Comprehensive Assessments in Bonding Energy of Plasma Assisted Si-SiO2 Direct Wafer Bonding after Low Temperature Rapid Thermal Annealing
Abstract
1. Introduction
2. Experimental
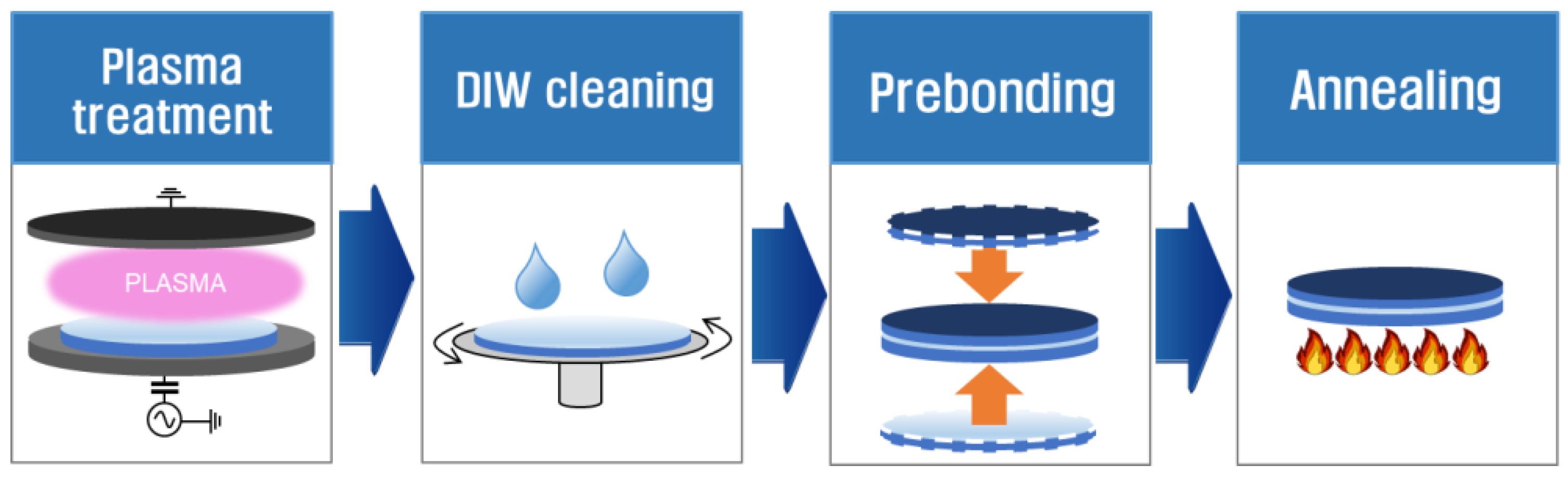
2.1. Plasma Treatment
2.2. DIW Cleaning
2.3. Prebonding
2.4. Annealing
2.5. DCB Evaluation
2.6. Characterization
3. Results and Discussion
3.1. Before the Plasma Treatment Step
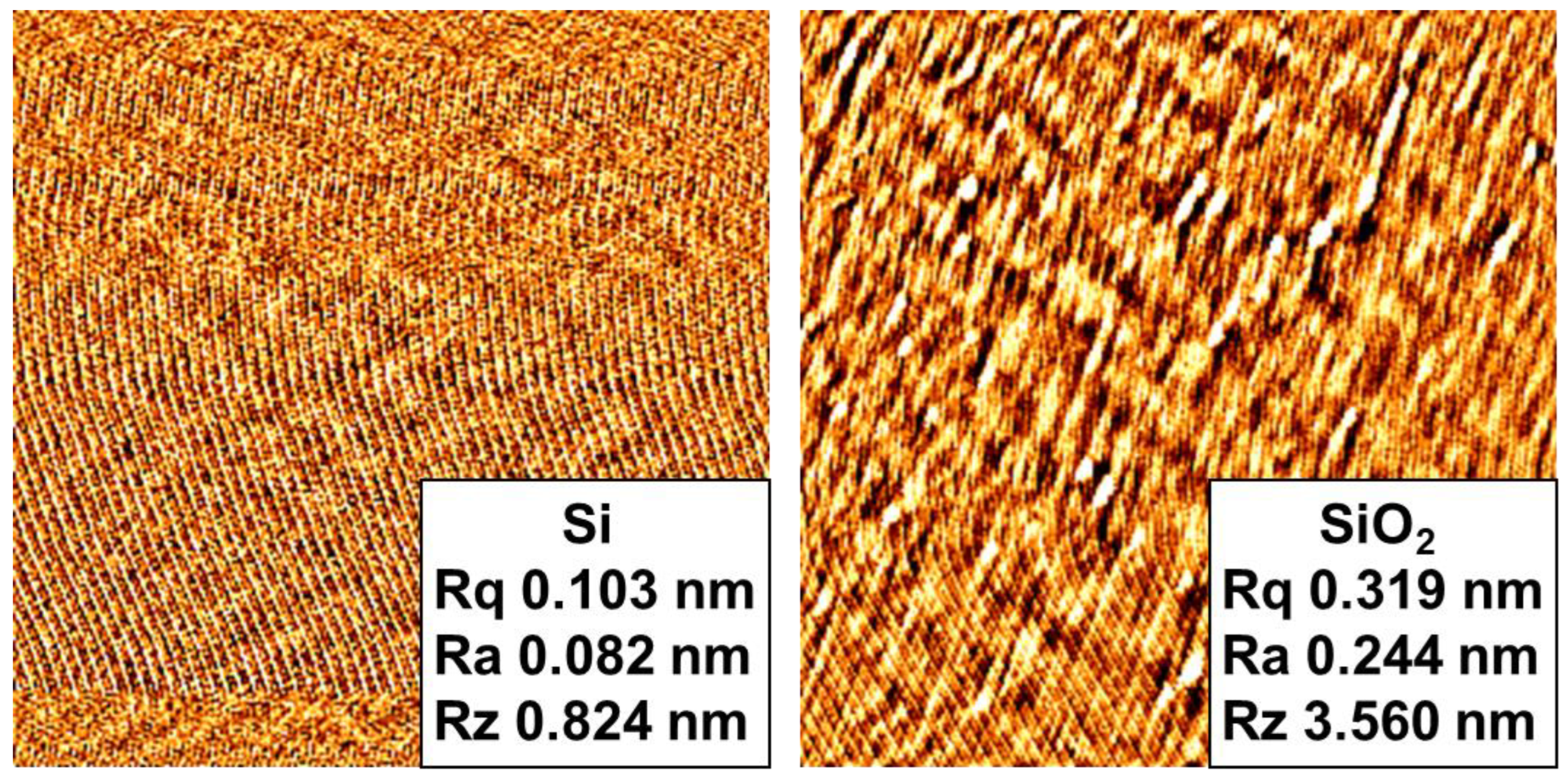
3.1.1. Surface Rinsing
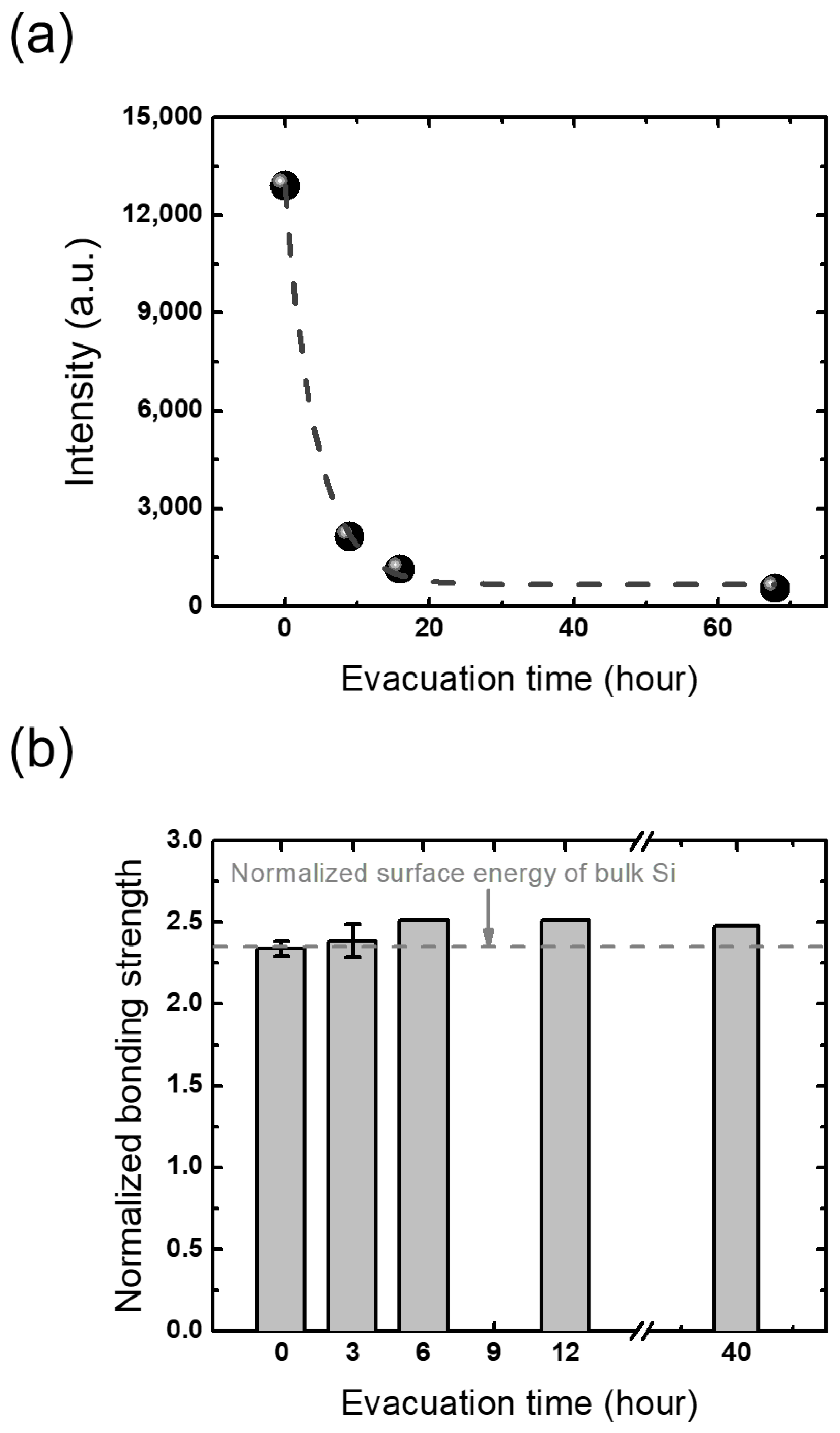
3.1.2. Moisture in Vacuum
3.2. Plasma Treatment Step
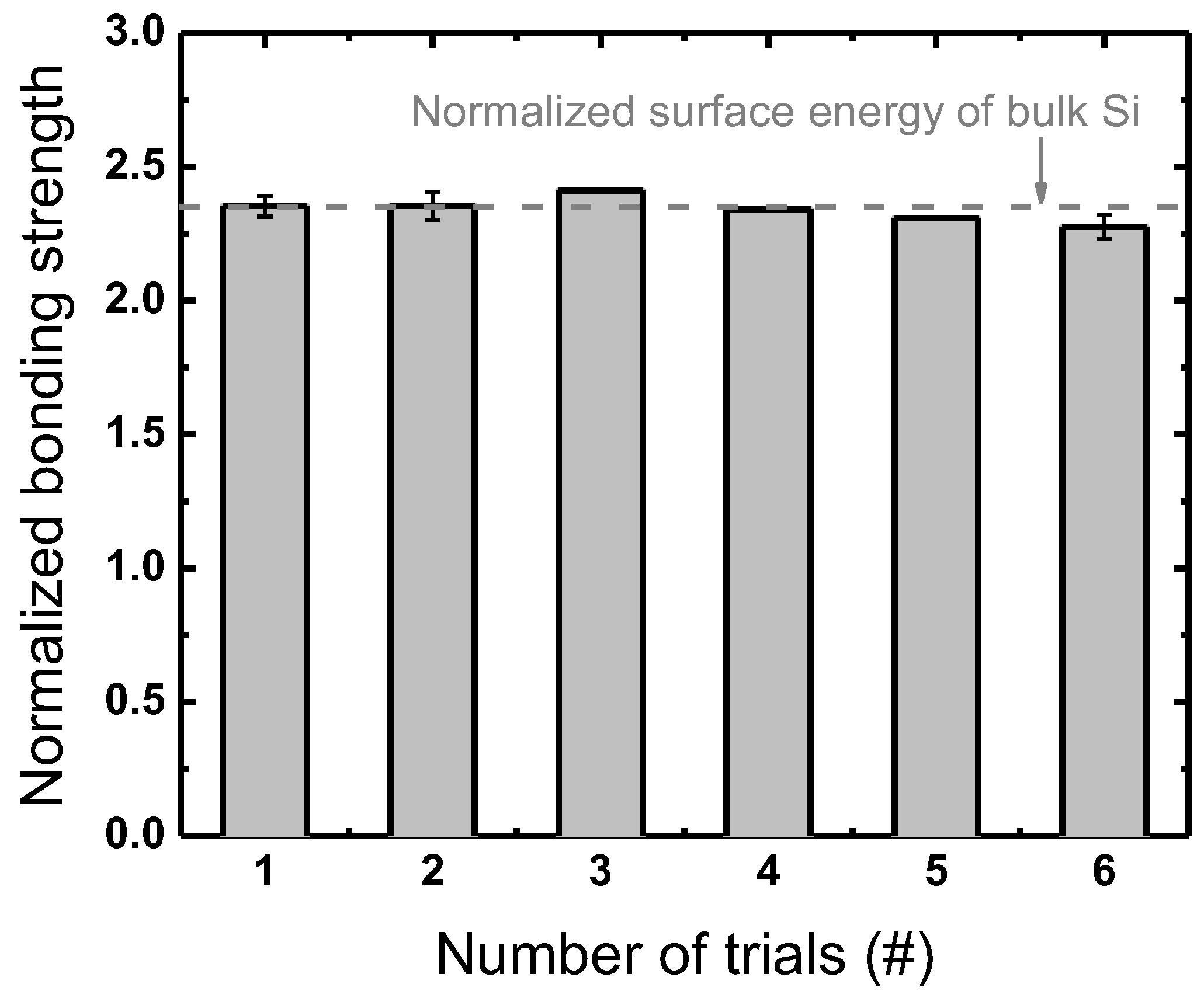
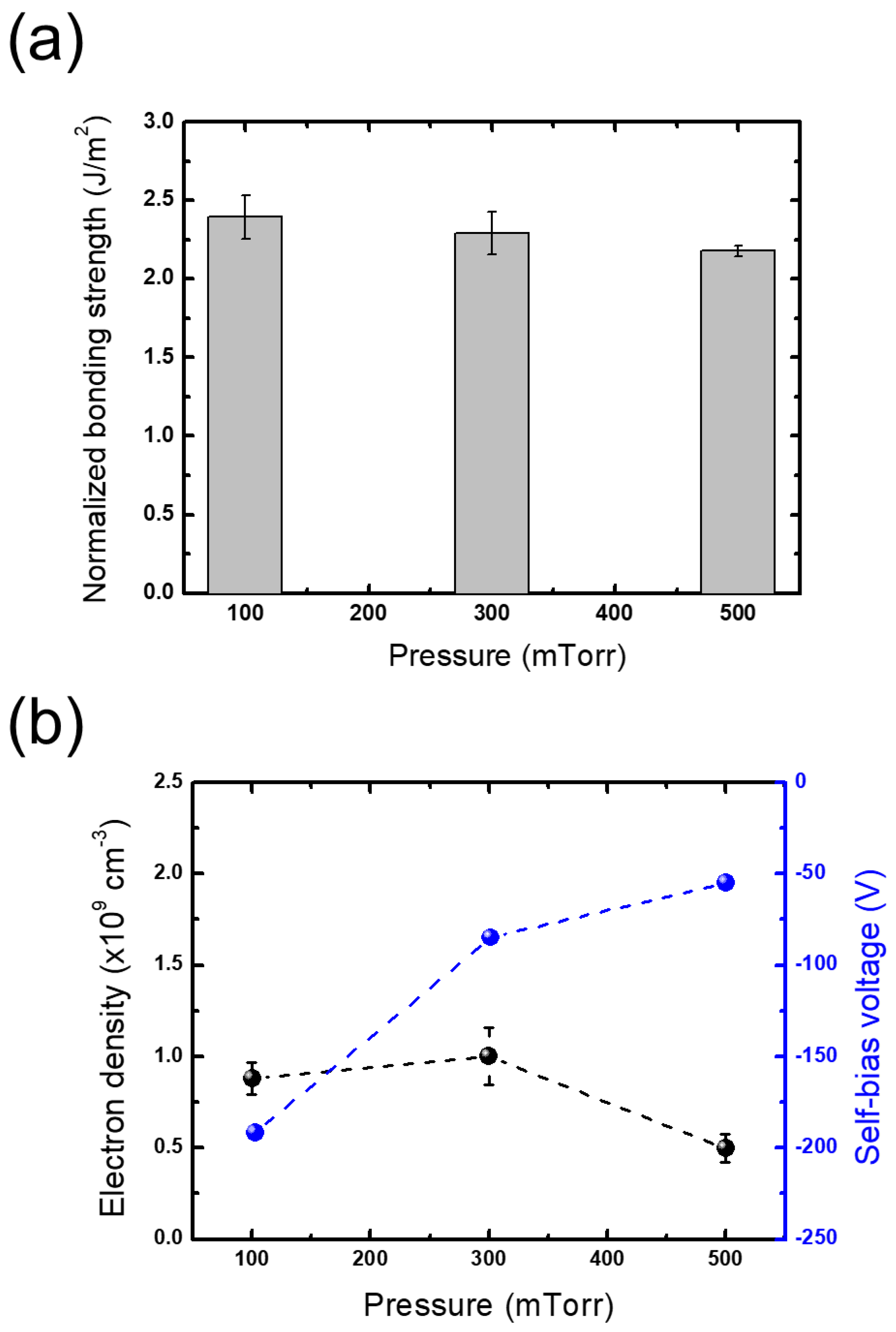
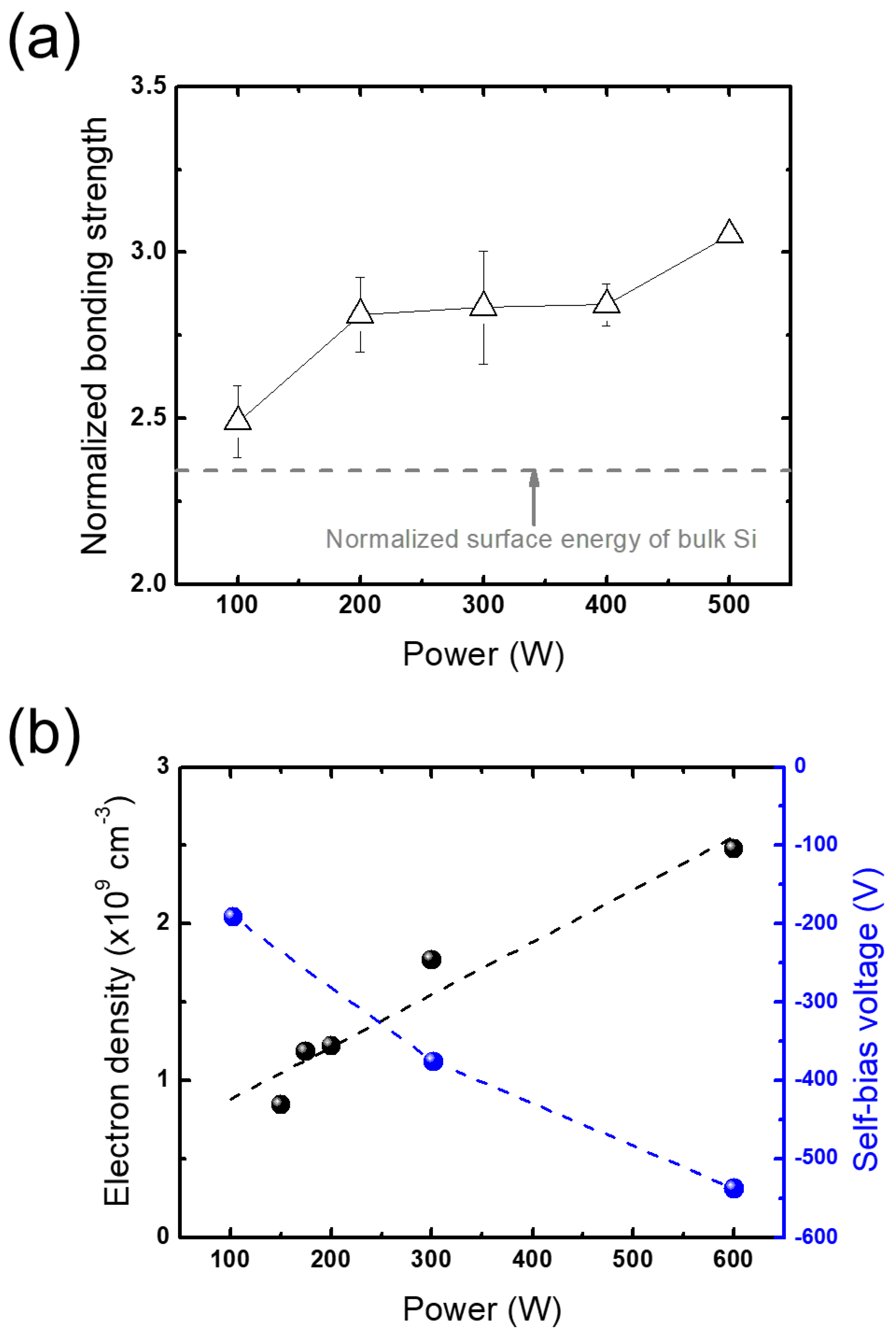
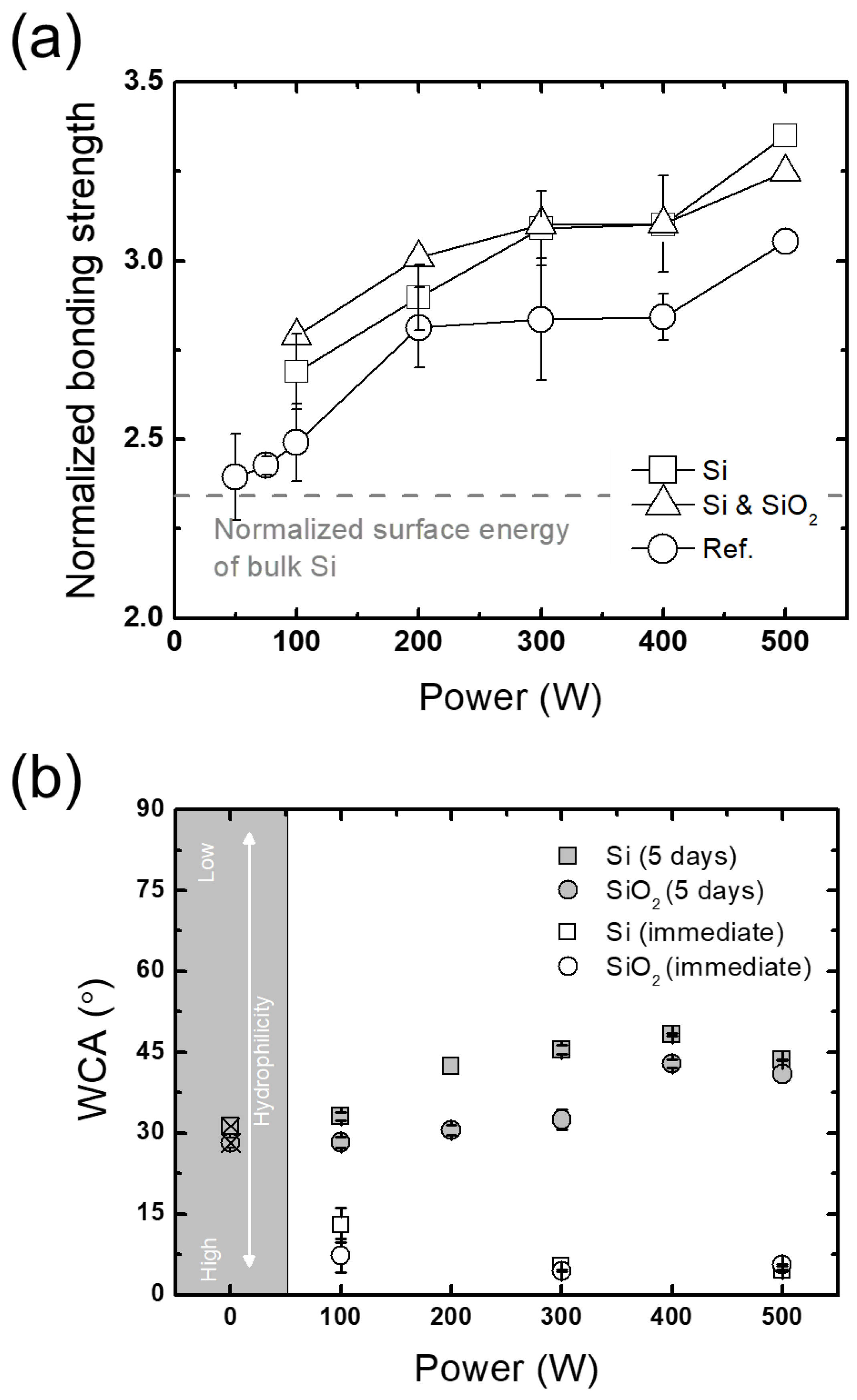
3.2.1. Plasma Parameters
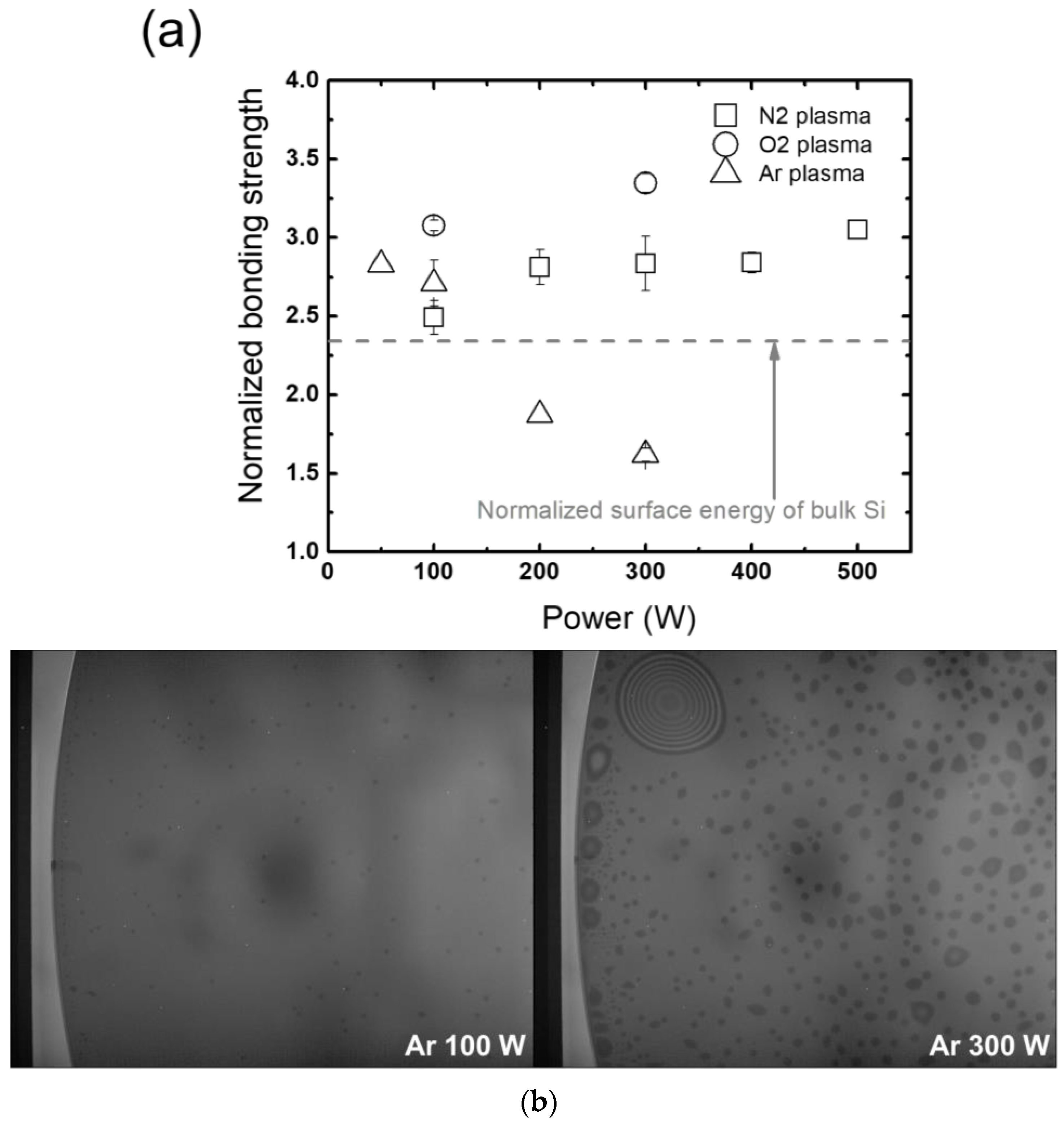
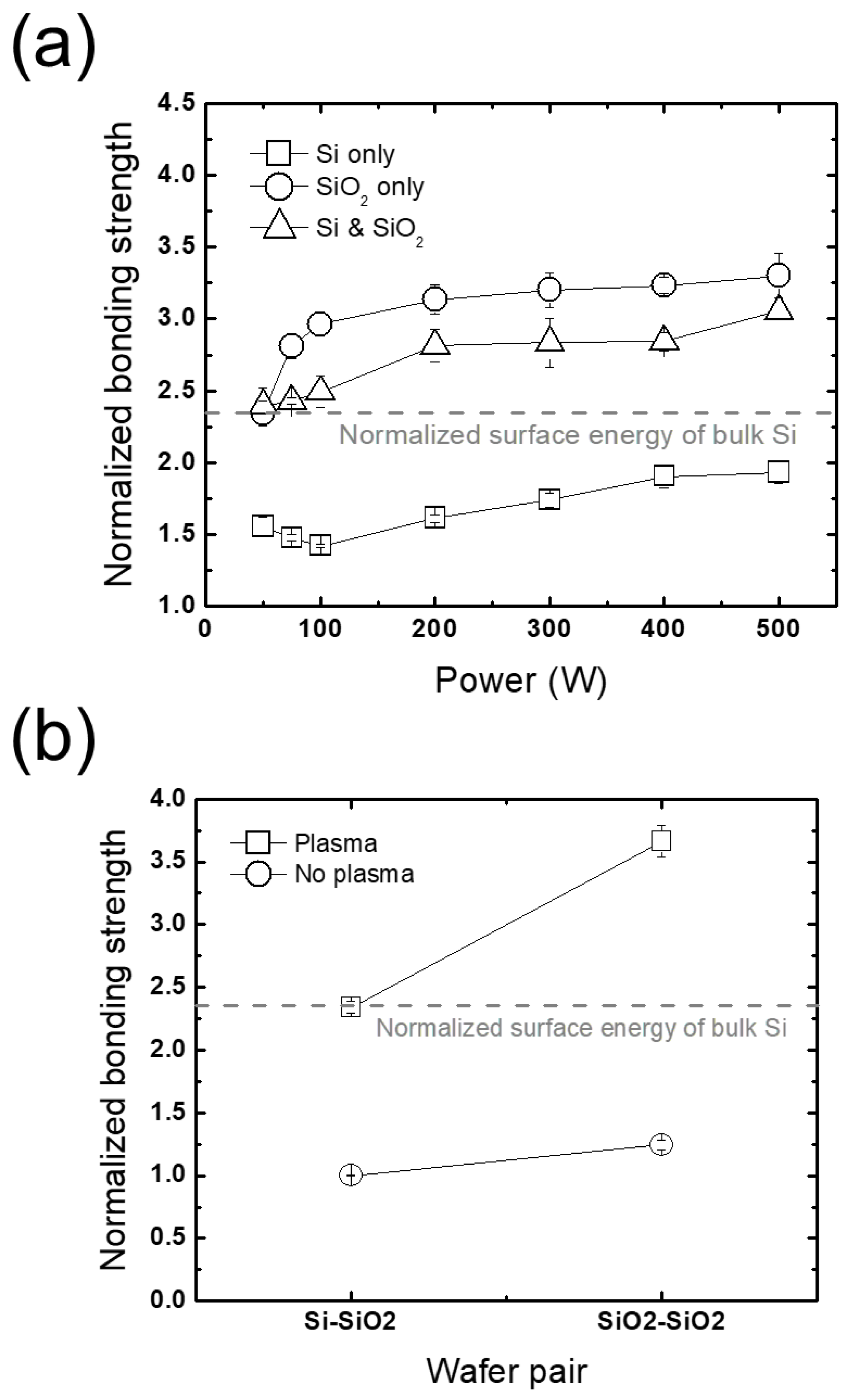
3.2.2. Wafer-Selective Treatment
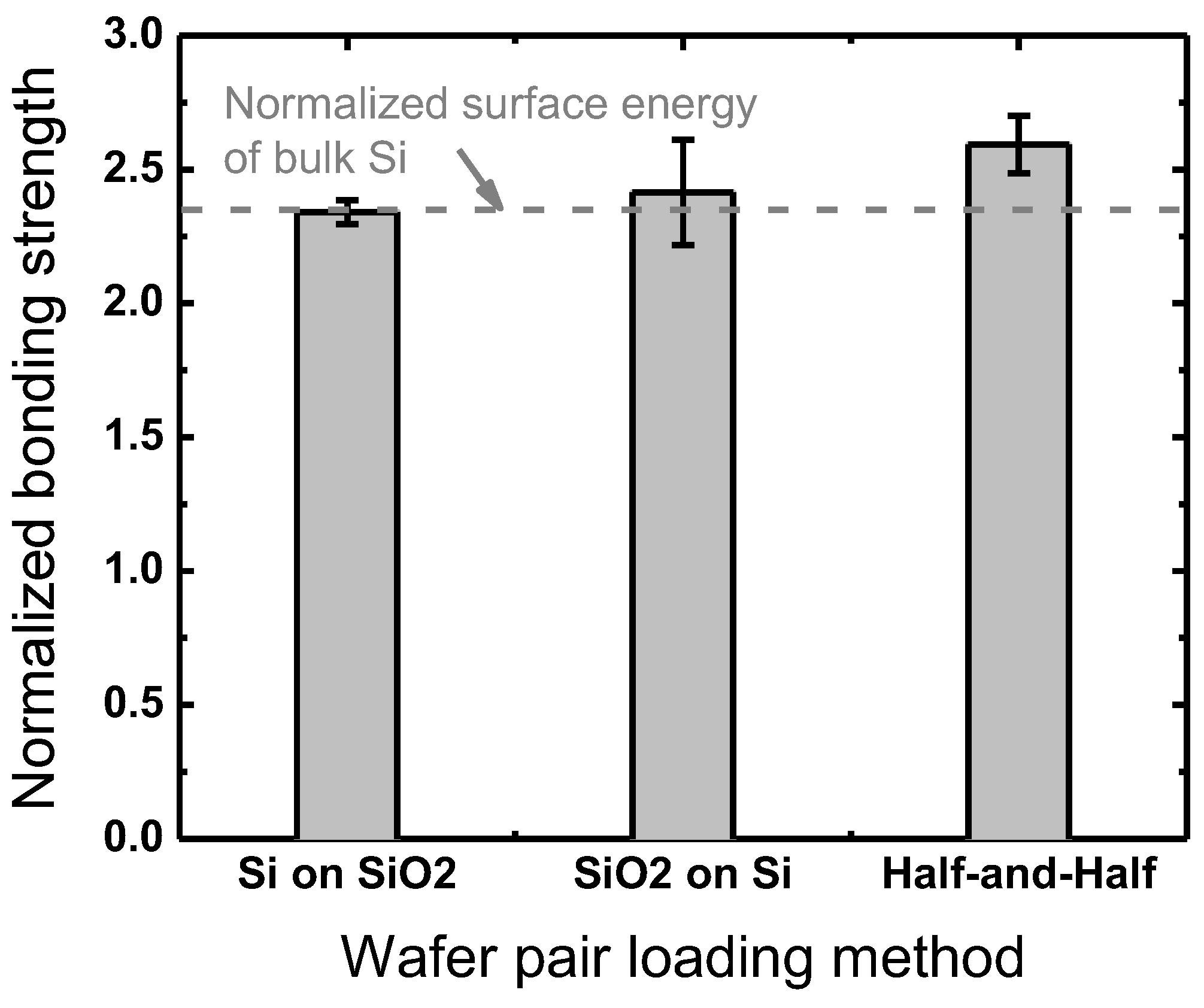
3.3. Between Plasma Treatment and Prebonding
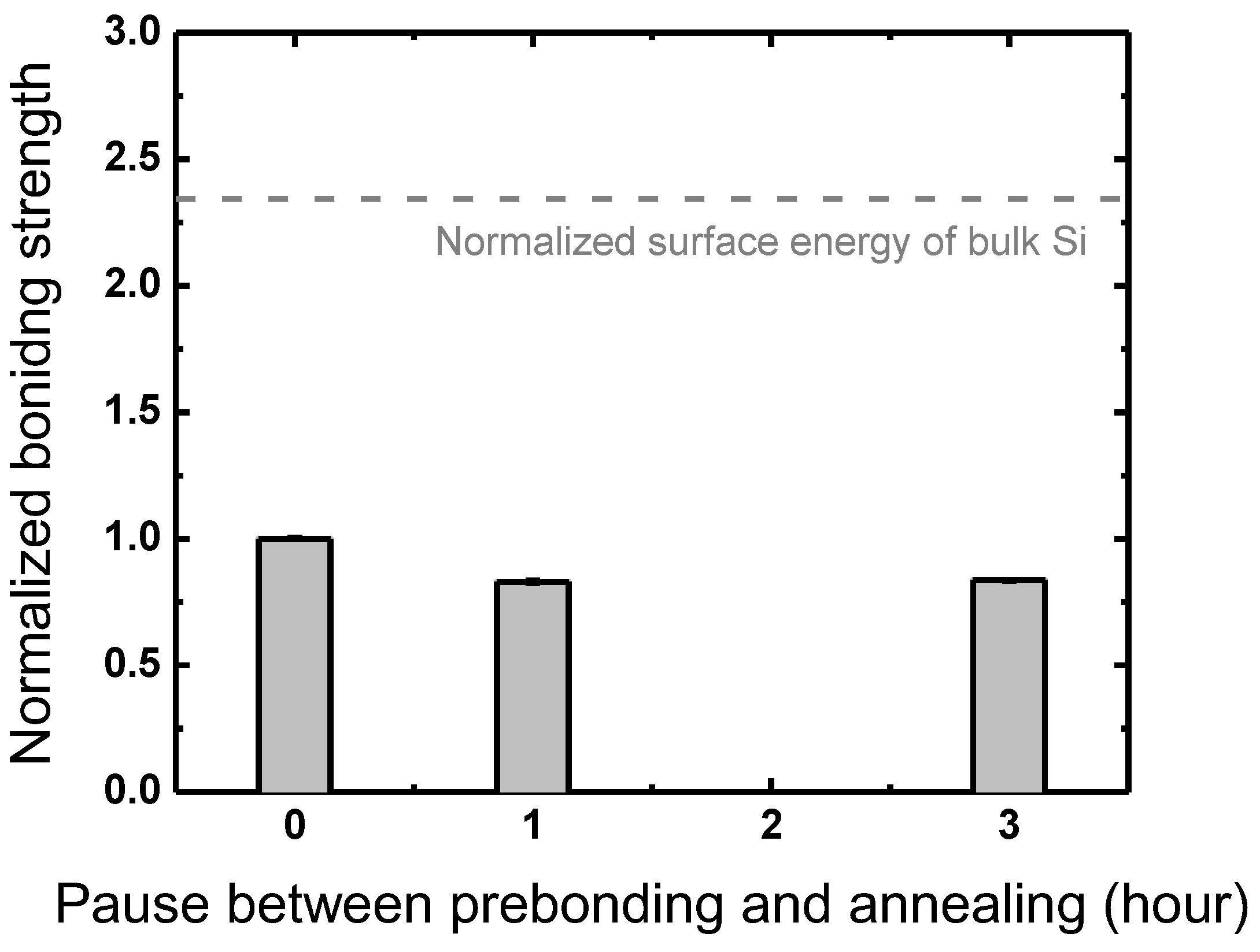
3.4. Between Prebonding and Annealing
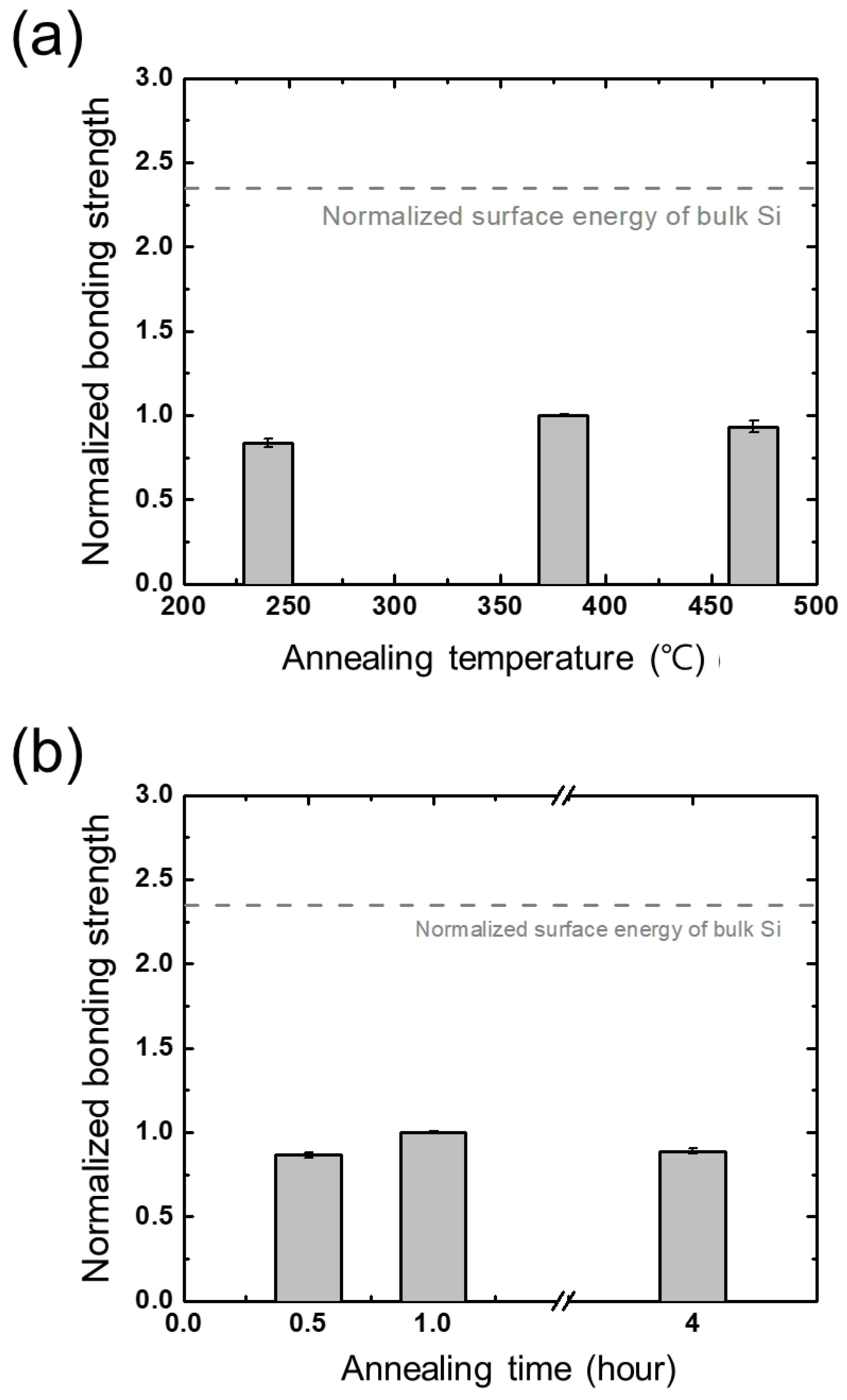
3.5. Annealing
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lau, J.H. Recent Advances and Trends in Advanced Packaging. IEEE Trans. Compon. Packag. Manuf. Technol. 2022, 12, 228–252. [Google Scholar] [CrossRef]
- Yeap, L. Meeting the Assembly Challenges in New Semiconductor Packaging Trend. In Proceedings of the 34th International Electronic Manufacturing Technology Conference, Melaka, Malaysia, 30 November–2 December 2010; pp. 1–5. [Google Scholar]
- Agarwal, R.; Kannan, S.; England, L.; Reed, R.; Song, Y.; Lee, W.; Lee, S.; Yoo, J. 3D Packaging Challenges for High-End Applications. In Proceedings of the 2017 IEEE 67th Electronic Components and Technology Conference (ECTC), Orlando, FL, USA, 30 May–2 June 2017; pp. 1249–1256. [Google Scholar]
- Manier, C.; Zoschke, K.; Wilke, M.; Oppermann, M.; Ruffieux, D.; Piazza, S.D.; Suni, T.; Dekker, J.; Allegato, G.; Lang, K.-D. Wafer Level Packaigng of MEMS and 3D Integration with CMOS for fabrication of Timing Microsystems. In Proceedings of the 2016 Symposium on Design, Test, Integration & Packaging of MEMS and MOEMS, Budapest, Hungary, 30 May–2 June 2016. [Google Scholar]
- Riso, C.-A.; Cardenas, S.; Galvin, S.; Warchall, J.; Fazzari, S.; Palmer, D.; Crum, D. Advanced Packaging Roadmaps and Government Needs. In Proceedings of the 2022 Government Microcircuit Applications and Critical Technology (GOMACTech), Miami, FL, USA, 21–24 March 2022. [Google Scholar]
- Inoue, R.; Takehara, N.; Naito, T.; Tanabe, K. Direct Semiconductor Wafer Bonding in Non-Cleanroom Environment: Understanding the Environmental Influences on Bonding. ACS Appl. Electron. Mater. 2019, 1, 936–944. [Google Scholar] [CrossRef]
- Nagano, F.; Iacovo, S.; Phommahaxay, A.; Inoue, F.; Sleeckx, E.; Beyer, G.; Beyne, E.; De Gendt, S. Film Characterization of Low-Temperature Silicon Carbon Nitride for Direct Bonding Applications. ECS J. Solid State Sci. Technol. 2020, 9, 123011. [Google Scholar] [CrossRef]
- Ke, S.; Li, D.; Chen, S. A review: Wafer bonding of Si-based semiconductors. J. Phys. D Appl. Phys. 2020, 53, 323001. [Google Scholar] [CrossRef]
- Bao, S.; Wang, Y.; Lina, K.; Zhang, L.; Wang, B.; Sasangka, W.A.; Lee, K.E.K.; Chua, S.J.; Michel, J.; Fitzgerald, E.; et al. A review of silicon-based wafer bonding processes, an approach to realize the monolithic integration of Si-CMOS and III–V-on-Si wafers. J. Semicond. 2021, 42, 023106. [Google Scholar] [CrossRef]
- Yamamoto, M.; Matsumae, T.; Kurashima, Y.; Takagi, H.; Suga, T.; Takamatsu, S.; Itoh, T.; Higurashi, E. Effect of Au Film Thickness and Surface Roughness on Room-Temperature Wafer Bonding and Wafer-Scale Vacuum Sealing by Au-Au Surface Activated Bonding. Micromachines 2020, 11, 454. [Google Scholar] [CrossRef]
- Kim, Y.S.; Nguyen, T.H.; Choa, S.H. Enhancement of the Bond Strength and Reduction of Wafer Edge Voids in Hybrid Bonding. Micromachines 2022, 13, 537. [Google Scholar] [CrossRef]
- Moriceau, H.; Rieutord, F.; Fournel, F.; Le Tiec, Y.; Di Cioccio, L.; Morales, C.; Charvet, A.M.; Deguet, C. Overview of recent direct wafer bonding advances and applications. Adv. Nat. Sci. Nanosci. Nanotechnol. 2011, 1, 043004. [Google Scholar] [CrossRef]
- Suga, T. Low Temperature Bonding for 3D Integration—A Review of the Surface Activated Bonding (SAB). In Proceedings of the 2012 3rd IEEE International Workshop on Low Temperature Bonding for 3D Integration, Tokyo, Japan, 22–23 May 2012; p. 4. [Google Scholar]
- Plach, T.; Hingerl, K.; Tollabimazraehno, S.; Hesser, G.; Dragoi, V.; Wimplinger, M. Mechanisms for room temperature direct wafer bonding. J. Appl. Phys. 2013, 113, 094905. [Google Scholar] [CrossRef]
- Suni, T.; Henttinen, K.; Suni, I.; Makinen, J. Effects of Plasma Activation on Hydrophilic Bonding of Si and SiO2. J. Electrochem. Soc. 2002, 149, G348–G351. [Google Scholar] [CrossRef]
- Xu, J.; Wang, C.; Wang, T.; Wang, Y.; Kang, Q.; Liu, Y.; Tian, Y. Mechanisms for low-temperature direct bonding of Si/Si and quartz/quartz via VUV/O3 activation. RSC Adv. 2018, 8, 11528–11535. [Google Scholar] [CrossRef] [PubMed]
- Fournel, F.; Martin-Cocher, C.; Radisson, D.; Larrey, V.; Beche, E.; Morales, C.; Delean, P.A.; Rieutord, F.; Moriceau, H. Water Stress Corrosion in Bonded Structures. ECS J. Solid State Sci. Technol. 2015, 4, P124–P130. [Google Scholar] [CrossRef]
- Fournel, F.; Continni, L.; Morales, C.; Da Fonseca, J.; Moriceau, H.; Rieutord, F.; Barthelemy, A.; Radu, I. Measurement of bonding energy in an anhydrous nitrogen atmosphere and its application to silicon direct bonding technology. J. Appl. Phys. 2012, 111, 104907. [Google Scholar] [CrossRef]
- Pasquariello, D.; Hedlund, C.; Hjort, K. Oxidation and Induced Damage in Oxygen plasma In Situ Wafer Bonding. J. Electrochem. Soc. 2000, 147, 2699–2703. [Google Scholar] [CrossRef]
- Rauch, N.; Andersen, E.; Vicente-Gabás, I.G.; Duchoslav, J.; Minenkov, A.; Gasiorowski, J.; Flötgen, C.; Hingerl, K.; Groiss, H. A model for spectroscopic ellipsometry analysis of plasma-activated Si surfaces for direct wafer bonding. Appl. Phys. Lett. 2022, 121, 081603. [Google Scholar] [CrossRef]
- Seong, I.H.; Lee, J.J.; Cho, C.H.; Lee, Y.S.; Kim, S.J.; You, S.J. Characterization of SiO2 Over Poly-Si Mask Etching in Ar/C4F8 Capacitively Coupled Plasma. Appl. Sci. Converg. Technol. 2021, 30, 176–182. [Google Scholar] [CrossRef]
- Cho, C.; You, K.; Kim, S.; Lee, Y.; Lee, J.; You, S. Characterization of SiO2 Etching Profiles in Pulse-Modulated Capacitively Coupled Plasmas. Materials 2021, 14, 5036. [Google Scholar] [CrossRef]
- Lee, Y.; Oh, S.; Lee, J.; Cho, C.; Kim, S.; You, S. A Quantification Method in Quadrupole Mass Spectrometer Measurement. Appl. Sci. Converg. Technol. 2021, 30, 50–53. [Google Scholar] [CrossRef]
- Maszara, W.P.; Goetz, G.; Caviglia, A.; McKitterick, J.B. Bonding of silicon wafers for silicon-on-insulator. J. Appl. Phys. 1988, 64, 4943–4950. [Google Scholar] [CrossRef]
- Masteika, V.; Kowal, J.; Braithwaite, N.S.J.; Rogers, T. A Review of Hydrophilic Silicon Wafer Bonding. ECS J. Solid State Sci. Technol. 2014, 3, Q42–Q54. [Google Scholar] [CrossRef]
- Kim, D.W.; You, S.J.; Kim, S.J.; Kim, J.H.; Oh, W.Y. Two-resonance probe for measuring electron density in low-pressure plasmas. Plasma Sources Sci. Technol. 2017, 26, 045015. [Google Scholar] [CrossRef]
- You, K.H.; You, S.J.; Kim, D.W.; Na, B.K.; Seo, B.H.; Kim, J.H.; Shin, Y.H.; Seong, D.J.; Chang, H.Y. A cutoff probe for the measurement of high density plasma. Thin Solid Film. 2013, 547, 250–255. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, J.J.; Kim, D.W.; Kim, J.H.; You, S.J. A transmission line model of the cutoff probe. Plasma Sources Sci. Technol. 2019, 28, 055014. [Google Scholar] [CrossRef]
- Kim, J.-H.; Choi, S.-C.; Shin, Y.-H.; Chung, K.-H. Wave cutoff method to measure absolute electron density in cold plasma. Rev. Sci. Instrum. 2004, 75, 2706–2710. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, J.J.; Lee, Y.S.; Kim, D.W.; You, S.J. Effect of an inhomogeneous electron density profile on the transmission microwave frequency spectrum of the cutoff probe. Plasma Sources Sci. Technol. 2020, 29, 125014. [Google Scholar] [CrossRef]
- Boris, D.R.; Fernsler, R.F.; Walton, S.G. The spatial profile of density in electron beam generated plasmas. Surf. Coat. Technol. 2014, 241, 13–18. [Google Scholar] [CrossRef]
- Kim, J.-H.; Seong, D.-J.; Lim, J.-Y.; Chung, K.-H. Plasma frequency measurements for absolute plasma density by means of wave cutoff method. Appl. Phys. Lett. 2003, 83, 4725–4727. [Google Scholar] [CrossRef]
- Tong, Q.Y.; Schmidt, E.; Gösele, U.; Reiche, M. Hydrophobic silicon wafer bonding. Appl. Phys. Lett. 1994, 64, 625–627. [Google Scholar] [CrossRef]
- Rezaei, F.; Gorbanev, Y.; Chys, M.; Nikiforov, A.; Van Hulle, S.W.H.; Cos, P.; Bogaerts, A.; De Geyter, N. Investigation of plasma-induced chemistry in organic solutions for enhanced electrospun PLA nanofibers. Plasma Process. Polym. 2018, 15, 1700226. [Google Scholar] [CrossRef]
- Ershov, A.; Borysow, J. Dynamics of OH (X2Π, v = 0) in high-energy atmospheric pressure electric pulsed discharge. J. Phys. D Appl. Phys. 1995, 28, 68–74. [Google Scholar] [CrossRef]
- Sanz-Velasco, P.A.; Bengtsson, S.; Colinge, C. Room Temperature Wafer Bonding Using Oxygen Plasma Treatment in Reactive Ion Etchers With and Without Inductively Coupled Plasma. J. Electrochem. Soc. 2003, 150, G155–G162. [Google Scholar] [CrossRef]
- Chen, L.; Ngo, D.; Luo, J.; Gong, Y.; Xiao, C.; He, X.; Yu, B.; Qian, L.; Kim, S.H. Dependence of water adsorption on the surface structure of silicon wafers aged under different environmental conditions. Phys. Chem. Chem. Phys. 2019, 21, 26041–26048. [Google Scholar] [CrossRef] [PubMed]
- Tedjini, M.; Fournel, F.; Moriceau, H.; Larrey, V.; Landru, D.; Kononchuk, O.; Tardif, S.; Rieutord, F. Interface water diffusion in silicon direct bonding. Appl. Phys. Lett. 2016, 109, 111603. [Google Scholar] [CrossRef]
- Min, K.S.; Kang, S.H.; Kim, J.K.; Yum, J.H.; Jhon, Y.I.; Hudnall, T.W.; Bielawski, C.W.; Banerjee, S.K.; Bersuker, G.; Jhon, M.S.; et al. Atomic layer etching of BeO using BCl3/Ar for the interface passivation layer of III–V MOS devices. Microelectron. Eng. 2014, 114, 121–125. [Google Scholar] [CrossRef]


| Processing Step | Conditions |
|---|---|
| Preparation | No surface cleaning 5 min evacuation |
| Plasma treatment | 100 sccm N2 flow rate 100 mTorr pressure 100 W RF power 15 s duration |
| DIW cleaning | 500 RPM for cleaning for 30 s 1800 RPM for drying for 60 s |
| Annealing | 10 min ramping up to 380 °C 60 min annealing up to 380 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.; You, Y.; Cho, C.; Kim, S.; Lee, J.; Kim, M.; Lee, H.; You, Y.; Kim, K.; You, S. Comprehensive Assessments in Bonding Energy of Plasma Assisted Si-SiO2 Direct Wafer Bonding after Low Temperature Rapid Thermal Annealing. Micromachines 2022, 13, 1856. https://doi.org/10.3390/mi13111856
Lee Y, You Y, Cho C, Kim S, Lee J, Kim M, Lee H, You Y, Kim K, You S. Comprehensive Assessments in Bonding Energy of Plasma Assisted Si-SiO2 Direct Wafer Bonding after Low Temperature Rapid Thermal Annealing. Micromachines. 2022; 13(11):1856. https://doi.org/10.3390/mi13111856
Chicago/Turabian StyleLee, Youngseok, Yebin You, Chulhee Cho, Sijun Kim, Jangjae Lee, Minyoung Kim, Hanglim Lee, Youngjun You, Kyungman Kim, and ShinJae You. 2022. "Comprehensive Assessments in Bonding Energy of Plasma Assisted Si-SiO2 Direct Wafer Bonding after Low Temperature Rapid Thermal Annealing" Micromachines 13, no. 11: 1856. https://doi.org/10.3390/mi13111856
APA StyleLee, Y., You, Y., Cho, C., Kim, S., Lee, J., Kim, M., Lee, H., You, Y., Kim, K., & You, S. (2022). Comprehensive Assessments in Bonding Energy of Plasma Assisted Si-SiO2 Direct Wafer Bonding after Low Temperature Rapid Thermal Annealing. Micromachines, 13(11), 1856. https://doi.org/10.3390/mi13111856







