A Flexible a-SiC-Based Neural Interface Utilizing Pyrolyzed-Photoresist Film (C) Active Sites
Abstract
:1. Introduction
2. Materials and Methods
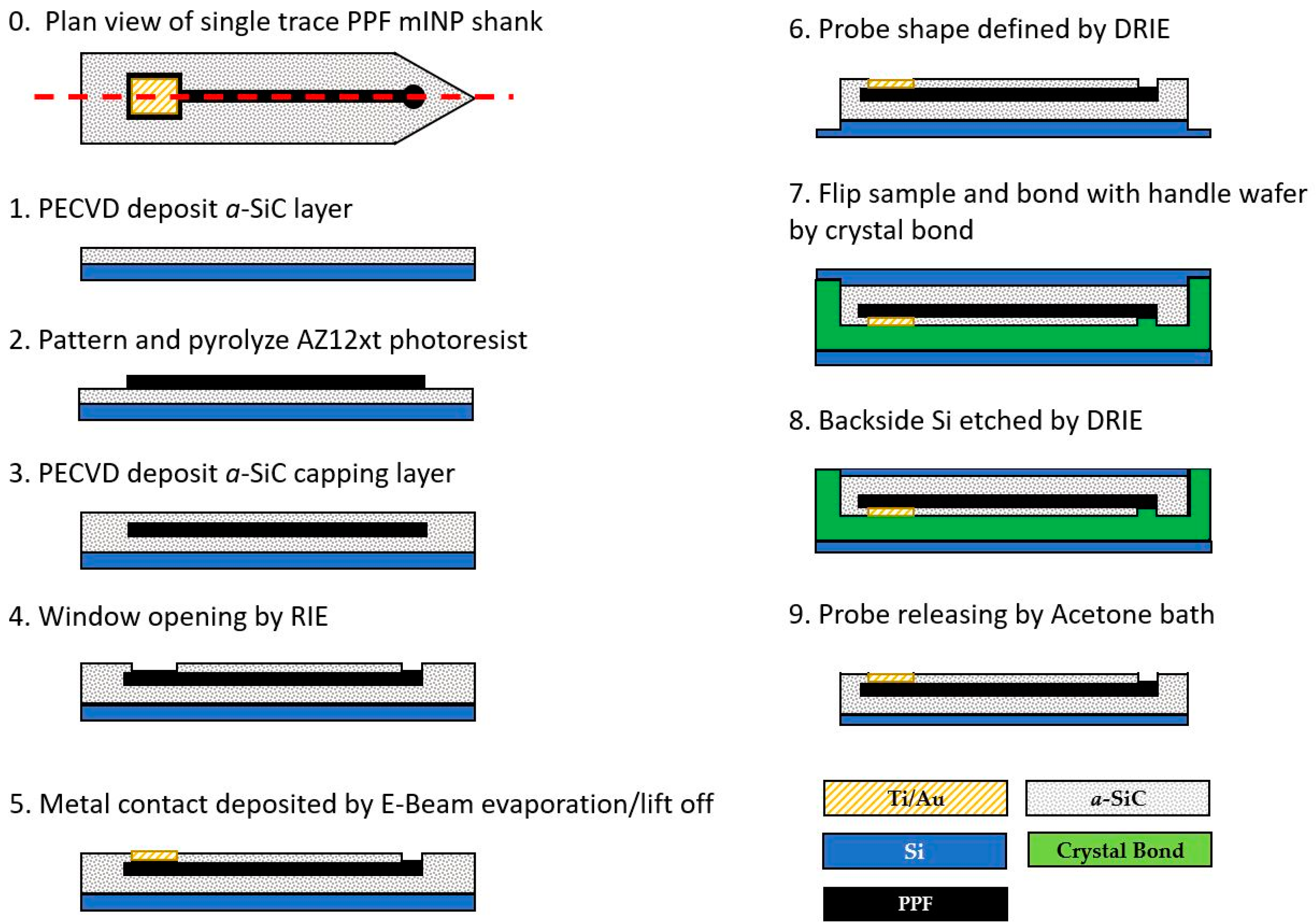
2.1. Device Fabrication Process
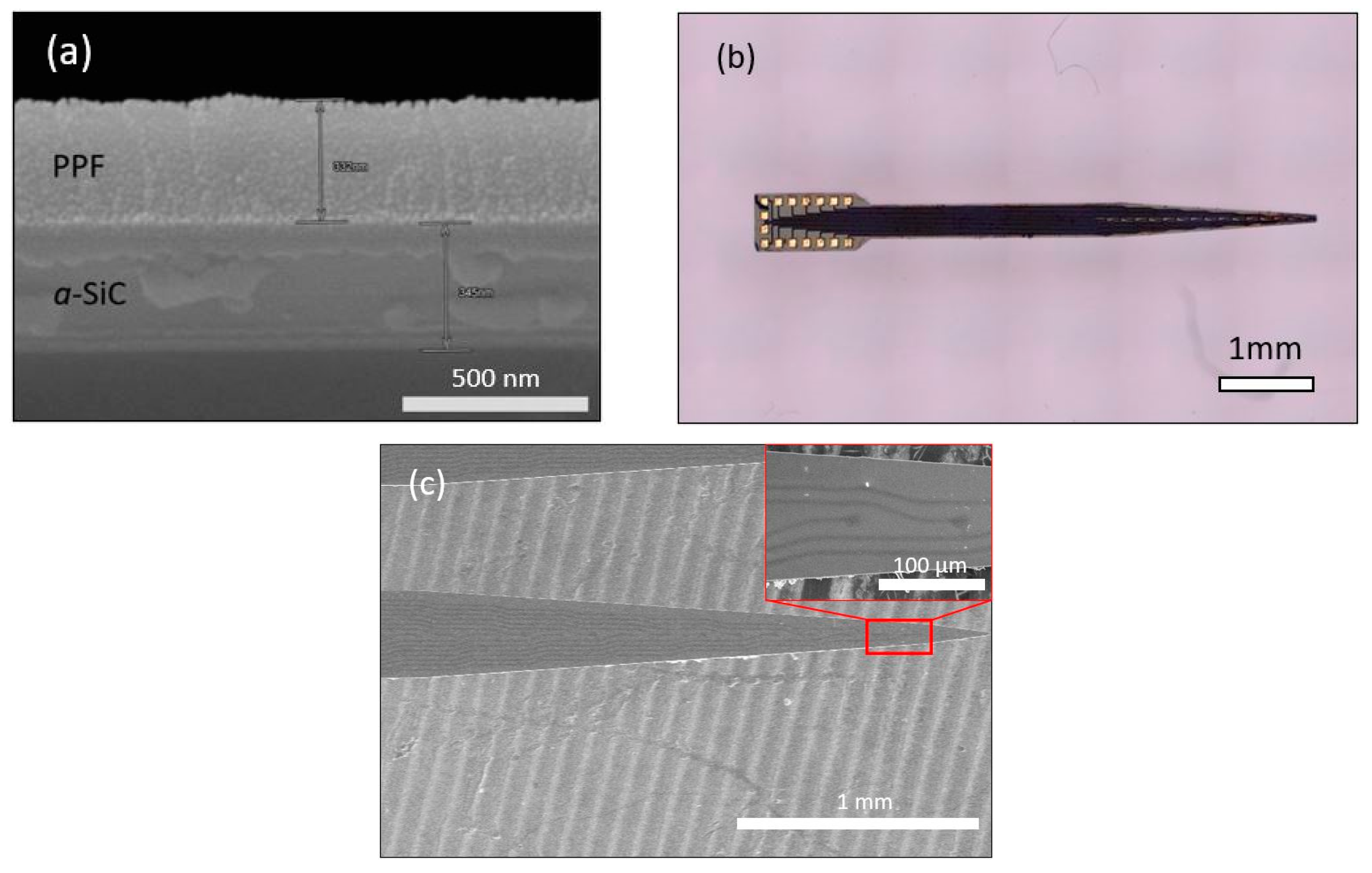
2.2. Surface Characterization of a-SiC Insulated PPF Probe
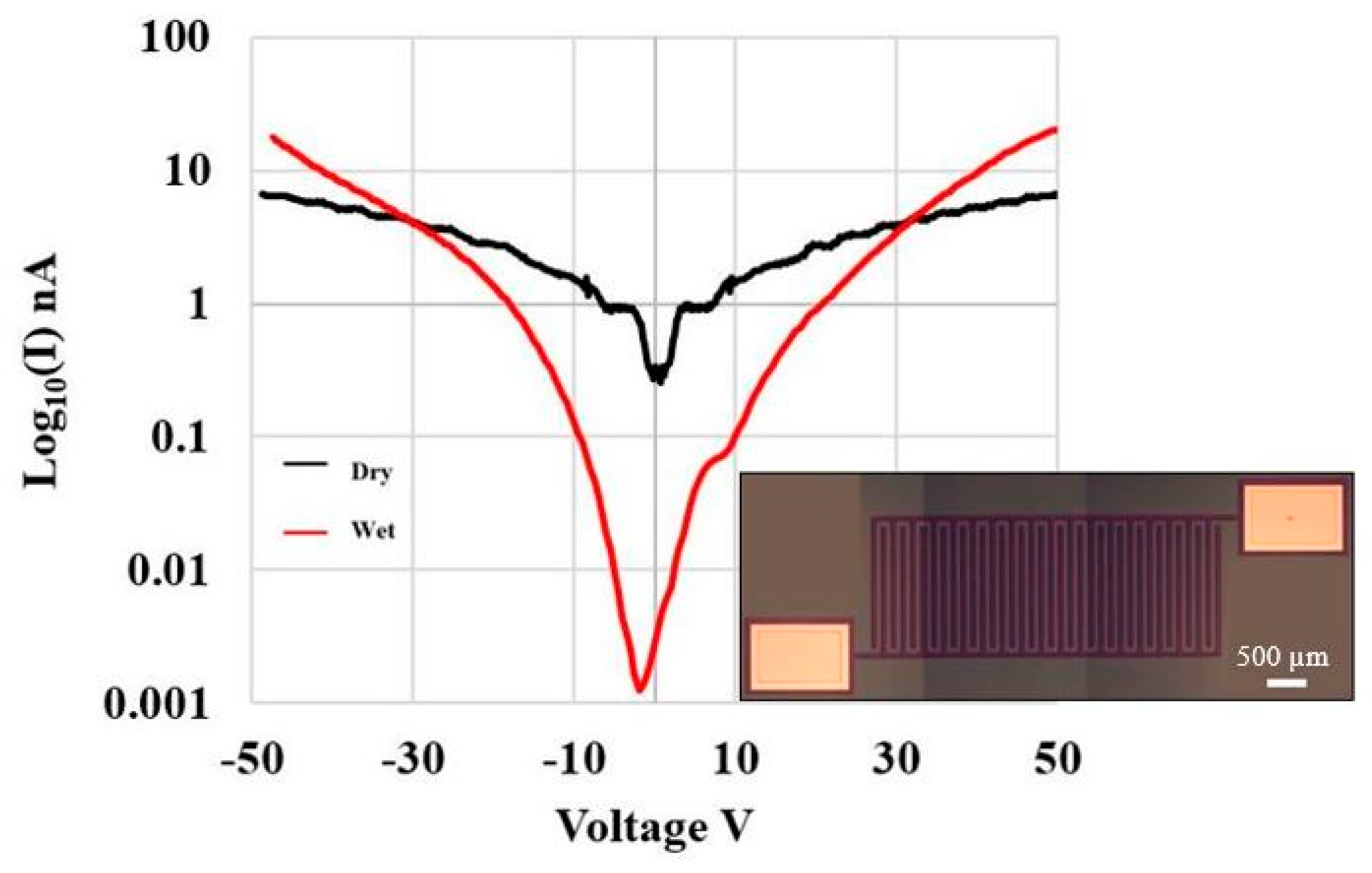
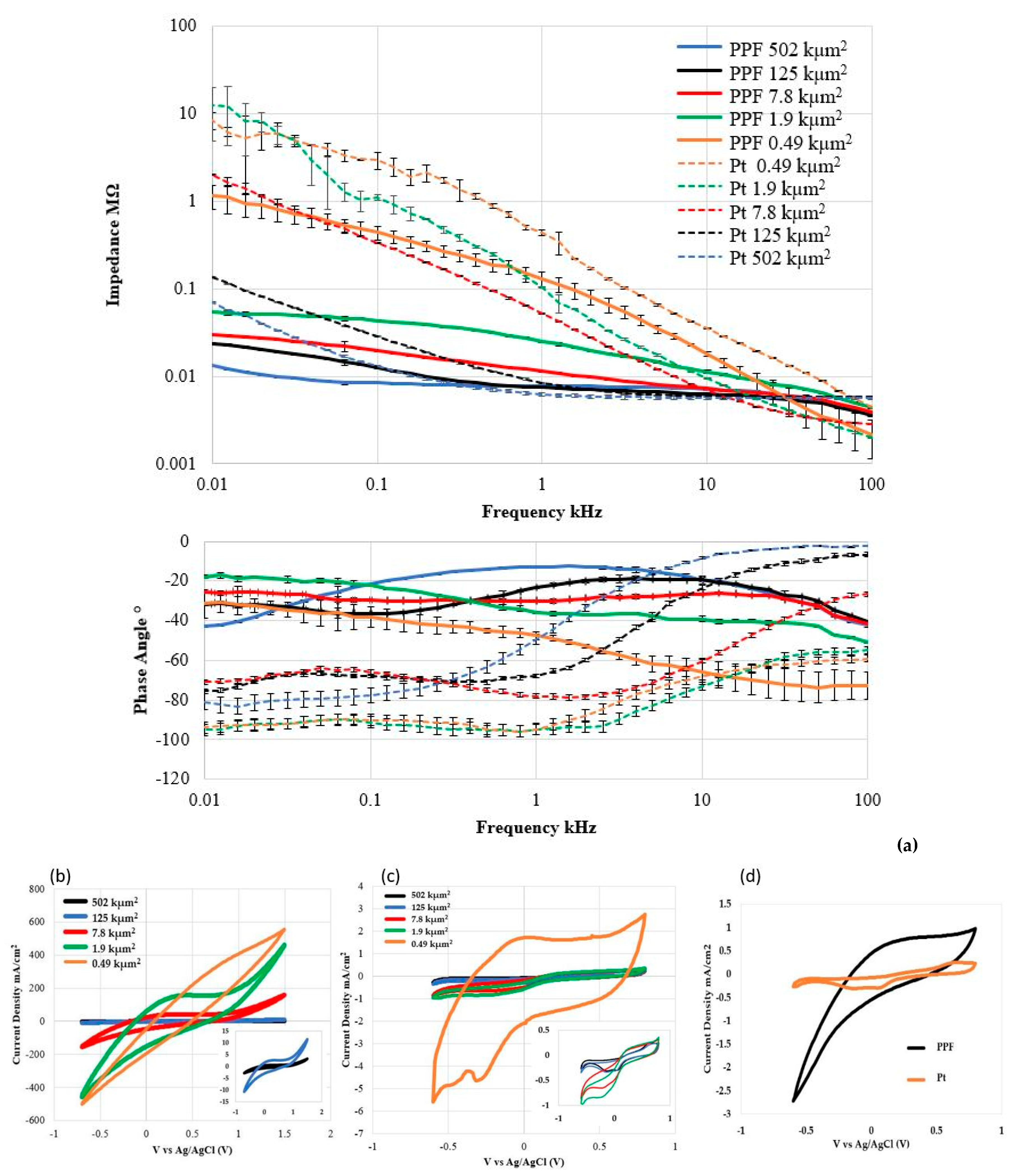
2.3. Electrical and Electrochemical Characterization of a-SiC Insulated PPF mINP
3. Results
3.1. Surface Characterization
3.2. Electrochemical and Electrical Characterization
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gooch, C.L.; Pracht, E.; Borenstein, A.R. The burden of neurological disease in the United States: A summary report and call to action. Ann. Neurol. 2017, 81, 479–484. [Google Scholar] [CrossRef]
- Introduction to Microfabrication, 2nd ed. Available online: https://www.wiley.com/en-us/Introduction+to+Microfabrication%2C+2nd+Edition-p-9781119991892 (accessed on 4 May 2021).
- Bong, J.; Attia, Z.I.; Vaidya, V.R.; Jung, Y.H.; Padmanabhan, D.; Lee, J.; Kim, H.; Ladewig, D.J.; Noseworthy, P.A.; Asirvatham, S.J.; et al. Radiolucent implantable electrocardiographic monitoring device based on graphene. Carbon N. Y. 2019, 152, 946–953. [Google Scholar] [CrossRef]
- Goshi, N.; Castagnola, E.; Vomero, M.; Gueli, C.; Cea, C.; Zucchini, E.; Bjanes, D.; Maggiolini, E.; Moritz, C.; Kassegne, S.; et al. Glassy carbon MEMS for novel origami-styled 3D integrated intracortical and epicortical neural probes. J. Micromech. Microeng. 2018, 28, 065009. [Google Scholar] [CrossRef] [Green Version]
- Barrese, J.C.; Rao, N.; Paroo, K.; Triebwasser, C.; Vargas-Irwin, C.; Franquemont, L.; Donoghue, J.P. Failure mode analysis of silicon-based intracortical microelectrode arrays in non-human primates. J. Neural Eng. 2013, 10, 066014. [Google Scholar] [CrossRef]
- Bernardin, E.K. Demonstration of Monolithic-Silicon Carbide (SiC) Neural Devices; University of South Florida: Tampa, FL, USA, 2018. [Google Scholar] [CrossRef]
- Barrese, J.C.; Aceros, J.; Donoghue, J.P. Scanning electron microscopy of chronically implanted intracortical microelectrode arrays in non-human primates. J. Neural Eng. 2016, 13. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Wang, L.; Lu, Y.; Du, X. Bioinspired microcone-array-based living biointerfaces: Enhancing the anti-inflammatory effect and neuronal network formation. Microsyst. Nanoeng. 2020, 6, 1–8. [Google Scholar] [CrossRef]
- Deku, F.; Frewin, C.L.; Stiller, A.; Cohen, Y.; Aqeel, S.; Joshi-Imre, A.; Black, B.; Gardner, T.J.; Pancrazio, J.J.; Cogan, S.F. Amorphous silicon carbide platform for next generation penetrating neural interface designs. Micromachines 2018, 9, 480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saddow, S.E.; Frewin, C.L.; Nezafati, M.; Oliveros, A.; Afroz, S.; Register, J.; Reyes, M.; Thomas, S. 3C-SiC on Si: A bio- and hemo-compatible material for advanced nano-bio devices. In Proceedings of the 2014 IEEE 9th Nanotechnology Materials and Devices Conference, Aci Castello, Italy, 12–15 October 2014; pp. 49–53. [Google Scholar] [CrossRef]
- Knaack, G.L.; Charkhkar, H.; Cogan, S.F.; Pancrazio, J.J. Chapter 8: Amorphous Silicon Carbide for Neural Interface Applications, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; ISBN 9780128029930. [Google Scholar]
- Feng, C.; Beygi, M.; Frewin, C.L.; Tanjil, M.R.-E.; Kumar, A.; Wang, M.C.; Saddow, S.E. Silicon Carbide Biotechnology: Carbon-Based Neural Interfaces. In Proceedings of the TMS 2021 150th Annual Meeting & Exhibition Supplemental Proceedings, Orlando, FL, USA, 15–18 March 2021; pp. 777–790, ISBN 9783030652609. [Google Scholar]
- Brassard, D.; El Khakani, M.A. Dielectric properties of amorphous hydrogenated silicon carbide thin films grown by plasma-enhanced chemical vapor deposition. J. Appl. Phys. 2003, 93, 4066–4071. [Google Scholar] [CrossRef]
- Li, X.; Wong, T.K.S.; Rusli; Yang, D. Structural and electronic properties of low dielectric constant carbon rich amorphous silicon carbide. Diam. Relat. Mater. 2003, 12, 963–967. [Google Scholar] [CrossRef]
- Cogan, S.F.; Edell, D.J.; Guzelian, A.A.; Liu, Y.P.; Edell, R. Plasma-enhanced chemical vapor deposited silicon carbide as an implantable dielectric coating. J. Biomed. Mater. Res. Part A 2003, 67, 856–867. [Google Scholar] [CrossRef]
- Beygi, M.; Bentley, J.T.; Frewin, C.L.; Kuliasha, C.A.; Takshi, A.; Bernardin, E.K.; La Via, F.; Saddow, S.E. Fabrication of a Monolithic Implantable Neural Interface from Cubic Silicon Carbide. Micromachines 2019, 10, 430. [Google Scholar] [CrossRef] [Green Version]
- Bernardin, E.K.; Frewin, C.L.; Everly, R.; Ul Hassan, J.; Saddow, S.E. Demonstration of a robust all-silicon-carbide intracortical neural interface. Micromachines 2018, 9, 412. [Google Scholar] [CrossRef] [Green Version]
- Beygi, M.; Dominguez-Viqueira, W.; Feng, C.; Mumcu, G.; Frewin, C.L.; La Via, F.; Saddow, S.E. Silicon Carbide and MRI: Towards Developing a MRI Safe Neural Interface. Micromachines 2021, 12, 126. [Google Scholar] [CrossRef] [PubMed]
- Baldrighi, M.; Trusel, M.; Tonini, R.; Giordani, S. Carbon nanomaterials interfacing with neurons: An in vivo perspective. Front. Neurosci. 2016, 10, 250. [Google Scholar] [CrossRef] [Green Version]
- David-Pur, M.; Bareket-Keren, L.; Beit-Yaakov, G.; Raz-Prag, D.; Hanein, Y. All-carbon-nanotube flexible multi-electrode array for neuronal recording and stimulation. Biomed. Microdevices 2014, 16, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Richardson, A.G.; Bink, H.; de Vries, J.; Cubukcu, E.; Shim, E.; Reed, J.C.; Lucas, T.H.; Takano, H.; Coulter, D.A.; Kuzum, D.; et al. Transparent and flexible low noise graphene electrodes for simultaneous electrophysiology and neuroimaging. Nat. Commun. 2014, 5, 5259. [Google Scholar] [CrossRef]
- Fairman, C.; Liu, G.; Hibbert, D.B.; Gooding, J.J. Comparing the electrochemical performance of pyrolysed photoresist film electrodes to glassy carbon electrodes for sensing applications. In Proceedings of the 2010 International Conference on Nanoscience and Nanotechnology, Sydney, Australia, 22–26 February 2010; pp. 308–311. [Google Scholar] [CrossRef]
- Lee, J.A.; Hwang, S.; Kwak, J.; Park, S.I.; Lee, S.S.; Lee, K.C. An electrochemical impedance biosensor with aptamer-modified pyrolyzed carbon electrode for label-free protein detection. Sens. Actuators B Chem. 2008, 129, 372–379. [Google Scholar] [CrossRef]
- Oliveros, A.; Coletti, C.; Saddow, S.E. Carbon Based Materials on SiC for Advanced Biomedical Applications. Silicon Carbide Biotechnol. 2012, 431–458. [Google Scholar] [CrossRef]
- Zachek, M.K.; Park, J.; Takmakov, P.; Wightman, R.M.; McCarty, G.S. Microfabricated FSCV-compatible microelectrode array for real-time monitoring of heterogeneous dopamine release. Analyst 2010, 135, 1556–1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Z.; Zhang, P.; Chetwynd, A.J.; Xie, H.Q.; Valsami-Jones, E.; Zhao, B.; Lynch, I. Elucidating the mechanism of the surface functionalization dependent neurotoxicity of graphene family nanomaterials. Nanoscale 2020, 12, 18600–18605. [Google Scholar] [CrossRef]
- Hansen, S.F.; Lennquist, A. Carbon nanotubes added to the SIN List as a nanomaterial of Very High Concern. Nat. Nanotechnol. 2020, 15, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Kaplas, T.; Matikainen, A.; Nuutinen, T.; Suvanto, S.; Vahimaa, P.; Svirko, Y. Scalable fabrication of the graphitic substrates for graphene-enhanced Raman spectroscopy. Sci. Rep. 2017, 7, 8561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Dersarl, J.J.; Mercanzini, A.; Renaud, P. Integration of 2D and 3D thin film glassy carbon electrode arrays for electrochemical dopamine sensing in flexible neuroelectronic implants. Adv. Funct. Mater. 2015, 25, 78–84. [Google Scholar] [CrossRef]
- Vomero, M.; Castagnola, E.; Ciarpella, F.; Maggiolini, E.; Goshi, N.; Zucchini, E.; Carli, S.; Fadiga, L.; Kassegne, S.; Ricci, D. Highly Stable Glassy Carbon Interfaces for Long-Term Neural Stimulation and Low-Noise Recording of Brain Activity. Sci. Rep. 2017, 7, 40332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deku, F.; Cohen, Y.; Joshi-Imre, A.; Kanneganti, A.; Gardner, T.J.; Cogan, S.F. Amorphous silicon carbide ultramicroelectrode arrays for neural stimulation and recording. J. Neural Eng. 2018, 15. [Google Scholar] [CrossRef]
- Nimbalkar, S.; Fuhrer, E.; Silva, P.; Nguyen, T.; Sereno, M.; Kassegne, S.; Korvink, J. Glassy carbon microelectrodes minimize induced voltages, mechanical vibrations, and artifacts in magnetic resonance imaging. Microsyst. Nanoeng. 2019, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vahidi, N.W.; Rudraraju, S.; Castagnola, E.; Cea, C.; Nimbalkar, S.; Hanna, R.; Arvizu, R.; Dayeh, S.A.; Gentner, T.Q.; Kassegne, S. Epi-Intra neural probes with glassy carbon microelectrodes help elucidate neural coding and stimulus encoding in 3D volume of tissue. J. Neural Eng. 2020, 17. [Google Scholar] [CrossRef]
- Register, J. SiC for Advanced Biological Applications; University of South Florida: Tampa, FL, USA, 2014. [Google Scholar]
- Chen, B.; Zhang, B.; Chen, C.; Hu, J.; Qi, J.; He, T.; Tian, P.; Zhang, X.; Ni, G.; Cheng, M.M.-C. Penetrating glassy carbon neural electrode arrays for brain-machine interfaces. Biomed. Microdevices 2020. [Google Scholar] [CrossRef]
- Nimbalkar, S.; Castagnola, E.; Balasubramani, A.; Scarpellini, A.; Samejima, S.; Khorasani, A.; Boissenin, A.; Thongpang, S.; Moritz, C.; Kassegne, S. Ultra-Capacitive Carbon Neural Probe Allows Simultaneous Long-Term Electrical Stimulations and High-Resolution Neurotransmitter Detection. Sci. Rep. 2018, 8, 6958. [Google Scholar] [CrossRef] [Green Version]
- Cogan, S.F. Neural Stimulation and Recording Electrodes. Annu. Rev. Biomed. Eng. 2008, 10, 275–309. [Google Scholar] [CrossRef] [Green Version]
- Hudak, E.M.; Kumsa, D.W.; Martin, H.B.; Mortimer, J.T. Electron transfer processes occurring on platinum neural stimulating electrodes: Calculated charge-storage capacities are inaccessible during applied stimulation. J. Neural Eng. 2017, 14, 046012. [Google Scholar] [CrossRef] [PubMed]
- Gerwig, R.; Fuchsberger, K.; Schroeppel, B.; Link, G.S.; Heusel, G.; Kraushaar, U.; Schuhmann, W.; Stett, A.; Stelzle, M. PEDOT-CNT composite microelectrodes for recording and electrostimulation applications: Fabrication, morphology, and electrical properties. Front. Neuroeng. 2012, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitale, F.; Summerson, S.R.; Aazhang, B.; Kemere, C.; Pasquali, M. Neural stimulation and recording with bidirectional, soft carbon nanotube fiber microelectrodes. ACS Nano 2015, 9, 4465–4474. [Google Scholar] [CrossRef]
- Wang, K.; Frewin, C.L.; Esrafilzadeh, D.; Yu, C.; Wang, C.; Pancrazio, J.J.; Romero-Ortega, M.; Jalili, R.; Wallace, G. High-Performance Graphene-Fiber-Based Neural Recording Microelectrodes. Adv. Mater. 2019, 31, 1805867. [Google Scholar] [CrossRef]
- Wilks, S. Poly(3,4-ethylene dioxythiophene) (PEDOT) as a micro-neural interface material for electrostimulation. Front. Neuroeng. 2009, 2, 7. [Google Scholar] [CrossRef] [Green Version]
- Kozai, T.D.Y.; Catt, K.; Li, X.; Gugel, Z.V.; Olafsson, V.T.; Vazquez, A.L.; Cui, X.T. Mechanical failure modes of chronically implanted planar silicon-based neural probes for laminar recording. Biomaterials 2015, 37, 25–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Species | Atomic Concentration |
|---|---|
| Carbon | 43.10 |
| Silicon | 48.37 |
| Oxygen | 8.53 |
| C:Si ratio | 0.9 |
| Annealing Temperature (°C) | PPF Thickness (nm) | Resistivity mΩ∙cm) |
|---|---|---|
| 500 | ~599 | 24 |
| 600 | ~498 | 11 |
| 700 | ~459 | 8 |
| 800 | ~424 | 4 |
| 900 | ~387 | 3 |
| Electrode * | Area (kµm2) | Impedance 1 kHz (kΩ) | CSCa (mC/cm2) | CSCc (mC/cm2) |
|---|---|---|---|---|
| PPF | 0.49 | 125.6 ± 25.7 | 5600 | 3000 |
| Pt | 0.49 | 437.8 ± 35.9 | 53.2 | 33.2 |
| PPF | 1.9 | 24.8 ± 0.4 | 8900 | 5260 |
| Pt | 1.9 | 103.8 ± 2.2 | 4.7 | 7.4 |
| PPF | 7.8 | 11.4 ± 0.04 | 2870 | 1600 |
| Pt | 7.8 | 51.8 ± 1.1 | 4.9 | 6.4 |
| PPF | 125 | 7.4 ± 0.04 | 193 | 107 |
| Pt | 125 | 8.4 ± 0.2 | 4.1 | 5.6 |
| PPF | 502 | 7.7 ± 0.01 | 50 | 27 |
| Pt | 502 | 6.3 ± 0.1 | 3.9 | 5.2 |
| Material | Recording Area (kµm2) | Impedance @1kHz (kΩ) | CSC * (mC/cm2) |
|---|---|---|---|
| PPF/a-SiC | 1.9 | 24.8 | 14,160 |
| Pt | 1.9 | 103.8 | 12 |
| PEDOT/CNT [39] | 2.83 | 15.0 | 6 |
| Carbon-nanotube fiber [40] | 1.450 | 14.1 | 372 |
| Graphene Fiber [41] | 0.749 | 37.9 | 798 |
| IrOx [42] | 0.177 | 132.9 | 29 |
| TiN [39] | 2.83 | 54.8 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, C.; Frewin, C.L.; Tanjil, M.R.-E.; Everly, R.; Bieber, J.; Kumar, A.; Wang, M.C.; Saddow, S.E. A Flexible a-SiC-Based Neural Interface Utilizing Pyrolyzed-Photoresist Film (C) Active Sites. Micromachines 2021, 12, 821. https://doi.org/10.3390/mi12070821
Feng C, Frewin CL, Tanjil MR-E, Everly R, Bieber J, Kumar A, Wang MC, Saddow SE. A Flexible a-SiC-Based Neural Interface Utilizing Pyrolyzed-Photoresist Film (C) Active Sites. Micromachines. 2021; 12(7):821. https://doi.org/10.3390/mi12070821
Chicago/Turabian StyleFeng, Chenyin, Christopher L. Frewin, Md Rubayat-E Tanjil, Richard Everly, Jay Bieber, Ashok Kumar, Michael Cai Wang, and Stephen E. Saddow. 2021. "A Flexible a-SiC-Based Neural Interface Utilizing Pyrolyzed-Photoresist Film (C) Active Sites" Micromachines 12, no. 7: 821. https://doi.org/10.3390/mi12070821
APA StyleFeng, C., Frewin, C. L., Tanjil, M. R.-E., Everly, R., Bieber, J., Kumar, A., Wang, M. C., & Saddow, S. E. (2021). A Flexible a-SiC-Based Neural Interface Utilizing Pyrolyzed-Photoresist Film (C) Active Sites. Micromachines, 12(7), 821. https://doi.org/10.3390/mi12070821







