3.1. PCM Fabrication
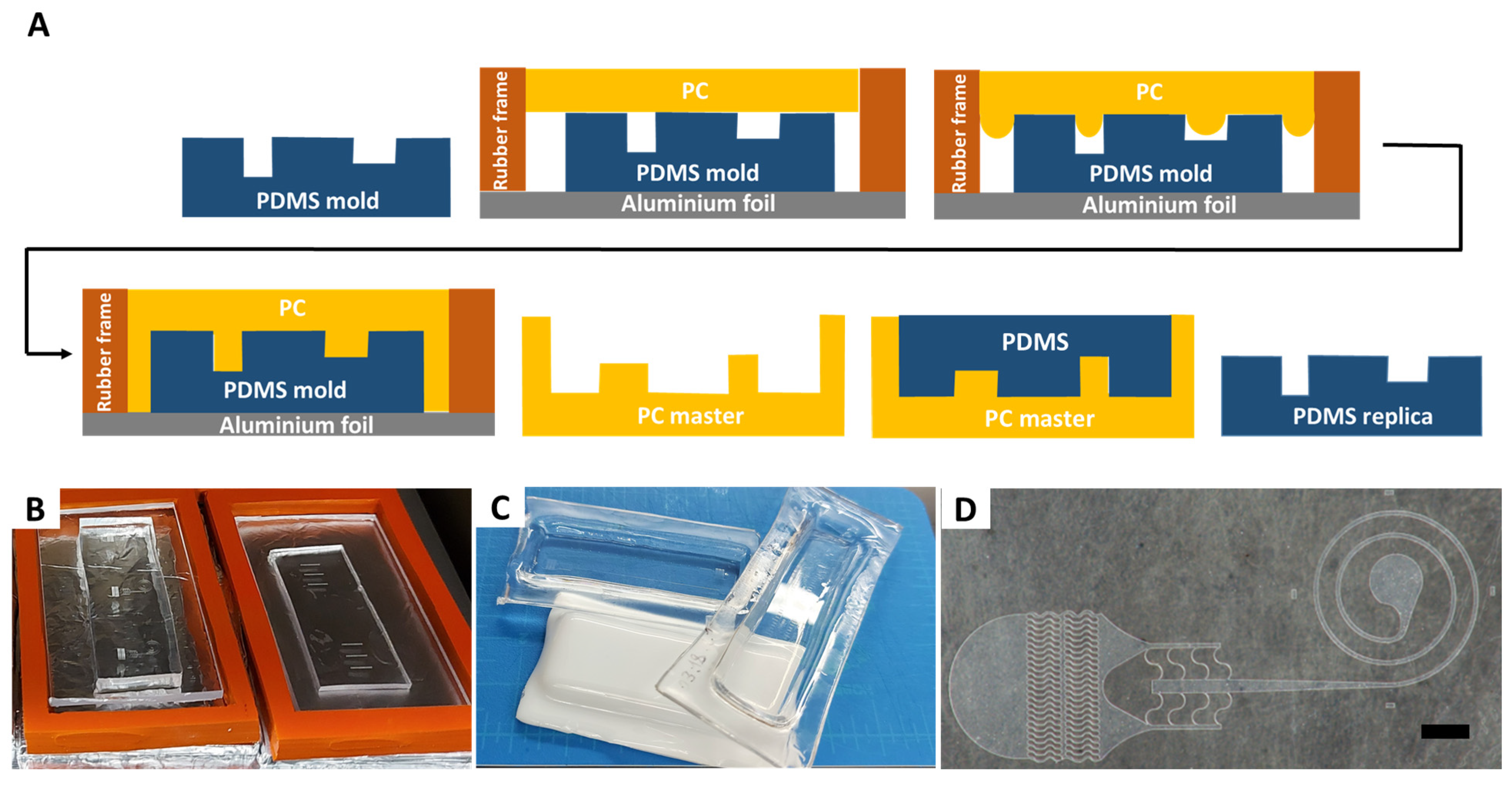
The PCM fabrication process starts with making a PDMS replica of the original master. The process is schematically illustrated in
Figure 1A. The approach yields a monolithic master, directly addressing the delamination and poor structural integrity of SPMs. Herein, we used SPMs fabricated by standard photolithographic techniques or by dry photoresist, as we reported earlier. However, instead of using SPM to generate device replicas in PDMS, as in a standard soft lithography process, we used the SPM to cast PDMS once, and then used it to fabricate a PCM that clones the original SPM. Alternatively, 3D printed or micromilled masters can be used to begin the process. Ultimately, multiple PCMs can be generated, allowing for cost-effective scale up of masters and devices.
A rectangular rubber gasket was placed around the PDMS replica to form perimeter of the mold (
Figure 1B). Gaskets of custom size and thickness can be cut from a sheet of rubber and reused in multiple processes. A piece of a cut PC sheet was then placed on top of the PDMS replica and heated in the vacuum oven. The baking time depended on the aspect ratio and on the minimum spacing of the mold micro features and ranged from a minimum of 2 h for low aspect ratios and spaced features, to 4 h for high aspect ratio and dense features. It is preferable to conduct the baking process in a well-ventilated room since PC fumes can build inside the oven and release when opening the oven door.
PCM fabrication can be used to generate masters of varying thickness and area. We have created plastic masters of a single device the size of a standard microscope glass slide (
Figure 1C) as well as masters encompassing a larger 3 in wafer area. For microfluidic applications, the form factor of a microscope glass slide (1 in × 3 in) is the most useful due to the sealing process of PDMS replicas subsequently formed using these masters. Both large area and small area topology can be replicated in a single master (
Figure 1D), giving the approach added flexibility. Furthermore, the fabricated PCMs have Rockwell R hardness of 118 and tensile strength >8900 psi, making them resistant to abrasion and fracture and sufficiently robust for daily handling in a laboratory [
15]. In addition, PCMs exhibit good chemical resistance against diluted acids, aliphatic hydrocarbons, and alcohols, but are attacked by diluted alkalis and aromatic and halogenated hydrocarbons [
16]. PCMs are also thermally stable up to 135 °C, making them compatible with the wide range of curing temperatures of the PDMS soft lithography process.
PCMs can be fabricated in a variety of colors, from natural (transparent) to black, stemming from polycarbonate sheets being available in a wide range of shades. In its natural form, PC is an extremely clear plastic that can transmit over 90% of light [
17], yielding PCMs that are as clear as glass and are convenient for observation of the PDMS patters during casting. The use of color (e.g., white PCM in
Figure 1C) can be advantageous for color-coding and simplicity of attributing masters to a particular project or to lab personnel. However, it must be noted that pigment additives can impact the mechanical and rheological properties of PC [
18,
19]. Indeed, recent work suggests that addition of ~5% of pigment (by volume) can modify the melt flow index (MFI)—a measure of the ease of flow of a thermoplastic polymer—by as much as 16% [
19]. Such changes in rheology would necessitate process optimization for each color. Most of the work herein was carried out using the natural PC sheets, as the transparent nature of the material also permitted better observation of the process.
The drying phase of the fabrication protocol proved to be one of the most critical steps since both PC [
20] and PDMS [
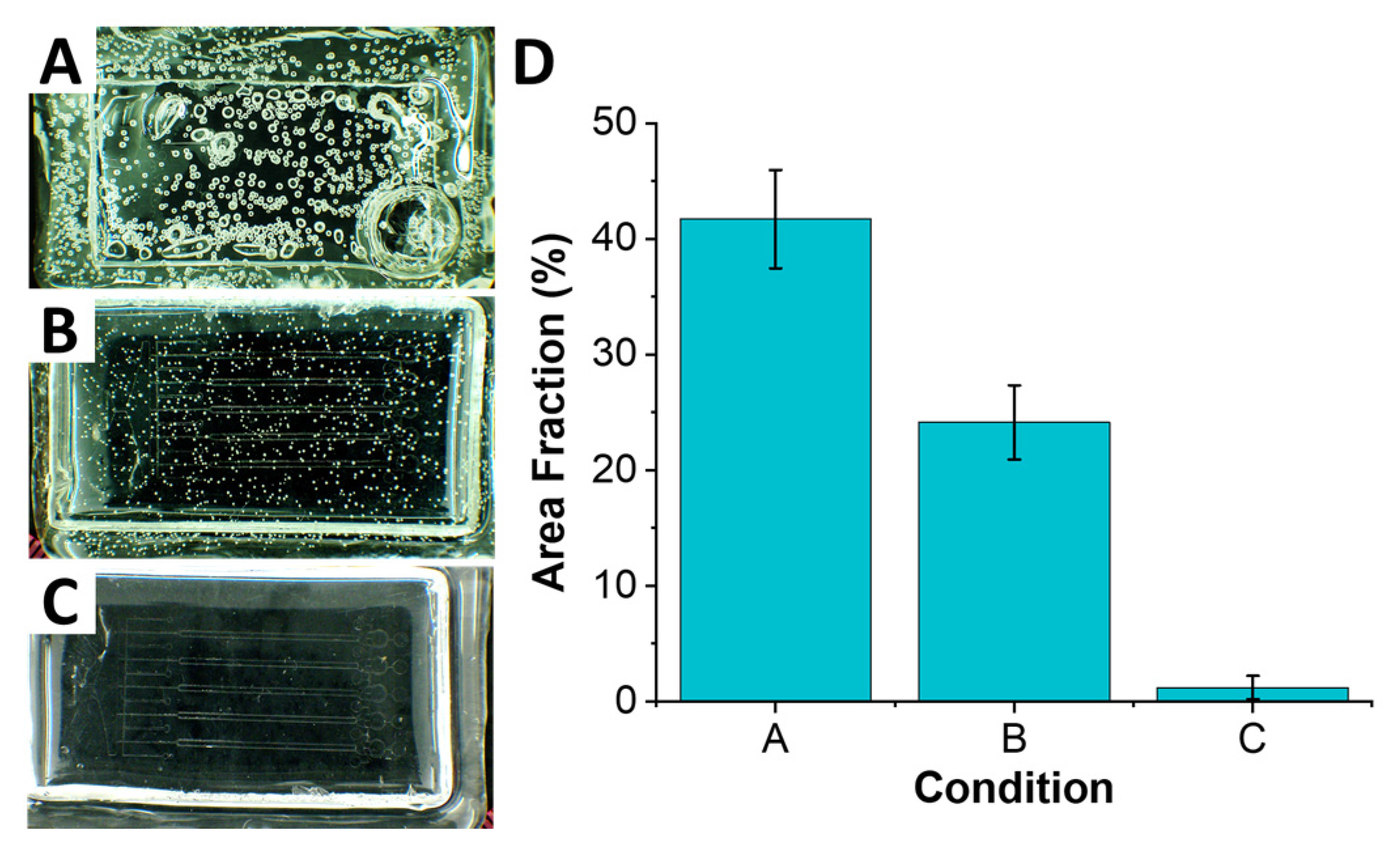
21] are hygroscopic and can absorb moisture from air. It is this absorbed moisture that is thought to be the cause of formation of air bubbles throughout the sheet when heated above 125 °C (
Figure 2A). These air bubbles can affect the correct replication of microfeatures, as it is well-known in hot embossing and soft lithography [
14,
22]. Thus, both PC sheets and PDMS molds were dried below Tg prior to the molding process. The drying step was performed for 2 h in a vacuum oven at 125 °C; a vacuum at ~49 mm Hg was used to aid the process. Sonmez et al. [
12] reported drying PDMS molds at 60 °C for 24 h; this substantially longer drying time was needed due to using base to curing agent ratio of 5:1 to fabricate PDMS molds. The higher fraction of curing agent yields a stiffer PDMS material, which is desirable for a molding process especially of higher aspect ratio structure. However, this also makes PDMS less gas permeable, making the drying process extremely long and not always effective [
23]. We found that higher gas permeability of the conventional 10:1 PDMS mixture allowed for a much more rapid drying process, while retaining ability to replicate high aspect ratio features (as we discuss in the next section).
In addition to the drying phase, we found that thermal stress plays a role in formation of air bubbles throughout the polymer. The thermal stress arises due to rapid change in temperature. In our case, placing PC sheet into oven preheated to 220 °C baking temperature yielded a significant and rapid change from room temperature, which resulted in formation of numerous air bubbles. One of the reasons for this, as discussed above, is the rapid vaporization of moisture trapped within the polymer, stemming from its hygroscopic nature. As water vapor expands and escapes from polymer above its glass temperature, it forms small bubble cavities in the compliant polymer. We found that starting the process below Tg, at 125 °C, and ramping it up to the 220 °C baking temperature (at ~5 °C/min) allowed vapor to escape with significantly fewer (nearly 2× fold) distortions to the polymer (
Figure 2B). Coupling the temperature ramping with the drying step yielded the best results (
Figure 2C) with nearly no bubbles forming.
The baking process was performed in the same vacuum oven as in the drying phase, without removing components. The baking temperature was set at 220 °C, selected to exceed the Tg of PC (~150 °C) but remain below the thermal degradation temperature of PDMS (~280 °C). The baking time was found to depend on the density and aspect ratio of the microfeatures, as it was desirable for the PC melt to fill the PDMS mold features. For the widely spaced and low aspect ratio features (AR < 1.5), a 2 h bake time yielded accurate replication. For higher aspect ratio or dense structures, a longer 4 h baking time was necessary.
After baking, the molded PCM was allowed to cool to room temperature for 30 min (~6.5 °C/min) and was separated from PDMS mold. Sonmez et al. [
12] coated PDMS molds prior to the drying and baking steps with tridecafluoro-1,1,2,2-tetrahydrooctyl-1-trichlorosilane (TFOCS) to lower surface energy and improve mold release. However, we found that a mold release coating was not necessary as PDMS molds and PCMs detached effortlessly. It is possible that higher pattern density or higher aspect ratio microfeatures than those testing in this work may necessitate use of a release agent. In such cases, Sigmacoat silanization agent can be used. Sigmacoat is a solution of a chlorinated organopolysiloxane in heptane that reacts with surface silanol (Si–OH) groups to produce a hydrophobic film and is commonly used to aid mold release is soft lithography. The coating process is rapid, only requiring a few minutes inside a fume hood, and is thus much faster than the 2 h coating process reported by Sonmez et al. [
12].
The formed PCMs can now be used to cast PDMS using the standard soft lithography process. We have used PCMs to generate PDMS replicas at least 20 times without any visible degradation. To ensure a dust-free surface, PCMs can be cleaned with IPA and dried using an air gun before each PDMS casting, due to the excellent chemical resistance of PC to IPA. When demolding the cured PDMS replicas from the PCM, it is best to avoid touching the patterned areas to minimize chance of scratches. Finally, we also found that PDMS molds themselves can be used multiple times to fabricate multiple PCM copies.
3.2. Cross-Sectional Characterization
Masters with different aspect ratios were fabricated to assess performance range.
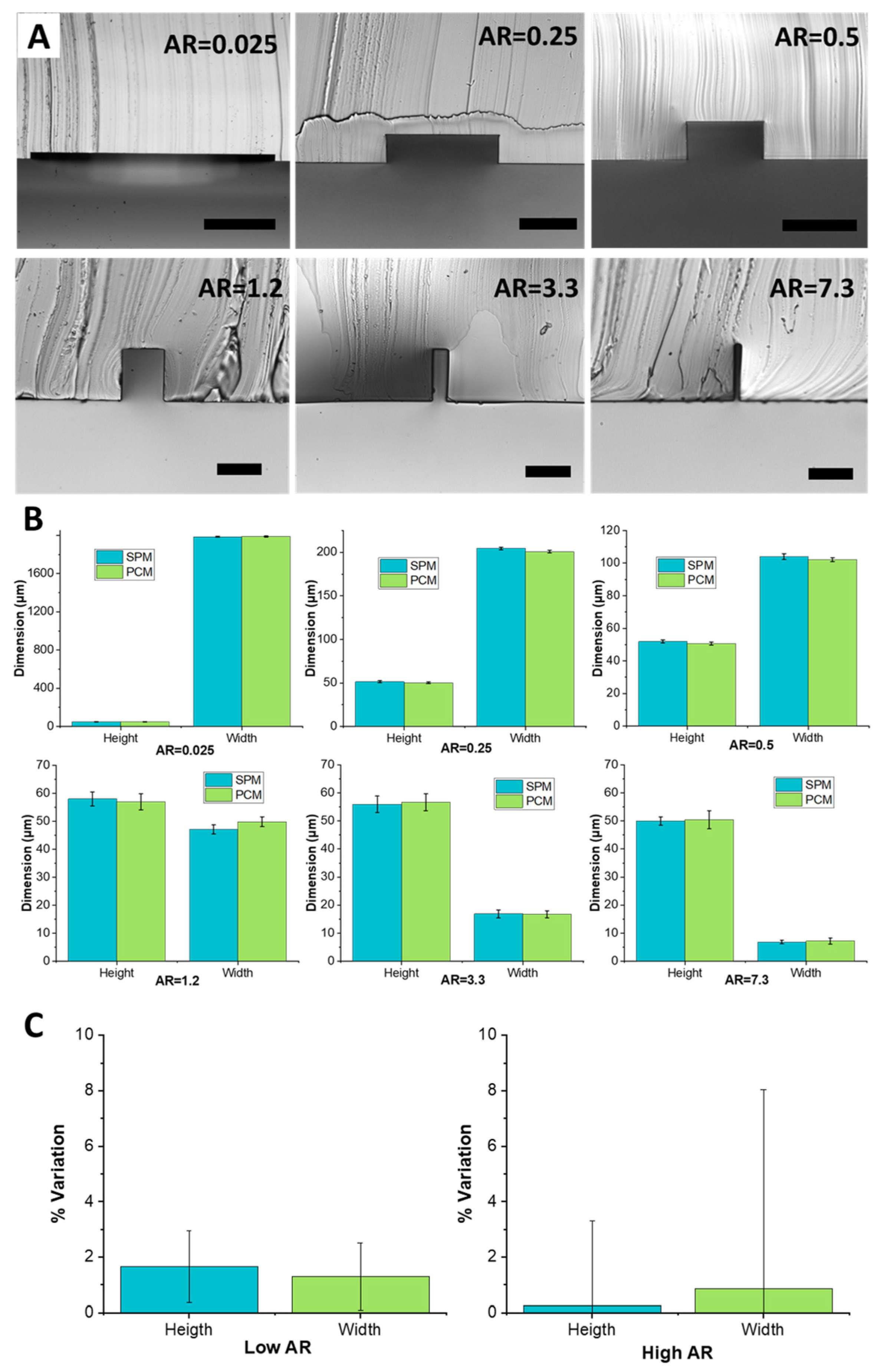
Figure 3A shows cross-sections of different aspect ratio channels, illustrating precise rectangular shape and perpendicular walls. The channels ranged from a 2 mm wide low aspect ratio channel to a 7 µm high aspect ratio channel. Specifically, the included channels were as follows: 2000 µm × 50 µm (AR = 0.025), 200 µm × 50 µm (AR = 0.25), 100 µm × 50 µm (AR = 0.5), 47 µm × 58 µm (AR = 1.2), 17 µm × 56 µm (AR = 3.3), and 7 µm × 50 µm (AR = 7.3). The images were obtained by forming PDMS replica of the PCM and then slicing through it to image its cross-section. Across all aspect ratios, geometries were faithfully replicated. The slender structures, at both low aspect ratio (AR = 0.025) and high aspect ratio (AR = 7.3) were fabricated without any distortion or deformation during the heat molding process.
Measurements from PDMS replicas show that the completed channels have dimensions within ±2 μm of the dimensions of the original PDMS mold (
Figure 3B). For low aspect ratios (0.025 to 0.5) variation of height and width stayed within 1–2% of the original SPM dimension. For AR = 1.2, a slight decrease in the height and a slight increase of width of a few microns was observed. This variation stays within the 2% of the original dimensions. For AR = 3.3 the channel height saw an increase of 1 µm while the width remained constant. For AR = 7.3, the channel height and width variations were limited to 1 µm. The high closeness of channel dimensions casted from the SPM master and from the PC master confirms the high fidelity of the replication abilities of the process and the absence of any detectable shrinkage of PC features during cool down. The standard deviation of the data was low, although increasing with aspect ratio, suggesting low variability and thus high quality of replication. In addition, channels replicated from PCMs were constantly slightly smaller (~1–2%) in width and height than the original SPMs. Additionally, the PDMS surface around the bottom of the channel was smooth, suggesting that quality of the subsequent bonding step necessary to seal microfluidic channels should be unaffected by the PCM process.
Downstream channel uniformity of the PCM is another important factor that can impact microfluidic device performance and is an indicator of replication quality. The uniformity was measured by slicing the 4 cm long PDMS channel replicas at 5 mm increments.
Figure 3C reports variation in channel height and width in the downstream direction. For low aspect ratio channels (AR < 1), the downstream variability in channel dimensions was <2%. For high aspect ratio channels (AR > 1), dimensional variability was even less, <1% on average, although variations from channel to channel were more significant as indicated by the error bars. Ultimately, the quality of replication was high.
3.3. Replication of Various Channel Geometries
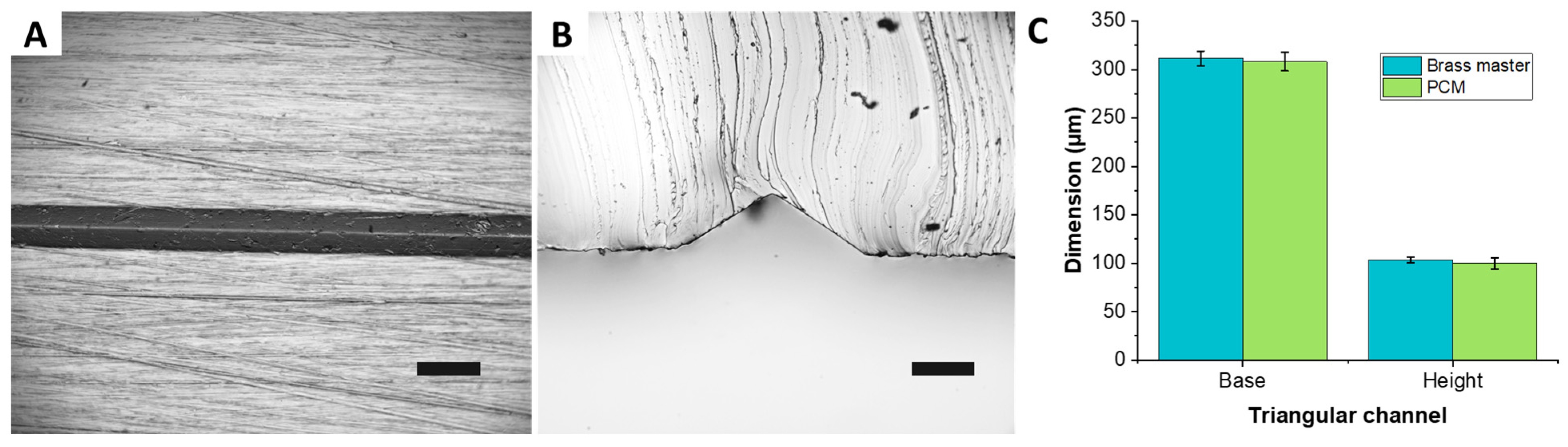
To assess the ability of the PCM approach to reproduce non-rectangular channel geometries, an 8 cm × 5 cm micromilled brass plate was used to cast a PDMS mold of a microchannel with a triangular cross-section. The surface was patterned using a conic engraving tool to realize 4 parallel straight channels having different depths. Here, the smallest channel (depth of ~100 µm, base of ~300 µm, and apex angle of ~113°) was considered to assess the replication capabilities of the PCM heat molding process. Microscope images of PDMS cast from the PCM shows a well-defined and straight channel having cross section with sharp edges (
Figure 4). Quantitative comparisons of the channel height and base show very low dimensional variations of four microns only (
Figure 4C). Directly heat molding PC on the brass master turned out to be impractical due to inability of the metal mold to absorb air that fills the microfeature, yielding pockets of air trapped in between the PC and the bottom of the triangular microchannel. These air pockets were detrimental to the channels cross-sectional geometry. Other limitation of using micromilled metal masters included the high surface roughness that could affect the bonding quality to the glass substrate during subsequent microfluidic experiments, and high thermal conductivity that impacted the polymer thermal cycle. Ultimately, forming PCM from PDMS replicas yielded channels that replicated the triangular cross-section with high accuracy.
To assess the PC replication capabilities of structures having large area, a microfluidic device with a low aspect ratio spiral channel was used. The spiral channel cross-section was 150 µm wide and 30 µm high (AR = 0.2). The spacing between the adjacent turns of the spiral was 350 µm, and the area covered by the spiral was 30 mm
2. The top view image of the PDMS replica cast from PCM shows well-defined and highly concentric spiral channels (
Figure 5A). The dimensional comparison of the PDMS replica of the PCM with the one cast from the original SPM shows that the spacing between adjacent turns variation stays within the 4% of the original dimensions (
Figure 5B).
To demonstrate replication of dense post arrays that PC could correctly copy, a number of test structures were fabricated. These included a microfluidic device having two arrays of microposts at 5 µm spacing, a matrix of diamond microposts that are 65 µm wide and high and at 8 µm spacing, and arrays of circular posts (100 µm diameter at 50 µm spacing, and 50µm diameter at 25 µm spacing). By analyzing the microscope images of the PDMS micropost arrays, it was confirmed that PC can flow in between features spaced by as little as 5 µm, replicating micropost arrays of different geometries and spacing correctly (
Figure 5C,E,G). Indeed, the averaged measurements show that the spacing between posts is replicated and varies within a reasonable experimental error of ~2–3 µm (
Figure 5D,F,H). While the post spacing of 5 µm corresponds to the smallest feature replicated here, it is not necessarily the resolution of the process. In this work we optimized the process in terms of the required time and instrumentation. Previous work [
12], however, demonstrated replication of ~1 µm features, which we believe is much closer to the resolution limit of our approach.
3.4. Fabrication of Rounded Features
Since during the baking process PC melt gradually flows into microfeatures to fill the mold shape, it may be possible to intentionally obtain rounded, partially-filled features by interrupting the process. One potential application of this is formation of microwells with rounded bottom for culture of cell spheroids or patient-derived organoids [
24]. Having wells with rounded U-shaped bottom rather than the traditional flat bottom ones has the advantage of promoting cell-to-cell adhesion and formation of spherical cell aggregates (spheroids). Previously, we had resorted to the use of 3D printing to generate U-shaped wells [
24], but were limited by printer resolution (~25 µm) and stepped cross-sectional profile. Outside cell culture, rounded features can be used as micro lenses in optical applications [
25]. Consequently, we explored the possibility of making such features.
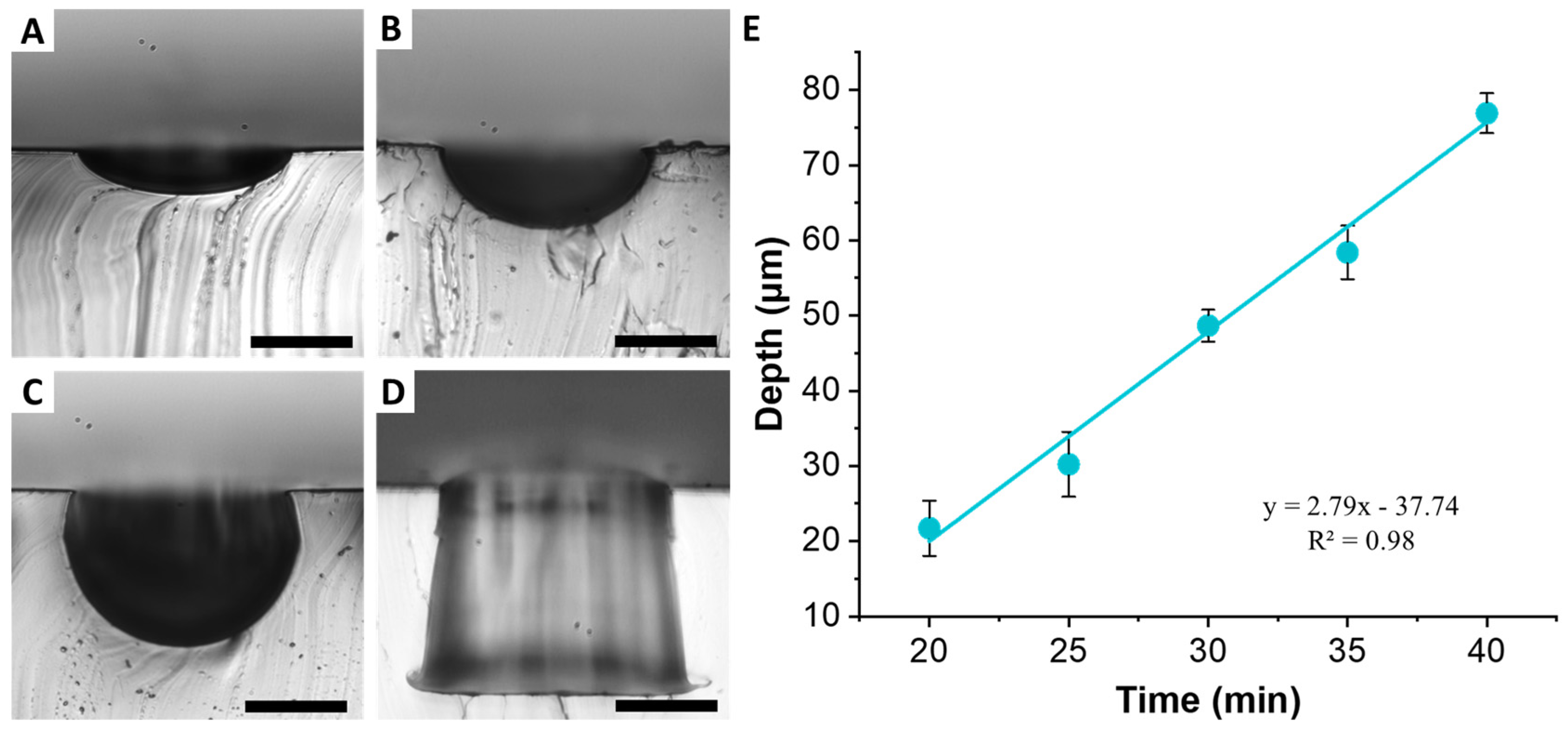
An array of cylindrical microwells 100 µm in diameter and 100 µm in depth was used to demonstrate formation of U-shaped structures. The baking time was the most important parameter to control, since it was necessary to stop the PC melt flow before the complete structure was replicated. Baking for 20 min allowed us to obtain shallow 21.7 ± 3.6 µm deep wells (
Figure 6A). The 100 µm diameter well structure was preserved at the top. However, the bottom of the structure was clearly rounded, indicating that PC melt just started to flow into the well structure. Increasing bake time of 30 min yielded depth of 48.6 ± 2.1 µm (
Figure 6B). Again, the top of the structure was well preserved, while the bottom exhibited a semicircular shape. Increasing time further to 40 min, resulted in depth of 76.9 ± 2.6 µm (
Figure 6C). The complete replication of the cylindrical well occurred around 60 min (
Figure 6D). Plotting the rounded well depth as a function of bake time reveals a linear relationship (
Figure 6E). Regardless of the baking time, the diameter of the wells remained constant, although increased slightly from the original PDMS mold to 105.8 ± 2.8 µm, which is most likely due to the slanted side walls of the original well. These results suggest that PCMs of wells of different depth can be obtained from a single PDMS mold by simply by tuning the baking time and without the need to re-fabricate SPMs with multiple thicknesses.
In addition to modifying the baking process time, the baking temperature was lowered to 190 °C to increase PC melt viscosity to improve uniformity and slow the flow of PC inside the features. The uniformity can also be improved by adding a weight on top of the PC sheet during the molding process to flatten the PDMS-PC contact area. Indeed, since the process must be stopped before PC entirely fills the wells, any slight difference in the initial relative position of PC and PDMS across the mold area can bring to differences in the wells’ depths of up to tens of microns. For this, an 8 cm × 5 cm brass plate with a mass of ~108 g was placed on top of a PC sheet at the start of the replication process. The added weight improved uniformity of wells considerably.