Rabia, M.; Shaban, M. Synthesis and Characterization of Biogenic Iron Oxides of Different Nanomorphologies from Pomegranate Peels for Efficient Solar Hydrogen Production. J. Mater. Res. Technol. 2020, 9, 4255–4271. [Google Scholar] [CrossRef]
- Shaban, M.; Rabia, M.; Fathallah, W.; El-Mawgoud, N.A.; Mahmoud, A.; Hussien, H.; Said, O. Preparation and Characterization of Polyaniline and Ag/ Polyaniline Composite Nanoporous Particles and Their Antimicrobial Activities. J. Polym. Environ. 2017, 26, 434–442. [Google Scholar] [CrossRef]
- Zheng, Z.X.; Wang, M.; Shi, X.Z.; Wang, C.M. Palladium Nanoparticles/Graphitic Carbon Nitride Nanosheets-Carbon Nanotubes as a Catalytic Amplification Platform for the Selective Determination of 17α-Ethinylestradiol in Feedstuffs. Sci. Rep. 2019, 9, 14162. [Google Scholar] [CrossRef]
- Santos, R.S.; Suresh Babu, R.; Devendiran, M.; Haddad, D.B.; de Barros, A.L.F. Facile Synthesis of Transition Metal (M = Cu, Co) Oxide Grafted Graphitic Carbon Nitride Nanosheets for High Performance Asymmetric Supercapacitors. Mater. Lett. 2022, 308, 131156. [Google Scholar] [CrossRef]
- Kumar Kuila, S.; Ghorai, A.; Midya, A.; Sekhar Tiwary, C.; Kumar Kundu, T. Chemisorption of Gadolinium Ions on 2D-Graphitic Carbon Nitride Nanosheet for Enhanced Solid-State Supercapacitor Performance. Chem. Phys. Lett. 2022, 796, 139572. [Google Scholar] [CrossRef]
- Rani, B.; Nayak, A.K.; Sahu, N.K. Electrochemical Supercapacitor Application of CoFe2O4 Nanoparticles Decorated over Graphitic Carbon Nitride. Diam. Relat. Mater. 2021, 120, 108671. [Google Scholar] [CrossRef]
- Nazari, M.; Rahmanifar, M.S.; Noori, A.; Li, W.; Zhang, C.; Mousavi, M.F. The Ordered Mesoporous Carbon Nitride-Graphene Aerogel Nanocomposite for High-Performance Supercapacitors. J. Power Sources 2021, 494, 229741. [Google Scholar] [CrossRef]
- Lin, R.; Li, Z.; Abou El Amaiem, D.I.; Zhang, B.; Brett, D.J.L.; He, G.; Parkin, I.P. A General Method for Boosting the Supercapacitor Performance of Graphitic Carbon Nitride/Graphene Hybrids. J. Mater. Chem. A 2017, 5, 25545–25554. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, Y.; Huang, X.; Chen, N.; Qu, L. Three-Dimensional Graphitic Carbon Nitride Functionalized Graphene-Based High-Performance Supercapacitors. J. Mater. Chem. A 2015, 3, 6761–6766. [Google Scholar] [CrossRef]
- Lu, C.; Chen, X. Carbon Nanotubes/Graphitic Carbon Nitride Nanocomposites for All-Solid-State Supercapacitors. Sci. China Technol. Sci. 2020, 63, 1714–1720. [Google Scholar] [CrossRef]
- Pilathottathil, S.; Kavil, J.; Shahin Thayyil, M. Boosting Ion Dynamics by Developing Graphitic Carbon Nitride/Carbon Hybrid Electrode Materials for Ionogel Supercapacitor. Mater. Sci. Eng. B 2022, 276, 115573. [Google Scholar] [CrossRef]
- Azizi-Toupkanloo, H.; Karimi-Nazarabad, M.; Shakeri, M.; Eftekhari, M. Photocatalytic Mineralization of Hard-Degradable Morphine by Visible Light-Driven Ag@g-C3N4 Nanostructures. Environ. Sci. Pollut. Res. 2019, 26, 30941–30953. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, P.; Joshi, C.; Manchanda, M.; Boukherroub, R.; Jain, S.L. Nickel Decorated on Phosphorous-Doped Carbon Nitride as an Efficient Photocatalyst for Reduction of Nitrobenzenes. Nanomaterials 2016, 6, 59. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.H.; Kang, Y.C. Electrochemical Properties of Cobalt Sulfide-Carbon Composite Powders Prepared by Simple Sulfidation Process of Spray-Dried Precursor Powders. Electrochim. Acta 2014, 137, 336–343. [Google Scholar] [CrossRef]
- Rajeevgandhi, C.; Sathiyamurthy, K.; Guganathan, L.; Savithiri, S.; Bharanidharan, S.; Mohan, K. Experimental and Theoretical Investigations on the Spinel Structure of Co2O3 Nanoparticles Synthesized via Simple Co-Precipitation Method. J. Mater. Sci. Mater. Electron. 2020, 31, 16769–16779. [Google Scholar] [CrossRef]
- Atta, A.; Abdelhamied, M.M.; Essam, D.; Shaban, M.; Alshammari, A.H.; Rabia, M. Structural and Physical Properties of Polyaniline/Silver Oxide/Silver Nanocomposite Electrode for Supercapacitor Applications. Int. J. Energy Res. 2021, 46, 6702–6710. [Google Scholar] [CrossRef]
- Gamal, A.; Shaban, M.; BinSabt, M.; Moussa, M.; Ahmed, A.M.; Rabia, M.; Hamdy, H. Facile Fabrication of Polyaniline/Pbs Nanocomposite for High-Performance Supercapacitor Application. Nanomaterials 2022, 12, 817. [Google Scholar] [CrossRef] [PubMed]
- Different Types of Supercapacitors, 978-620-2-67505-5, 6202675055, 9786202675055 by Mohamed Mosaad Hamid, Eman Ali, Mohamed Rabia. Available online: https://www.morebooks.de/store/gb/book/different-types-of-supercapacitors/isbn/978-620-2-67505-5 (accessed on 23 January 2021).
- Sayyah, E.S.M.; Shaban, M.; Rabia, M. A sensor of m-cresol nanopolymer/Pt-electrode film for detection of lead ions by potentiometric methods. Adv. Polym. Technol. 2018, 37, 1296–1304. [Google Scholar]
- Sayyah, S.M.; Shaban, M.; Rabia, M. Electropolymerization of m-toluidin on platinum electrode from aqueous acidic solution and character of the obtained polymer. Adv. Polym. Technol. 2018, 37, 126–136. [Google Scholar]
- Hao, J.; Huang, Y.; He, C.; Xu, W.; Yuan, L.; Shu, D.; Song, X.; Meng, T. Bio-Templated Fabrication of Three-Dimensional Network Activated Carbons Derived from Mycelium Pellets for Supercapacitor Applications. Sci. Rep. 2018, 8, 562. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, M.; Abdellah, A.M.; Mohamed, S.G.; Allam, N.K. 3D Interconnected Binder-Free Electrospun MnO@C Nanofibers for Supercapacitor Devices. Sci. Rep. 2018, 8, 7988. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.K.; Stimming, U.; Lee, A.A. Impedance-Based Forecasting of Lithium-Ion Battery Performance amid Uneven Usage. Nat. Commun. 2022, 13, 4806. [Google Scholar] [CrossRef]
- Qu, D.; Ji, W.; Qu, H. Probing Process Kinetics in Batteries with Electrochemical Impedance Spectroscopy. Commun. Mater. 2022, 3, 61. [Google Scholar] [CrossRef]
- Yu, J.; Fu, N.; Zhao, J.; Liu, R.; Li, F.; Du, Y.; Yang, Z. High Specific Capacitance Electrode Material for Supercapacitors Based on Resin-Derived Nitrogen-Doped Porous Carbons. ACS Omega 2019, 4, 15904–15911. [Google Scholar] [CrossRef] [PubMed]
- Naveed ur Rehman, M.; Munawar, T.; Nadeem, M.S.; Mukhtar, F.; Maqbool, A.; Riaz, M.; Manzoor, S.; Ashiq, M.N.; Iqbal, F. Facile Synthesis and Characterization of Conducting Polymer-Metal Oxide Based Core-Shell PANI-Pr2O–NiO–Co3O4 Nanocomposite: As Electrode Material for Supercapacitor. Ceram. Int. 2021, 47, 18497–18509. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).