Mixing Mechanism of Microfluidic Mixer with Staggered Virtual Electrode Based on Light-Actuated AC Electroosmosis
Abstract
:1. Introduction
2. Theory and Methods
2.1. Micromixer Structure
2.2. Governing Equations
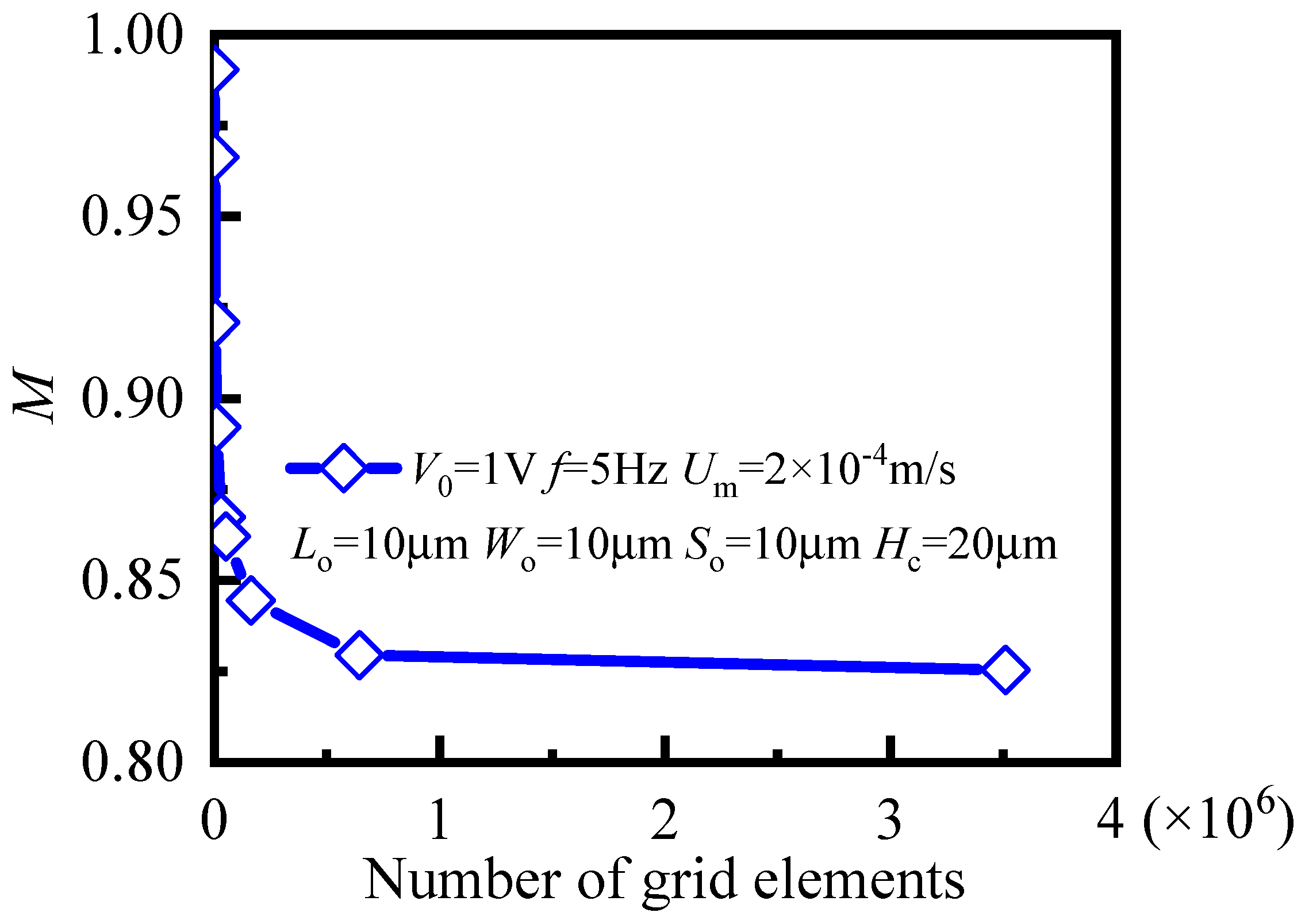
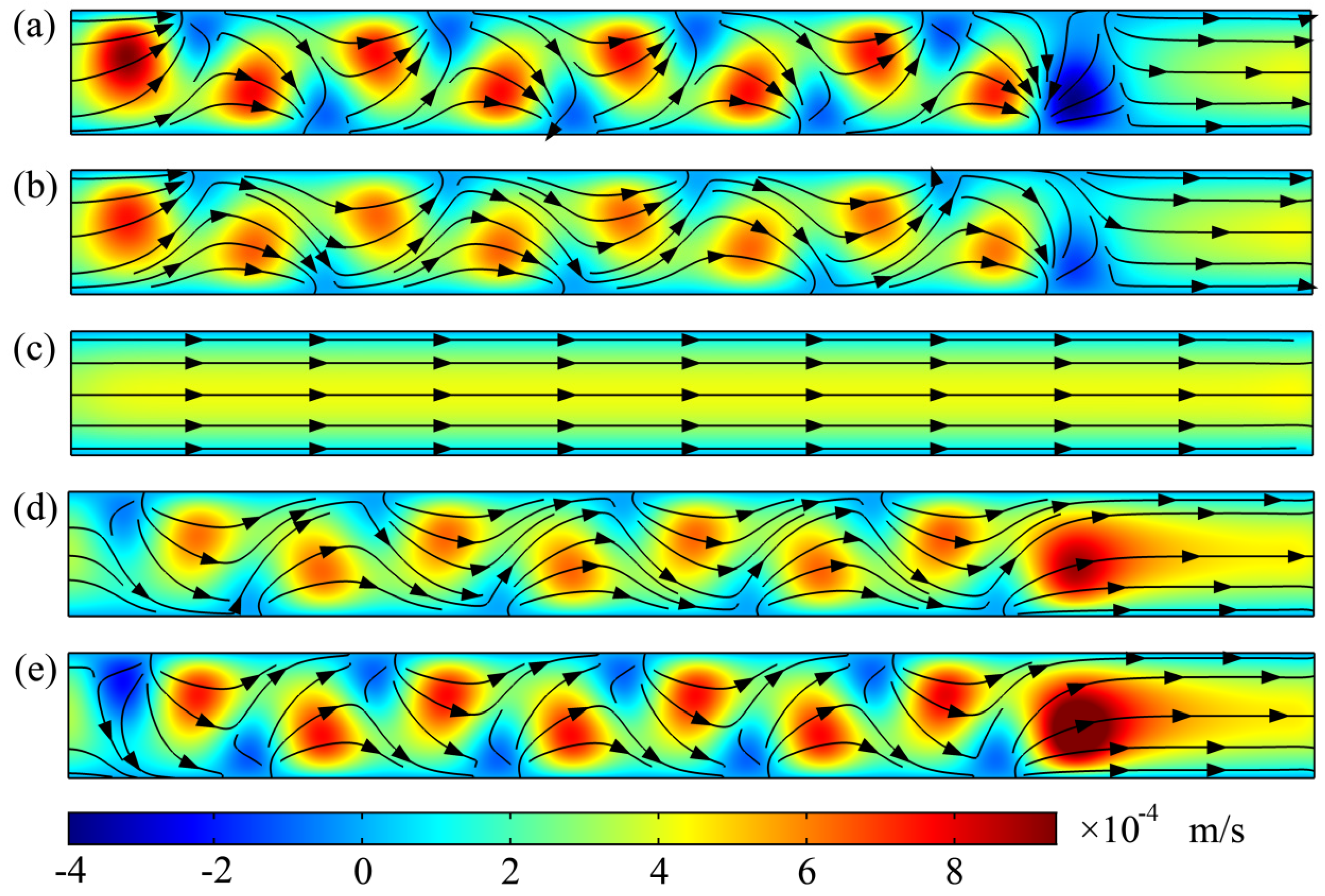
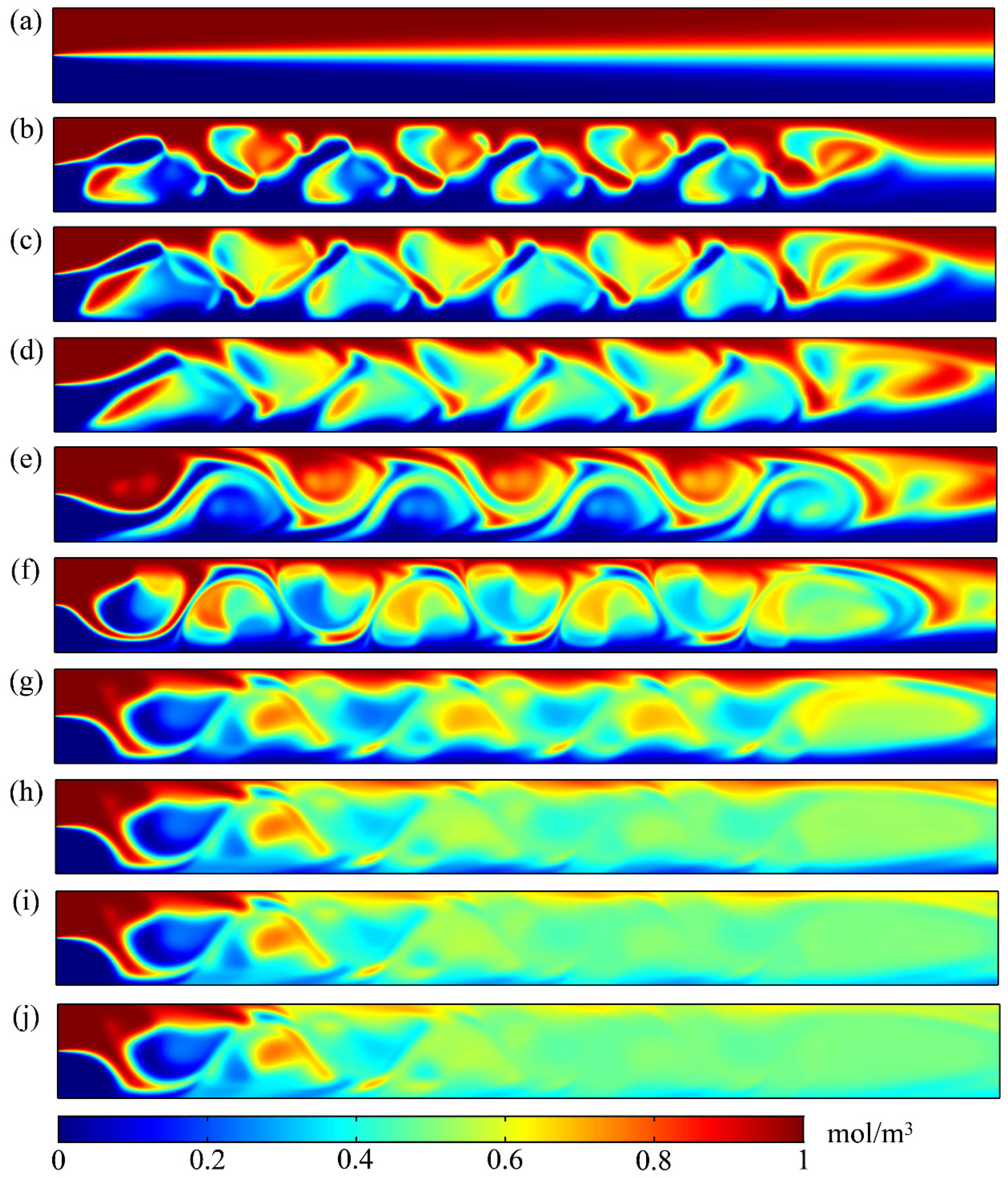
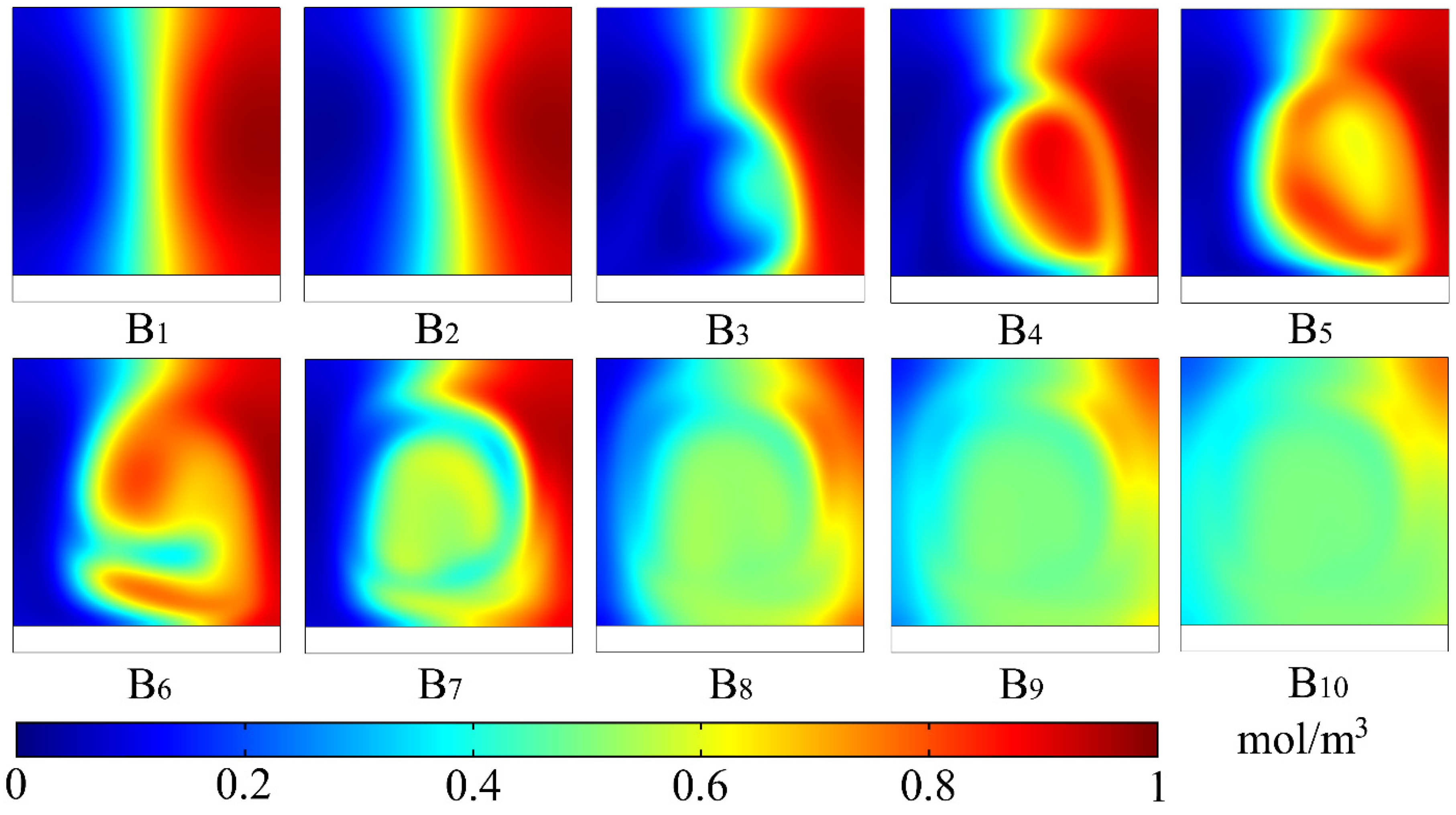
3. Results and Discussion
3.1. Mixing Process and Mechanism
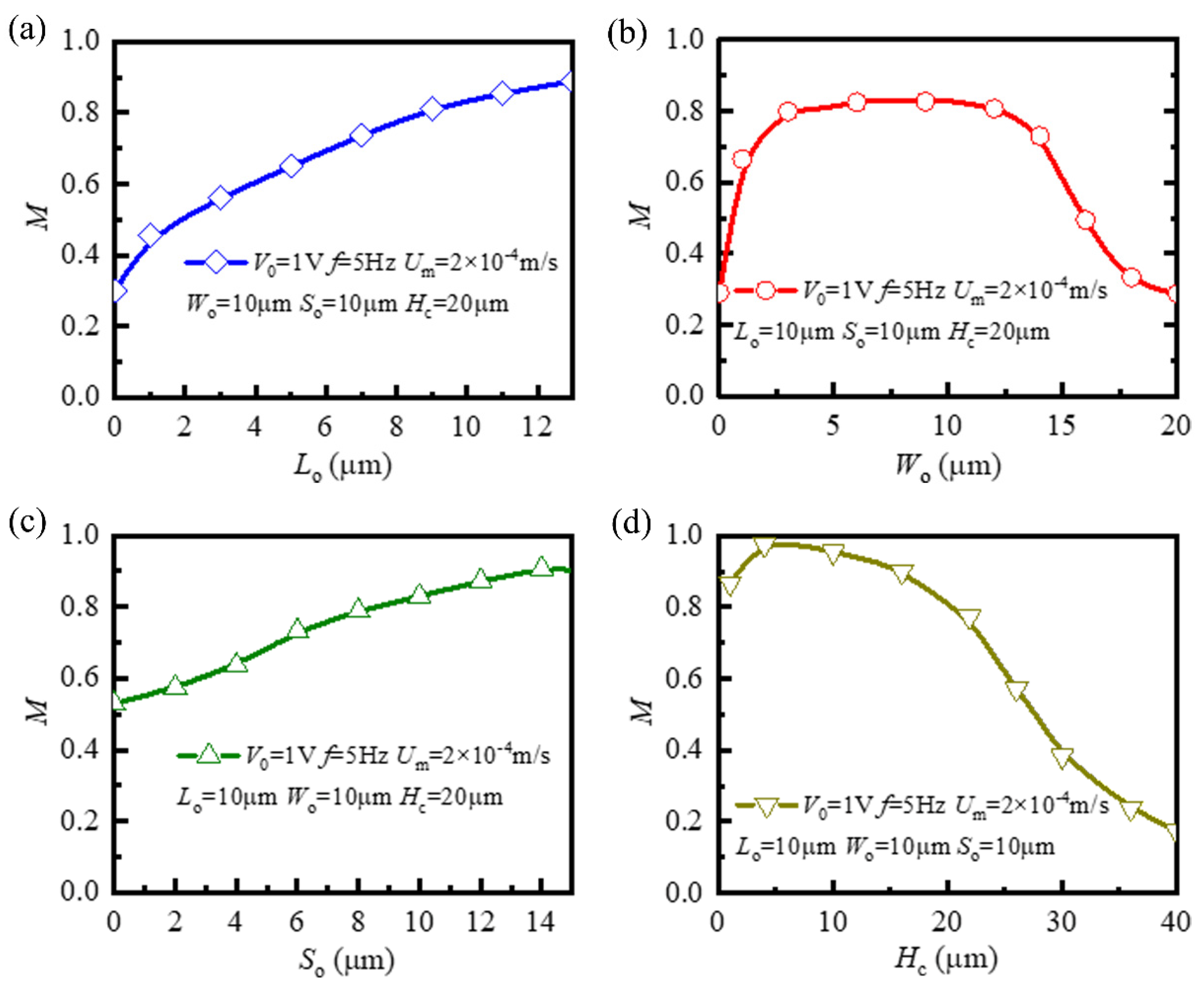
3.2. Influence of Key Geometric Parameters on Mixing Performance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jeong, G.S.; Chung, S.; Kim, C.B.; Lee, S.H. Applications of micromixing technology. Analyst 2010, 135, 460–473. [Google Scholar] [CrossRef]
- Manz, A.; Graber, N.; Widmer, H.M. Miniaturized total chemical analysis systems a novel concept for chemical sensing. Sens. Actuators B1 1990, 1, 244–248. [Google Scholar] [CrossRef]
- Harrison, D.J.; Manz, A.; Fan, Z.; Luedi, H.; Widmer, H.M.; Manz, J.A.; Fan, Z.; Ludi, H.; Widmer, H.M. Capillary electrophoresis and sample injection systems integrated on a planar glass chip. Anal. Chem. 1992, 64, 1926–1932. [Google Scholar] [CrossRef]
- Alam, M.K.; Koomson, E.; Zou, H.; Yi, C.; Li, C.W.; Xu, T.; Yang, M. Recent advances in microfluidic technology for manipulation and analysis of biological cells (2007–2017). Anal. Chim. Acta 2018, 1044, 29–65. [Google Scholar] [CrossRef]
- Sasaki, N.; Kitamori, T.; Kim, H.B. AC electroosmotic micromixer for chemical processing in a microchannel. Lab Chip 2006, 6, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Kim, G.Y.; Seo, T.S. An integrated passive micromixer-magnetic separation-capillary electrophoresis microdevice for rapid and multiplex pathogen detection at the single-cell level. Lab Chip 2011, 11, 3465–3470. [Google Scholar] [CrossRef]
- Lee, C.Y.; Chang, C.L.; Wang, Y.N.; Fu, L.M. Microfluidic mixing: A review. Int. J. Mol. Sci. 2011, 12, 3263–3287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haeberle, S.; Zengerle, R. Microfluidic platforms for lab-on-a-chip applications. Lab Chip 2007, 7, 1094–1110. [Google Scholar] [CrossRef] [PubMed]
- Mark, D.; Haeberle, S.; Roth, G.; von Stetten, F.; Zengerle, R. Microfluidic lab-on-a-chip platforms: Requirements, characteristics and applications. Chem. Soc. Rev. 2010, 39, 1153–1182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Lin, Q.; Mukherjee, T. A model for laminar diffusion-based complex electrokinetic passive micromixers. Lab Chip 2005, 5, 877–887. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, H.; Shi, L.; Liu, Z.; Joo, S.W. An enhanced electroosmotic micromixer with an efficient asymmetric lateral structure. Micromachines 2016, 7, 218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayareh, M.; Ashani, M.N.; Usefian, A. Active and passive micromixers: A comprehensive review. Chem. Eng. Process.-Process Intensif. 2020, 147, 107771. [Google Scholar] [CrossRef]
- Zhou, T.; Xu, Y.; Liu, Z.; Joo, S.W. An enhanced one-layer passive microfluidic mixer with an optimized lateral structure with the dean effect. J. Fluids Eng. 2015, 137. [Google Scholar] [CrossRef]
- Hossain, S.; Kim, K.-Y. Mixing analysis in a three-dimensional serpentine split and recombine micromixer. Chem. Eng. Res. Des. 2015, 100, 95–103. [Google Scholar] [CrossRef]
- Sheu, T.S.; Chen, S.J.; Chen, J.J. Mixing of a split and recombine micromixer with tapered curved microchannels. Chem. Eng. Sci. 2012, 71, 321–332. [Google Scholar] [CrossRef]
- Chew, Y.T.; Xia, H.M.; Shu, C.; Wan, S.Y.M. Techniques to enhance fluid micro-mixing and chaotic micromixers. Mod. Phys. Lett. B 2005, 19, 1567–1570. [Google Scholar] [CrossRef]
- Wang, L.; Liu, D.; Wang, X.; Han, X. Mixing enhancement of novel passive microfluidic mixers with cylindrical grooves. Chem. Eng. Sci. 2012, 81, 157–163. [Google Scholar] [CrossRef]
- Liu, A.L.; He, F.Y.; Wang, K.; Zhou, T.; Lu, Y.; Xia, X.H. Rapid method for design and fabrication of passive micromixers in microfluidic devices using a direct-printing process. Lab Chip 2005, 5, 974–978. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, J.; Kwon, S. A novel micro-mixer with a quasi-active rotor: Fabrication and design improvement. J. Micromech. Microeng. 2009, 19, 105028. [Google Scholar] [CrossRef]
- Ahmed, D.; Mao, X.; Shi, J.; Juluri, B.K.; Huang, T.J. A millisecond micromixer via single-bubble-based acoustic streaming. Lab Chip 2009, 9, 2738–2741. [Google Scholar] [CrossRef]
- Chen, H.; Chen, C.; Bai, S.; Gao, Y.; Metcalfe, G.; Cheng, W.; Zhu, Y. Multiplexed detection of cancer biomarkers using a microfluidic platform integrating single bead trapping and acoustic mixing techniques. Nanoscale 2018, 10, 20196–20206. [Google Scholar] [CrossRef]
- Cartier, C.A.; Drews, A.M.; Bishop, K.J. Microfluidic mixing of nonpolar liquids by contact charge electrophoresis. Lab Chip 2014, 14, 4230–4236. [Google Scholar] [CrossRef]
- Harnett, C.K.; Templeton, J.; Dunphy-Guzman, K.A.; Senousy, Y.M.; Kanouff, M.P. Model based design of a microfluidic mixer driven by induced charge electroosmosis. Lab Chip 2008, 8, 565–572. [Google Scholar] [CrossRef]
- Wu, Y.; Ren, Y.; Tao, Y.; Hou, L.; Hu, Q.; Jiang, H. A novel micromixer based on the alternating current-flow field effect transistor. Lab Chip 2016, 17, 186–197. [Google Scholar] [CrossRef]
- Yap, L.W.; Chen, H.; Gao, Y.; Petkovic, K.; Liang, Y.; Si, K.J.; Wang, H.; Tang, Z.; Zhu, Y.; Cheng, W. Bifunctional plasmonic-magnetic particles for an enhanced microfluidic sers immunoassay. Nanoscale 2017, 9, 7822–7829. [Google Scholar] [CrossRef]
- Wen, C.Y.; Liang, K.P.; Chen, H.; Fu, L.M. Numerical analysis of a rapid magnetic microfluidic mixer. Electrophoresis 2011, 32, 3268–3276. [Google Scholar] [CrossRef]
- Park, S.; Chuang, H.-S.; Kwon, J.-S. Numerical study and taguchi optimization of fluid mixing by a microheater-modulated alternating current electrothermal flow in a y-shape microchannel. Sens. Actuators B Chem. 2021, 329, 129242. [Google Scholar] [CrossRef]
- Khakpour, A.; Ramiar, A. Numerical investigation of the effect of electrode arrangement and geometry on electrothermal fluid flow pumping and mixing in microchannel. Chem. Eng. Process.-Process Intensif. 2020, 150, 107864. [Google Scholar] [CrossRef]
- Kunti, G.; Bhattacharya, A.; Chakraborty, S. Analysis of micromixing of non-newtonian fluids driven by alternating current electrothermal flow. J. Non-Newton. Fluid Mech. 2017, 247, 123–131. [Google Scholar] [CrossRef]
- Ng, W.Y.; Goh, S.; Lam, Y.C.; Yang, C.; Rodriguez, I. Dc-biased ac-electroosmotic and ac-electrothermal flow mixing in microchannels. Lab Chip 2009, 9, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Kim, S.J. Pulsatile micromixing using water-head-driven microfluidic oscillators. Chem. Eng. J. 2017, 313, 1364–1369. [Google Scholar] [CrossRef]
- Rashidi, S.; Bafekr, H.; Valipour, M.S.; Esfahani, J.A. A review on the application, simulation, and experiment of the electrokinetic mixers. Chem. Eng. Process.-Process Intensif. 2018, 126, 108–122. [Google Scholar] [CrossRef]
- Zambrano, H.A.; Vasquez, N.; Wagemann, E. Wall embedded electrodes to modify electroosmotic flow in silica nanoslits. Phys. Chem. Chem. Phys. 2016, 18, 1202–1211. [Google Scholar] [CrossRef]
- Song, H.; Cai, Z.; Noh, H.M.; Bennett, D.J. Chaotic mixing in microchannels via low frequency switching transverse electroosmotic flow generated on integrated microelectrodes. Lab Chip 2010, 10, 734–740. [Google Scholar] [CrossRef]
- Bag, N.; Bhattacharyya, S. Electroosmotic flow of a non-newtonian fluid in a microchannel with heterogeneous surface potential. J. Non-Newton. Fluid Mech. 2018, 259, 48–60. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Bera, S. Combined electroosmosis-pressure driven flow and mixing in a microchannel with surface heterogeneity. Appl. Math. Model. 2015, 39, 4337–4350. [Google Scholar] [CrossRef]
- Kateb, M.; Kolahdouz, M.; Fathipour, M. Modulation of heterogeneous surface charge and flow pattern in electrically gated converging-diverging nanochannel. Int. Commun. Heat Mass Transf. 2018, 91, 103–108. [Google Scholar] [CrossRef] [Green Version]
- Nayak, A.K. Analysis of mixing for electroosmotic flow in micro/nano channels with heterogeneous surface potential. Int. J. Heat Mass Transf. 2014, 75, 135–144. [Google Scholar] [CrossRef]
- Mahapatra, B.; Bandopadhyay, A. Electroosmosis of a viscoelastic fluid over non-uniformly charged surfaces: Effect of fluid relaxation and retardation time. Phys. Fluids 2020, 32, 032005. [Google Scholar] [CrossRef]
- Ahmed, F.; Kim, K.Y. Parametric study of an electroosmotic micromixer with heterogeneous charged surface patches. Micromachines 2017, 8, 199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, H.; Park, J.K. Optoelectrofluidic platforms for chemistry and biology. Lab Chip 2011, 11, 33–47. [Google Scholar] [CrossRef]
- Baigl, D. Photo-actuation of liquids for light-driven microfluidics: State of the art and perspectives. Lab Chip 2012, 12, 3637–3653. [Google Scholar] [CrossRef]
- Han, D.; Park, J.K. Optoelectrofluidic enhanced immunoreaction based on optically-induced dynamic ac electroosmosis. Lab Chip 2016, 16, 1189–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pei-Yu, C.; Ohta, A.T.; Jamshidi, A.; Hsin-Yi, H.; Wu, M.C. Light-actuated ac electroosmosis for nanoparticle manipulation. J. Microelectromech. Syst. 2008, 17, 525–531. [Google Scholar] [CrossRef]
- Chiou, P.Y.; Ohta, A.T.; Wu, M.C. Massively parallel manipulation of single cells and microparticles using optical images. Nature 2005, 436, 370–372. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.H.; Zhong, X.T.; Liu, B.; Shi, L.Y.; Zhou, T.; Zhu, Y.G. Mixing mechanism of a straight channel micromixer based on light-actuated oscillating electroosmosis in low-frequency sinusoidal ac electric field. Microfluid. Nanofluidics 2021, 25, 1–15. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, S.; Wang, Q. Simulation and analysis of particle trajectory caused by the optical-induced dielectrophoresis force. Microfluid. Nanofluidics 2013, 16, 533–540. [Google Scholar] [CrossRef]
- Zhu, X.; Yin, Z.; Ni, Z. Dynamics simulation of positioning and assembling multi-microparticles utilizing optoelectronic tweezers. Microfluid. Nanofluidics 2011, 12, 529–544. [Google Scholar] [CrossRef]
- Fu, H.; Liu, X.; Li, S. Mixing indexes considering the combination of mean and dispersion information from intensity images for the performance estimation of micromixing. RSC Adv. 2017, 7, 10906–10914. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, L.; Ding, H.; Zhong, X.; Yin, B.; Liu, Z.; Zhou, T. Mixing Mechanism of Microfluidic Mixer with Staggered Virtual Electrode Based on Light-Actuated AC Electroosmosis. Micromachines 2021, 12, 744. https://doi.org/10.3390/mi12070744
Shi L, Ding H, Zhong X, Yin B, Liu Z, Zhou T. Mixing Mechanism of Microfluidic Mixer with Staggered Virtual Electrode Based on Light-Actuated AC Electroosmosis. Micromachines. 2021; 12(7):744. https://doi.org/10.3390/mi12070744
Chicago/Turabian StyleShi, Liuyong, Hanghang Ding, Xiangtao Zhong, Binfeng Yin, Zhenyu Liu, and Teng Zhou. 2021. "Mixing Mechanism of Microfluidic Mixer with Staggered Virtual Electrode Based on Light-Actuated AC Electroosmosis" Micromachines 12, no. 7: 744. https://doi.org/10.3390/mi12070744
APA StyleShi, L., Ding, H., Zhong, X., Yin, B., Liu, Z., & Zhou, T. (2021). Mixing Mechanism of Microfluidic Mixer with Staggered Virtual Electrode Based on Light-Actuated AC Electroosmosis. Micromachines, 12(7), 744. https://doi.org/10.3390/mi12070744







