Multilayer Thin-Film Optical Filters for Reflectance-Based Malaria Diagnostics
Abstract
:1. Introduction
2. Methods
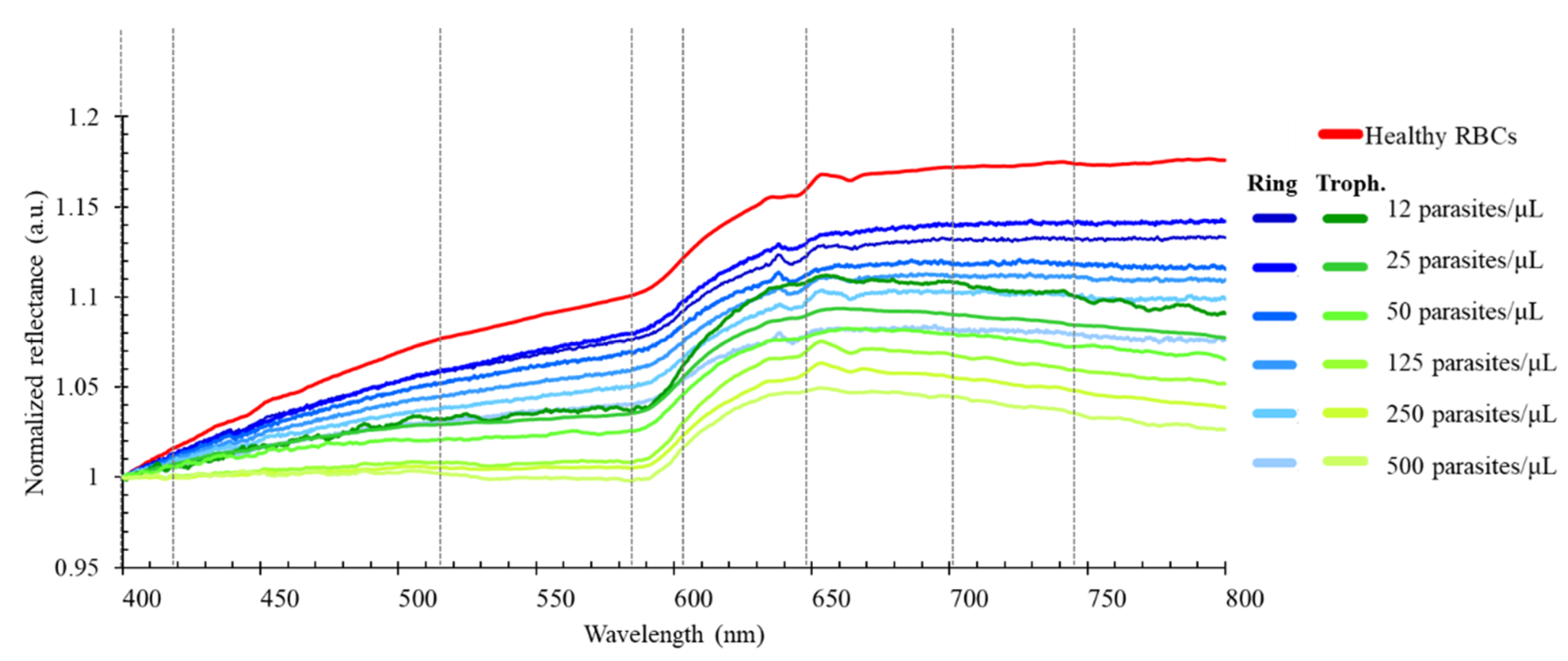
2.1. Optical Reflectance Spectra of Uninfected and Parasite Infected RBCs
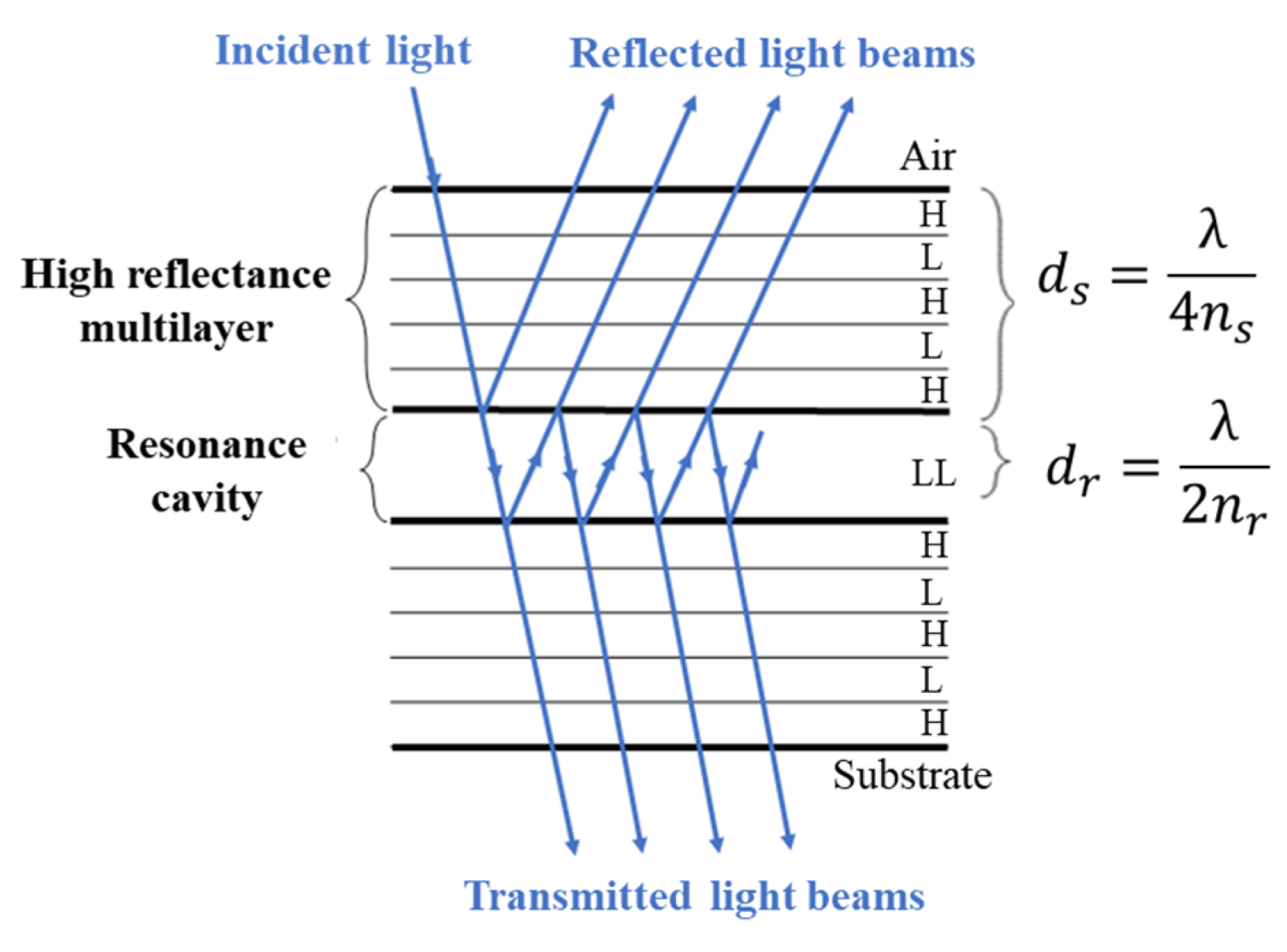
2.2. Design and Simulation of the Thin-Film Optical Filters
2.3. Experimental Setup
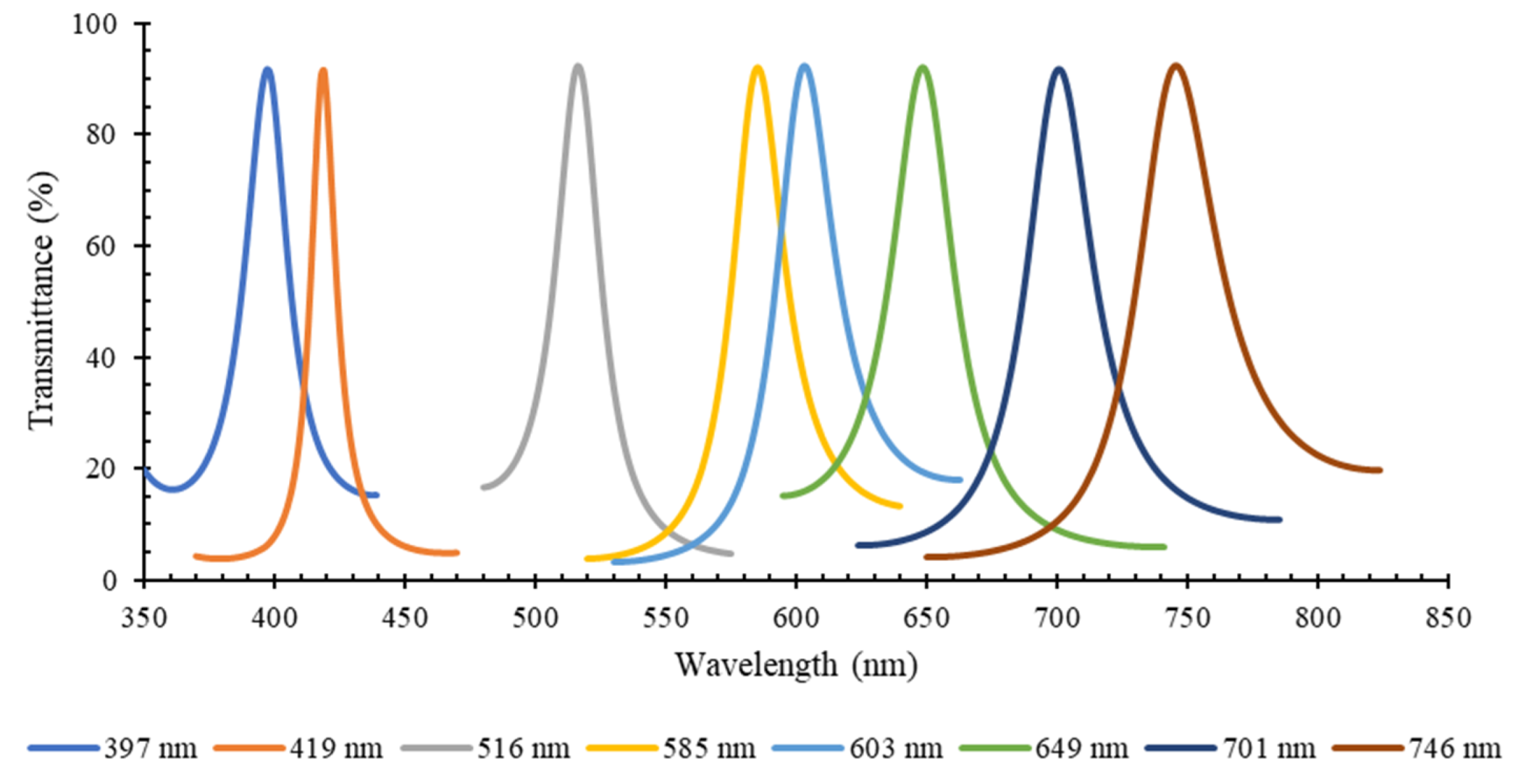
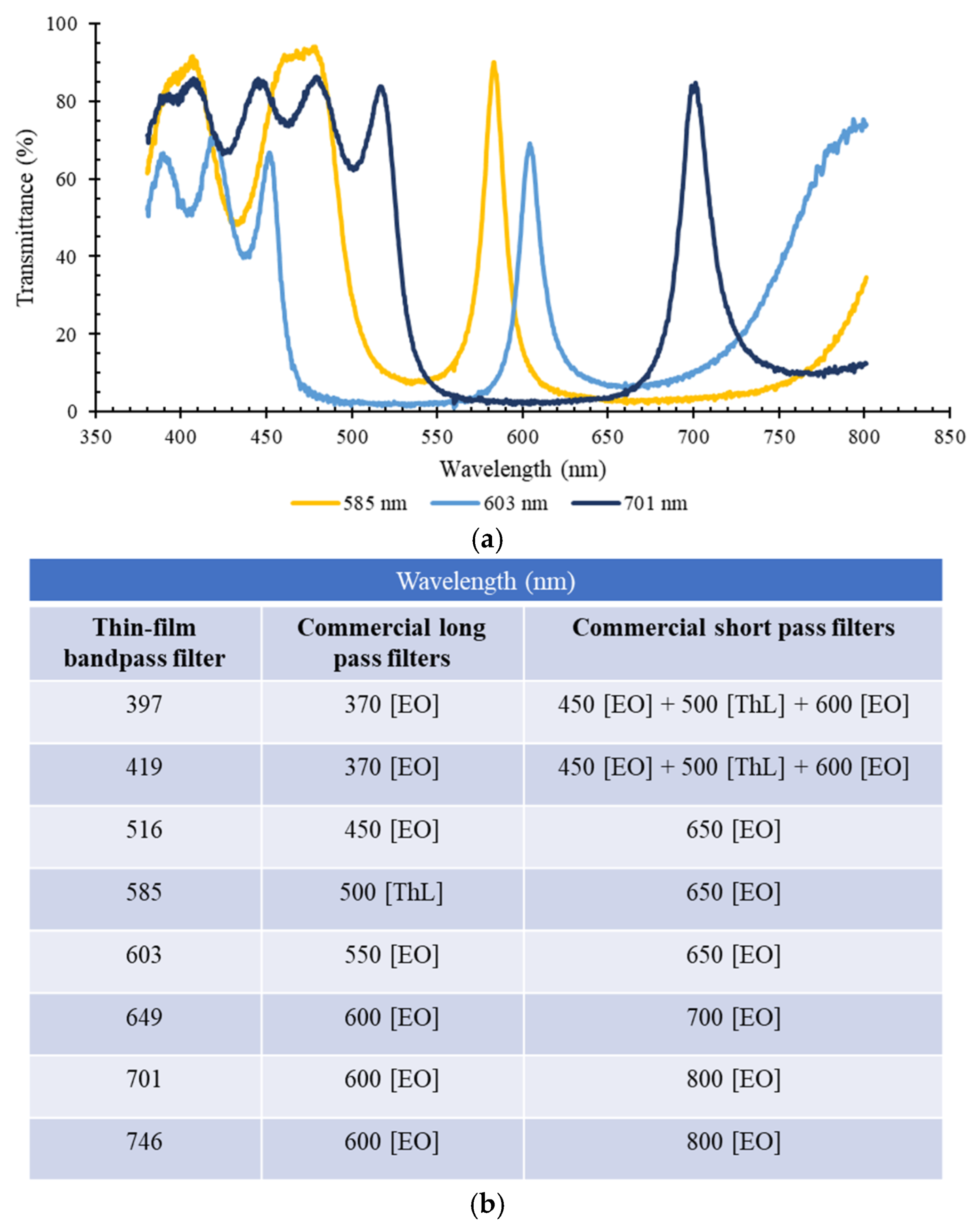
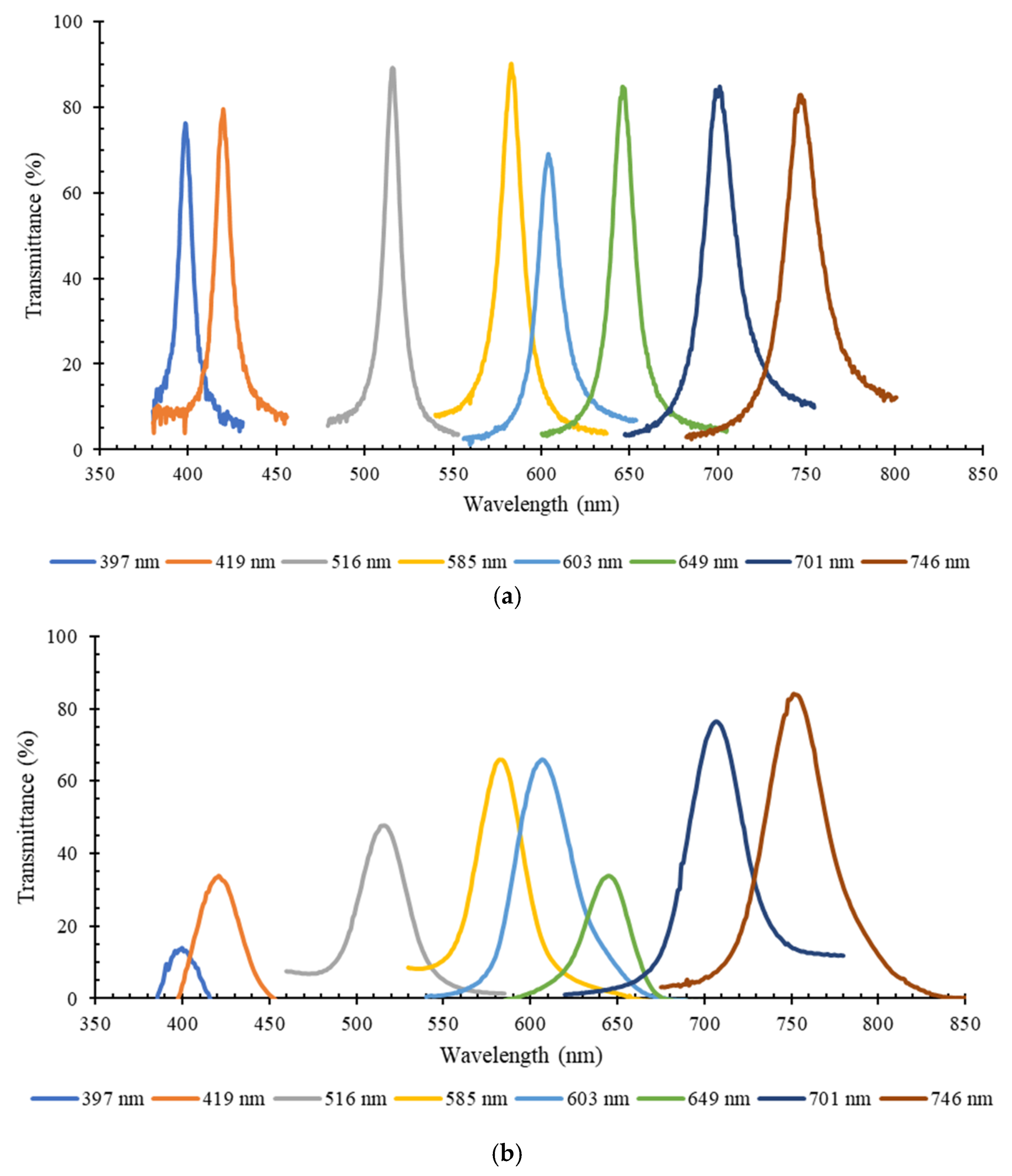
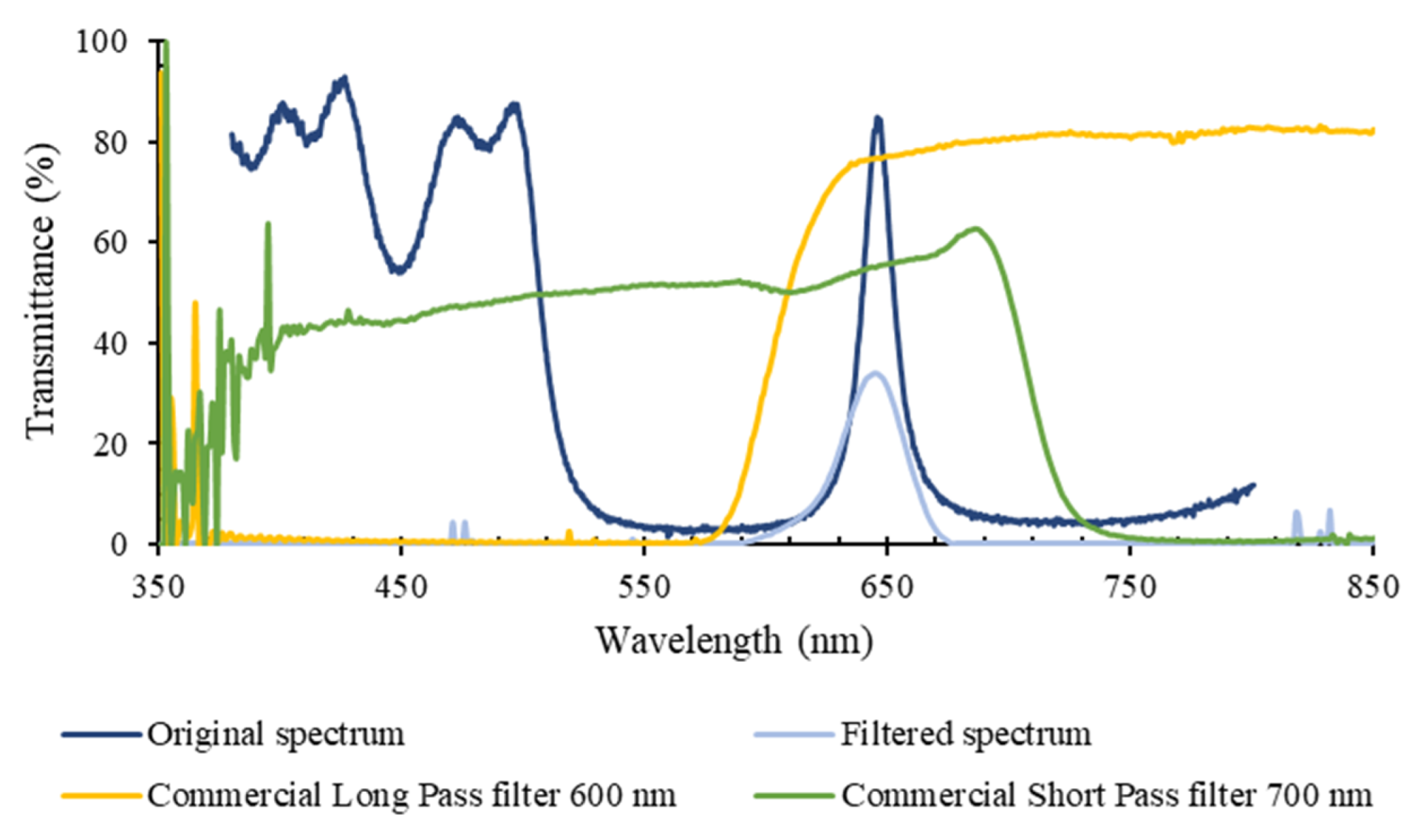
3. Results
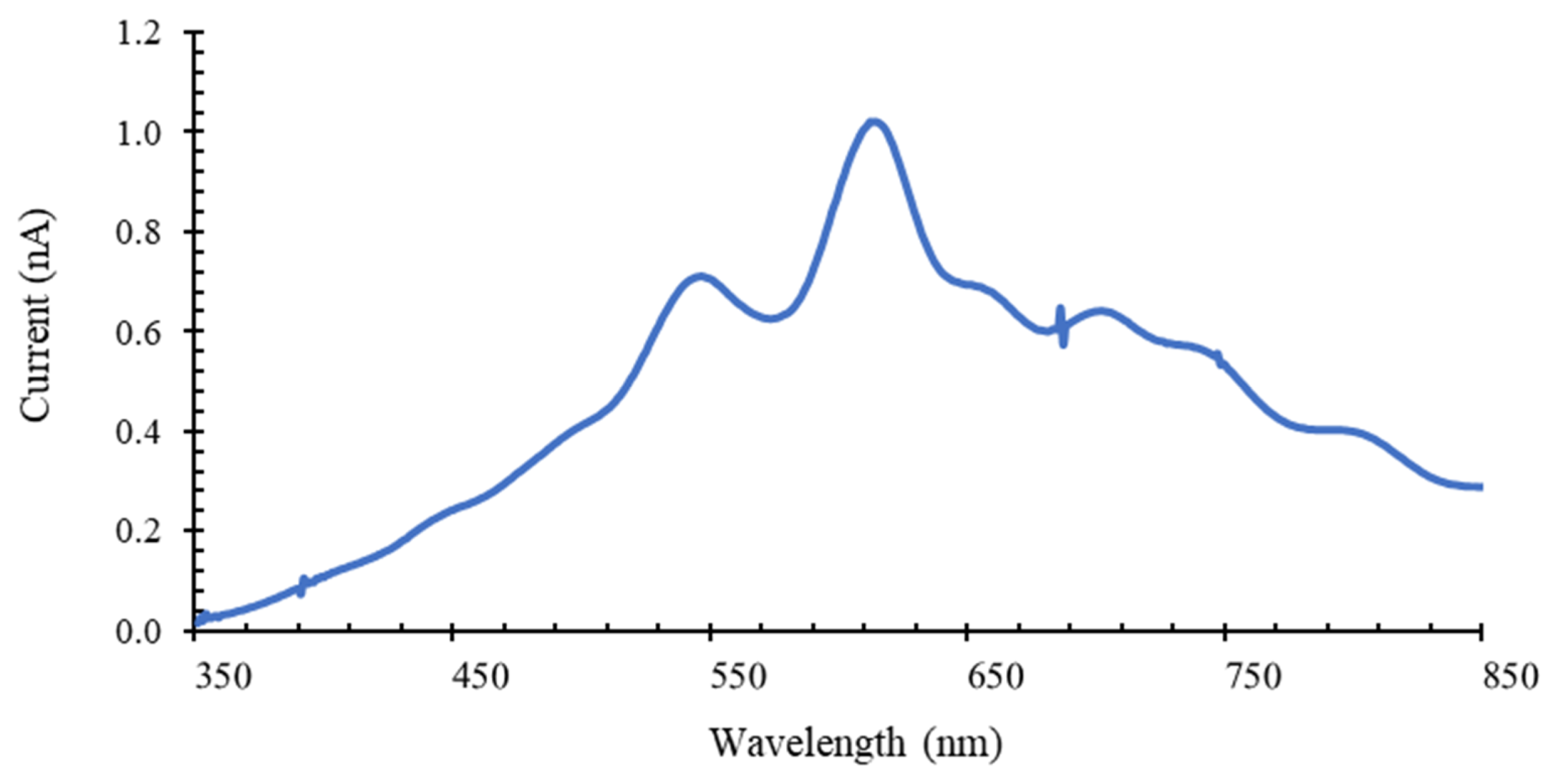
3.1. Optical Filters Characterization
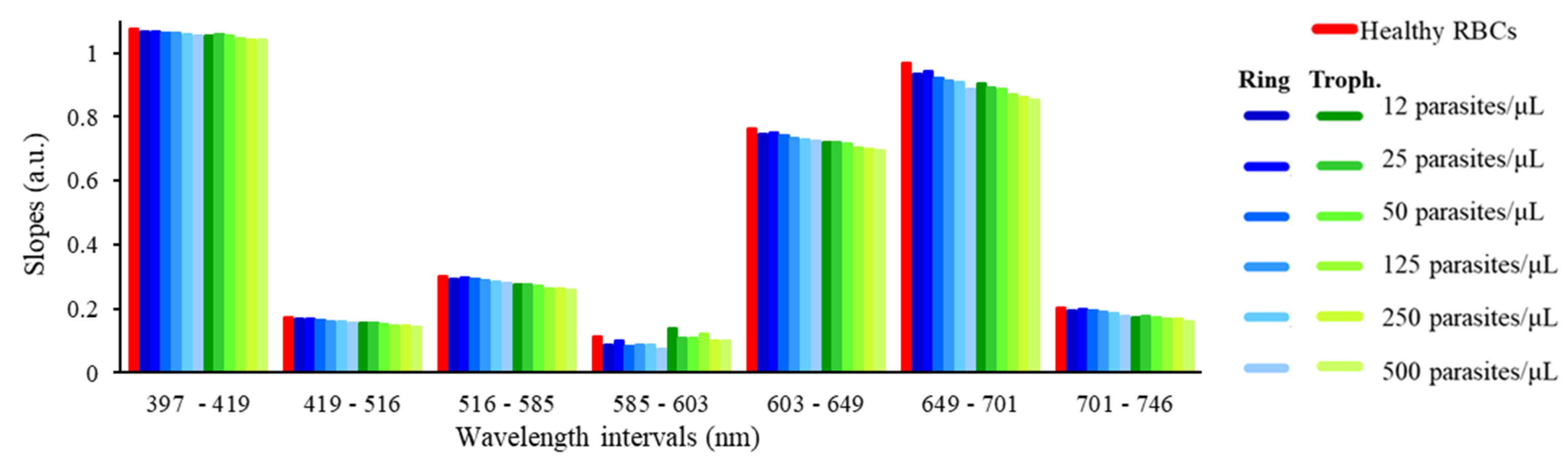
3.2. Discrete Reconstruction of the Samples’ Original Spectra: Application to Malaria Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pham, N.M.; Karlen, W.; Beck, H.P.; Delamarche, E. Malaria and the ‘last’ parasite: How can technology help? Malar. J. 2018, 17, 260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. World Malaria Report 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Mer, M.; Dünser, M.W.; Giera, R.; Dondorp, A.M. Severe malaria. Current concepts and practical overview: What every intensivist should know. Intensive Care Med. 2020, 46, 907–918. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. UNICEF/UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases, “Microscopy for the Detection, Identification and Quantification of Malaria Parasites on Stained Thick and Thin Blood Films in Research Settings (Version 1.0)” Procedure, Methods Manual. 2015. Available online: https://apps.who.int/iris/handle/10665/163782 (accessed on 5 July 2021).
- Berzosa, P.; de Lucio, A.; Romay-Barja, M.; Herrador, Z.; González, V.; García, L.; Fernández-Martínez, A.; Santana-Morales, M.; Ncogo, P.; Valladares, B.; et al. Comparison of three diagnostic methods (microscopy, RDT, and PCR) for the detection of malaria parasites in representative samples from Equatorial Guinea. Malar. J. 2018, 17, 333. [Google Scholar] [CrossRef] [PubMed]
- Echeverry, D.F.; Deason, N.A.; Davidson, J.; Makuru, V.; Xiao, H.; Niedbalski, J.; Kern, M.; Russell, T.L.; Burkot, T.R.; Collins, F.H.; et al. Human malaria diagnosis using a single-step direct-PCR based on the Plasmodium cytochrome oxidase III gene. Malar. J. 2016, 15, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krampa, F.D.; Aniweh, Y.; Kanyong, P.; Awandare, G.A. Recent advances in the development of biosensors for malaria diagnosis. Sensors 2020, 20, 799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francis, S.E.; Sullivan, D.J.; Goldberg, D.E. Hemoglobin metabolism in the malaria parasite Plasmodium falciparium. Annu. Rev. Microbiol. 1997, 51, 97–123. [Google Scholar] [CrossRef] [PubMed]
- Baptista, V.; Calçada, C.; Silva, M.; Teixeira, M.; Ferreira, P.; Minas, G.; Peng, W.K.; Catarino, S.O.; Veiga, M.I. Hemozoin: The future in malaria diagnosis. In Proceedings of the MAM 2020—Molecular Approaches to Malaria Conference, Lorne, Australia, 23–27 February 2020. [Google Scholar]
- Silva, I.; Lima, R.; Minas, G.; Catarino, S.O. Spectrophotometric characterization of hemozoin as a malaria biomarker. In Proceedings of the SPIE 10453, Third International Conference on Applications of Optics and Photonics, Faro, Portugal, 8–12 May 2017. [Google Scholar] [CrossRef]
- Silva, I.; Lima, R.; Minas, G.; Catarino, S.O. Hemozoin and hemoglobin characterization by optical absorption towards a miniaturized spectrophotometric malaria diagnostic system. In Proceedings of the 2017 IEEE 5th Portuguese Meeting on Bioengineering (ENBENG), Coimbra, Portugal, 16–18 February 2017; pp. 1–4. [Google Scholar] [CrossRef]
- Catarino, S.O.; Felix, P.; Sousa, P.J.; Pinto, V.; Veiga, M.I.; Minas, G. Portable Device for Optical Quantification of Hemozoin in Diluted Blood Samples. IEEE Trans. Biomed. Eng. 2020, 67, 365–371. [Google Scholar] [CrossRef]
- Ridley, R.G. Haemozoin formation in malaria parasites: Is there a haem polymerase? Trends Microbiol. 1996, 4, 253–254. [Google Scholar] [CrossRef]
- Costa, M.S.; Baptista, V.; Minas, G.; Veiga, M.I.; Catarino, S.O. Effect of the materials’ properties in the design of high transmittance and low FWHM SiO2/TiO2 thin film optical filters for integration in a malaria diagnostics device. In Proceedings of the 14th International Joint Conference on Biomedical Engineering Systems and Technologies, Biodevices 2021, Vienna, Austria, 11–13 February 2021; Volume 1, pp. 21–31. [Google Scholar] [CrossRef]
- Minas, G.; Wolffenbuttel, R.F.; Correia, J.H. An array of highly selective Fabry-Perot optical channels for biological fluid analysis by optical absorption using a white light source for illumination. J. Opt. A Pure Appl. Opt. 2006, 8, 272–278. [Google Scholar] [CrossRef] [Green Version]
- Minas, G.; Ribeiro, J.C.; Martins, J.S.; Wolffenbuttel, R.F.; Correia, J.H. An array of Fabry-Perot optical-channels for biological fluids analysis. Sens. Actuators A Phys. 2004, 115, 362–367. [Google Scholar] [CrossRef] [Green Version]
- Pimenta, S.; Cardoso, S.; Miranda, A.; de Beule, P.; Castanheira, E.M.S.; Minas, G. Design and fabrication of SiO2/TiO2 and MgO/TiO2 based high selective optical filters for diffuse reflectance and fluorescence signals extraction. Biomed. Opt. Express 2015, 6, 3084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, D.S.; Mirkovic, J.; Wolffenbuttel, R.F.; Correia, J.H.; Feld, M.S.; Minas, G. Narrow-band pass filter array for integrated opto-electronic spectroscopy detectors to assess esophageal tissue. Biomed. Opt. Express 2011, 2, 1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pimenta, S.; Cardoso, S.; Castanheira, E.M.S.; Minas, G. Advances towards a miniaturized optical system for gastrointestinal cancer detection using diffuse reflectance and fluorescence spectroscopies. In Gastrointestinal Cancers: Prevention, Detection and Treatment; Tyagi, A.K., Prasad, S., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2016; Volume 1, Chapter 13. [Google Scholar]
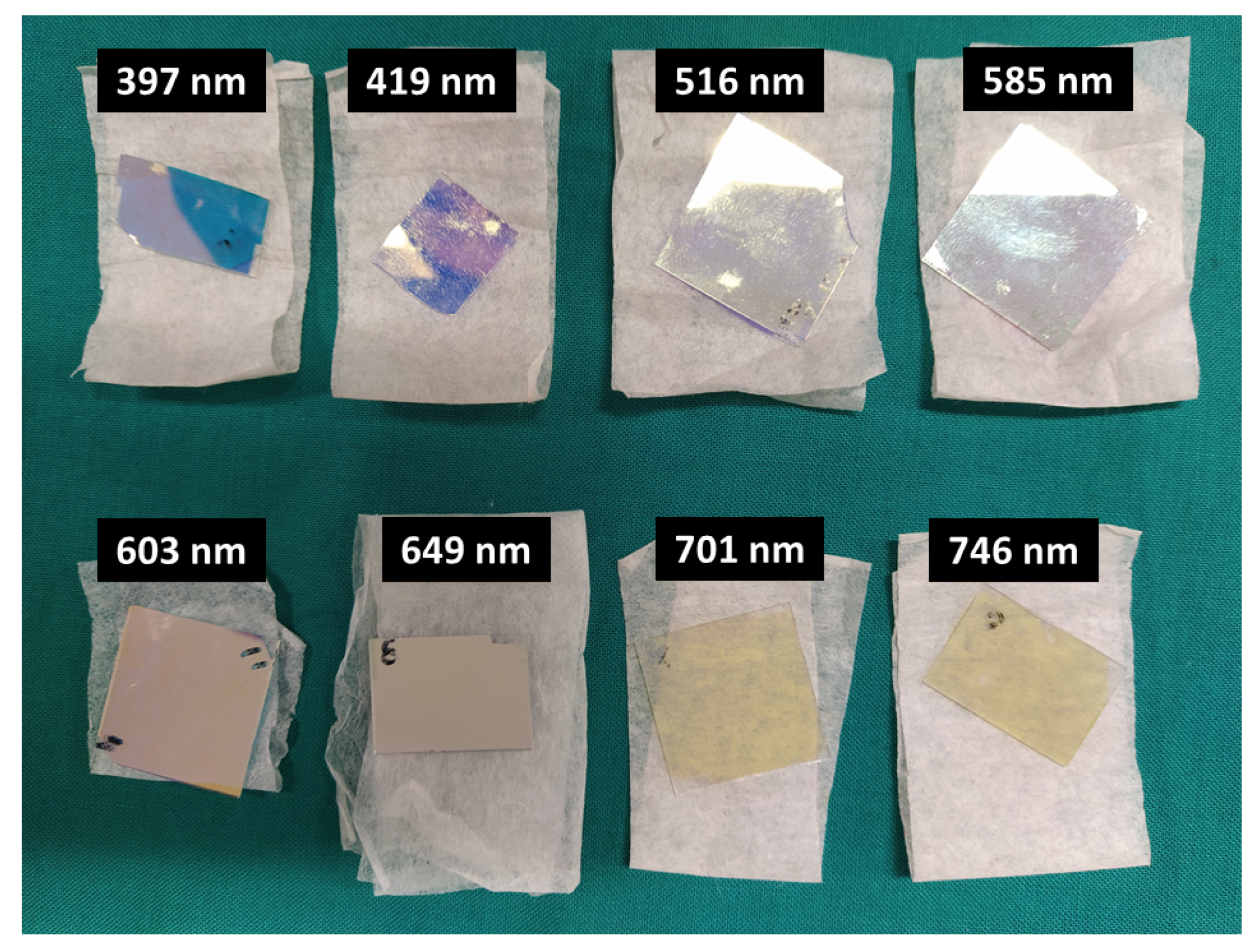
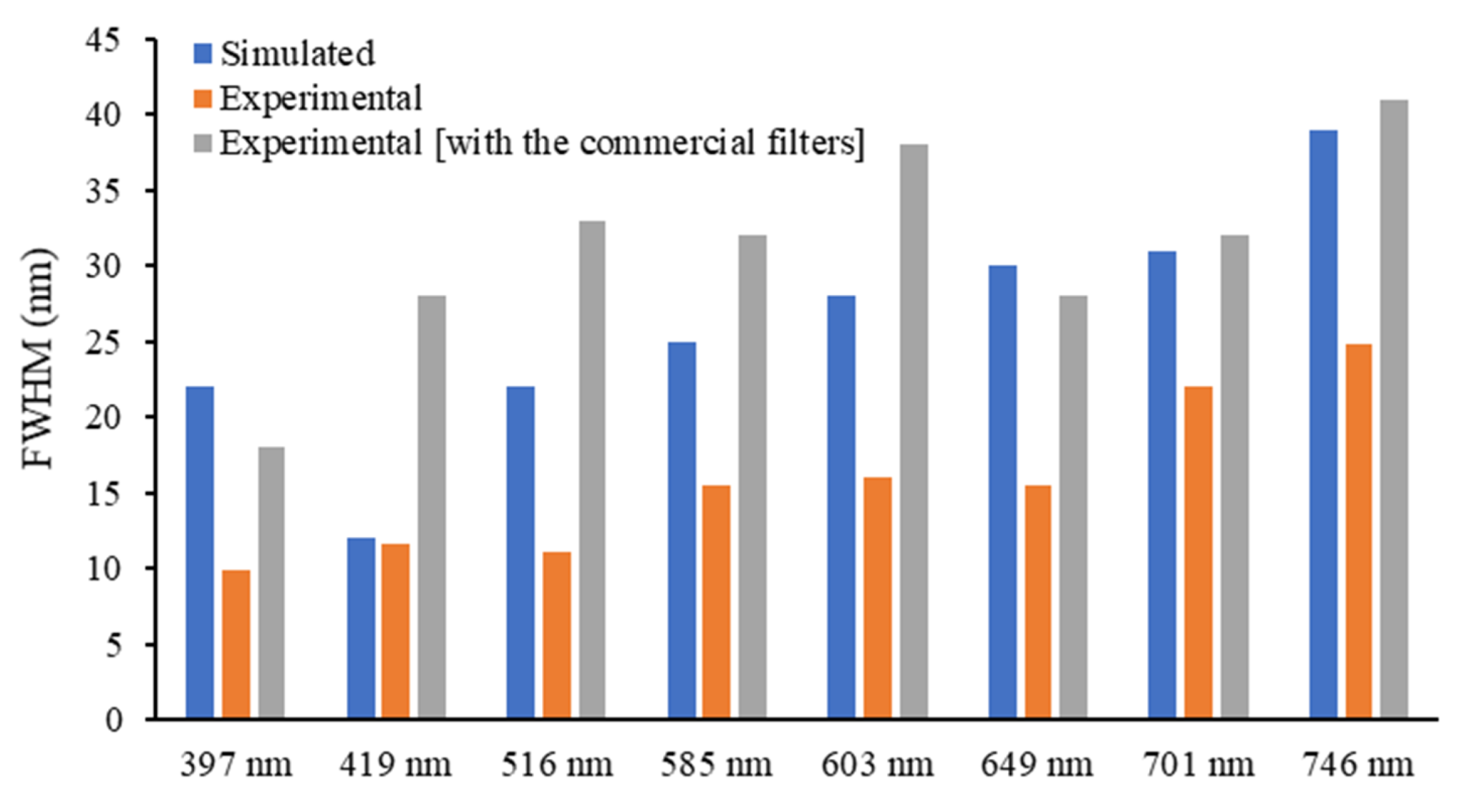
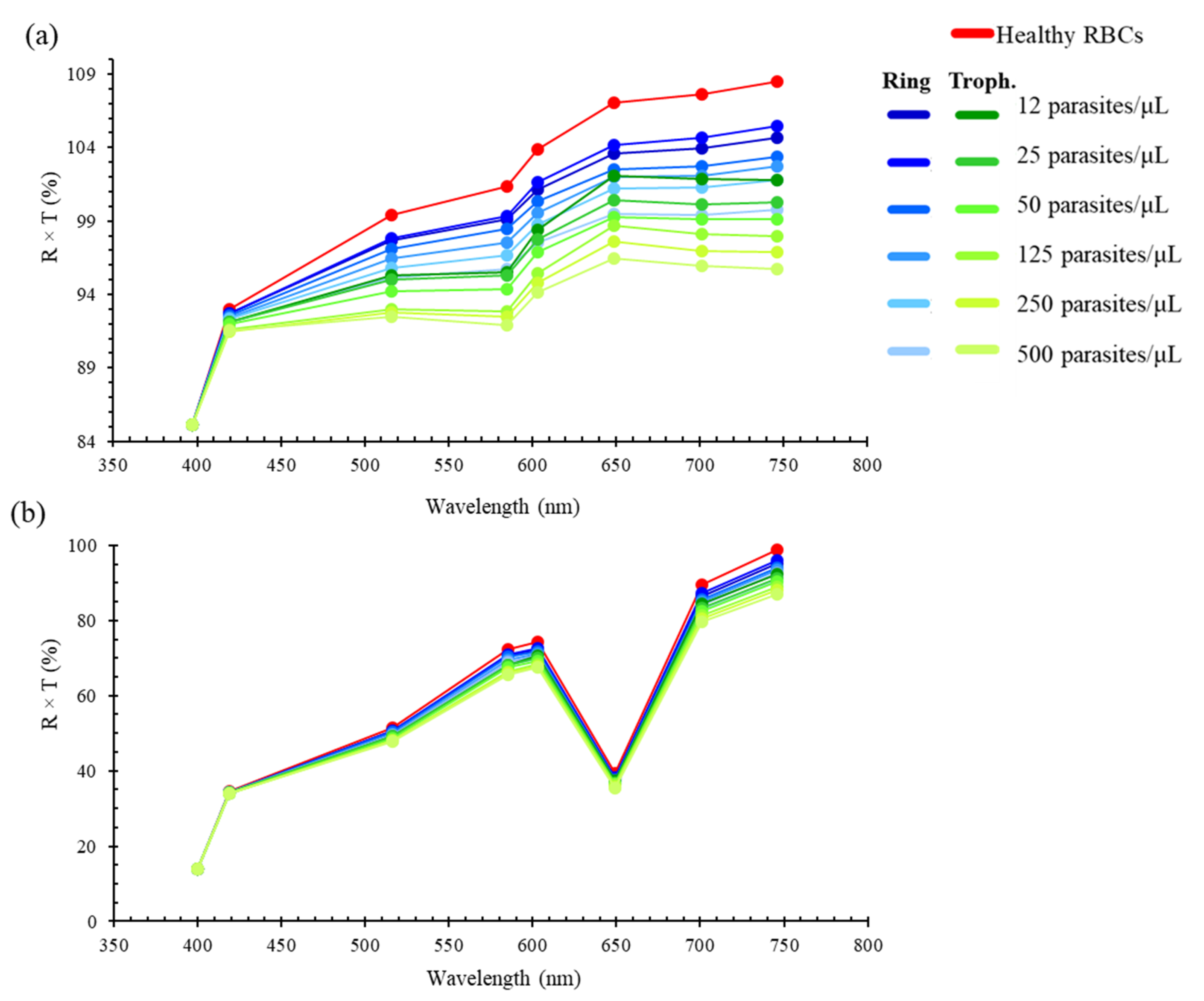
| Maximum Transmittance Peaks Per λ (nm) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 397 | 419 | 516 | 585 | 603 | 649 | 701 | 746 | ||
| Thickness layer (nm) | |||||||||
| TiO2 | 40 | TiO2 | 44 | 63 | 80 | ||||
| MgO | 63 | SiO2 | 75 | 97 | 118 | ||||
| TiO2 | 40 | TiO2 | 44 | 63 | 80 | ||||
| MgO | 63 | SiO2 | 75 | 97 | 118 | ||||
| TiO2 | 40 | TiO2 | 44 | 63 | 80 | ||||
| MgO (RC) | 110 | SiO2 (RC) | 141 | 150 | 218 | 236 | 198 | 249 | 294 |
| TiO2 | 40 | TiO2 | 44 | 63 | 80 | ||||
| MgO | 63 | SiO2 | 75 | 97 | 118 | ||||
| TiO2 | 40 | TiO2 | 44 | 63 | 80 | ||||
| MgO | 63 | SiO2 | 75 | 97 | 118 | ||||
| TiO2 | 40 | TiO2 | 44 | 63 | 80 | ||||
| Slope Variation (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rings (Parasites/μL) | Trophozoites (Parasites/μL) | |||||||||||
| Wavelength Interval (nm) | 12 | 25 | 50 | 125 | 250 | 500 | 12 | 25 | 50 | 125 | 250 | 500 |
| 397–419 | 0.5 | 0.5 | 1.0 | 1.0 | 1.3 | 1.8 | 2.0 | 1.6 | 1.9 | 2.9 | 3.0 | 2.9 |
| 419–516 | 4.5 | 4.3 | 5.9 | 7.8 | 9.4 | 10.7 | 10.2 | 11.5 | 13.6 | 16.0 | 16.6 | 17.6 |
| 516–585 | 3.5 | 2.7 | 4.0 | 5.6 | 6.8 | 8.4 | 9.8 | 9.6 | 10.6 | 13.2 | 13.6 | 15.1 |
| 585–603 | 22.8 | 12.0 | 26.9 | 22.9 | 24.1 | 32.7 | −22.7 | 2.3 | 5.4 | −5.9 | 11.1 | 9.6 |
| 603–649 | 2.4 | 1.8 | 2.8 | 3.9 | 4.7 | 5.3 | 5.7 | 5.7 | 6.5 | 8.3 | 8.7 | 9.0 |
| 649–701 | 3.7 | 2.9 | 5.0 | 5.6 | 6.4 | 8.4 | 6.7 | 7.9 | 8.7 | 10.1 | 11.1 | 12.2 |
| 701–746 | 4.9 | 3.5 | 5.4 | 7.0 | 8.9 | 11.9 | 14.2 | 12.9 | 14.3 | 16.8 | 17.9 | 20.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, M.S.; Baptista, V.; Ferreira, G.M.; Lima, D.; Minas, G.; Veiga, M.I.; Catarino, S.O. Multilayer Thin-Film Optical Filters for Reflectance-Based Malaria Diagnostics. Micromachines 2021, 12, 890. https://doi.org/10.3390/mi12080890
Costa MS, Baptista V, Ferreira GM, Lima D, Minas G, Veiga MI, Catarino SO. Multilayer Thin-Film Optical Filters for Reflectance-Based Malaria Diagnostics. Micromachines. 2021; 12(8):890. https://doi.org/10.3390/mi12080890
Chicago/Turabian StyleCosta, Mariana S., Vitória Baptista, Gabriel M. Ferreira, Duarte Lima, Graça Minas, Maria Isabel Veiga, and Susana O. Catarino. 2021. "Multilayer Thin-Film Optical Filters for Reflectance-Based Malaria Diagnostics" Micromachines 12, no. 8: 890. https://doi.org/10.3390/mi12080890
APA StyleCosta, M. S., Baptista, V., Ferreira, G. M., Lima, D., Minas, G., Veiga, M. I., & Catarino, S. O. (2021). Multilayer Thin-Film Optical Filters for Reflectance-Based Malaria Diagnostics. Micromachines, 12(8), 890. https://doi.org/10.3390/mi12080890









