Dynamic Behaviours of Monodisperse Double Emulsion Formation in a Tri-Axial Capillary Device
Abstract
1. Introduction
2. Experimental Materials and Methods
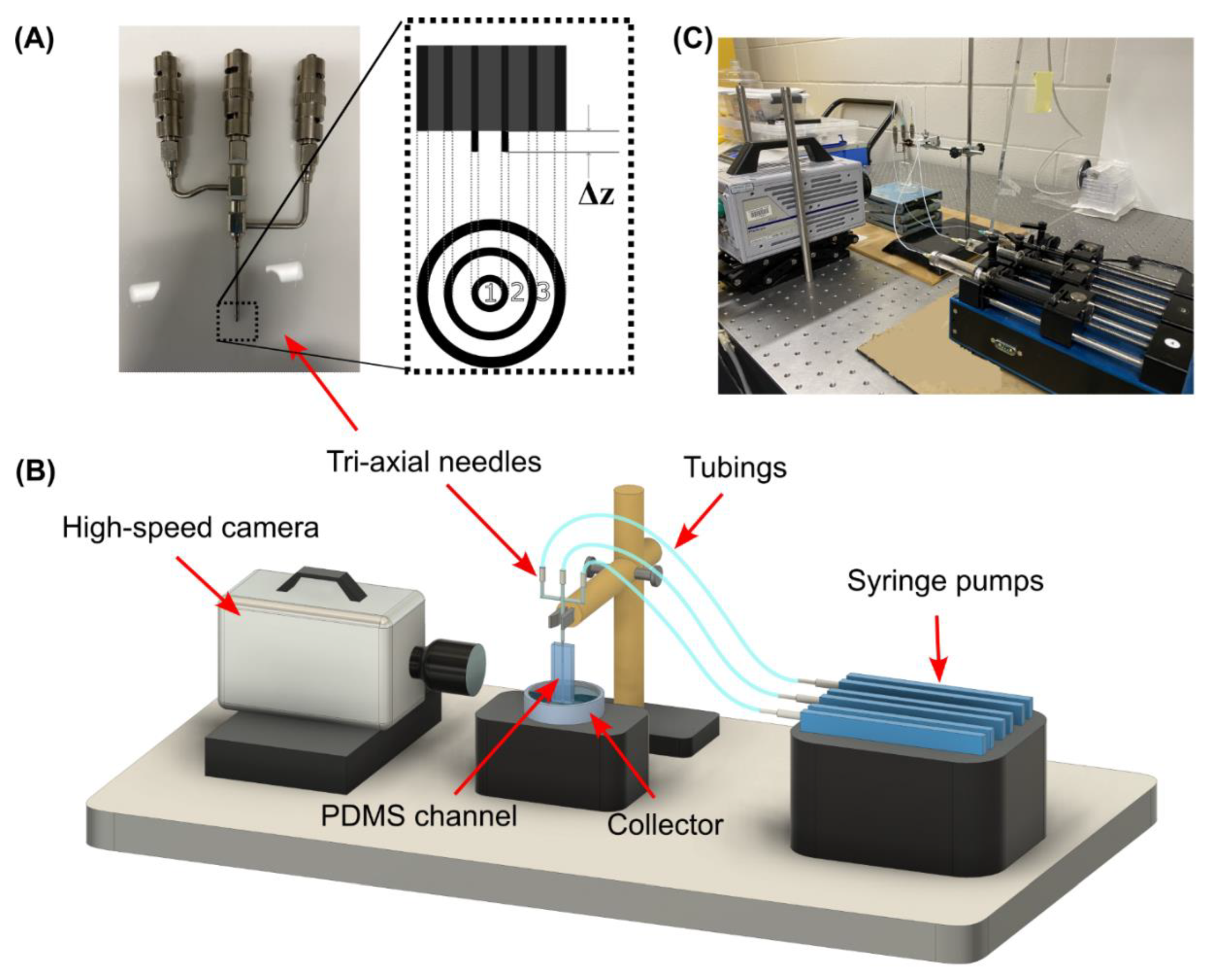
2.1. Design and Fabrication of the Capillary Device
2.2. Materials
2.3. Experimental Setup and Data Analysis
3. Numerical Methodology
3.1. Governing Equations
3.2. Computational Domain and Boundary Conditions
4. Analytical Model
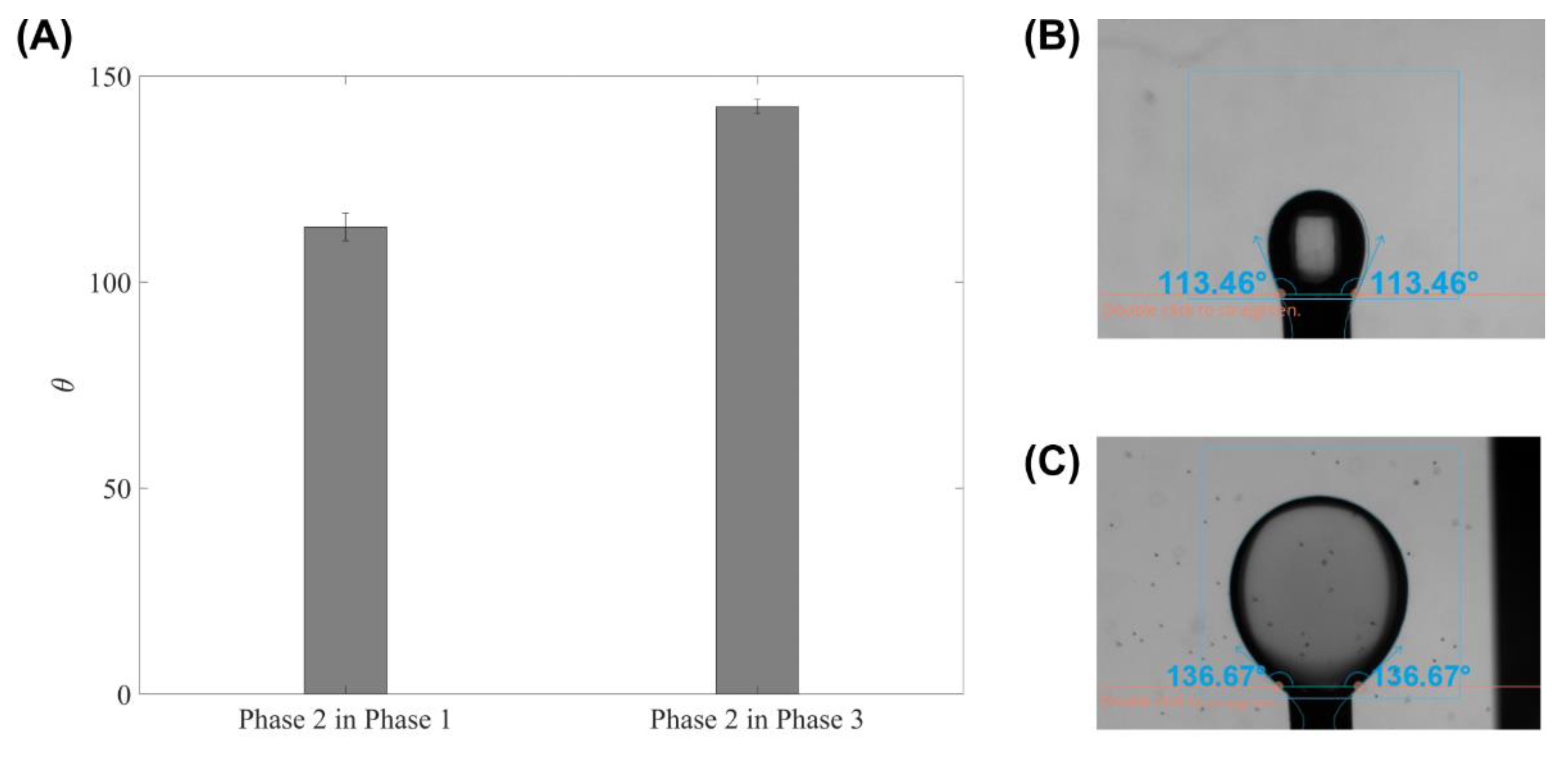
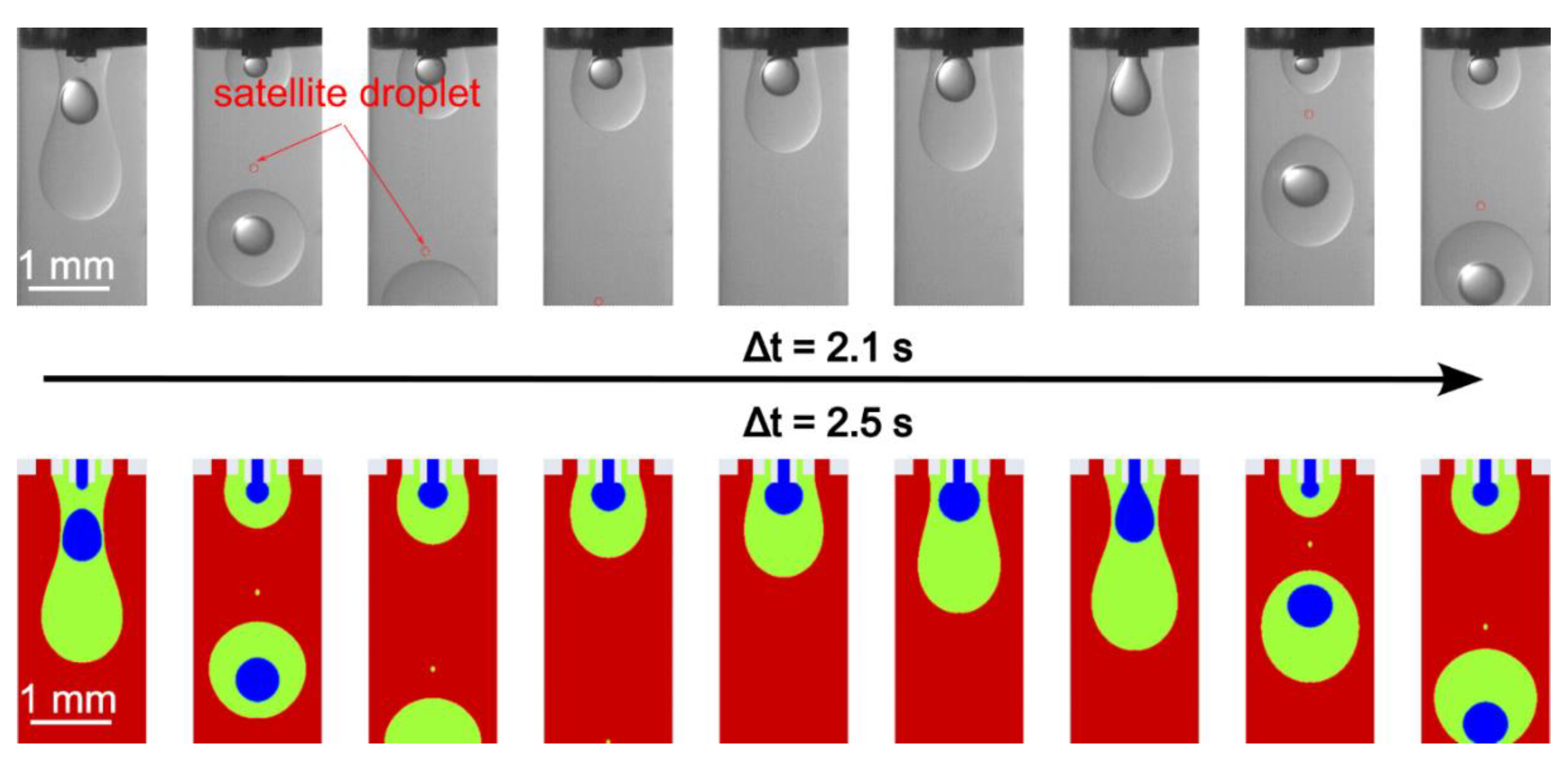
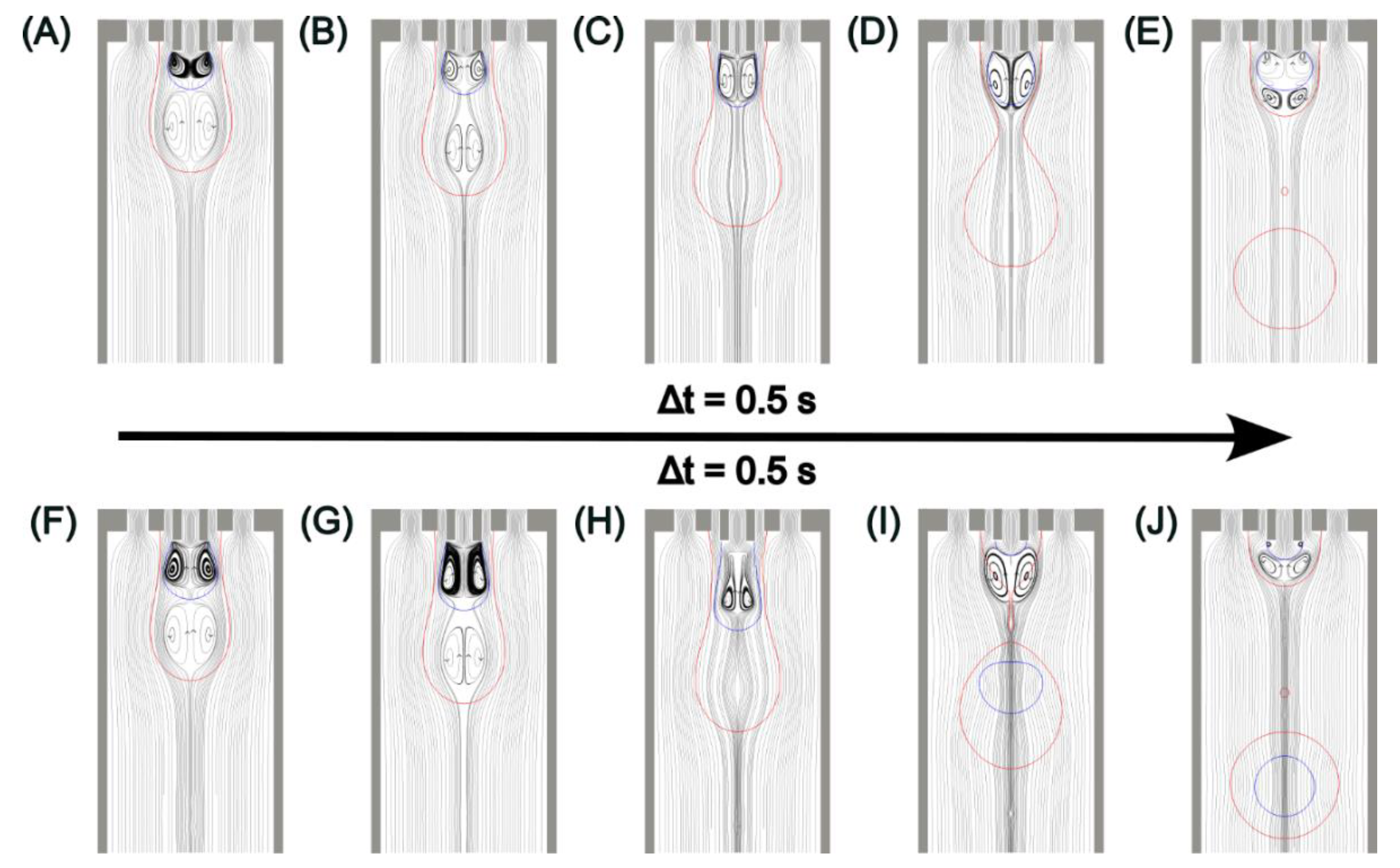
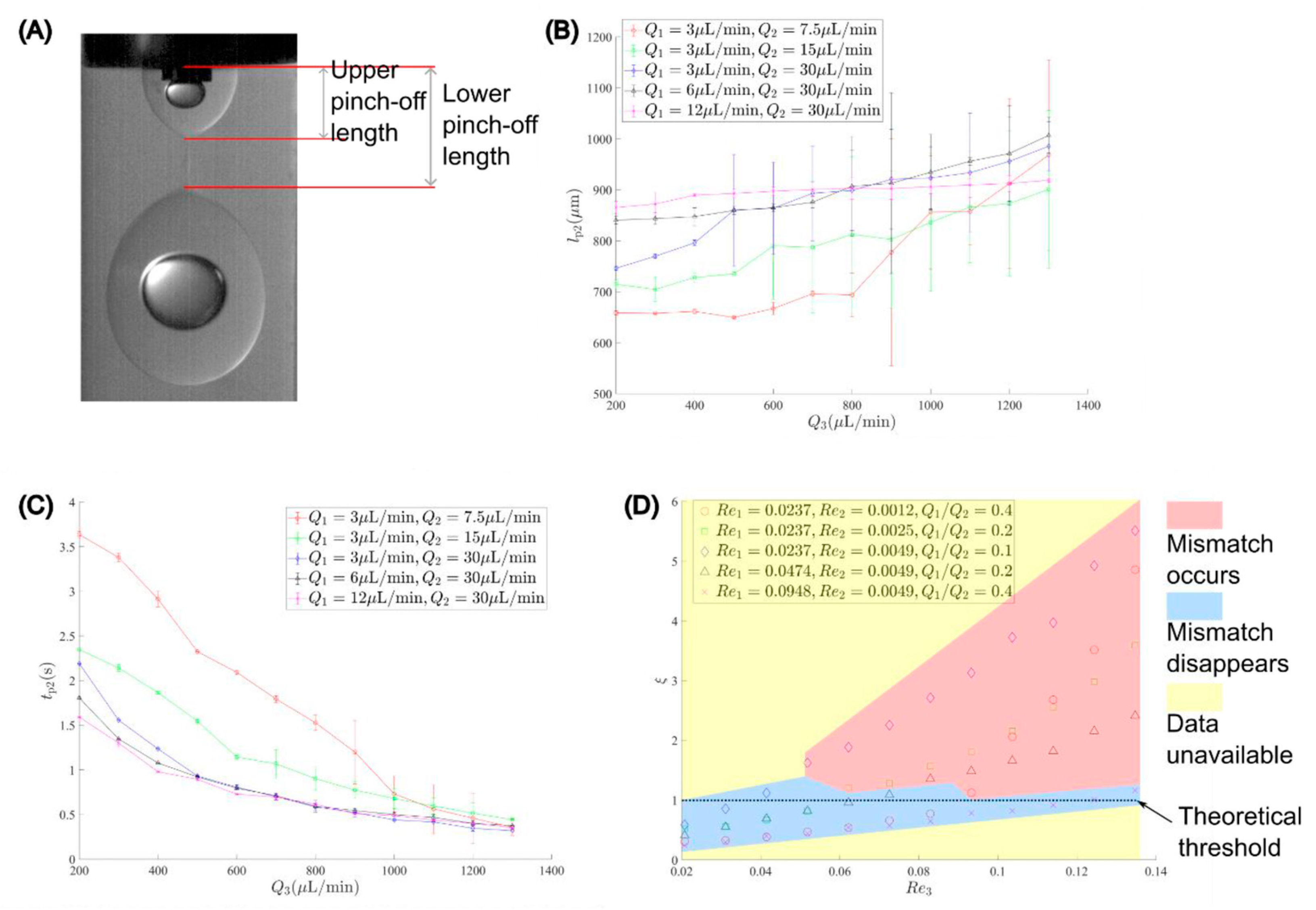
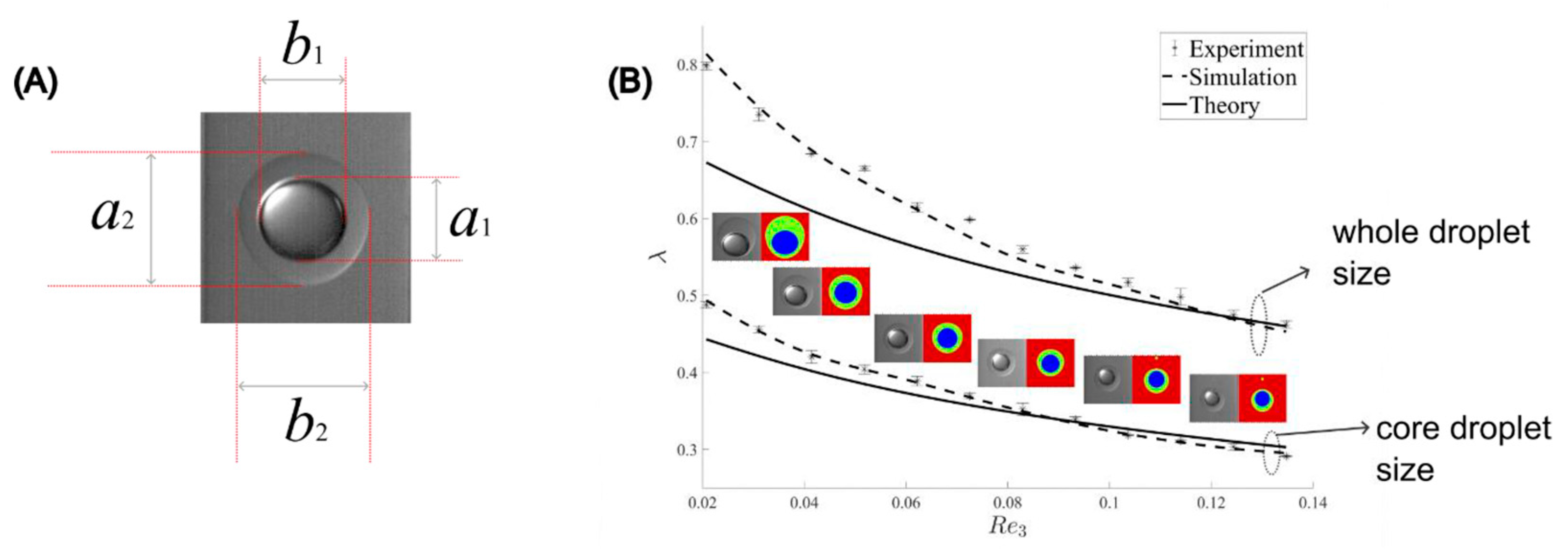
5. Results and Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Clegg, P.S.; Tavacoli, J.W.; Wilde, P.J. One-step production of multiple emulsions: Microfluidic, polymer-stabilized and particle-stabilized approaches. Soft Matter 2016, 12, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Serra, C.A.; Vandamme, T.F.; Yu, W.; Anton, N. Double emulsions prepared by two–step emulsification: History, state-of-the-art and perspective. J. Control. Release 2019, 295, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.I. The formation of emulsions in definable fields of flow. In Proceedings of the Royal Society of London, Series A, Containing Papers of a Mathematical and Physical Character, London, UK, 1 October 1934; pp. 501–523. [Google Scholar]
- Tomotika, S. On the instability of a cylindrical thread of a viscous liquid surrounded by another viscous fluid. In Proceedings of the Royal Society of London, Series A—Mathematical and Physical Sciences, London, UK, 1 June 1935; pp. 322–337. [Google Scholar]
- Utada, A.S.; Lorenceau, E.; Link, D.R.; Kaplan, P.D.; Stone, H.A.; Weitz, D.A. Monodisperse Double Emulsions Generated from a Microcapillary Device. Science 2005, 308, 537–541. [Google Scholar] [CrossRef]
- Nie, Z.; Xu, S.; Seo, M.; Lewis, P.C.; Kumacheva, E. Polymer Particles with Various Shapes and Morphologies Produced in Continuous Microfluidic Reactors. J. Am. Chem. Soc. 2005, 127, 8058–8063. [Google Scholar] [CrossRef] [PubMed]
- Galogahi, F.M.; Zhu, Y.; An, H.; Nguyen, N.-T. Core-shell microparticles: Generation approaches and applications. J. Sci. Adv. Mater. Devices 2020, 5, 417–435. [Google Scholar] [CrossRef]
- Galogahi, F.M.; Zhu, Y.; An, H.; Nguyen, N.-T. Formation of core–shell droplets for the encapsulation of liquid contents. Microfluid Nanofluidics 2021, 25, 1–11. [Google Scholar] [CrossRef]
- Lewińska, D.; Bukowski, J.; Kożuchowski, M.; Kinasiewicz, J.; Weryński, A. Electrostatic microencapsulation of living cells. Biocybern. Biomed. Eng. 2008, 28, 69–84. [Google Scholar]
- Vadivelu, R.K.; Kamble, H.; Shiddiky, M.J.A.; Nguyen, N.-T. Microfluidic Technology for the Generation of Cell Spheroids and Their Applications. Micromachines 2017, 8, 94. [Google Scholar] [CrossRef]
- Bremond, N.; Santanach-Carreras, E.; Chu, L.-Y.; Bibette, J. Formation of liquid-core capsules having a thin hydrogel membrane: Liquid pearls. Soft Matter 2010, 6, 2484–2488. [Google Scholar] [CrossRef]
- Sreejith, K.R.; Gorgannezhad, L.; Jin, J.; Ooi, C.H.; Takei, T.; Hayase, G.; Stratton, H.; Lamb, K.; Shiddiky, M.; Dao, D.V. Core-Shell Beads Made by Composite Liquid Marble Technology as A Versatile Microreactor for Polymerase Chain Reaction. Micromachines 2020, 11, 242. [Google Scholar] [CrossRef]
- Chaurasia, A.S.; Sajjadi, S. Millimetric core–shell drops via buoyancy assisted non-confined microfluidics. Chem. Eng. Sci. 2015, 129, 260–270. [Google Scholar] [CrossRef]
- Nguyen, N.-T.; Wereley, S.T.; Shaegh, S.A.M. Fundamentals and Applications of Microfluidics; Artech House: Norwood, MA, USA, 2019. [Google Scholar]
- Shao, T.; Feng, X.; Jin, Y.; Cheng, Y. Controlled production of double emulsions in dual-coaxial capillaries device for millimeter-scale hollow polymer spheres. Chem. Eng. Sci. 2013, 104, 55–63. [Google Scholar] [CrossRef]
- Schmit, A.; Courbin, L.; Marquis, M.; Renard, D.; Panizza, P. A pendant drop method for the production of calibrated double emulsions and emulsion gels. RSC Adv. 2014, 4, 28504–28510. [Google Scholar] [CrossRef]
- Zhou, C.; Yue, P.; Feng, J.J. Formation of simple and compound drops in microfluidic devices. Phys. Fluids 2006, 18, 092105. [Google Scholar] [CrossRef]
- Park, J.M.; Anderson, P.D. A ternary model for double-emulsion formation in a capillary microfluidic device. Lab Chip 2012, 12, 2672–2677. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.V.; Homma, S.; Tryggvason, G.; Wells, J.C.; Takakura, H. Computations of breakup modes in laminar compound liquid jets in a coflowing fluid. Int. J. Multiph. Flow 2012, 49, 58–69. [Google Scholar] [CrossRef]
- Tryggvason, G.; Bunner, B.; Esmaeeli, A.; Juric, D.; Al-Rawahi, N.; Tauber, W.; Han, J.; Nas, S.; Jan, Y.-J. A Front-Tracking Method for the Computations of Multiphase Flow. J. Comput. Phys. 2001, 169, 708–759. [Google Scholar] [CrossRef]
- Fu, Y.; Zhao, S.; Bai, L.; Jin, Y.; Cheng, Y. Numerical study of double emulsion formation in microchannels by a ternary Lattice Boltzmann method. Chem. Eng. Sci. 2016, 146, 126–134. [Google Scholar] [CrossRef]
- Herrada, M.A.; Montanero, J.M.; Ferrera, C.; Gañán-Calvo, A.M. Analysis of the dripping–jetting transition in compound capillary jets. J. Fluid Mech. 2010, 649, 523–536. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, L.; Zhang, L. Dynamic behaviors of double emulsion formation in a flow-focusing device. Int. J. Heat Mass Transf. 2015, 82, 42–50. [Google Scholar] [CrossRef]
- Nabavi, S.A.; Vladisavljević, G.T.; Gu, S.; Ekanem, E.E. Double emulsion production in glass capillary microfluidic device: Parametric investigation of droplet generation behaviour. Chem. Eng. Sci. 2015, 130, 183–196. [Google Scholar] [CrossRef]
- Azarmanesh, M.; Farhadi, M.; Azizian, P. Double emulsion formation through hierarchical flow-focusing microchannel. Phys. Fluids 2016, 28, 032005. [Google Scholar] [CrossRef]
- Yang, Z.; Ma, X.; Wang, S.; Liu, D. Generation and evolution of double emulsions in a circular microchannel. Chem. Eng. Sci. 2022, 255, 117683. [Google Scholar] [CrossRef]
- Umbanhowar, P.B.; Prasad, V.; Weitz, D.A. Monodisperse Emulsion Generation via Drop Break Off in a Coflowing Stream. Langmuir 1999, 16, 347–351. [Google Scholar] [CrossRef]
- Wang, X.; Riaud, A.; Wang, K.; Luo, G. Pressure drop-based determination of dynamic interfacial tension of droplet generation process in T-junction micro-channel. Microfluid. Nanofluidics 2015, 18, 503–512. [Google Scholar] [CrossRef]
- Murshed, S.M.S.; Tan, S.H.; Nguyen, N.-T.; Wong, T.N.; Yobas, L. Microdroplet formation of water and nanofluids in heat-induced microfluidic T-junction. Microfluid. Nanofluidics 2008, 6, 253–259. [Google Scholar] [CrossRef]
- Wu, P.; Luo, Z.; Liu, Z.; Li, Z.; Chen, C.; Feng, L.; He, L. Drag-induced breakup mechanism for droplet generation in dripping within flow focusing microfluidics. Chin. J. Chem. Eng. 2014, 23, 7–14. [Google Scholar] [CrossRef]
- Happel, J.; Brenner, H. Low Reynolds Number Hydrodynamics: With Special Applications to Particulate Media; Springer Science & Business Media: New York, NY, USA, 2012. [Google Scholar]
- Tan, S.-H.; Murshed, S.M.S.; Nguyen, N.; Wong, T.N.; Yobas, L. Thermally controlled droplet formation in flow focusing geometry: Formation regimes and effect of nanoparticle sus-pension. J. Phys. D Appl. Phys. 2008, 41, 165501. [Google Scholar] [CrossRef]
- Yap, Y.F.; Tan, S.H.; Nguyen, N.-T.; Murshed, S.M.S.; Wong, T.N.; Yobas, L. Thermally mediated control of liquid microdroplets at a bifurcation. J. Phys. D Appl. Phys. 2009, 42, 065503. [Google Scholar] [CrossRef]
- Chang, Z.; Serra, C.A.; Bouquey, M.; Prat, L.; Hadziioannou, G. Co-axial capillaries microfluidic device for synthesizing size-and morphology-controlled polymer core-polymer shell particles. Lab Chip 2009, 9, 3007–3011. [Google Scholar] [CrossRef]
- Teo, A.J.; Malekpour-Galogahi, F.; Sreejith, K.R.; Takei, T.; Nguyen, N. Surfactant-free, UV-curable core–shell microcapsules in a hydrophilic PDMS microfluidic device. Aip Adv. 2020, 10, 065101. [Google Scholar] [CrossRef]
- Takamura, K.; Fischer, H.; Morrow, N.R. Physical properties of aqueous glycerol solutions. J. Pet. Sci. Eng. 2012, 98, 50–60. [Google Scholar] [CrossRef]
- Brackbill, J.; Kothe, D.; Zemach, C. A continuum method for modeling surface tension. J. Comput. Phys. 1992, 100, 335–354. [Google Scholar] [CrossRef]
- Bergman, T.L.; Incropera, F.P.; DeWitt, D.P.; Lavine, A.S. Fundamentals of Heat and Mass Transfer; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Erb, R.M.; Obrist, D.; Chen, P.W.; Studer, J.; Studart, A.R. Predicting sizes of droplets made by microfluidic flow-induced dripping. Soft Matter 2011, 7, 8757–8761. [Google Scholar] [CrossRef]
- Bohlin, T. On the Drag on a Rigid Sphere Moving in a Viscous Liquid Inside a Cylindrical Tube; Elanders: 1960. Available online: https://jglobal.jst.go.jp/en/detail?JGLOBAL_ID=201602000920293570 (accessed on 12 October 2022).
- Haberman, W.L.; Sayre, R.M. Motion of Rigid and Fluid Spheres in Stationary and Moving Liquids Inside Cylindrical Tubes. David Taylor Model Basin Washington DC. 1958. Available online: https://apps.dtic.mil/sti/pdfs/AD0206307.pdf (accessed on 12 October 2022).
- Dai, Y.; Cha, H.; Simmonds, M.J.; Fallahi, H.; An, H.; Ta, H.T.; Nguyen, N.-T.; Zhang, J.; McNamee, A.P. Enhanced Blood Plasma Extraction Utilising Viscoelastic Effects in a Serpentine Microchannel. Biosensors 2022, 12, 120. [Google Scholar] [CrossRef]


| Needle/Phase | Inner Diameter (µm) | Outer Diameter (µm) | Thickness (µm) |
|---|---|---|---|
| 1 | 178 | 356 | 89 |
| 2 | 508 | 813 | 152 |
| 3 | 1190 | 1650 | 229 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, Y.; Cha, H.; Nguyen, N.-K.; Ouyang, L.; Galogahi, F.; Yadav, A.S.; An, H.; Zhang, J.; Ooi, C.H.; Nguyen, N.-T. Dynamic Behaviours of Monodisperse Double Emulsion Formation in a Tri-Axial Capillary Device. Micromachines 2022, 13, 1877. https://doi.org/10.3390/mi13111877
Dai Y, Cha H, Nguyen N-K, Ouyang L, Galogahi F, Yadav AS, An H, Zhang J, Ooi CH, Nguyen N-T. Dynamic Behaviours of Monodisperse Double Emulsion Formation in a Tri-Axial Capillary Device. Micromachines. 2022; 13(11):1877. https://doi.org/10.3390/mi13111877
Chicago/Turabian StyleDai, Yuchen, Haotian Cha, Nhat-Khuong Nguyen, Lingxi Ouyang, Fariba Galogahi, Ajeet Singh Yadav, Hongjie An, Jun Zhang, Chin Hong Ooi, and Nam-Trung Nguyen. 2022. "Dynamic Behaviours of Monodisperse Double Emulsion Formation in a Tri-Axial Capillary Device" Micromachines 13, no. 11: 1877. https://doi.org/10.3390/mi13111877
APA StyleDai, Y., Cha, H., Nguyen, N.-K., Ouyang, L., Galogahi, F., Yadav, A. S., An, H., Zhang, J., Ooi, C. H., & Nguyen, N.-T. (2022). Dynamic Behaviours of Monodisperse Double Emulsion Formation in a Tri-Axial Capillary Device. Micromachines, 13(11), 1877. https://doi.org/10.3390/mi13111877












