Flexohand: A Hybrid Exoskeleton-Based Novel Hand Rehabilitation Device
Abstract
:1. Introduction
- Isolated and combined digit motion of fingers: (index, middle, ring, small) and thumb;
- Isolated and combined digit joints flexion–extension (fingers: DIP, PIP, MCP; thumb: IP, MCP);
- The device should not restrict wrist motion;
- The device should accommodate natural motion during finger flexion–extension by compensating MCP abduction–adduction motion;
- Easy donning and doffing;
- Lower added weight burden to the user’s hand.
2. Anatomically Inspired Design
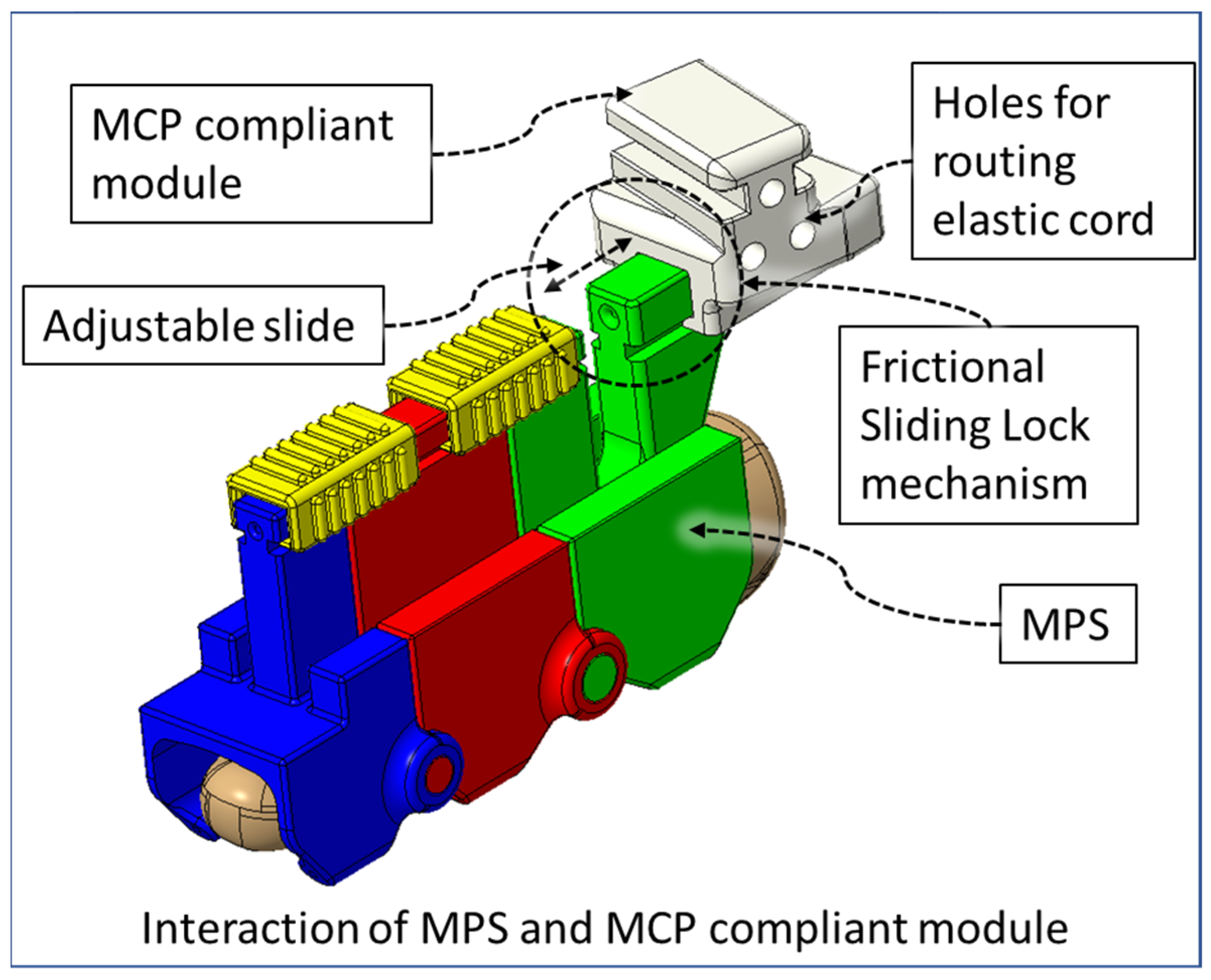
2.1. Compliant Mechanism
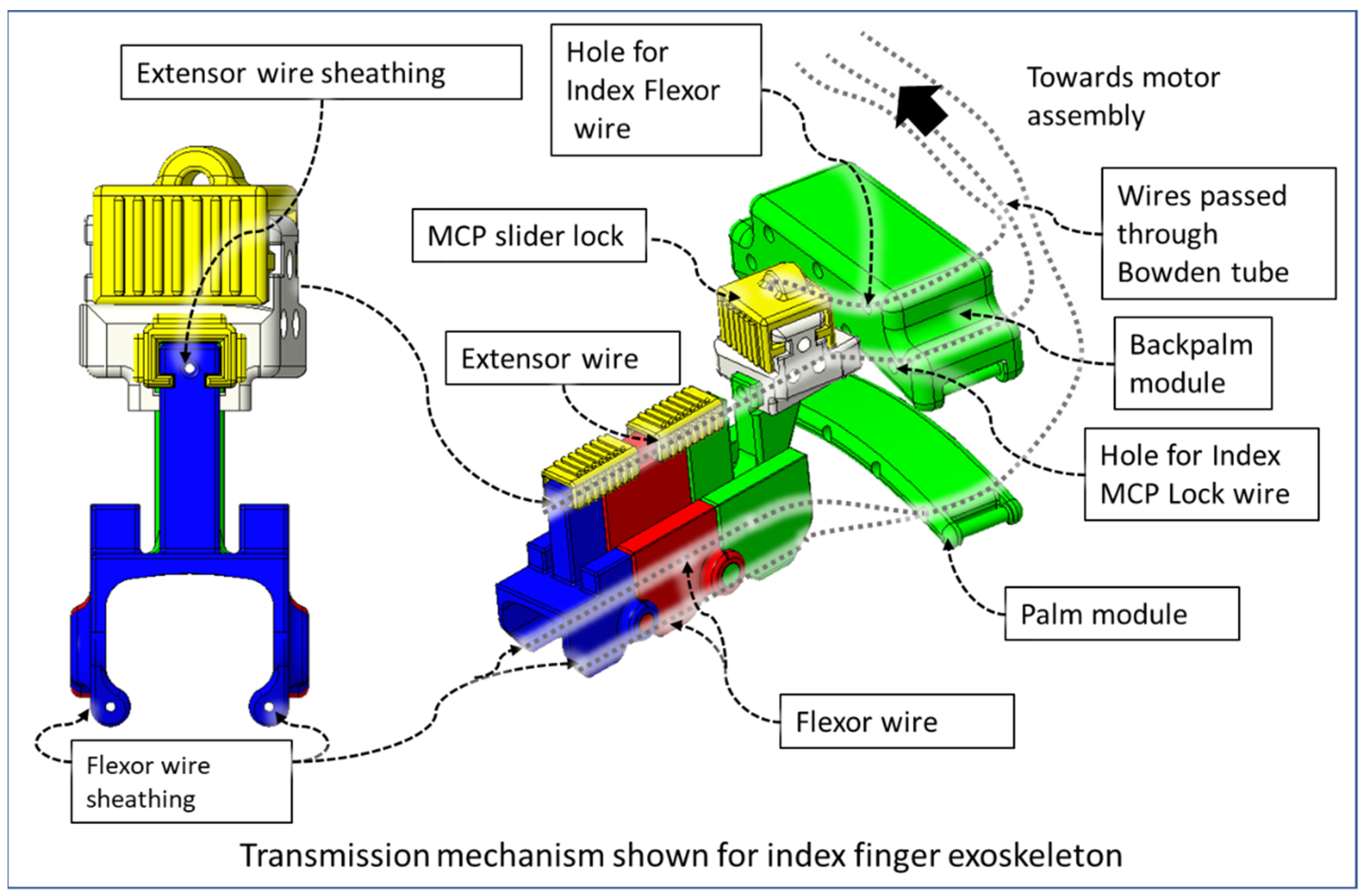
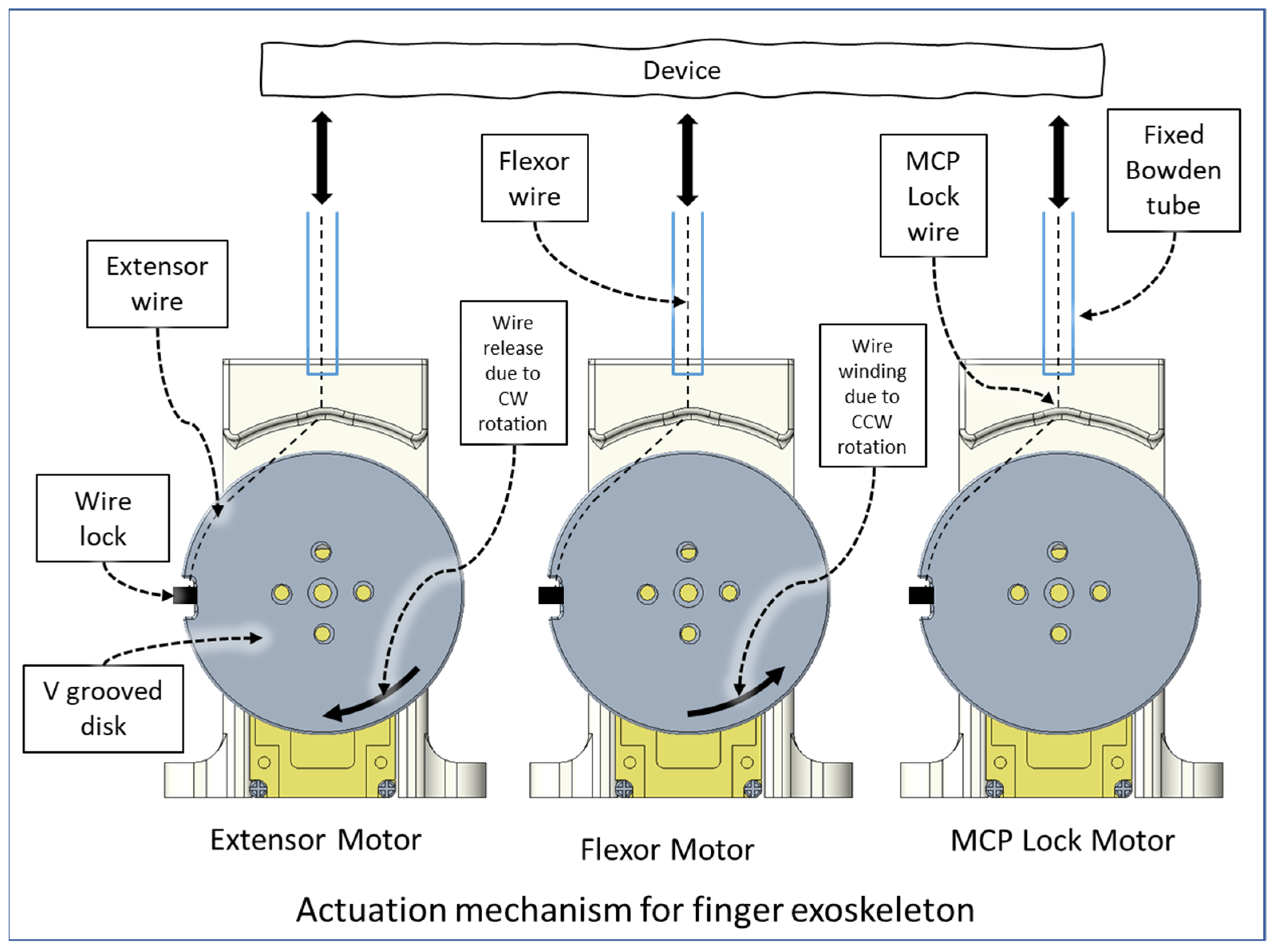
2.2. Transmission and Actuation Mechanism
2.3. Isolated Digit and Digital Joint Motion
2.4. Modelling of Structural Parts
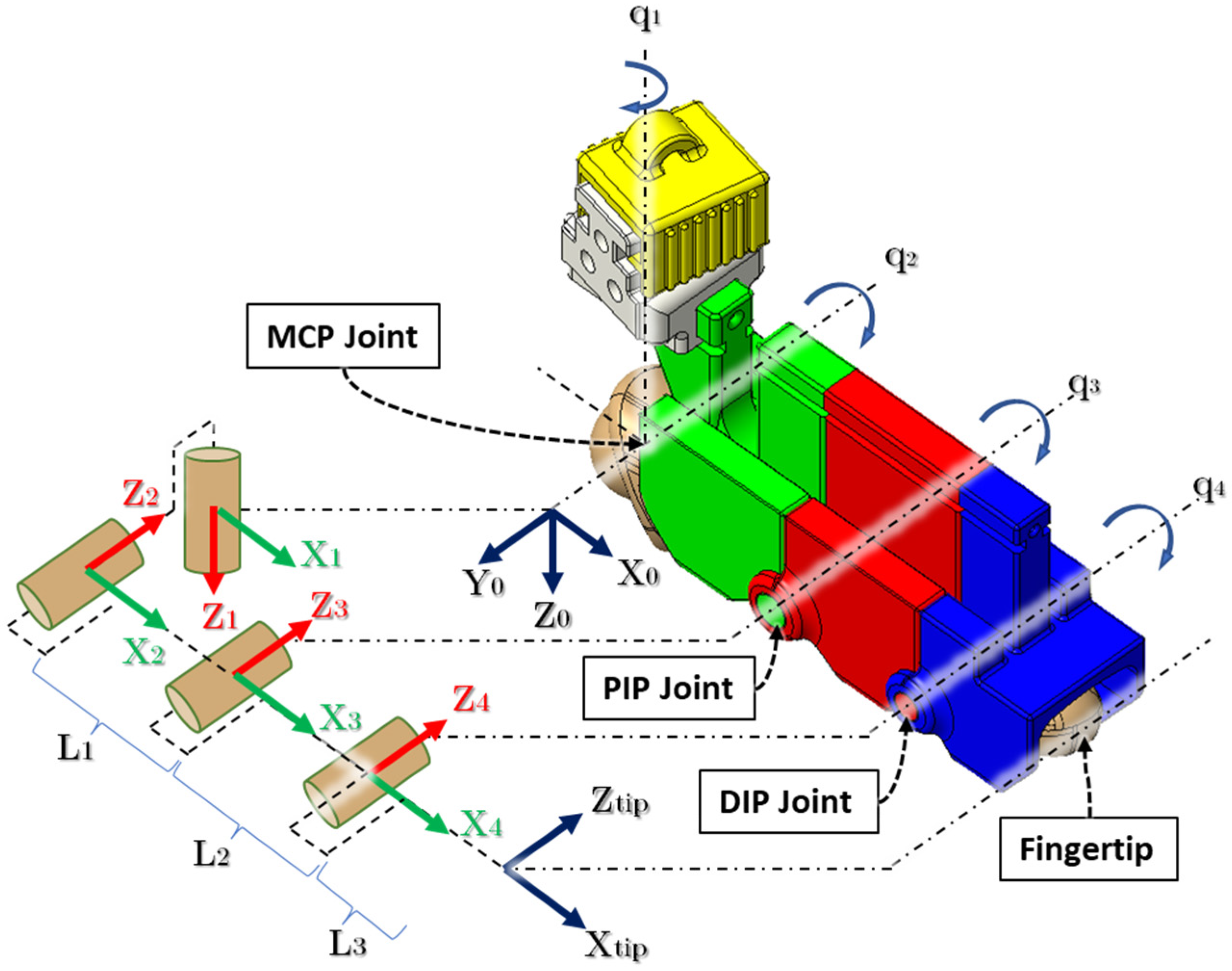
3. Kinematic Analysis
4. Donning and Doffing of the Device
5. Experimental Evaluation
5.1. Fabrication abd Experimental Setup
5.2. Actuation Calibration
5.3. Experiments with Flexohand
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
References
- Cooper, C. Fundamentals. In Fundamentals of Hand Therapy; Wietlisbach, C., Ed.; Mosby: St. Louis, MO, USA, 2014; pp. 1–14. [Google Scholar]
- Feehan, L.M.; Sheps, S.B. Incidence and Demographics of Hand Fractures in British Columbia, Canada: A Population-Based Study. J. Hand Surg. 2006, 31, 1068.e1–1068.e9. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.R.C. Examination of the Hand and Wrist. In The Journal of Hand Surgery: British & European Volume; Tubina, R., Thomine, J.M., Mackin, E., Eds.; Elsevier: Amsterdam, The Netherlands, 1996; Volume 21, pp. 289–290. [Google Scholar]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2020 Update: A Report from the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef] [PubMed]
- Warlow, C.P. Epidemiology of stroke. Lancet 1998, 352, S1–S4. [Google Scholar] [CrossRef]
- Lopez, A.D.; Mathers, C.D.; Ezzati, M.; Jamison, D.T.; Murray, C.J. Global and regional burden of disease and risk factors, 2001: Systematic analysis of population health data. Lancet 2006, 367, 1747–1757. [Google Scholar] [CrossRef]
- Caplan, L.R. Introduction and Perspective. In Caplan’s Stroke; Caplan, L.R., Ed.; W.B. Saunders: Philadelphia, PA, USA, 2009; pp. 3–21. [Google Scholar]
- Pak, S.; Patten, C. Strengthening to promote functional recovery poststroke: An evidence-based review. Top. Stroke Rehabil. 2008, 15, 177–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, M.H. NSF, I-Corps: Enhancing Regional Technology Commercialization, Powered Hand Rehab Glove, NSF Award# 1450586 (sub-awards), 02/14/18–02/13/2019.
- Brahmi, B.; El Bojairami, I.; Ahmed, T.; Rahman, M.H.; Swapnil, A.A.Z.; De Caro, J.S. New Adaptive Sliding Mode for Unperturbed Forearm and Wrist Rehabilitation Robot. In Proceedings of the 2021 18th International Multi-Conference on Systems, Signals & Devices (SSD), Monastir, Tunisia, 22–25 March 2021; pp. 1160–1165. [Google Scholar]
- Swapnil, A.A.Z. Intelligent Therapeutic Robot: Design, Development, and Control. Ph.D. Thesis, University of Wisconsin-Milwaukee, Milwaukee, WI, USA, December 2020. [Google Scholar]
- Brahmi, B.; Ahmed, T.; Elbojairami, I.; Swapnil, A.A.Z.; Assaduzzaman, M.; Schultz, K.; Mcgonigle, E.; Rahman, M.H. Flatness Based Control of a Novel Smart Exoskeleton Robot. IEEE/ASME Trans. Mechatron. 2021. [Google Scholar] [CrossRef]
- Islam, M.R.; Assad-Uz-Zaman, M.; Swapnil, A.A.Z.; Ahmed, T.; Rahman, M.H. An ergonomic shoulder for robot-aided rehabilitation with hybrid control. Microsyst. Technol. 2020, 27, 159–172. [Google Scholar] [CrossRef]
- Rahman, M.H.; Cristobal, O.L.; Saad, M.; Kenné, J.P.; Archambault, P.S. Development of a whole arm wearable robotic exoskeleton for rehabilitation and to assist upper limb movements. Robotica 2015, 33, 19–39. [Google Scholar] [CrossRef] [Green Version]
- Kiguchi, K.; Rahman, M.H.; Sasaki, M.; Teramoto, K. Development of a 3DOF mobile exoskeleton robot for human upper-limb motion assist. Robot. Auton. Syst. 2008, 56, 678–691. [Google Scholar] [CrossRef]
- Hays, P.L.; Rozental, T.D. Rehabilitative Strategies Following Hand Fractures. Hand Clin. 2013, 29, 585–600. [Google Scholar] [CrossRef] [PubMed]
- Islam, R.; Rahmani, M.; Rahman, M.H. A Novel Exoskeleton with Fractional Sliding Mode Control for Upper Limb Rehabilitation. Robotica 2020, 38, 2099–2120. [Google Scholar] [CrossRef]
- Brahmi, B.; Bojairami, I.E.; Ahmed, T.; Swapnil, A.A.Z.; AssadUzZaman, M.; Wang, I.; McGonigle, E.; Rahman, M.H. A Novel Modified Super-Twisting Control Augmented Feedback Linearization for Wearable Robotic Systems Using Time Delay Estimation. Micromachines 2021, 12, 597. [Google Scholar] [CrossRef] [PubMed]
- Nef, T.; Guidali, M.; Riener, R. ARMin III—Arm therapy exoskeleton with an ergonomic shoulder actuation. Appl. Bionics Biomech. 2009, 6, 127–142. [Google Scholar] [CrossRef] [Green Version]
- Schabowsky, C.N.; Godfrey, S.B.; Holley, R.J.; Lum, P.S. Development and pilot testing of HEXORR: Hand EXOskeleton Rehabilitation Robot. J. Neuroeng. Rehabil. 2010, 7, 36. [Google Scholar] [CrossRef] [Green Version]
- Yoo, D.H.; Kim, S.Y. Effects of upper limb robot-assisted therapy in the rehabilitation of stroke patients. J. Phys. Ther. Sci. 2015, 27, 677–679. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.H.; Saad, M.; Kenné, J.P.; Archambault, P.S.; Ouimet, T.K. Development of a 4DoFs exoskeleton robot for passive arm movement assistance. Int. J. Mechatron. Autom. 2012, 2, 34–50. [Google Scholar] [CrossRef]
- Spiewak, C.; Islam, M.R.; Rahaman, M.A.; Rahman, M.H.; Smith, R.; Saad, M. Modeling and Control of a 4DoF Robotic Assistive Device for Hand Rehabilitation. Int. J. Mech. Mechatron. 2016, 10, 1414–1418. [Google Scholar] [CrossRef]
- Assad-Uz-Zaman, M.; Islam, M.R.; Rahman, M.H.; Wang, Y.-C.; McGonigle, E. Kinect Controlled NAO Robot for Telerehabilitation. J. Intell. Syst. 2021, 30, 224–239. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, S.; Sun, Q. Development and Assist-As-Needed Control of an End-Effector Upper Limb Rehabilitation Robot. Appl. Sci. 2020, 10, 6684. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Ji, L.; Bi, S.; Zhang, X.; Huo, J.; Ji, R. Development and Implementation of an End-Effector Upper Limb Rehabilitation Robot for Hemiplegic Patients with Line and Circle Tracking Training. J. Healthc. Eng. 2017, 2017, 4931217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- BIONIK Laboratories Corp. InMotion ARM® Robotic Rehabilitation Therapy for Stroke, SCI and More. 2021. Available online: https://www.bioniklabs.com/products/inmotion-arm (accessed on 21 July 2021).
- Ahmed, T.; Swapnil, A.A.Z.; Islam, R.; Wang, I.; Rahman, M. An Exoskeleton Based Robotic Device for Providing Rehabilitative Therapies to Human Forearm and Wrist Joints (UWM-FWRR). Arch. Phys. Med. Rehabil. 2020, 101, e56. [Google Scholar] [CrossRef]
- Brahmi, B.; Saad, M.; Lam, J.T.A.T.; Luna, C.O.; Archambault, P.S.; Rahman, M.H. Adaptive control of a 7-DOF exoskeleton robot with uncertainties on kinematics and dynamics. Eur. J. Control. 2018, 42, 77–87. [Google Scholar] [CrossRef]
- Rahman, M.H.; Kiguchi, K.; Rahman, M.M.; Sasaki, M. Robotic Exoskeleton for Rehabilitation and Motion Assist. In Proceedings of the First International Conference on Industrial and Information Systems, Tirtayasa, Indonesia, 8–11 August 2006. [Google Scholar]
- Zimmermann, Y.; Forino, A.; Riener, R.; Hutter, M. ANYexo: A versatile and dynamic upper-limb rehabilitation robot. IEEE Robot. Autom. Lett. 2019, 4, 3649–3656. [Google Scholar] [CrossRef] [Green Version]
- Lambercy, O.; Dovat, L.; Gassert, R.; Burdet, E.; Teo, C.L.; Milner, T. A Haptic Knob for Rehabilitation of Hand Function. IEEE Trans. Neural Syst. Rehabil. Eng. 2007, 15, 356–366. [Google Scholar] [CrossRef]
- Bouzit, M.; Burdea, G.; Popescu, G.; Boian, R. The Rutgers Master II-new design force-feedback glove. IEEE/ASME Trans. Mechatron. 2002, 7, 256–263. [Google Scholar] [CrossRef] [Green Version]
- Almusawi, H.; Husi, G. Design and Development of Continuous Passive Motion (CPM) for Fingers and Wrist Grounded-Exoskeleton Rehabilitation System. Appl. Sci. 2021, 11, 815. [Google Scholar] [CrossRef]
- Waveflex CPM | Remington Medical. 2021. Available online: https://www.remingtonmedical.com/product/waveflex-cpm/ (accessed on 30 July 2021).
- Maestra Kinetec USA. 2021. Available online: https://www.kinetecusa.com/shop/cpm-active/hand-wrist-cpms/kinetec-maestra/ (accessed on 30 July 2021).
- Reha-Stim Medtec GmbH & Co. Reha-Digit. 2021. Available online: https://www.neurorehabdirectory.com/rehab-products/reha-digit/ (accessed on 30 July 2021).
- AMADEO®: The pioneer in Finger-Hand-Rehabilitation| Tyrotherapy. 2021. Available online: https://tyromotion.com/en/products/amadeo/ (accessed on 30 July 2021).
- Janarthanan, V.; Zaman, A.-U.; Rahman, M.H.; Mcgonigle, E.; Wang, I. Design and development of a sensored glove for home-based rehabilitation. J. Hand Ther. 2020, 33, 209–219. [Google Scholar] [CrossRef]
- SaeboGlove. 2021. Available online: https://www.saebo.com/shop/saeboglove/ (accessed on 5 August 2021).
- Order the MusicGlove Hand Therapy Glove for Stroke Patients. 2021. Available online: https://www.flintrehab.com/product/musicglove-hand-therapy/ (accessed on 5 August 2021).
- Aggogeri, F.; Mikolajczyk, T.; O’Kane, J. Robotics for rehabilitation of hand movement in stroke survivors. Adv. Mech. Eng. 2019, 11. [Google Scholar] [CrossRef]
- Sandoval-Gonzalez, O.; Jacinto-Villegas, J.M.; Herrera-Aguilar, I.; Portillo-Rodriguez, O.; Tripicchio, P.; Hernandez-Ramos, M.; Cuautle, J.J.A.F.; Avizzano, C. Design and Development of a Hand Exoskeleton Robot for Active and Passive Rehabilitation. Int. J. Adv. Robot. Syst. 2016, 13. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zheng, R.; Zhang, Y.; Yao, J. iHandRehab: An interactive hand exoskeleton for active and passive rehabilitation. In Proceedings of the 2011 IEEE International Conference on Rehabilitation Robotics, Zurich, Switzerland, 29 June–1 July 2011. [Google Scholar] [CrossRef]
- Rowe, J.B.; Chan, V.; Ingemanson, M.L.; Cramer, S.C.; Wolbrecht, E.; Reinkensmeyer, D.J. Robotic Assistance for Training Finger Movement Using a Hebbian Model: A Randomized Controlled Trial. Neurorehabilit. Neural Repair 2017, 31, 769–780. [Google Scholar] [CrossRef]
- Hsu, T.-H.; Chiang, Y.-C.; Chan, W.-T.; Chen, S.-J. A Finger Exoskeleton Robot for Finger Movement Rehabilitation. Inventions 2017, 2, 12. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.M.; Wang, Y.; Zi, B.; Cao, Z.; Ding, H. Development of an active and passive finger rehabilitation robot using pneumatic muscle and magnetorheological damper. Mech. Mach. Theory 2020, 147, 103762. [Google Scholar] [CrossRef]
- Polygerinos, P.; Wang, Z.; Galloway, K.C.; Wood, R.J.; Walsh, C.J. Soft robotic glove for combined assistance and at-home rehabilitation. Robot. Auton. Syst. 2015, 73, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Gong, L.; Wei, L.; Yeh, S.-C.; Da Xu, L.; Zheng, L.; Zou, Z. A Wearable Hand Rehabilitation System with Soft Gloves. IEEE Trans. Ind. Inform. 2021, 17, 943–952. [Google Scholar] [CrossRef]
- Bernocchi, P.; Mulè, C.; Vanoglio, F.; Taveggia, G.; Luisa, A.; Scalvini, S. Home-based hand rehabilitation with a robotic glove in hemiplegic patients after stroke: A pilot feasibility study. Top. Stroke Rehabil. 2018, 25, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Portable Rehabilitation Robotic Gloves: SIFREHAB-1.0 | SIFSOF. 2021. Available online: https://sifsof.com/product/portable-rehabilitation-robotic-gloves-sifrehab-1-0 (accessed on 10 August 2021).
- Marconi, D.; Baldoni, A.; McKinney, Z.; Cempini, M.; Crea, S.; Vitiello, N. A novel hand exoskeleton with series elastic actuation for modulated torque transfer. Mechatronics 2019, 61, 69–82. [Google Scholar] [CrossRef]
- Chiri, A.; Giovacchini, F.; Vitiello, N.; Cattin, E.; Roccella, S.; Vecchi, F.; Carrozza, M.C. HANDEXOS: Towards an exoskeleton device for the rehabilitation of the hand. In Proceedings of the IEEE/RSJ International Conference on Intelligent Robots and Systems, St. Louis, MO, USA, 10–15 October 2009; pp. 1106–1111. [Google Scholar] [CrossRef]
- Moshaei, A.A.; Moghaddam, M.M.; Niestanak, V.D. Fuzzy sliding mode control of a wearable rehabilitation robot for wrist and finger. Ind. Robot. Int. J. 2019, 46, 839–850. [Google Scholar] [CrossRef]
- Hiwonder LX-16A Full Metal Gear Serial Bus Servo with Real-Time Feedback Function for RC Robot (Control Angle 240). 2021. Available online: https://www.hiwonder.hk/products/hiwonder-lx-16a-serial-bus-servo (accessed on 18 August 2021).
- Rahman, M.H.; Kittel-Ouimet, T.; Saad, M.; Kenné, J.-P.; Archambault, P.S. Dynamic modeling and evaluation of a robotic exoskeleton for upper-limb rehabilitation. Int. J. Inf. Acquis. 2011, 8, 83–102. [Google Scholar] [CrossRef]
- ELEGOO Mars 2 Pro Mono LCD MSLA Resin 3D Printer with Air Purifier. 2021. Available online: https://www.elegoo.com/products/elegoo-mars-2-pro-mono-lcd-3d-printer (accessed on 20 August 2021).
- ELEGOO Water Washable Rapid Resin LCD UV-Curing Resin for 3D Printers. 2021. Available online: https://www.elegoo.com/products/elegoo-water-washable-resin (accessed on 20 August 2021).
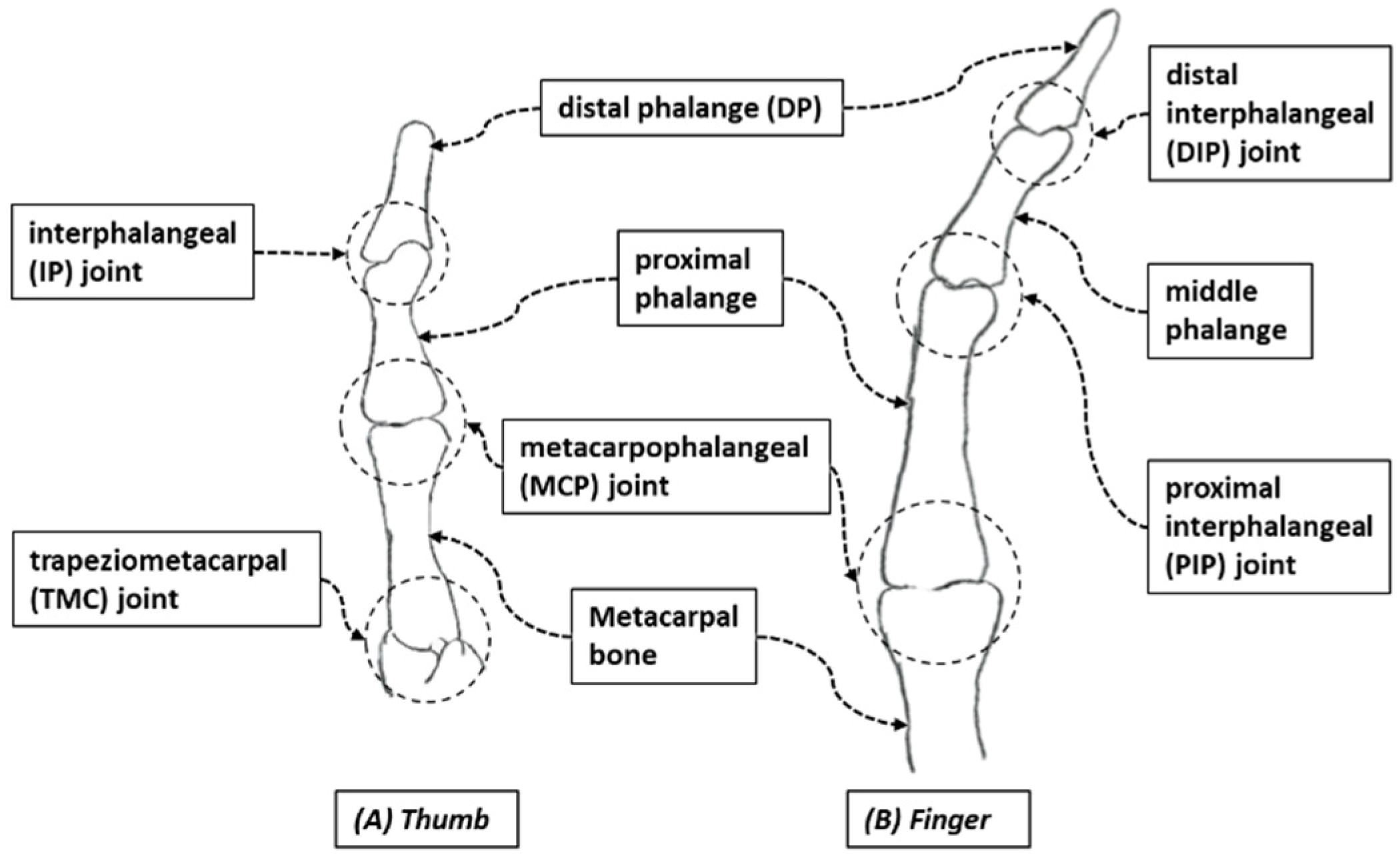
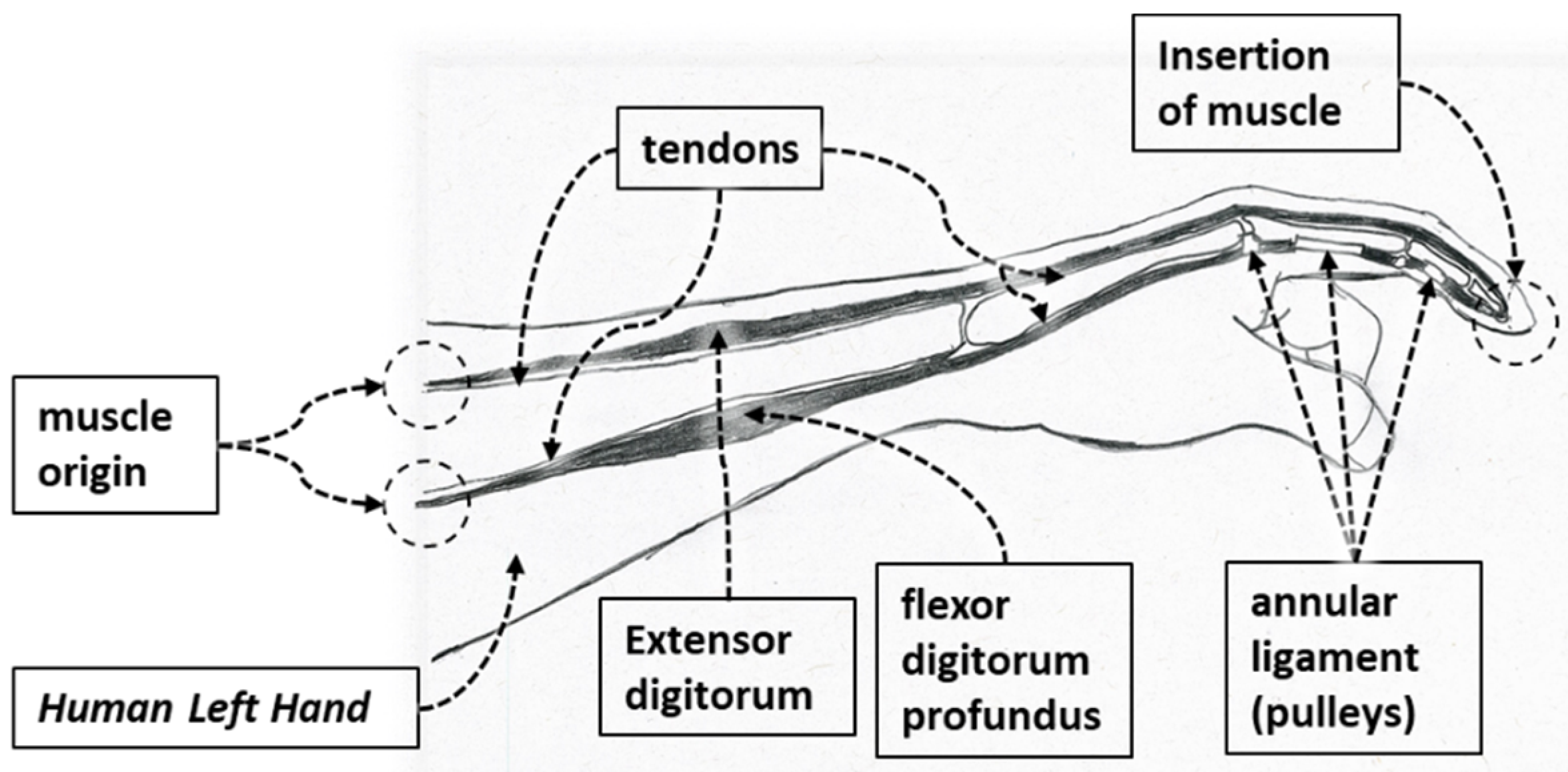

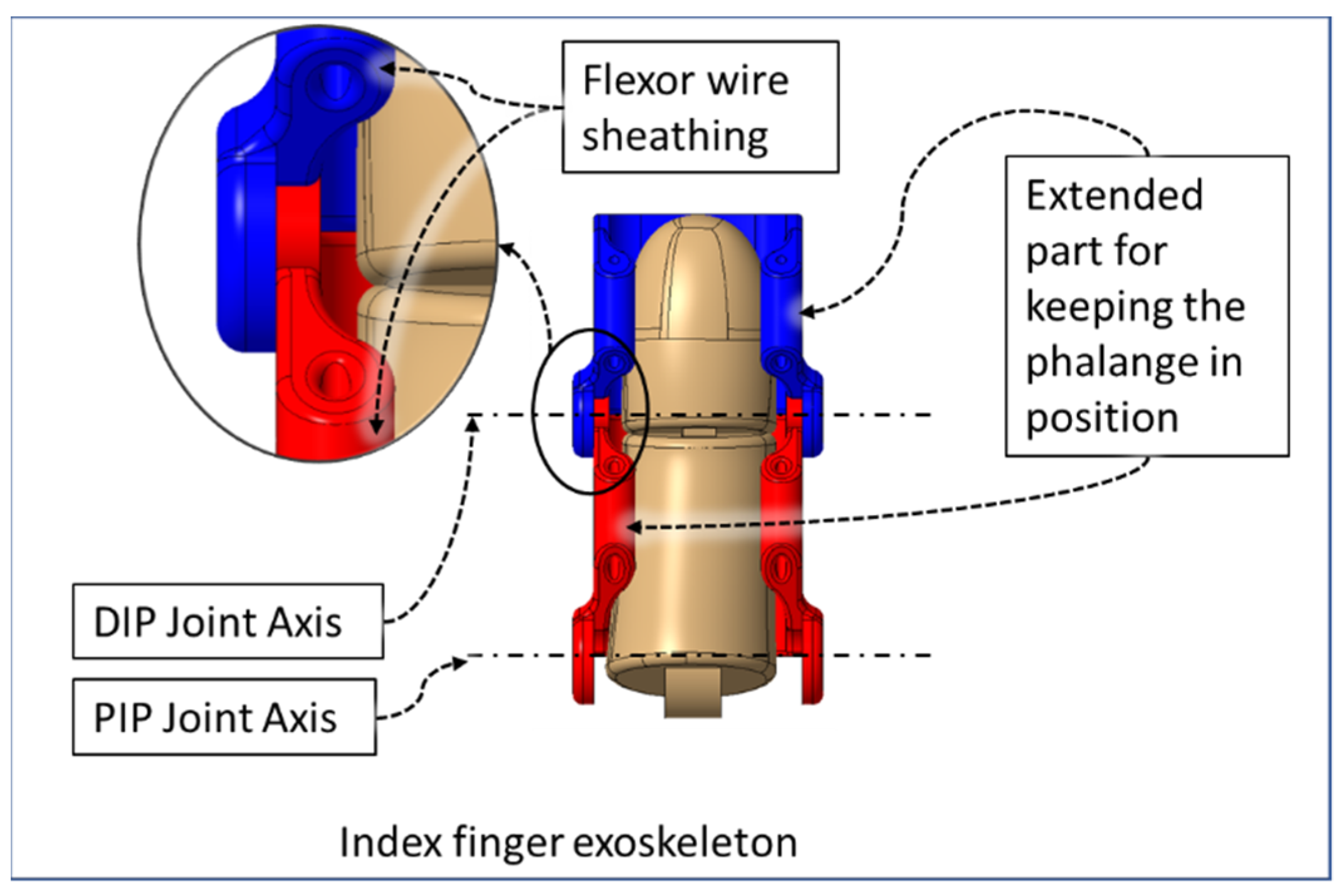
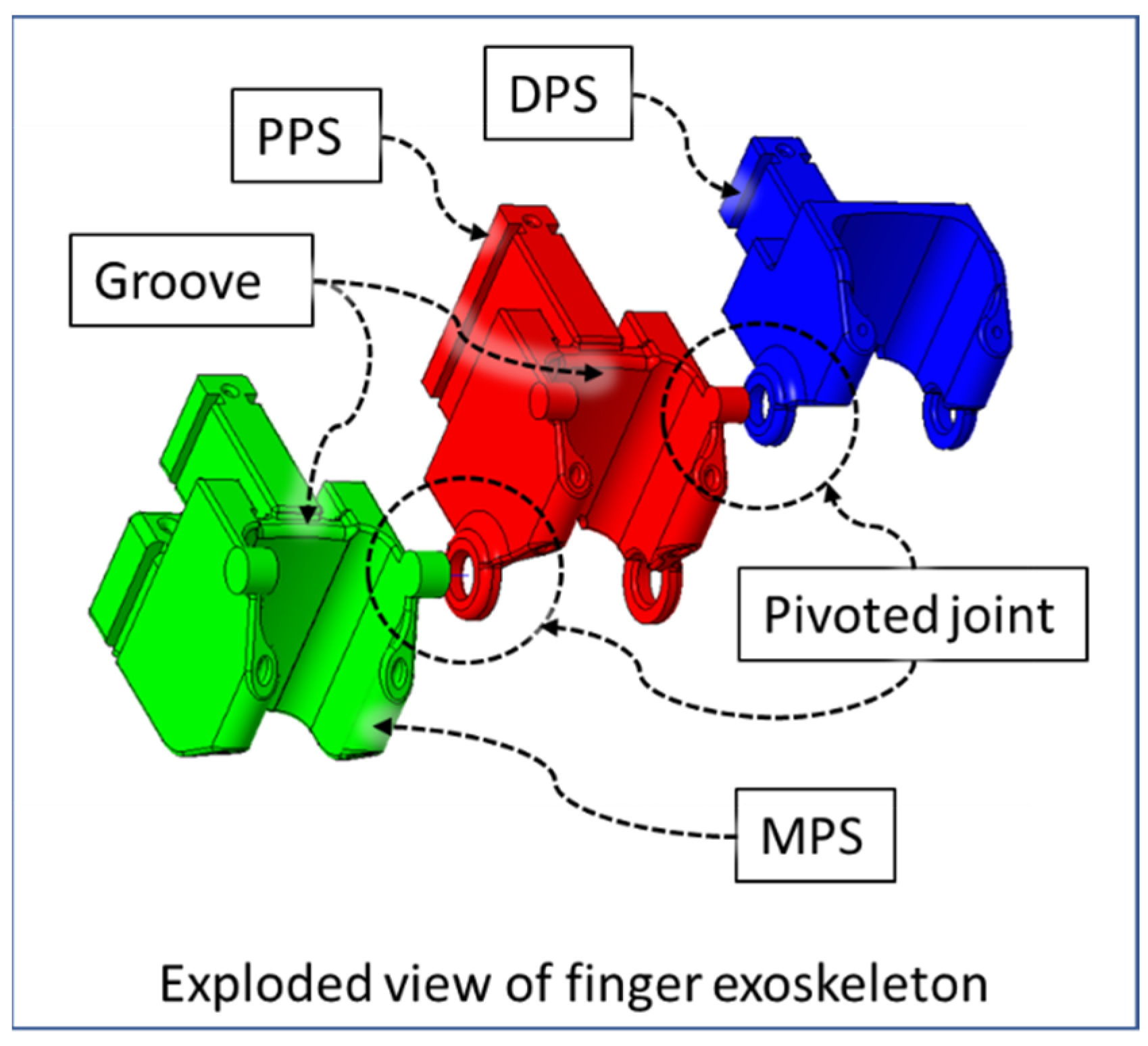

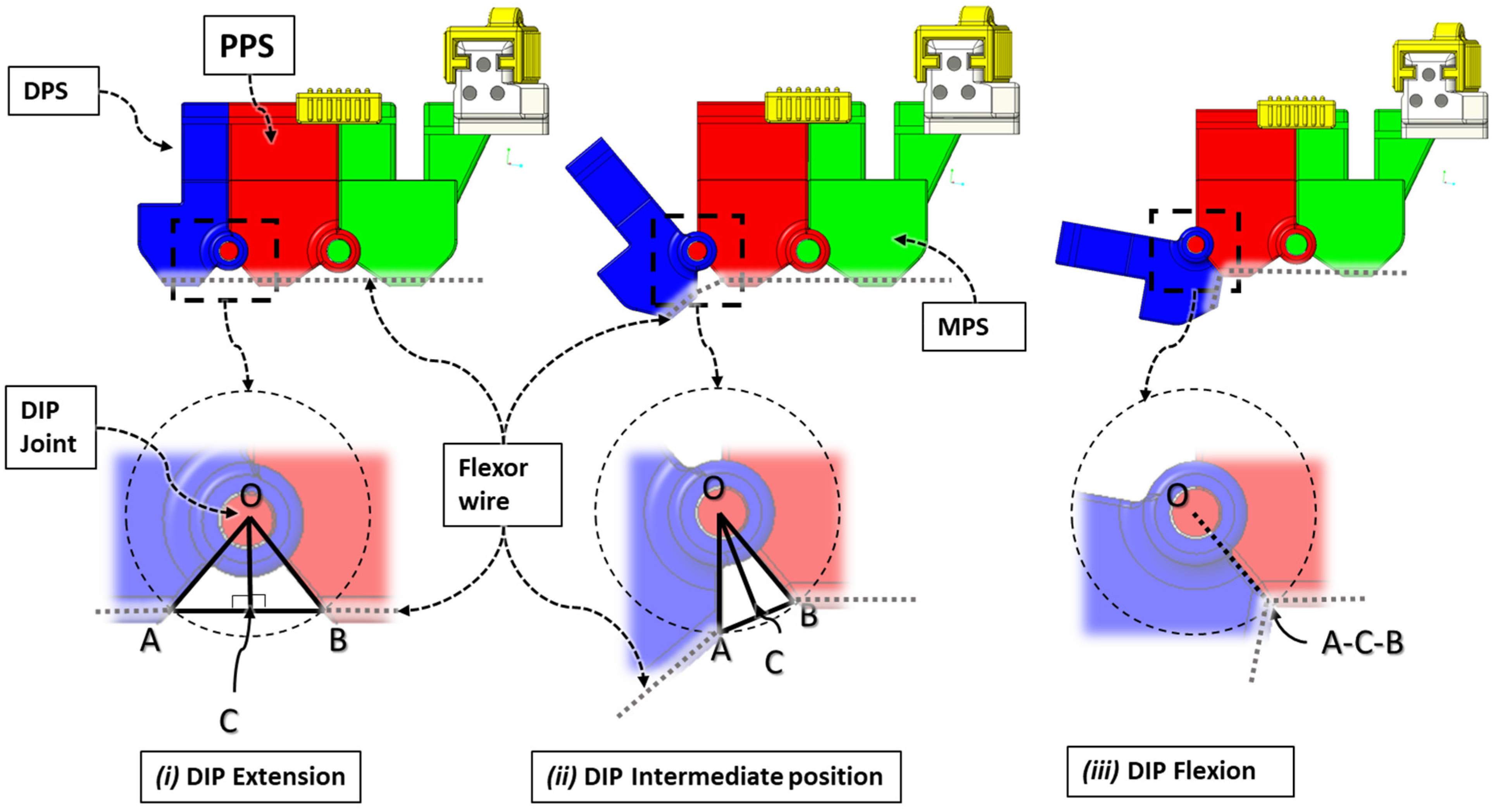
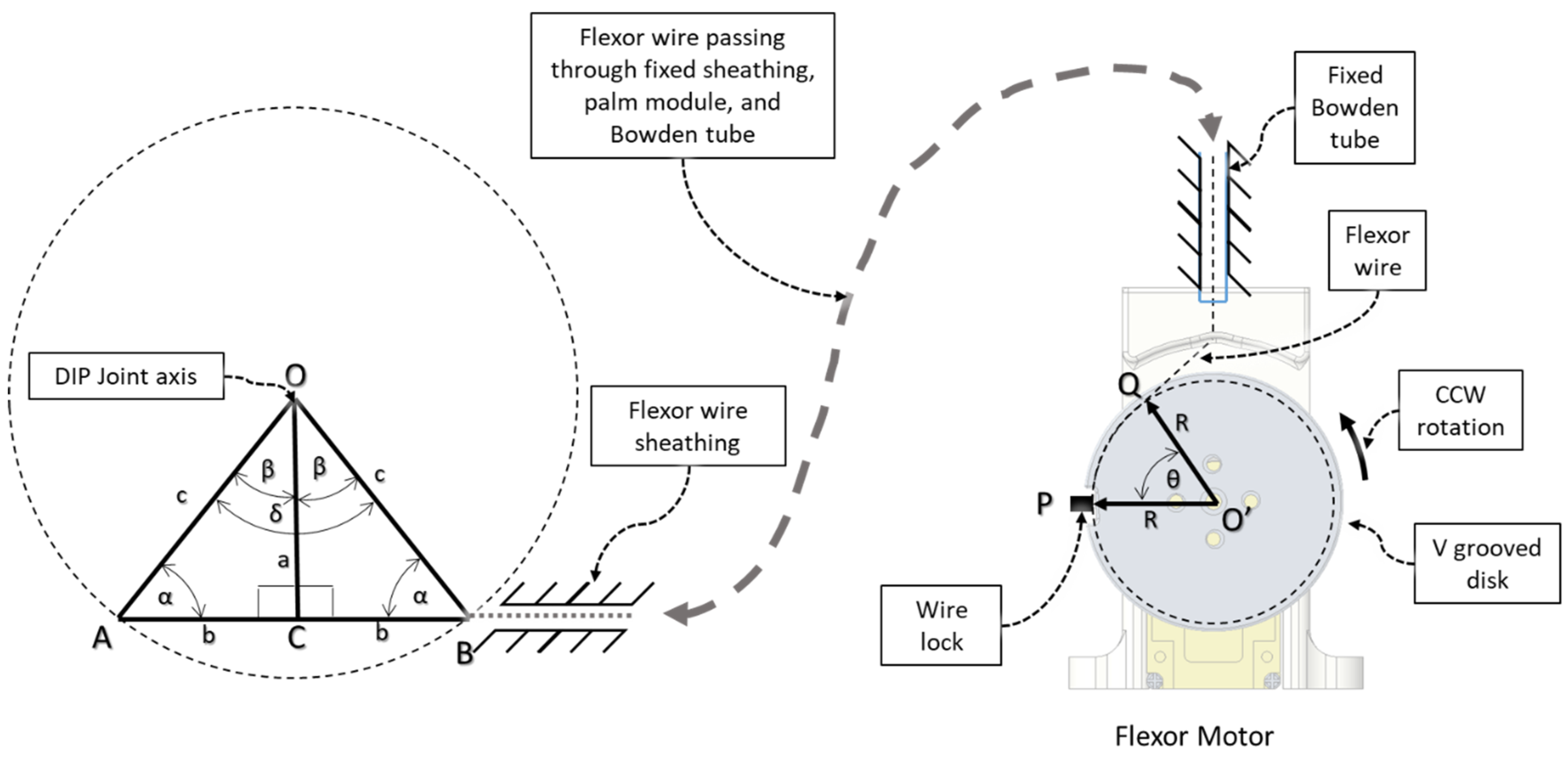
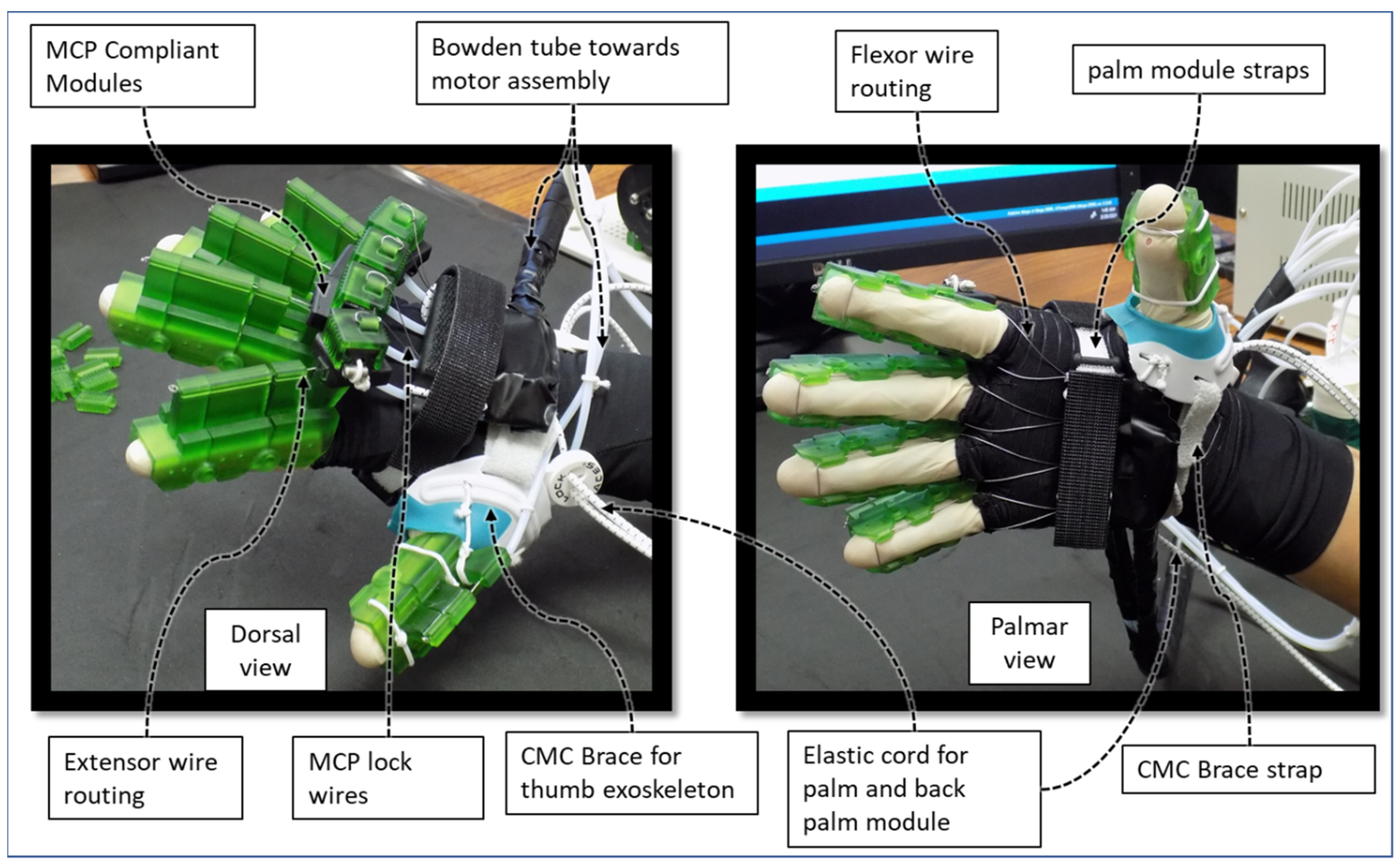
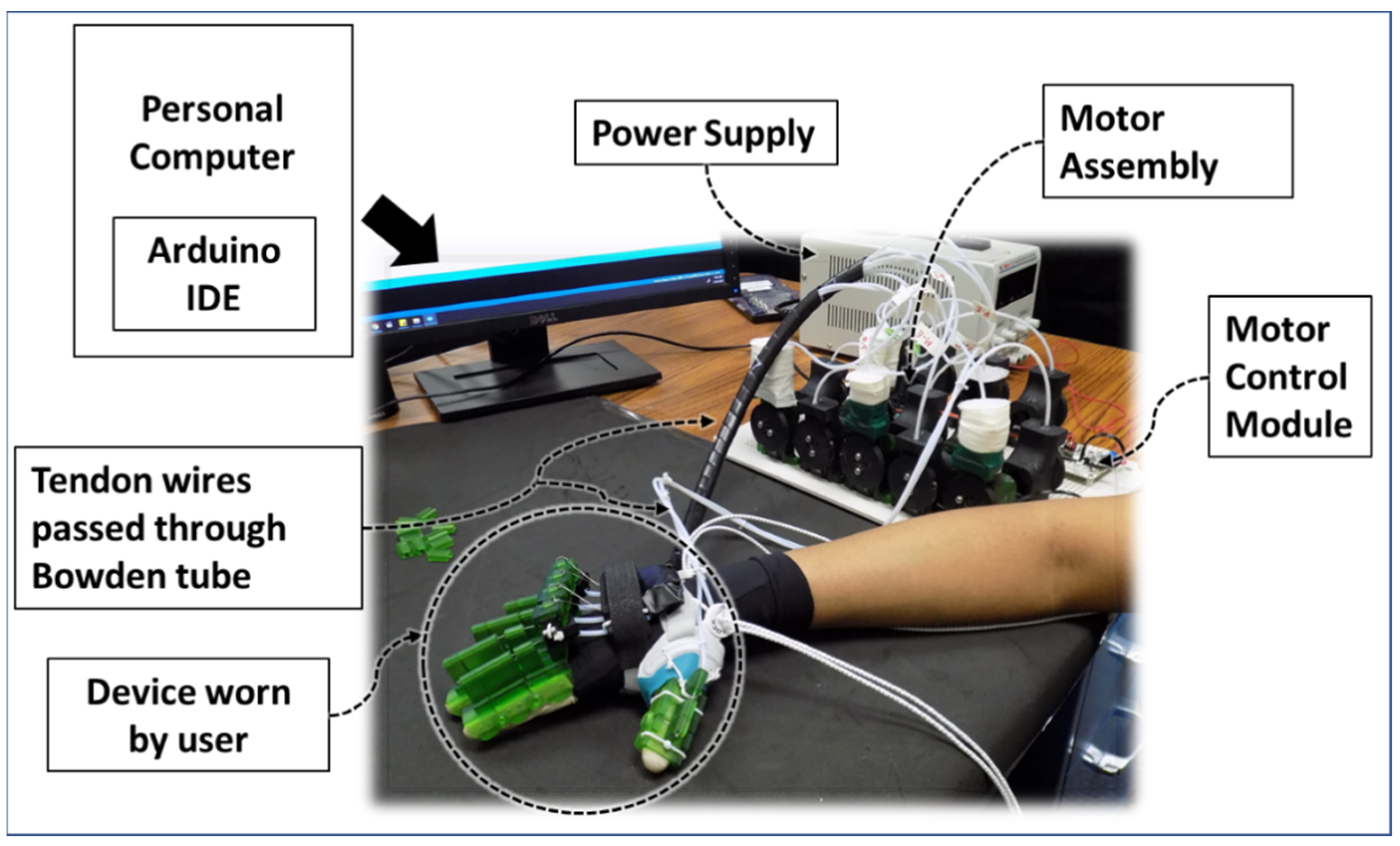
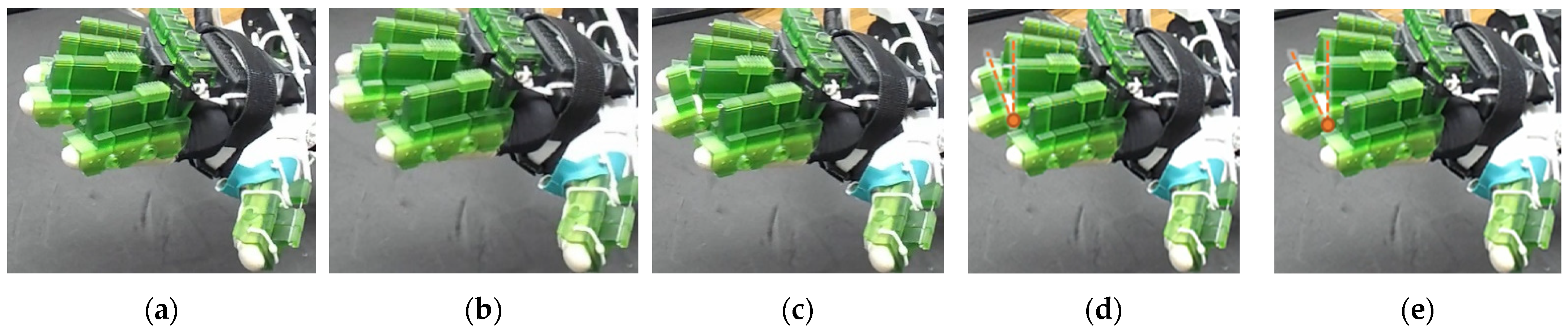
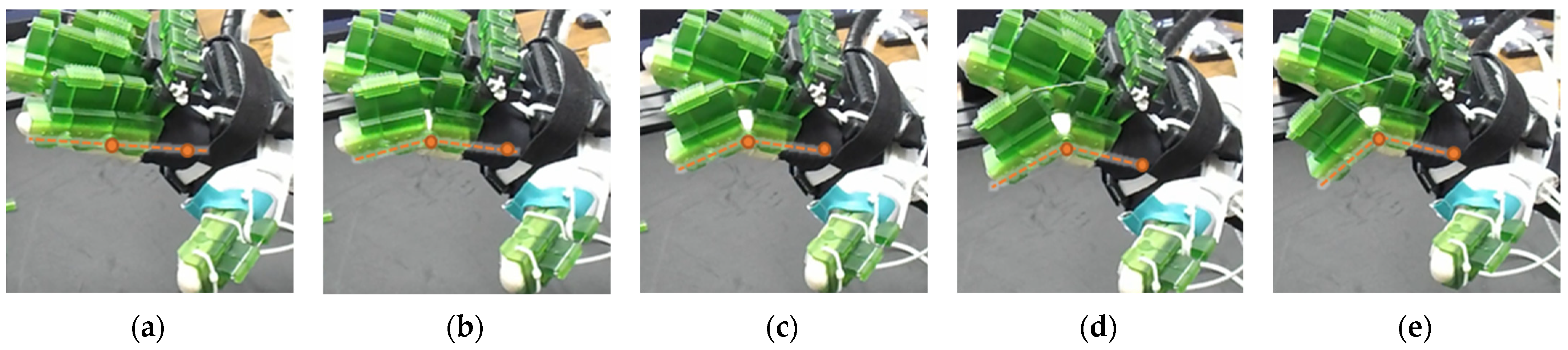
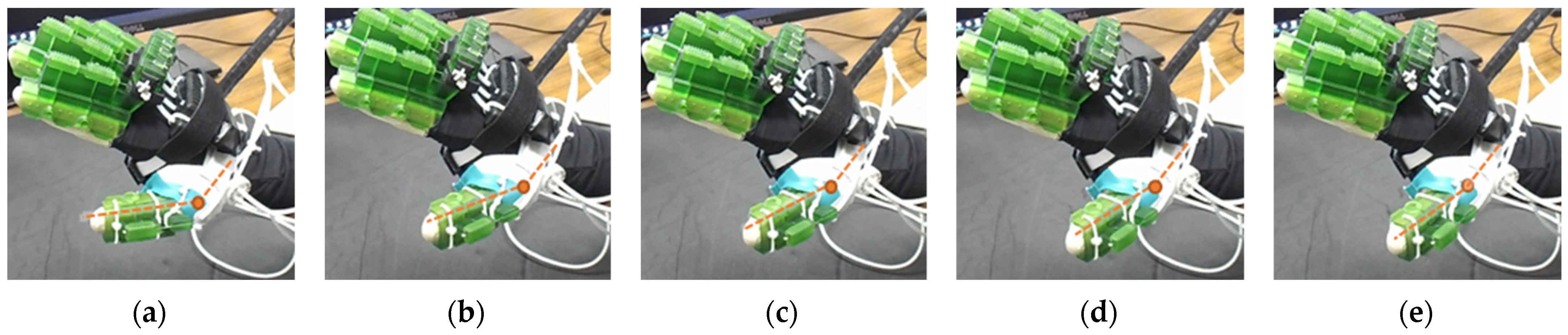



| Hand Exercises | Associated Digits | Associated Digit Joint Motions |
|---|---|---|
| Knuckle bend | Index, middle, ring, and small finger | MCP F/E |
| Hook Fist | DIP-PIP F/E | |
| Straight fist | PIP-MCP F/E | |
| Imaginary ball squeeze/claw hand | Index, middle, ring, and small finger and thumb | DIP-PIP-MCP (fingers), IP-MCP (thumb) F/E |
| Isolated DIP F/E | Index/middle/ring/small | DIP F/E |
| Isolated PIP F/E | Index/middle/ring/small/thumb | PIP (fingers), IP (thumb) F/E |
| Composite F/E | Isolated or combination of index, middle, ring, small, and thumb | Combination of DIP, PIP, MCP (fingers); IP, MCP (thumb) F/E |
| F/E: flexion/extension; MCP: metacarpophalangeal joint; DIP: distal interphalangeal joint; PIP: proximal interphalangeal joint; IP: interphalangeal joint. | ||
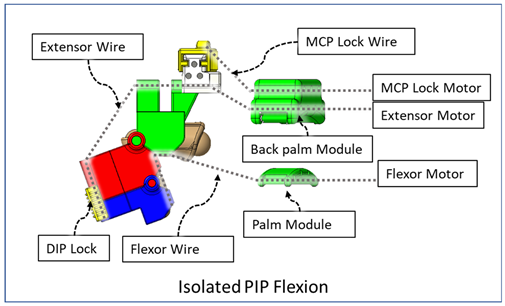
| Finger Joint Motions | Configuration Description |
|---|---|
 | The figure shown in the right column shows the nominal/zero position of the device while the user is wearing it. The same configuration is achieved during extension exercises as such: the extensor motor pulls the extensor wire at a slower rate while the MCP lock motor (if moved from zero position) and flexor motors release associated wires. The DIP and PIP locks can be slid off from the exoskeletal shells. |
 | The DIP lock has been removed while the PIP lock stays. The MCP lock motor stays at zero position throughout DIP flexion to prevent MCP joint motion. The extensor motor releases the extensor wire at a faster rate while the flexor motor pulls the flexor wire. |
 | The DIP lock has been removed; the PIP lock has been slid to the DIP lock’s position and used as the DIP lock. The motor configuration is the same as that of the isolated DIP flexion. |
 | Both DIP and PIP locks stay at the locking position. During this motion, the extensor and MCP lock motors release the extensor and MCP lock wire, respectively, faster than the flexor motor pulls the flexor wire. |
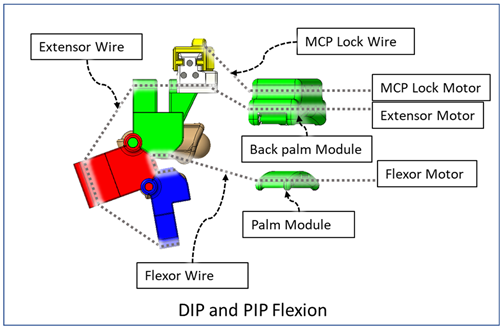 | During this composite joint motion, both the DIP and PIP locks are slid off. During this motion, motor configurations are the same as the that of the isolated MCP flexion exercise. |
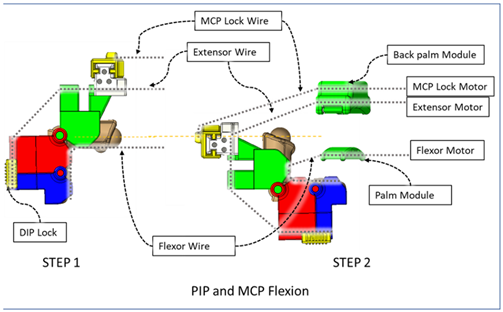 | One sliding lock is kept to lock the DIP joint’s motion and is thus named as the DIP lock. At Step 1, PIP flexion is achieved during this composite joint motion through the same motor configuration as that of the isolated PIP flexion. At Step 2, the MCP lock wire is released simultaneously so that MCP flexion is performed. |
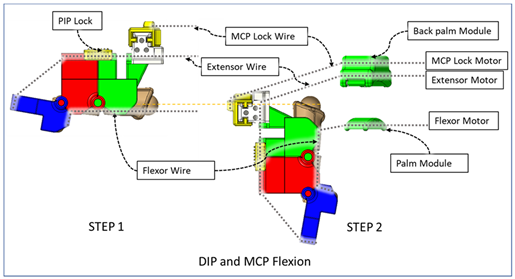 | The PIP lock is kept at the joint locking position. At Step 1, DIP flexion is achieved during this composite joint motion through the same motor configuration as that of the isolated DIP flexion. At Step 2, the MCP lock wire is released simultaneously so that MCP flexion is achieved. |
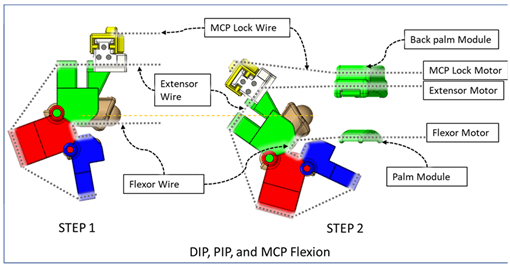 | At Step 1, DIP and PIP flexion is achieved by keeping the MCP lock motor at zero position until the extensor and flexor motors have achieved DIP and PIP flexion. At Step 2, the MCP lock wire is released simultaneously with the extensor motor to achieve MCP flexion. |
| Link. | αi−1 | ai−1 | di−1 | θi | Joint Axis Associated Motions |
|---|---|---|---|---|---|
| 1 | 0 | 0 | 0 | q1 | MCP abd/add |
| 2 | π/2 | 0 | 0 | q2 | MCP flex/ext |
| 3 | 0 | L1 | 0 | q3 | PIP flex/ext |
| 4 | 0 | L2 | 0 | q4 | DIP flex/ext |
| Fingertip (f) | 0 | L3 | 0 | 0 | - |
| (i) DIP Flexion–Extension of Finger | (ii) PIP Flexion–Extension of Finger | ||||||
| Flexor motor’s angular position θF (°) | Extensor motor’s angular position θE (°) | MCP lock motor’s angular position θML (°) | DIP joint angle (approx.) θd (°) | Flexor motor’s angular position θF (°) | Extensor motor’s angular position θE (°) | MCP lock motor’s angular position θML (°) | PIP joint angle (approx.) θp (°) |
| 51 | 0 | 0 | 60 | 80 | 0 | 0 | 90 |
| 48 | 7 | 0 | 55 | 75 | 6 | 0 | 85 |
| 44 | 12 | 0 | 52.5 | 70 | 12 | 0 | 80 |
| 41 | 16 | 0 | 50 | 65 | 18 | 0 | 75 |
| 38 | 21 | 0 | 45 | 60 | 24 | 0 | 70 |
| 34 | 26 | 0 | 40 | 55 | 30 | 0 | 62.5 |
| 31 | 28 | 0 | 35 | 50 | 37 | 0 | 47.5 |
| 28 | 33 | 0 | 32.5 | 45 | 43 | 0 | 42 |
| 25 | 35 | 0 | 25 | 40 | 49 | 0 | 37.5 |
| 21 | 40 | 0 | 20 | 35 | 55 | 0 | 32.5 |
| 18 | 45 | 0 | 17 | 30 | 61 | 0 | 27.5 |
| 15 | 50 | 0 | 14 | 25 | 67 | 0 | 22.5 |
| 11 | 54 | 0 | 10 | 20 | 73 | 0 | 17.5 |
| 8 | 57 | 0 | 8 | 15 | 79 | 0 | 12.5 |
| 5 | 61 | 0 | 5 | 10 | 85 | 0 | 7.5 |
| 3 | 64 | 0 | 2.5 | 5 | 91 | 0 | 2.5 |
| 0 | 67 | 0 | 0 | 0 | 97 | 0 | 0 |
| (iii) DIP and PIP Flexion-Extension of Finger | (iv) MCP Flexion-Extension of Finger | ||||||
| Flexor motor’s angular position θF (°) | Extensor motor’s angular position θE (°) | MCP lock motor’s angular position θML (°) | DIP + PIP joint angle (approx.) θdp (°) | Flexor motor’s angular position θF (°) | Extensor motor’s angular position θE (°) | MCP lock motor’s angular position θML (°) | MCP joint angle (approx.) θm (°) |
| 98 | 13 | 0 | 95 | 110 | 0 | 0 | 65 |
| 91 | 24 | 0 | 90 | 103 | 4 | 9 | 60 |
| 85 | 35 | 0 | 85 | 95 | 7 | 13 | 58 |
| 78 | 45 | 0 | 79 | 88 | 9 | 18 | 55 |
| 71 | 56 | 0 | 70 | 81 | 11 | 22 | 50 |
| 65 | 65 | 0 | 56 | 73 | 13 | 26 | 40 |
| 58 | 75 | 0 | 50 | 66 | 15 | 31 | 33 |
| 52 | 84 | 0 | 43 | 59 | 18 | 35 | 30 |
| 45 | 95 | 0 | 36 | 51 | 20 | 40 | 25 |
| 38 | 105 | 0 | 31 | 44 | 22 | 44 | 20 |
| 32 | 117 | 0 | 25 | 37 | 24 | 48 | 18 |
| 25 | 127 | 0 | 19 | 29 | 26 | 53 | 16 |
| 19 | 137 | 0 | 14 | 22 | 29 | 57 | 12 |
| 12 | 146 | 0 | 9 | 15 | 31 | 62 | 8 |
| 7 | 156 | 0 | 3 | 7 | 33 | 66 | 4 |
| 0 | 164 | 0 | 0 | 0 | 35 | 70 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, T.; Assad-Uz-Zaman, M.; Islam, M.R.; Gottheardt, D.; McGonigle, E.; Brahmi, B.; Rahman, M.H. Flexohand: A Hybrid Exoskeleton-Based Novel Hand Rehabilitation Device. Micromachines 2021, 12, 1274. https://doi.org/10.3390/mi12111274
Ahmed T, Assad-Uz-Zaman M, Islam MR, Gottheardt D, McGonigle E, Brahmi B, Rahman MH. Flexohand: A Hybrid Exoskeleton-Based Novel Hand Rehabilitation Device. Micromachines. 2021; 12(11):1274. https://doi.org/10.3390/mi12111274
Chicago/Turabian StyleAhmed, Tanvir, Md Assad-Uz-Zaman, Md Rasedul Islam, Drew Gottheardt, Erin McGonigle, Brahim Brahmi, and Mohammad Habibur Rahman. 2021. "Flexohand: A Hybrid Exoskeleton-Based Novel Hand Rehabilitation Device" Micromachines 12, no. 11: 1274. https://doi.org/10.3390/mi12111274
APA StyleAhmed, T., Assad-Uz-Zaman, M., Islam, M. R., Gottheardt, D., McGonigle, E., Brahmi, B., & Rahman, M. H. (2021). Flexohand: A Hybrid Exoskeleton-Based Novel Hand Rehabilitation Device. Micromachines, 12(11), 1274. https://doi.org/10.3390/mi12111274









