An Isomorphic Interactive Device for the Interventional Surgical Robot after In Vivo Study
Abstract
:1. Introduction
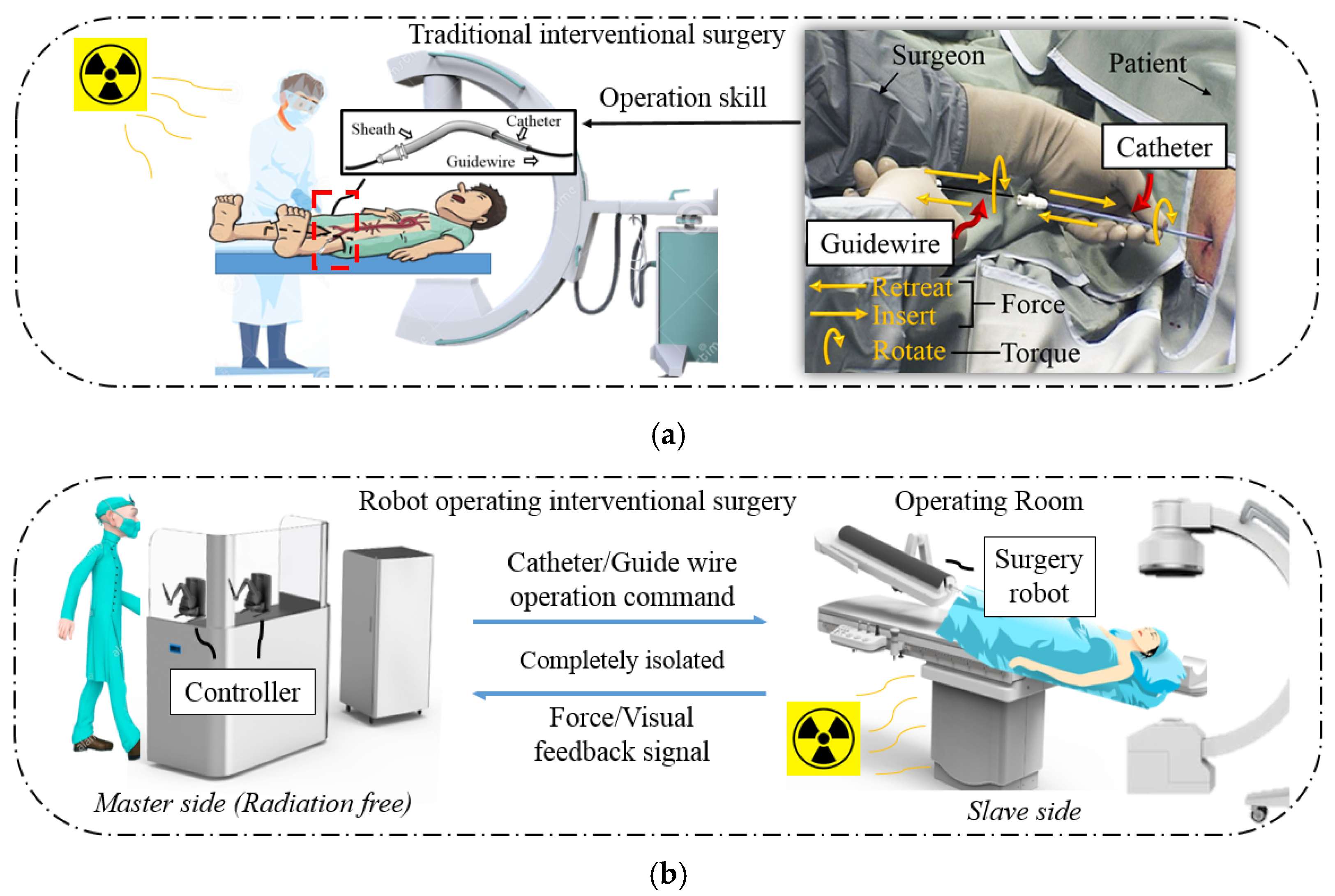
2. Robot System Description
- (1)
- Enter the wrong blood vessel. For the health of the patient and the surgeon, the surgeon cannot always observe the position of the catheter and guidewire inside the patient through x-rays during an operation. Therefore, there may be cases in which the catheter enters the wrong path during the operation. At this time, the surgeon needs to retreat the catheter and the guidewire.
- (2)
- Select the target blood vessel branch. When multiple vascular branches simultaneously appear in the surgical path, the surgeon needs to operate the guidewire to assist the catheter. At this time, the surgeon needs to rotate the catheter and the guidewire, point the curved tip of the guidewire toward the target blood vessel, and deliver it forward. After the guidewire enters the target branch, it supports and guides the catheter into the target branch. The operation at this time requires the coordinated operation of the catheter and the guidewire. This job relies heavily on the clinical surgical skills of the surgeon, and the duration can also be used to judge novices and experienced surgeons.
- (1)
- The previously used master controller has a limited linear operating range, which cannot meet the catheter and guidewire movement requirement;
- (2)
- The previously used controller cannot operate a 360° rotation in a single movement for the catheter and guidewire;
- (3)
- The control method of the previously used controller differs from the clinical surgery. Therefore, the surgeon cannot apply their catheter and guidewire operation skills in the master side control.
2.1. Master Controller Design Overview
2.2. Force Feedback Structure Design
3. System Control Strategy
3.1. Surgical Operation Control Strategy
3.2. Force Feedback Control Strategy
4. Evaluation Experiments and Results
4.1. Eccentric Force Compensation
4.2. Controller Passive Force Feedback Accuracy Evaluation
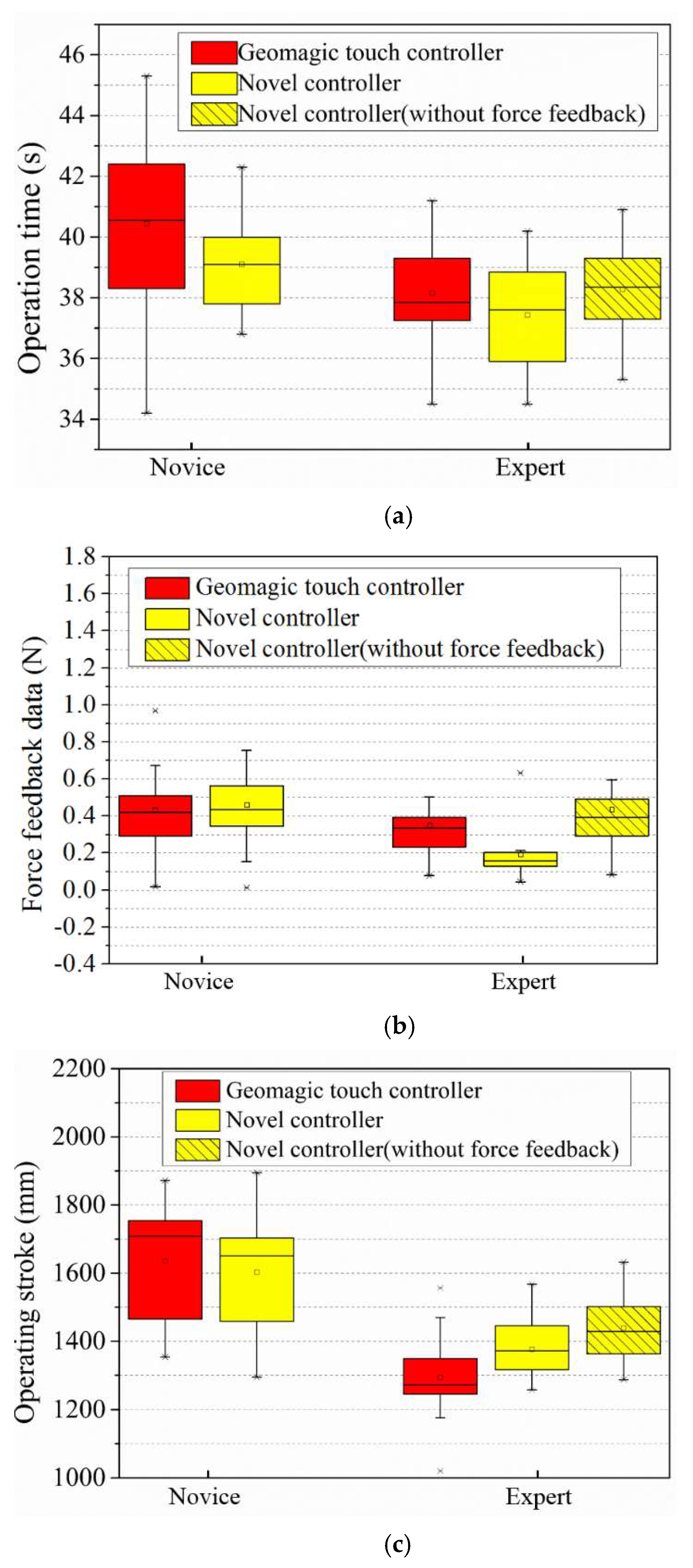
4.3. Robot System Performance Evaluation and Result on a Vascular Model
5. Discussion
- (1)
- On the master side, when the controller performs a reciprocating operation, the following errors will occur due to the master–slave transmission delay. This is more significant when the guidewire is frequently operated, such as the data in Figure 13b,d. It can be seen that the rapid operation of 20–35 s caused a large amount of follow-up error in both linear and rotational motion.
- (2)
- On the slave side, because the catheter and guidewire at the slave side will bend or deflect during operation, they may not move according to the expected operation instructions, which will cause difficulties for the surgeon to operate.
6. Conclusions
- (1)
- Proposed an isomorphic master controller to control the interventional surgical robot, mimicking the surgeon’s actual operation of the catheter and guidewire; thus, surgeons can apply their surgical skills during an operation.
- (2)
- A primary force feedback control method was designed to improve the safety of the operator’s remote operation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Statistics 2020: Monitoring Health for the SDGs, Sustainable Development Goals; World Health Organization: Geneva, Switzerland, 2020; Available online: www.who.int/publications/i/item/9789240005105 (accessed on 20 December 2021).
- Goyal, M.; Sutherland, G.R.; Lama, S.; Cimflova, P.; Kashani, N.; Mayank, A.; Psychogios, M.N.; Spelle, L.; Costalat, V.; Sakai, N.; et al. Neurointerventional Robotics: Challenges and Opportunities. Clin. Neuroradiol. 2020, 30, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Dos Reis, J.E.; Soullie, P.; Battaglia, A.; Petitmangin, G.; Hoyland, P.; Josseaume, L.; de Chillou, C.; Odille, F.; Felblinger, J. Electrocardiogram Acquisition during Remote Magnetic Catheter Navigation. Ann. Biomed. Eng. 2019, 47, 1141–1152. [Google Scholar] [CrossRef] [PubMed]
- Sajja, K.C.; Sweid, A.; Al Saiegh, F.; Chalouhi, N.; Avery, M.B.; Schmidt, R.F.; Tjoumakaris, S.I.; Gooch, M.R.; Herial, N.; Abbas, R.; et al. Endovascular robotic: Feasibility and proof of principle for diagnostic cerebral angiography and carotid artery stenting. J. Neuro. Interv. Surg. 2020, 12, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Kanagaratnam, P.; Koa-Wing, M.; Wallace, D.T.; Goldenberg, A.S.; Peters, N.S.; Davies, D.W. Experience of robotic catheter ablation in humans using a novel remotely steerable catheter sheath. J. Interv. Card. Electrophysiol. 2008, 21, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Schwein, A.; Kramer, B.; Chinnadurai, P.; Virmani, N.; Walker, S.; O’Malley, M.; Lumsden, A.B.; Bismuth, J. Electromagnetic tracking of flexible robotic catheters enables “assisted navigation” and brings automation to endovascular navigation in an in vitro study. J. Vasc. Surg. 2018, 67, 1274–1280. [Google Scholar] [CrossRef] [Green Version]
- Khan, E.M.; Frumkin, W.; Ng, G.A.; Neelagaru, S.; Abi-Samra, F.M.; Lee, J.; Giudici, M.; Gohn, D.; Winkle, R.A.; Sussman, J.; et al. First experience with a novel robotic remote catheter system: Amigo (TM) mapping trial. J. Interv. Card. Electrophysiol. 2013, 37, 121–129. [Google Scholar] [CrossRef]
- Hooshiar, A.; Najarian, S.; Dargahi, J. Haptic Telerobotic Cardiovascular Intervention: A Review of Approaches, Methods, and Future Perspectives. IEEE Rev. Biomed. Eng. 2020, 13, 32–50. [Google Scholar] [CrossRef] [PubMed]
- Lo, N.; Gutierrez, J.; Swaminathan, R. Robotic-assisted percutaneous coronary intervention. Curr. Treat. Options Cardiovasc. Med. 2018, 20, 14–23. [Google Scholar] [CrossRef]
- Wang, K.; Lu, Q.; Chen, B.; Shen, Y.; Li, H.; Liu, M.; Xu, Z. Endovascular intervention robot with multi-manipulators for surgical procedures: Dexterity, adaptability, and practicability. Robot. Comput. Integr. Manuf. 2019, 56, 75–84. [Google Scholar] [CrossRef]
- Bao, X.; Guo, S.; Xiao, N.; Li, Y.; Yang, C.; Jiang, Y. A cooperation of catheters and guidewires-based novel remote-controlled vascular interventional robot. Biomed. Microdevices 2018, 20, 20. [Google Scholar] [CrossRef]
- Bao, X.; Guo, S.; Xiao, N.; Zhao, Y.; Shen, R. Toward cooperation of catheter and guidewire for remote-controlled vascular interventional robot. In Proceedings of the IEEE International Conference on Mechatronics and Automation, Kagawa, Japan, 8 August 2017. [Google Scholar]
- Bao, X.; Guo, S.; Xiao, N.; Li, Y.; Yang, C.; Shen, R.; Cui, J.; Jiang, Y.; Liu, X.; Liu, K. Operation evaluation in-human of a novel remote-controlled vascular interventional robot. Biomed. Microdevices 2018, 20, 34. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Guo, S.; Bao, X.; Xiao, N.; Shi, L.; Li, Y.; Jiang, Y. A Vascular Interventional Surgical Robot Based on Surgeon’s Operating Skills. Med. Biol. Eng. Comput. 2019, 57, 1999–2010. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Guo, S.; Shi, L.; Xiao, N. Design and Evaluation of Sensorized Robot for Minimally Vascular Interventional Surgery. Microsyst. Technol. 2019, 25, 2759–2766. [Google Scholar] [CrossRef]
- Norouzi-Ghazbi, S.; Mehrkish, A.; Abdulhafiz, I.; Abbasi-Hashemi, T.; Mahdi, A.; Janabi-Sharifi, F. Design and experimental evaluation of an automated catheter operating system. Artif. Organs. 2021, 45, E171–E186. [Google Scholar] [CrossRef]
- Sankaran, N.; Chembrammel, P.; Kesavadas, T. Force calibration for an endovascular robotic system with proximal force measurement. Int. J. Med. Robot. Comput. Assist. Surg. 2020, 16, e2045. [Google Scholar] [CrossRef]
- Dalvand, M.; Nahavandi, S.; Fielding, M.; Mullins, J.; Najdovski, Z.; Howe, R.D. Modular instrument for a haptically enabled robotic surgical system (HeroSurg). IEEE Access 2018, 6, 31974–31982. [Google Scholar] [CrossRef]
- Kundrat, D.; Dagnino, G.; Kwok, T.; Abdelaziz, M.; Chi, W.; Nguyen, A.; Riga, C.; Yang, G. An MR-Safe endovascular robotic platform: Design, control, and ex-vivo evaluation. IEEE Trans. Biomed. Eng. 2021, 68, 31974–31982. [Google Scholar] [CrossRef] [PubMed]
- Seixas-Mikelus, S.; Stegemann, A.; Kesavadas, T.; Srimathveeravalli, G.; Sathyaseelan, G.; Chandrasekhar, R.; Wilding, G.; Peabody, J.; Guru, K. Content validation of a novel robotic surgical simulator. BJU Int. 2011, 107, 130–1135. [Google Scholar] [CrossRef]
- Matsunaga, T.; Tomizuka, D.; Yu, K.; Mizoguchi, T.; Ohnishi, K. Construction of motion reproduction system using haptic forceps robots for needle insertion. In Proceedings of the IEEE Industrial Electronics Society, Florence, Italy, 22 December 2016. [Google Scholar]
- Zhao, Y.; Xing, H.; Guo, S.; Wang, Y.; Cui, J.; Ma, Y.; Liu, Y.; Liu, Y.; Feng, J.; Li, Y. A novel noncontact detection method of surgeon’s operation for a master-slave endovascular surgery robot. Med. Biol. Eng. Comput. 2020, 58, 871–885. [Google Scholar] [CrossRef] [PubMed]
- Khaled, E.; Sandra, O. Introduction to Diagnostic Radiology, 1st ed.; McGraw-Hill Education: New York, NY, USA, 2014; pp. 485–486. [Google Scholar]
- Eppinger, D.; Seering, P. Introduction to dynamic models for robot force control. IEEE Control. Syst. Mag. 1987, 7, 45–82. [Google Scholar] [CrossRef]
- Peng, W.F.; Li, G.H.; Wu, P.; Tan, G.Y. Linear Motor Velocity and Acceleration Motion Control Study Based on PID plus Velocity and Acceleration Feedforward Parameters Adjustment. In Proceedings of the 14th International Manufacturing Conference, Tianjin, China, 15 October 2011. [Google Scholar]
- Jones, L. Kinesthetic sensing. In Human and Machine Haptics, 1st ed.; MIT Press: Cambridge, MA, USA, 2000; pp. 23–28. [Google Scholar]
- Wang, Y.; Guo, S.; Xiao, N.; Li, Y.; Jiang, Y. Surgeons’ Operation Skill-Based Control Strategy and Preliminary Evaluation for a Vascular Interventional Surgical Robot. J. Med. Biol. Eng. 2019, 39, 653–664. [Google Scholar] [CrossRef]
- Girerd, C.; Morimoto, T. Design and Control of a Hand-Held Concentric Tube Robot for Minimally Invasive Surgery. IEEE Trans. Robot. 2021, 37, 1022–1038. [Google Scholar] [CrossRef]
- Wang, K.; Chen, B.; Lu, Q.; Li, H.; Liu, M.; Shen, Y.; Xu, Z. Design and Performance Evaluation of Real-time Endovascular Interventional Surgical Robotic System with High Accuracy. Int. J. Med. Robot. Comput. Assist. Surg. 2018, 14, e1915. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.; Hu, Z.; Won, J.; Park, S.; Lee, H.; You, H.; Nam, G.B.; Choi, J. Novel Design of Master Manipulator for Robotic Catheter System. Int. J. Control Autom. Syst. 2018, 16, 2924–2934. [Google Scholar] [CrossRef]













| Force (N) | Maximum Error (N) | Average Error (N) | Maximum Relative Error | Average Relative Error |
|---|---|---|---|---|
| 0.5 | 0.071 | 0.049 | 14.2% | 9.8% |
| 1.0 | 0.112 | 0.082 | 11.2% | 8.2% |
| 1.5 | 0.151 | 0.118 | 10.1% | 7.9% |
| 2.0 | 0.207 | 0.174 | 10.4% | 8.7% |
| 2.5 | 0.221 | 0.180 | 8.8% | 7.2% |
| 3.0 | 0.258 | 0.231 | 8.6% | 7.7% |
| 3.5 | 0.419 | 0.312 | 12.0% | 8.9% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Guo, S.; Bao, X. An Isomorphic Interactive Device for the Interventional Surgical Robot after In Vivo Study. Micromachines 2022, 13, 111. https://doi.org/10.3390/mi13010111
Yang C, Guo S, Bao X. An Isomorphic Interactive Device for the Interventional Surgical Robot after In Vivo Study. Micromachines. 2022; 13(1):111. https://doi.org/10.3390/mi13010111
Chicago/Turabian StyleYang, Cheng, Shuxiang Guo, and Xianqiang Bao. 2022. "An Isomorphic Interactive Device for the Interventional Surgical Robot after In Vivo Study" Micromachines 13, no. 1: 111. https://doi.org/10.3390/mi13010111
APA StyleYang, C., Guo, S., & Bao, X. (2022). An Isomorphic Interactive Device for the Interventional Surgical Robot after In Vivo Study. Micromachines, 13(1), 111. https://doi.org/10.3390/mi13010111







