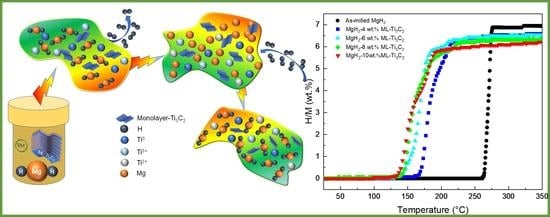
The Improvement in Hydrogen Storage Performance of MgH2 Enabled by Multilayer Ti3C2
Abstract
:1. Introduction
2. Experimental Details
2.1. Preparation of Material
2.2. Characterization Methods
2.3. De/Hydrogenation Characterization
3. Results and Discussion
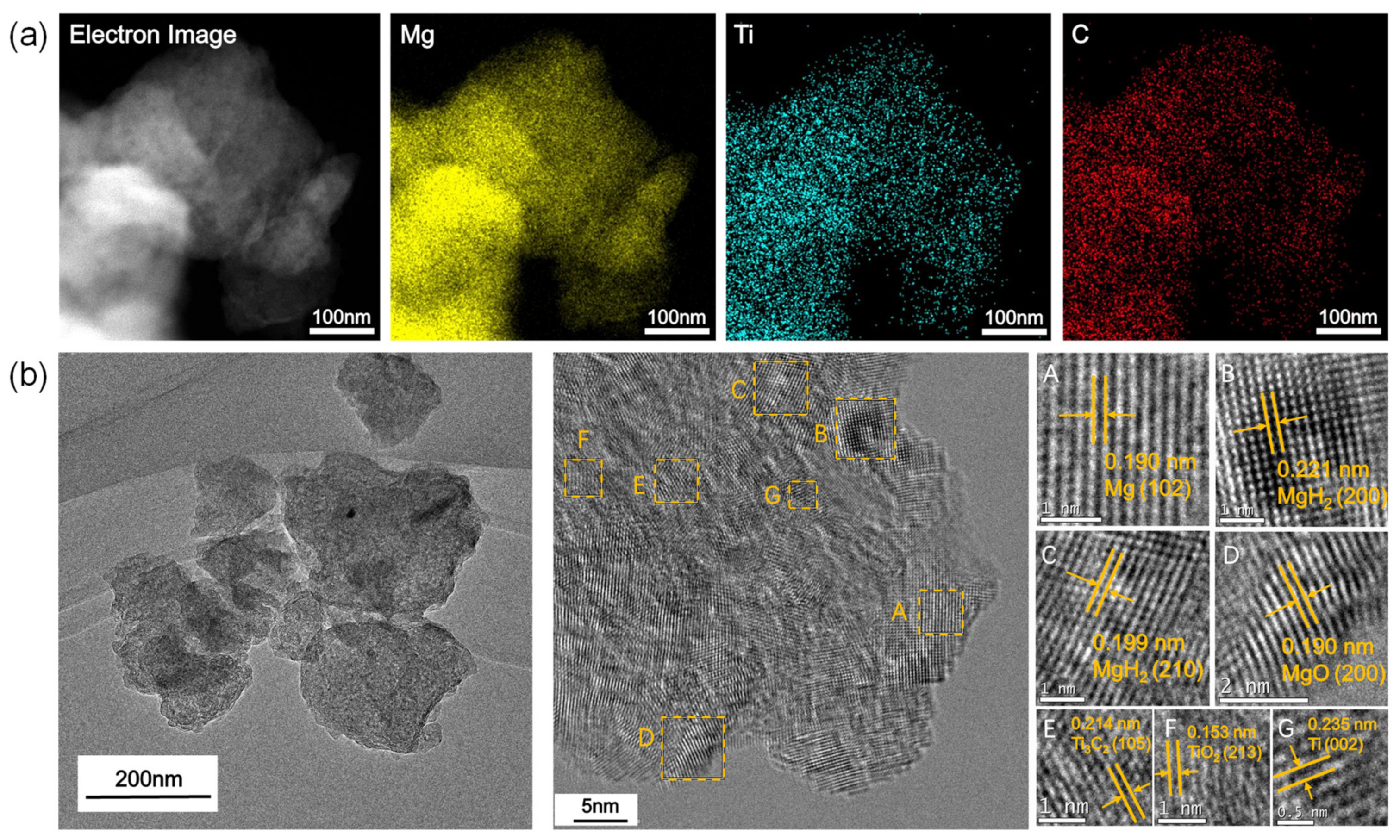
3.1. Characterization of ML-Ti3C2
3.2. De/Hydrogenation Performance of MgH2+ML-Ti3C2
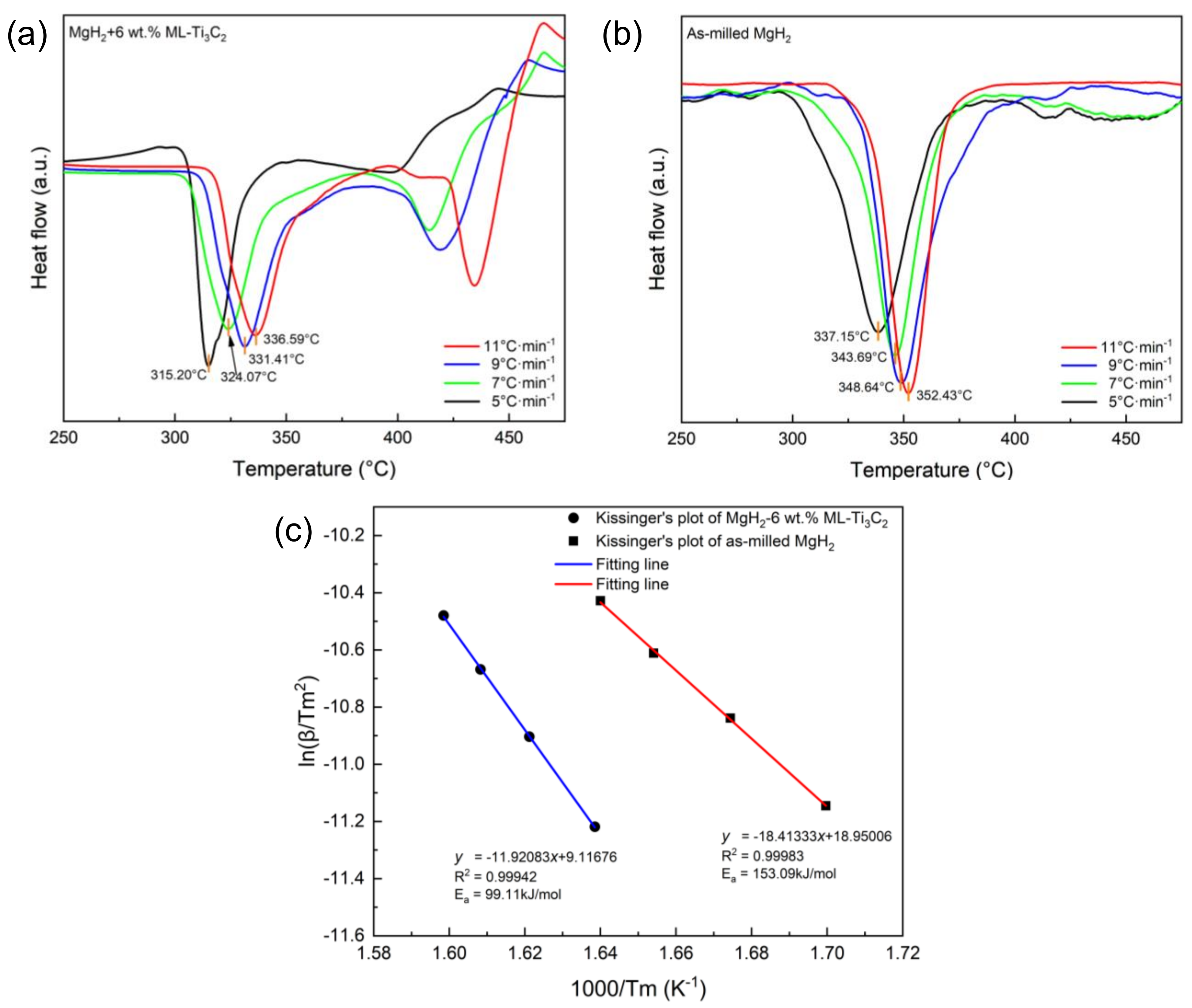
3.3. Kinetics and Thermodynamics of Hydrogen Desorption
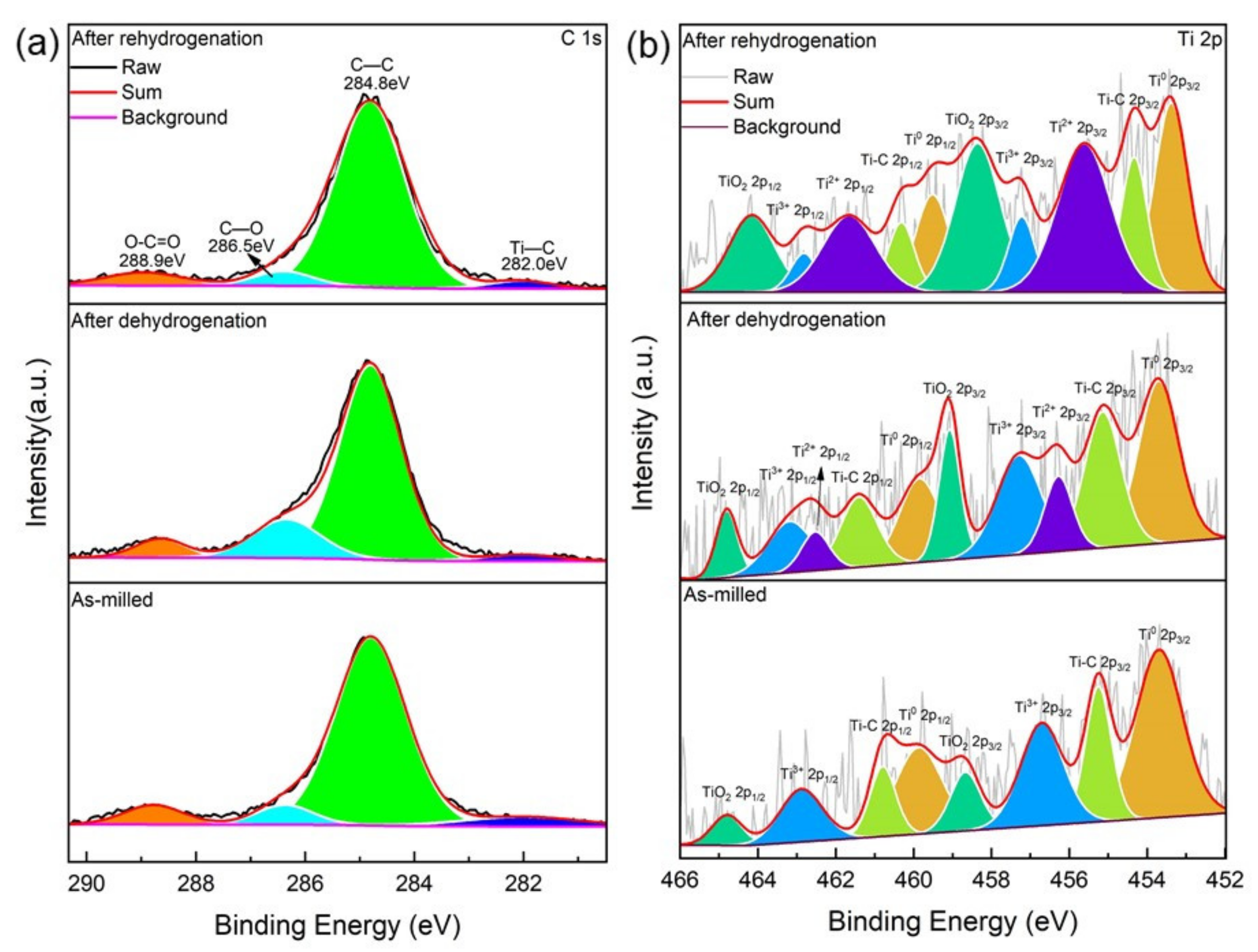
3.4. The Mechanisms for Improving the Hydrogen Storage Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Schapbach, L.; Zuttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef]
- Jia, Y.; Sun, C.; Shen, S.; Zou, J.; Mao, S.S.; Yao, X. Combination of nanosizing and interfacial effect: Future perspective for designing Mg-based nanomaterials for hydrogen storage. Renew. Sustain. Energy Rev. 2015, 44, 289–303. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Y.; Chen, J.; Guo, X.; Zhu, Y.; Li, L. Enhancing hydrogen storage performances of MgH2 by Ni nano-particles over mesoporous carbon CMK-3. Nanotechnology 2018, 29, 265705. [Google Scholar] [CrossRef]
- Fan, M.-Q.; Liu, S.-S.; Zhang, Y.; Zhang, J.; Sun, L.-X.; Xu, F. Superior hydrogen storage properties of MgH2-10 wt.% TiC composite. Energy 2010, 35, 3417–3421. [Google Scholar] [CrossRef]
- Ma, T.; Isobe, S.; Wang, Y.; Hashimoto, N.; Ohnuki, S. Catalytic Effect of Nb2O5 in MgH2-Nb2O5 Ball-Milled Composites. Catalysts 2012, 2, 344–351. [Google Scholar] [CrossRef] [Green Version]
- Malka, I.E.; Czujko, T.; Bystrzycki, J. Catalytic effect of halide additives ball milled with magnesium hydride. Int. J. Hydrogen Energy 2010, 35, 1706–1712. [Google Scholar] [CrossRef]
- Mao, J.; Guo, Z.; Yu, X.; Liu, H.; Wu, Z.; Ni, J. Enhanced hydrogen sorption properties of Ni and Co-catalyzed MgH2. Int. J. Hydrogen Energy 2010, 35, 4569–4575. [Google Scholar] [CrossRef]
- De Lima Andreani, G.F.; Martins Triques, M.R.; Kiminami, C.S.; Botta, W.J.; Roche, V.; Jorge, A.M., Jr. Characterization of hydrogen storage properties of Mg-Fe-CNT composites prepared by ball milling, hot-extrusion and severe plastic deformation. Int. J. Hydrogen Energy 2016, 41, 23092–23098. [Google Scholar] [CrossRef]
- Gkanas, E.I.; Damian, A.; Ioannidou, A.; Stoian, G.; Lupu, N.; Gjoka, M.; Makridis, S.S. Synthesis, characterisation and hydrogen sorption properties of mechanically alloyed Mg(Ni1-xMnx)(2). Mater. Today Energy 2019, 13, 186–194. [Google Scholar] [CrossRef]
- Luo, Q.; Li, J.; Li, B.; Liu, B.; Shao, H.; Li, Q. Kinetics in Mg-based hydrogen storage materials: Enhancement and mechanism. J. Magnes. Alloy. 2019, 7, 58–71. [Google Scholar] [CrossRef]
- Skryabina, N.; Aptukov, V.; Romanov, P.; Fruchart, D.; de Rango, P.; Girard, G.; Grandini, C.; Sandim, H.; Huot, J.; Lang, J.; et al. Microstructure Optimization of Mg-Alloys by the ECAP Process Including Numerical Simulation, SPD Treatments, Characterization, and Hydrogen Sorption Properties. Molecules 2019, 24, 89. [Google Scholar] [CrossRef] [Green Version]
- Yong, H.; Guo, S.; Yuan, Z.; Qi, Y.; Zhao, D.; Zhang, Y. Phase transformation, thermodynamics and kinetics property of Mg90Ce5RE5 (RE = La, Ce, Nd) hydrogen storage alloys. J. Mater. Sci. Technol. 2020, 51, 84–93. [Google Scholar] [CrossRef]
- Liu, Y.; Zou, J.; Zeng, X.; Wu, X.; Tian, H.; Ding, W.; Wang, J.; Walter, A. Study on hydrogen storage properties of Mg nanoparticles confined in carbon aerogels. Int. J. Hydrogen Energy 2013, 38, 5302–5308. [Google Scholar] [CrossRef]
- Schneemann, A.; White, J.L.; Kang, S.; Jeong, S.; Wan, L.F.; Cho, E.S.; Heo, T.W.; Prendergast, D.; Urban, J.J.; Wood, B.C.; et al. Nanostructured Metal Hydrides for Hydrogen Storage. Chem. Rev. 2018, 118, 10775–10839. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ma, T.; Aguey-Zinsou, K.-F. Magnesium Supported on Nickel Nanobelts for Hydrogen Storage: Coupling Nanosizing and Catalysis. Acs. Appl. Nano Mater. 2018, 1, 1272–1279. [Google Scholar] [CrossRef]
- Vajo, J.; Pinkerton, F.; Stetson, N. Nanoscale phenomena in hydrogen storage. Nanotechnology 2009, 20, 200201. [Google Scholar] [CrossRef] [PubMed]
- Xia, G.; Tan, Y.; Chen, X.; Sun, D.; Guo, Z.; Liu, H.; Ouyang, L.; Zhu, M.; Yu, X. Monodisperse Magnesium Hydride Nanoparticles Uniformly Self-Assembled on Graphene. Adv. Mater. 2015, 27, 5981–5988. [Google Scholar] [CrossRef]
- Xia, G.; Tan, Y.; Wu, F.; Fang, F.; Sun, D.; Guo, Z.; Huang, Z.; Yu, X. Graphene-wrapped reversible reaction for advanced hydrogen storage. Nano Energy 2016, 26, 488–495. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Tang, Z.; Sun, D.; Ouyang, L.; Zhu, M. Recent advances and remaining challenges of nanostructured materials for hydrogen storage applications. Prog. Mater. Sci. 2017, 88, 1–48. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, Y.; Xu, L.; Zang, L.; Guo, H.; Jiao, L.; Yuan, H.; Wang, Y. Highly Dispersed MgH2 Nanoparticle-Graphene Nanosheet Composites for Hydrogen Storage. Acs. Appl. Nano Mater. 2019, 2, 3828–3835. [Google Scholar] [CrossRef]
- Aktekin, B.; Eyovge, C.; Ozturk, T. Carbon coating of magnesium particles. J. Alloy. Compd. 2017, 720, 17–21. [Google Scholar] [CrossRef]
- Huang, Y.; An, C.; Zhang, Q.; Zang, L.; Shao, H.; Liu, Y.; Zhang, Y.; Yuan, H.; Wang, C.; Wang, Y. Cost-effective mechanochemical synthesis of highly dispersed supported transition metal catalysts for hydrogen storage. Nano Energy 2021, 80, 105535. [Google Scholar] [CrossRef]
- Liu, J.; Ma, Z.; Liu, Z.; Tang, Q.; Zhu, Y.; Lin, H.; Zhang, Y.; Zhang, J.; Liu, Y.; Li, L. Synergistic effect of rGO supported Ni3Fe on hydrogen storage performance of MgH2. Int. J. Hydrogen Energy 2020, 45, 16622–16633. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, J.; Zhu, Y.; Lin, H.; Liu, Y.; Zhang, Y.; Zhu, D.; Li, L. Facile Synthesis of Carbon Supported Nano-Ni Particles with Superior Catalytic Effect on Hydrogen Storage Kinetics of MgH2. Acs. Appl. Energy Mater. 2018, 1, 1158–1165. [Google Scholar] [CrossRef]
- Meena, P.; Singh, R.; Sharma, V.K.; Jain, I.P. Role of NiMn9.3Al4.0Co14.1Fe3.6 alloy on dehydrogenation kinetics of MgH2. J. Magnes. Alloy. 2018, 6, 318–325. [Google Scholar] [CrossRef]
- Sulaiman, N.N.; Juahir, N.; Mustafa, S.; Yap, F.A.H.; Ismail, M. Improved hydrogen storage properties of MgH2 catalyzed with K2NiF6. J. Energy Chem. 2016, 25, 832–839. [Google Scholar] [CrossRef]
- Yahya, M.S.; Ismail, M. Catalytic effect of SrTiO3 on the hydrogen storage behaviour of MgH2. J. Energy Chem. 2019, 28, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Yao, P.; Jiang, Y.; Liu, Y.; Wu, C.; Chou, K.-C.; Lyu, T.; Li, Q. Catalytic effect of Ni@rGO on the hydrogen storage properties of MgH2. J. Magnes. Alloy. 2020, 8, 461–471. [Google Scholar] [CrossRef]
- Grzech, A.; Lafont, U.; Magusin, P.C.M.M.; Mulder, F.M. Microscopic Study of TiF3 as Hydrogen Storage Catalyst for MgH2. J. Phys. Chem. C 2012, 116, 26027–26035. [Google Scholar] [CrossRef]
- Shao, H.; Felderhoff, M.; Schueth, F.; Weidenthaler, C. Nanostructured Ti-catalyzed MgH2 for hydrogen storage. Nanotechnology 2011, 22, 235401. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Zhang, W.; Han, S.M.; Qin, F. Improvement in hydrogen storage properties of MgH2 catalyzed with BaTiO3 additive. In Proceedings of the 2nd International Conference on New Material and Chemical Industry, Sanya, China, 13–15 November 2021. [Google Scholar]
- Zhang, L.; Chen, L.; Fan, X.; Xiao, X.; Zheng, J.; Huang, X. Enhanced hydrogen storage properties of MgH2 with numerous hydrogen diffusion channels provided by Na2Ti3O7 nanotubes. J. Mater. Chem. A 2017, 5, 6178–6185. [Google Scholar] [CrossRef]
- Cui, J.; Wang, H.; Liu, J.; Ouyang, L.; Zhang, Q.; Sun, D.; Yao, X.; Zhu, M. Remarkable enhancement in dehydrogenation of MgH2 by a nano-coating of multi-valence Ti-based catalysts. J. Mater. Chem. A 2013, 1, 5603–5611. [Google Scholar] [CrossRef]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y.G. Two-Dimensional Materials: 25th Anniversary Article: MXenes: A New Family of Two-Dimensional Materials (Adv. Mater. 7/2014). Adv. Mater. 2014, 26, 982. [Google Scholar] [CrossRef]
- Hu, Q.; Sun, D.; Wu, Q.; Wang, H.; Wang, L.; Liu, B.; Zhou, A.; He, J. MXene: A New Family of Promising Hydrogen Storage Medium. J. Phys. Chem. A 2013, 117, 14253–14260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xue, M.; Yang, X.; Wang, Z.; Luo, G.; Huang, Z.; Sui, X.; Li, C. Preparation and tribological properties of Ti3C2(OH)(2) nanosheets as additives in base oil. Rsc Adv. 2015, 5, 2762–2767. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Chen, Q.; Li, P.; Zhou, A.; Cao, X.; Hu, Q. Preparation, mechanical and anti-friction performance of MXene/polymer composites. Mater. Des. 2016, 92, 682–689. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, J.; Wu, D.; Zhao, X.; Jing, Y.; Zhou, Z. A Ti-anchored Ti2CO2 monolayer (MXene) as a single-atom catalyst for CO oxidation. J. Mater. Chem. A 2016, 4, 4871–4876. [Google Scholar] [CrossRef]
- Xie, Y.; Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y.; Yu, X.; Nam, K.-W.; Yang, X.-Q.; Kolesnikov, A.I.; Kent, P.R.C. Role of Surface Structure on Li-Ion Energy Storage Capacity of Two-Dimensional Transition-Metal Carbides. J. Am. Chem. Soc. 2014, 136, 6385–6394. [Google Scholar] [CrossRef]
- Yan, P.; Zhang, R.; Jia, J.; Wu, C.; Zhou, A.; Xu, J.; Zhang, X. Enhanced supercapacitive performance of delaminated two-dimensional titanium carbide/carbon nanotube composites in alkaline electrolyte. J. Power Sources 2015, 284, 38–43. [Google Scholar] [CrossRef]
- Yun, T.; Kim, H.; Iqbal, A.; Cho, Y.S.; Lee, G.S.; Kim, M.-K.; Kim, S.J.; Kim, D.; Gogotsi, Y.; Kim, S.O.; et al. Electromagnetic Shielding of Monolayer MXene Assemblies. Adv. Mater. 2020, 32, e1906769. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, H.-B.; Sun, R.; Liu, Y.; Liu, Z.; Zhou, A.; Yu, Z.-Z. Hydrophobic, Flexible, and Lightweight MXene Foams for High-Performance Electromagnetic-Interference Shielding. Adv. Mater. 2017, 29, 1702367. [Google Scholar] [CrossRef]
- Shen, Z.; Wang, Z.; Zhang, M.; Gao, M.; Hu, J.; Du, F.; Liu, Y.; Pan, H. A novel solid-solution MXene (Ti0.5V0.5)3C2 with high catalytic activity for hydrogen storage in MgH2. Materialia 2018, 1, 114–120. [Google Scholar] [CrossRef]
- Gao, H.; Liu, Y.; Zhu, Y.; Zhang, J.; Li, L. Catalytic effect of sandwich-like Ti3C2/TiO2(A)-C on hydrogen storage performance of MgH2. Nanotechnology 2020, 31, 115404. [Google Scholar] [CrossRef]
- Liu, H.Z.; Lu, C.L.; Wang, X.C.; Xu, L.; Huang, X.T.; Wang, X.H.; Ning, H.; Lan, Z.Q.; Guo, J. Combinations of V2C and Ti3C2 MXenes for Boosting the Hydrogen Storage Performances of MgH2. Acs Appl. Mater. Interfaces 2021, 13, 13235–13247. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Shao, Y.; Shi, R.; Liu, Y.; Zhu, J.; Liu, J.; Zhu, Y.; Zhang, J.; Li, L.; Hu, X. Effect of Few-Layer Ti3C2Tx Supported Nano-Ni via Self-Assembly Reduction on Hydrogen Storage Performance of MgH2. Acs. Appl. Mater. Interfaces 2020, 12, 47684–47694. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Y.; Cheng, H.; Zhu, Y.; Li, L.; Lin, H. Effects of two-dimension MXene Ti3C2 on hydrogen storage performances of MgH2-LiAlH4 composite. Chem. Phys. 2019, 522, 178–187. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, K.; Feng, Y.; Qi, R.; Ren, J.; Zhu, Z. VO2(p)-V2C(MXene) Grid Structure as a Lithium Polysulfide Catalytic Host for High-Performance Li–S Battery. ACS Appl. Mater. Interfaces 2019, 11, 44282–44292. [Google Scholar] [CrossRef] [PubMed]
- Shang, C.X.; Guo, Z.X. Effect of carbon on hydrogen desorption and absorption of mechanically milled MgH 2. J. Power Sources 2004, 129, 73–80. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; An, C.; Wang, Y.; Chen, C.; Jiao, L.; Yuan, H. Facile synthesis of TiN decorated graphene and its enhanced catalytic effects on dehydrogenation performance of magnesium hydride. Nanoscale 2014, 6, 6684. [Google Scholar] [CrossRef]
- Blaine, R.L.; Kissinger, H.E. Homer Kissinger and the Kissinger equation. Thermochim. Acta 2012, 540, 1–6. [Google Scholar] [CrossRef]
- Liu, Y.F.; Du, H.F.; Zhang, X.; Yang, Y.X.; Gao, M.X.; Pan, H.G. Superior catalytic activity derived from a two-dimensional Ti3C2 precursor towards the hydrogen storage reaction of magnesium hydride. Chem. Commun. 2016, 52, 705–708. [Google Scholar] [CrossRef]
- Zhu, W.; Panda, S.; Lu, C.; Ma, Z.; Khan, D.; Dong, J.; Sun, F.; Xu, H.; Zhang, Q.; Zou, J. Using a Self-Assembled Two-Dimensional MXene-Based Catalyst (2D-Ni@Ti3C2) to Enhance Hydrogen Storage Properties of MgH2. Acs. Appl. Mater. Interfaces 2020, 12, 50333–50343. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Zhang, X.L.; Ren, Z.H.; Liu, Y.; Hu, J.J.; Li, H.W.; Gao, M.X.; Pan, H.G.; Liu, Y.F. In situ formed ultrafine NbTi nanocrystals from a NbTiC solid-solution MXene for hydrogen storage in MgH2. J. Mater. Chem. A 2019, 7, 14244–14252. [Google Scholar] [CrossRef]
- Huang, X.; Xiao, X.; Zhang, W.; Fan, X.; Zhang, L.; Cheng, C.; Li, S.; Ge, H.; Wang, Q.; Chen, L. Transition metal (Co, Ni) nanoparticles wrapped with carbon and their superior catalytic activities for the reversible hydrogen storage of magnesium hydride. Phys. Chem. Chem. Phys. 2017, 19, 4019–4029. [Google Scholar] [CrossRef] [PubMed]
- Narayanasamy, M.; Kirubasankar, B.; Shi, M.; Velayutham, S.; Wang, B.; Angaiah, S.; Yan, C. Morphology restrained growth of V(2)O(5)by the oxidation of V-MXenes as a fast diffusion controlled cathode material for aqueous zinc ion batteries. Chem. Commun. 2020, 56, 6412–6415. [Google Scholar] [CrossRef]
- Halim, J.; Cook, K.M.; Naguib, M.; Eklund, P.; Gogotsi, Y.; Rosen, J.; Barsoum, M.W. X-ray photoelectron spectroscopy of select multi-layered transition metal carbides (MXenes). Appl. Surf. Sci. 2016, 362, 406–417. [Google Scholar] [CrossRef] [Green Version]
- Xian, K.; Gao, M.; Li, Z.; Gu, J.; Shen, Y.; Wang, S.; Yao, Z.; Liu, Y.; Pan, H. Superior Kinetic and Cyclic Performance of a 2D Titanium Carbide Incorporated 2LiH+MgB2 Composite toward Highly Reversible Hydrogen Storage. Acs. Appl. Energy Mater. 2019, 2, 4853–4864. [Google Scholar] [CrossRef]
- Zhang, X.; Leng, Z.; Gao, M.; Hu, J.; Du, F.; Yao, J.; Pan, H.; Liu, Y. Enhanced hydrogen storage properties of MgH2 catalyzed with carbon-supported nanocrystalline TiO2. J. Power Sources 2018, 398, 183–192. [Google Scholar] [CrossRef]
- Tan, Y.; Zhu, Y.; Li, L. Excellent catalytic effects of multi-walled carbon nanotube supported titania on hydrogen storage of a Mg-Ni alloy. Chem. Commun. 2015, 51, 2368–2371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, J.; Liu, J.; Wang, H.; Ouyang, L.; Sun, D.; Zhu, M.; Yao, X. Mg-TM (TM: Ti, Nb, V, Co, Mo or Ni) core-shell like nanostructures: Synthesis, hydrogen storage performance and catalytic mechanism. J. Mater. Chem. A 2014, 2, 9645–9655. [Google Scholar] [CrossRef]
- Chen, M.; Xiao, X.; Zhang, M.; Liu, M.; Huang, X.; Zheng, J.; Zhang, Y.; Jiang, L.; Chen, L. Excellent synergistic catalytic mechanism of in-situ formed nanosized Mg2Ni and multiple valence titanium for improved hydrogen desorption properties of magnesium hydride. Int. J. Hydrogen Energy 2019, 44, 1750–1759. [Google Scholar] [CrossRef]

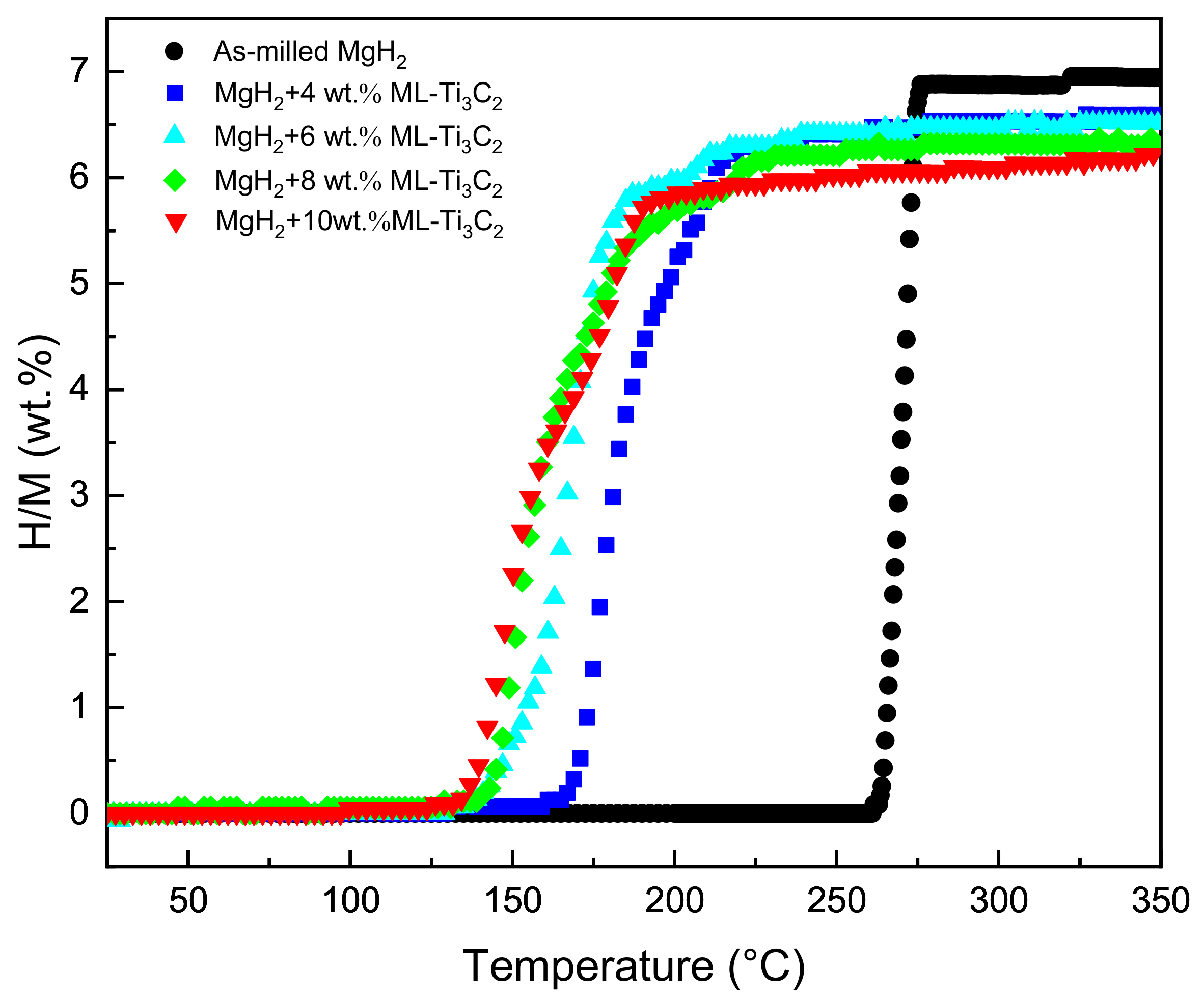


| Hydrogenation Temperature (°C) | Hydrogenation Capacity (wt.%) | Dehydrogenation Temperature (°C) | Dehydrogenation Capacity (wt.%) |
|---|---|---|---|
| 150 | 6.47 | 240 | 6.45 |
| 125 | 6.20 | 200 | 6.29 |
| 100 | 4.86 | 160 | 6.08 |
| 75 | 4.20 | 140 | 1.95 (3.63 wt.% in 1 h) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Fang, J.; Liu, N.; Wu, J.; Kong, L. The Improvement in Hydrogen Storage Performance of MgH2 Enabled by Multilayer Ti3C2. Micromachines 2021, 12, 1190. https://doi.org/10.3390/mi12101190
Wu Z, Fang J, Liu N, Wu J, Kong L. The Improvement in Hydrogen Storage Performance of MgH2 Enabled by Multilayer Ti3C2. Micromachines. 2021; 12(10):1190. https://doi.org/10.3390/mi12101190
Chicago/Turabian StyleWu, Zhaojie, Jianhua Fang, Na Liu, Jiang Wu, and Linglan Kong. 2021. "The Improvement in Hydrogen Storage Performance of MgH2 Enabled by Multilayer Ti3C2" Micromachines 12, no. 10: 1190. https://doi.org/10.3390/mi12101190
APA StyleWu, Z., Fang, J., Liu, N., Wu, J., & Kong, L. (2021). The Improvement in Hydrogen Storage Performance of MgH2 Enabled by Multilayer Ti3C2. Micromachines, 12(10), 1190. https://doi.org/10.3390/mi12101190