Next-Generation Wearable Biosensors Developed with Flexible Bio-Chips
Abstract
:1. Introduction
2. Current Biosensor Technologies
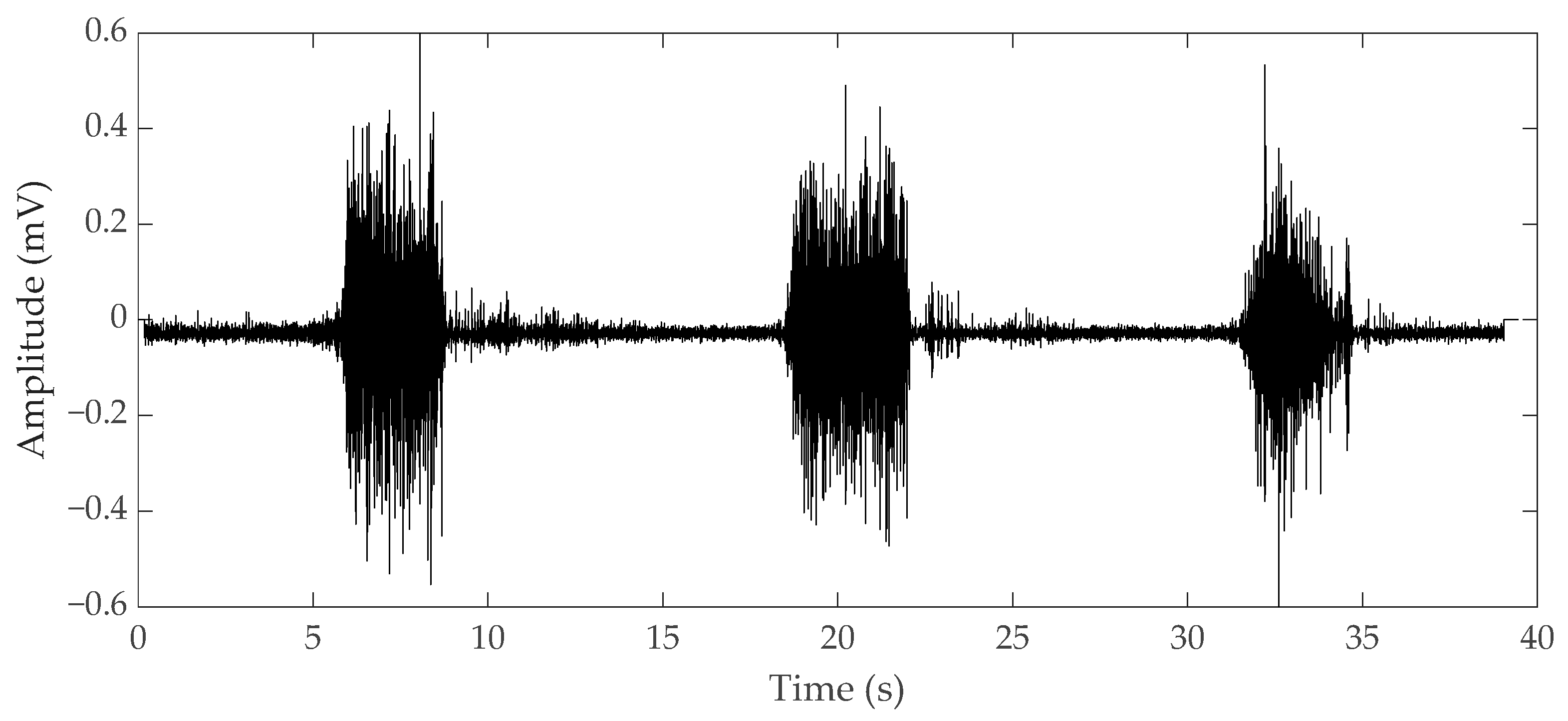
2.1. Electromyography
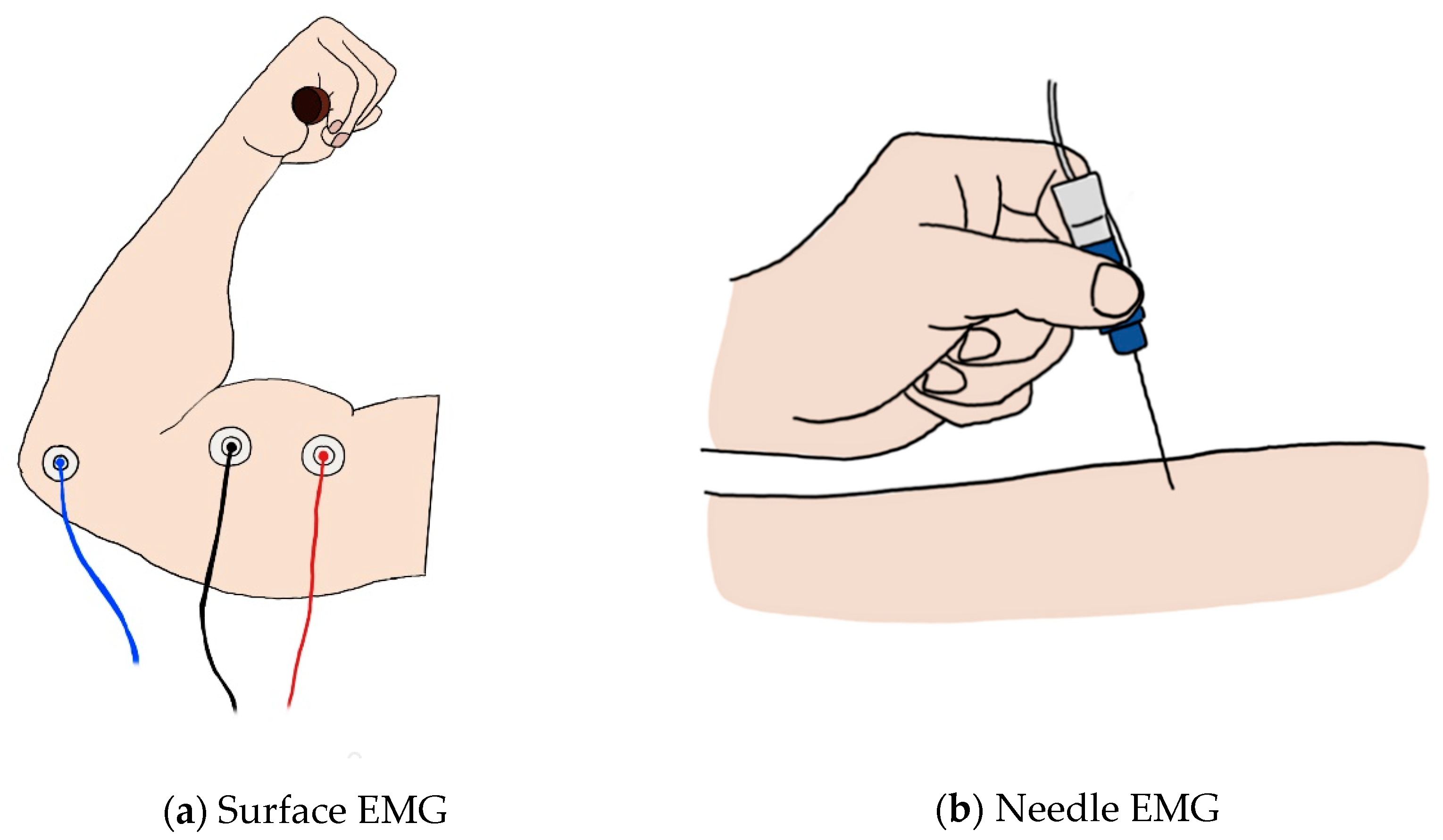
2.1.1. Surface EMG
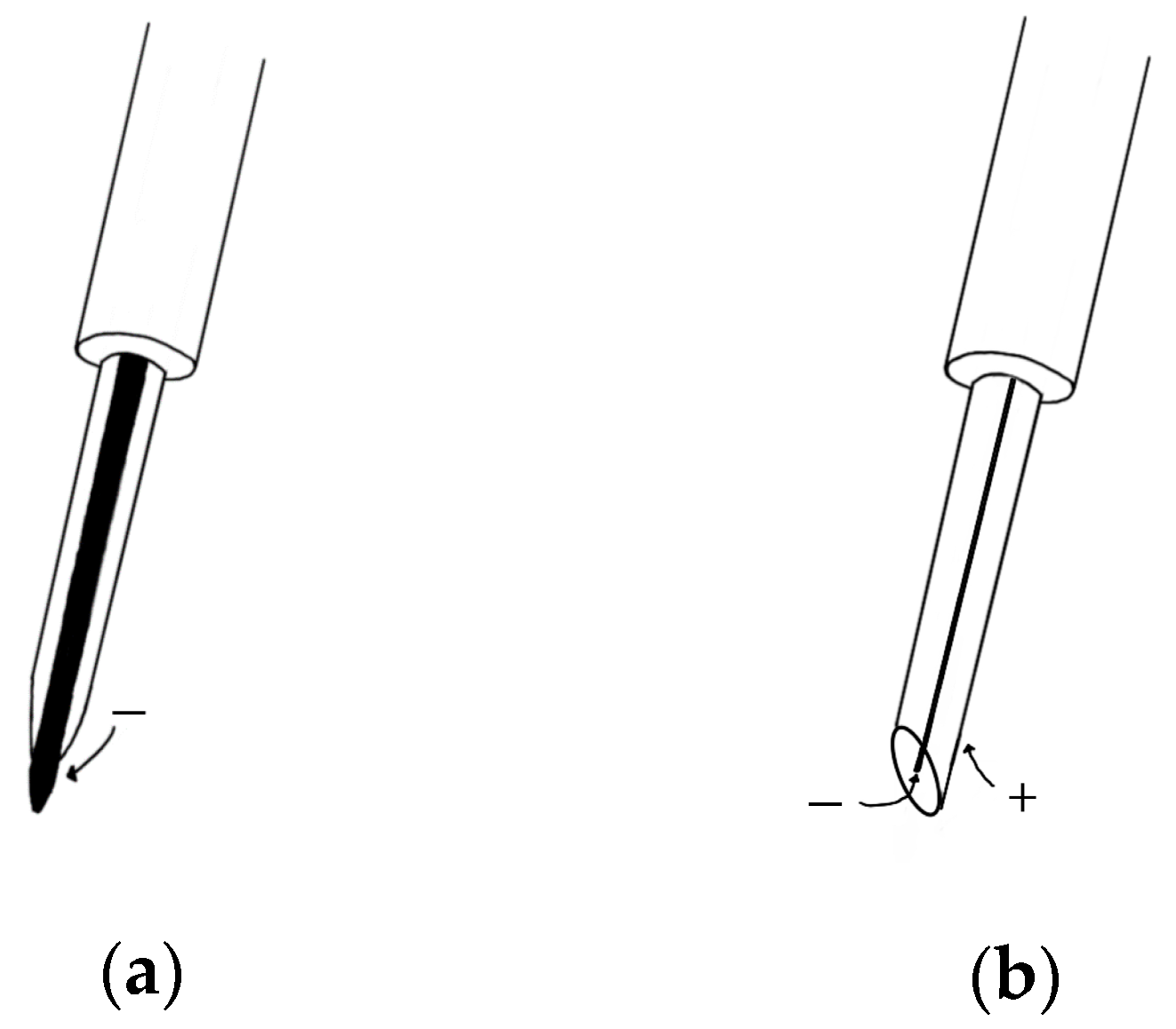
2.1.2. Needle EMG
2.2. Electrocardiography
2.3. Photoplethysmography
2.4. Electroencephalography
3. Limitations of Current Biosensors
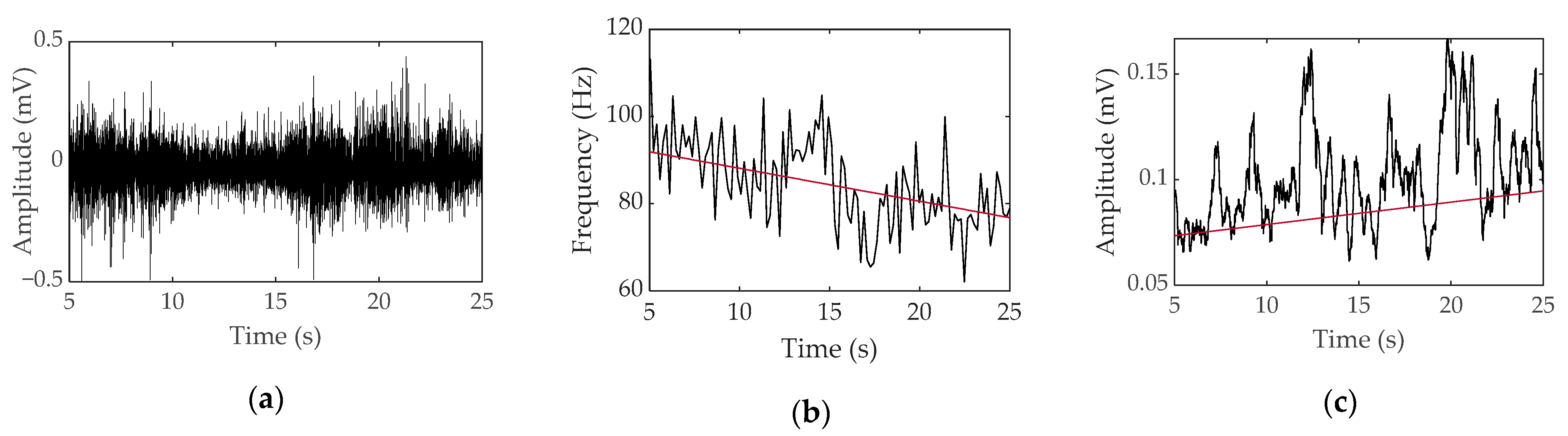
3.1. Transducer Noise
3.2. Electrode Artifacts during Body Movement
4. Technology for the Next Generation of Flexible Wearable Biosensors
4.1. Noise Elimination
4.2. New Types of Biosensors
5. Concluding Remarks and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Burns, A.; Greene, B.R.; McGrath, M.J.; O’Shea, T.J.; Kuris, B.; Ayer, S.M.; Stroiescu, F.; Cionca, V. SHIMMER™–A wireless sensor platform for noninvasive biomedical research. IEEE Sens. J. 2010, 10, 1527–1534. [Google Scholar] [CrossRef]
- Bonato, P. Advances in wearable technology and applications in physical medicine and rehabilitation. BioMed Central 2005, 2, 1–4. [Google Scholar]
- Reisner, A.; Shaltis, P.; McCombie, D.; Asada, H. A critical appraisal of opportunities for wearable medical sensors. In Proceedings of the 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Francisco, CA, USA, 1–5 September 2004; pp. 2149–2152. [Google Scholar]
- Ebashi, S.; Endo, M.; Ohtsuki, I. Control of muscle contraction. Q. Rev. Biophys. 1969, 2, 351–384. [Google Scholar] [CrossRef] [PubMed]
- Sandow, A. Excitation-contraction coupling in muscular response. Yale J. Biol. Med. 1952, 25, 176–201. [Google Scholar] [PubMed]
- De Luca, C.J. The use of surface electromyography in biomechanics. J. Appl. Biomech. 1997, 13, 135–163. [Google Scholar] [CrossRef] [Green Version]
- Farina, D.; Merletti, R.; Enoka, R.M. The extraction of neural strategies from the surface EMG: An update. J. Appl. Physiol. 2014, 117, 1215–1230. [Google Scholar] [CrossRef] [Green Version]
- Merletti, R.; Parker, P.J. Electromyography: Physiology, Engineering, and Non-Invasive Applications; John Wiley & Sons: Hoboken, NJ, USA, 2004; Volume 11. [Google Scholar]
- Travell, J.G. Myofascial trigger points: Clinical view. Adv. Pain Res. Ther. 1976, 1, 919–926. [Google Scholar]
- Chen, J.-T.; Chen, S.-M.; Kuan, T.-S.; Chung, K.-C.; Hong, C.-Z. Phentolamine effect on the spontaneous electrical activity of active loci in a myofascial trigger spot of rabbit skeletal muscle. Arch. Phys. Med. Rehabil. 1998, 79, 790–794. [Google Scholar] [CrossRef]
- Simons, D.G.; Dexter, J.R. Comparison of local twitch responses elicited by palpitation and needling of myofascial trigger points. J. Musculoskelet. Pain 1995, 3, 49–61. [Google Scholar] [CrossRef]
- Campbell, W.W. Essentials of Electrodiagnostic Medicine; Demos Medical Publishing: New York, NY, USA, 2013. [Google Scholar]
- Bunzynski, T.H.; Stoyva, J.M.; Adler, C.S.; Mullaney, D.J. EMG biofeedback and tension headache: A controlled outcome study. Psychosom. Med. 1973, 35, 484–496. [Google Scholar] [CrossRef] [Green Version]
- Glazer, H.I.; Rodke, G.; Swencionis, C.; Hertz, R.; Young, A.W. Treatment of vulvar vestibulitis syndrome with electromyographic biofeedback of pelvic floor musculature. Obstet. Gynecol. Surv. 1995, 50, 658–659. [Google Scholar] [CrossRef]
- Milner-Brown, H.; Stein, R. The relation between the surface electromyogram and muscular force. J. Physiol. 1975, 246, 549–569. [Google Scholar] [CrossRef] [PubMed]
- Mathiassen, S.; Winkel, J.; Hägg, G. Normalization of surface EMG amplitude from the upper trapezius muscle in ergonomic studies—A review. J. Electromyogr. Kinesiol. 1995, 5, 197–226. [Google Scholar] [CrossRef]
- De Luca, C.J. Myoelectrical manifestations of localized muscular fatigue in humans. Crit. Rev. Biomed. Eng. 1984, 11, 251. [Google Scholar] [PubMed]
- Nazmi, N.; Abdul Rahman, M.A.; Yamamoto, S.-I.; Ahmad, S.A.; Zamzuri, H.; Mazlan, S.A. A review of classification techniques of EMG signals during isotonic and isometric contractions. Sensors 2016, 16, 1304. [Google Scholar] [CrossRef] [Green Version]
- Naeije, M.; Zorn, H. Relation between EMG power spectrum shifts and muscle fibre action potential conduction velocity changes during local muscular fatigue in man. Eur. J. Appl. Physiol. Occup. Physiol. 1982, 50, 23–33. [Google Scholar] [CrossRef]
- Gerdle, B.; Larsson, B.; Karlsson, S. Criterion validation of surface EMG variables as fatigue indicators using peak torque: A study of repetitive maximum isokinetic knee extensions. J. Electromyogr. Kinesiol. 2000, 10, 225–232. [Google Scholar] [CrossRef]
- Kollmitzer, J.; Ebenbichler, G.R.; Kopf, A. Reliability of surface electromyographic measurements. Clin. Neurophysiol. 1999, 110, 725–734. [Google Scholar] [CrossRef]
- Dedering, Å.; Németh, G.; Harms-Ringdahl, K. Correlation between electromyographic spectral changes and subjective assessment of lumbar muscle fatigue in subjects without pain from the lower back. Clin. Biomech. 1999, 14, 103–111. [Google Scholar] [CrossRef]
- Petrofsky, J.S.; Glaser, R.M.; Phillips, C.A.; Lind, A.R.; Williams, C. Evaluation of the amplitude and frequency components of the surface EMG as an index of muscle fatigue. Ergonomics 1982, 25, 213–223. [Google Scholar] [CrossRef]
- Komi, P.V.; Tesch, P. EMG frequency spectrum, muscle structure, and fatigue during dynamic contractions in man. Eur. J. Appl. Physiol. Occup. Physiol. 1979, 42, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Moritani, T.; Muro, M.; Nagata, A. Intramuscular and surface electromyogram changes during muscle fatigue. J. Appl. Physiol. 1986, 60, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Mannion, A.F.; Dolan, P. Electromyographic median frequency changes during isometric contraction of the back extensors to fatigue. Spine 1994, 19, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.H.; De Luca, C.J.; Schneider, J. Effects of electrode location on myoelectric conduction velocity and median frequency estimates. J. Appl. Physiol. 1986, 61, 1510–1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rainoldi, A.; Melchiorri, G.; Caruso, I. A method for positioning electrodes during surface EMG recordings in lower limb muscles. J. Neurosci. Methods 2004, 134, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.; Kippers, V.; Richardson, C. Muscle fibre orientation of abdominal muscles and suggested surface EMG electrode positions. Electromyogr. Clin. Neurophysiol. 1998, 38, 51–58. [Google Scholar]
- Hermens, H.J.; Freriks, B.; Merletti, R.; Stegeman, D.; Blok, J.; Rau, G.; Disselhorst-Klug, C.; Hägg, G. European recommendations for surface electromyography. Roessingh Res. Dev. 1999, 8, 13–54. [Google Scholar]
- Ament, W.; Bonga, G.J.; Hof, A.L.; Verkerke, G.J. EMG median power frequency in an exhausting exercise. J. Electromyogr. Kinesiol. 1993, 3, 214–220. [Google Scholar] [CrossRef]
- Minning, S.; Eliot, C.A.; Uhl, T.L.; Malone, T.R. EMG analysis of shoulder muscle fatigue during resisted isometric shoulder elevation. J. Electromyogr. Kinesiol. 2007, 17, 153–159. [Google Scholar] [CrossRef]
- Dimitrov, G.V.; Arabadzhiev, T.I.; Mileva, K.N.; Bowtell, J.L.; Crichton, N.; Dimitrova, N.A. Muscle fatigue during dynamic contractions assessed by new spectral indices. Med. Sci. Sports Exerc. 2006, 38, 1971. [Google Scholar] [CrossRef]
- Roy, S.H.; Bonato, P.; Knaflitz, M. EMG assessment of back muscle function during cyclical lifting. J. Electromyogr. Kinesiol. 1998, 8, 233–245. [Google Scholar] [CrossRef]
- Hoozemans, M.J.; Van Dieen, J.H. Prediction of handgrip forces using surface EMG of forearm muscles. J. Electromyogr. Kinesiol. 2005, 15, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Van Dieën, J.; De Looze, M.; Hermans, V. Effects of dynamic office chairs on trunk kinematics, trunk extensor EMG and spinal shrinkage. Ergonomics 2001, 44, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Akin, M.; Kurt, M.B.; Sezgin, N.; Bayram, M. Estimating vigilance level by using EEG and EMG signals. Neural Comput. Appl. 2008, 17, 227–236. [Google Scholar] [CrossRef]
- Lal, S.K.; Craig, A. Driver fatigue: Electroencephalography and psychological assessment. Psychophysiology 2002, 39, 313–321. [Google Scholar] [CrossRef]
- Fu, R.; Wang, H. Detection of driving fatigue by using noncontact EMG and ECG signals measurement system. Int. J. Neural Syst. 2014, 24, 1450006. [Google Scholar] [CrossRef]
- Rubin, D.I. Needle electromyography: Basic concepts and patterns of abnormalities. Neurol. Clin. 2012, 30, 429–456. [Google Scholar] [CrossRef]
- Strommen, J.A.; Daube, J.R. Determinants of pain in needle electromyography. Clin. Neurophysiol. 2001, 112, 1414–1418. [Google Scholar] [CrossRef]
- Boe, S.G.; Stashuk, D.W.; Doherty, T.J. Motor unit number estimation by decomposition-enhanced spike-triggered averaging: Control data, test-retest reliability, and contractile level effects. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 2004, 29, 693–699. [Google Scholar] [CrossRef]
- Nandedkar, S.D.; Barkhaus, P.E.; Sanders, D.B.; Stålberg, E.V. Analysis of amplitude and area of concentric needle EMG motor unit action potentials. Electroencephalogr. Clin. Neurophysiol. 1988, 69, 561–567. [Google Scholar] [CrossRef]
- Stålberg, E.; Falck, B.; Sonoo, M.; Stålberg, S.; Åström, M. Multi-MUP EMG analysis—A two year experience in daily clinical work. Electroencephalogr. Clin. Neurophysiol. Electromyogr. Mot. Control 1995, 97, 145–154. [Google Scholar] [CrossRef]
- Watanabe, K.; Akima, H. Validity of surface electromyography for vastus intermedius muscle assessed by needle electromyography. J. Neurosci. Methods 2011, 198, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, J.; Rafiee, M.; Yavari, F.; Schoen, M. Feature extraction of forearm EMG signals for prosthetics. Expert Syst. Appl. 2011, 38, 4058–4067. [Google Scholar] [CrossRef]
- Stålberg, E. Jitter analysis with concentric needle electrodes. Ann. New York Acad. Sci. 2012, 1274, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Ertaş, M.; Baslo, M.B.; Yildiz, N.; Yazici, J.; Öge, A.E. Concentric needle electrode for neuromuscular jitter analysis. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 2000, 23, 715–719. [Google Scholar] [CrossRef]
- Sarrigiannis, P.G.; Kennett, R.P.; Read, S.; Farrugia, M.E. Single-fiber EMG with a concentric needle electrode: Validation in myasthenia gravis. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 2006, 33, 61–65. [Google Scholar] [CrossRef]
- Finsterer, J.; Fuglsang-Frederiksen, A.; Mamoli, B. Needle EMG of the tongue: Motor unit action potential versus peak ratio analysis in limb and bulbar onset amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 1997, 63, 175–180. [Google Scholar] [CrossRef] [Green Version]
- Fye, W.B. A history of the origin, evolution, and impact of electrocardiography. Am. J. Cardiol. 1994, 73, 937–949. [Google Scholar] [CrossRef]
- Einthoven, W.; Fahr, G.; De Waart, A. Über die Richtung und die manifeste Grösse der Potentialschwankungen im menschlichen Herzen und über den Einfluss der Herzlage auf die Form des Elektrokardiogramms. Pflüger’s Arch. Für Die Gesamte Physiol. Des Menschen Und Der Tiere 1913, 150, 275–315. [Google Scholar] [CrossRef]
- Kligfield, P.; Gettes, L.S.; Bailey, J.J.; Childers, R.; Deal, B.J.; Hancock, E.W.; Van Herpen, G.; Kors, J.A.; Macfarlane, P.; Mirvis, D.M. Recommendations for the standardization and interpretation of the electrocardiogram: Part I: The electrocardiogram and its technology a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society endorsed by the International Society for Computerized Electrocardiology. J. Am. Coll. Cardiol. 2007, 49, 1109–1127. [Google Scholar]
- Holmes, J.; Kubo, S.H.; Cody, R.J.; Kligfield, P. Arrhythmias in ischemic and nonischemic dilated cardiomyopathy: Prediction of mortality by ambulatory electrocardiography. Am. J. Cardiol. 1985, 55, 146–151. [Google Scholar] [CrossRef]
- Algra, A.; Tijssen, J.; Roelandt, J.; Pool, J.; Lubsen, J. QTc prolongation measured by standard 12-lead electrocardiography is an independent risk factor for sudden death due to cardiac arrest. Circulation 1991, 83, 1888–1894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleg, J.L.; Kennedy, H.L. Cardiac arrhythmias in a healthy elderly population: Detection by 24-hour ambulatory electrocardiography. Chest 1982, 81, 302–307. [Google Scholar] [CrossRef]
- Wang, Y.; Agrafioti, F.; Hatzinakos, D.; Plataniotis, K.N. Analysis of human electrocardiogram for biometric recognition. Eurasip J. Adv. Signal Process. 2007, 2008, 148658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biel, L.; Pettersson, O.; Philipson, L.; Wide, P. ECG analysis: A new approach in human identification. IEEE Trans. Instrum. Meas. 2001, 50, 808–812. [Google Scholar] [CrossRef] [Green Version]
- Challoner, A. Non-Invasive Physiological Measurements; Rolfe, P., Ed.; Academic: London, UK, 1979. [Google Scholar]
- Kamal, A.; Harness, J.; Irving, G.; Mearns, A. Skin photoplethysmography—A review. Comput. Methods Programs Biomed. 1989, 28, 257–269. [Google Scholar] [CrossRef]
- Allen, J. Photoplethysmography and its application in clinical physiological measurement. Physiol. Meas. 2007, 28, R1. [Google Scholar] [CrossRef] [Green Version]
- Shelley, K.H. Photoplethysmography: Beyond the calculation of arterial oxygen saturation and heart rate. Anesth. Analg. 2007, 105, S31–S36. [Google Scholar] [CrossRef] [Green Version]
- Phan, D.; Siong, L.Y.; Pathirana, P.N.; Seneviratne, A. Smartwatch: Performance evaluation for long-term heart rate monitoring. In Proceedings of the 2015 International Symposium on Bioelectronics and Bioinformatics (ISBB), Beijing, China, 14–17 October 2015; pp. 144–147. [Google Scholar]
- Sun, Y.; Thakor, N. Photoplethysmography revisited: From contact to noncontact, from point to imaging. Ieee Trans. Biomed. Eng. 2015, 63, 463–477. [Google Scholar] [CrossRef] [Green Version]
- Selvaraj, N.; Jaryal, A.; Santhosh, J.; Deepak, K.K.; Anand, S. Assessment of heart rate variability derived from finger-tip photoplethysmography as compared to electrocardiography. J. Med Eng. Technol. 2008, 32, 479–484. [Google Scholar] [CrossRef]
- Yan, Y.; Poon, C.C.Y.; Zhang, Y. Reduction of motion artifact in pulse oximetry by smoothed pseudo Wigner-Ville distribution. J. Neuroeng. Rehabil. 2005, 2, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, K.; Zhang, Y. Adaptive reduction of motion artifact from photoplethysmographic recordings using a variable step-size LMS filter. In Proceedings of the SENSORS, 2002 IEEE, Orlando, FL, USA, 12–14 June 2002; pp. 1343–1346. [Google Scholar]
- Seyedtabaii, S.; Seyedtabaii, L. Kalman filter based adaptive reduction of motion artifact from photoplethysmographic signal. Int. J. Electr. Comput. Eng. 2008, 3, 597–600. [Google Scholar]
- Haas, L.F. Hans berger (1873–1941), richard caton (1842–1926), and electroencephalography. J. Neurol. Neurosurg. Psychiatry 2003, 74, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teplan, M. Fundamentals of EEG measurement. Meas. Sci. Rev. 2002, 2, 1–11. [Google Scholar]
- Al Naqeeb, N.; Edwards, A.D.; Cowan, F.M.; Azzopardi, D. Assessment of neonatal encephalopathy by amplitude-integrated electroencephalography. Pediatrics 1999, 103, 1263–1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, W.W. The hyperkinetic child: A neurological appraisal. Neurology 1963, 13, 968. [Google Scholar] [CrossRef]
- Casson, A.J.; Yates, D.C.; Smith, S.J.; Duncan, J.S.; Rodriguez-Villegas, E. Wearable electroencephalography. IEEE Eng. Med. Biol. Mag. 2010, 29, 44–56. [Google Scholar] [CrossRef] [Green Version]
- Huigen, E.; Peper, A.; Grimbergen, C. Investigation into the origin of the noise of surface electrodes. Med Biol. Eng. Comput. 2002, 40, 332–338. [Google Scholar] [CrossRef]
- Puurtinen, M.M.; Komulainen, S.M.; Kauppinen, P.K.; Malmivuo, J.A.; Hyttinen, J.A. Measurement of noise and impedance of dry and wet textile electrodes, and textile electrodes with hydrogel. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; pp. 6012–6015. [Google Scholar]
- Bokareva, T.; Hu, W.; Kanhere, S.; Ristic, B.; Gordon, N.; Bessell, T.; Rutten, M.; Jha, S. Wireless sensor networks for battlefield surveillance. In Proceedings of the Land Warfare Conference, Brisbane, QLD, Australia, 24–27 October 2006; pp. 1–8. [Google Scholar]
- Roy, S.H.; De Luca, G.; Cheng, M.S.; Johansson, A.; Gilmore, L.D.; De Luca, C.J. Electro-mechanical stability of surface EMG sensors. Med Biol. Eng. Comput. 2007, 45, 447–457. [Google Scholar] [CrossRef]
- Webster, J.G. Reducing motion artifacts and interference in biopotential recording. IEEE Trans. Biomed. Eng. 1984, 31, 823–826. [Google Scholar] [CrossRef]
- Agante, P.; De Sá, J.M. ECG noise filtering using wavelets with soft-thresholding methods. In Proceedings of the Computers in Cardiology, Hannover, Germany, 26–29 September 1999; Volume 26, pp. 535–538. [Google Scholar]
- Phinyomark, A.; Limsakul, C.; Phukpattaranont, P. A novel feature extraction for robust EMG pattern recognition. arXiv 2009, arXiv:0912.3973. [Google Scholar]
- Coosemans, J.; Hermans, B.; Puers, R. Integrating wireless ECG monitoring in textiles. Sens. Actuators A Phys. 2006, 130, 48–53. [Google Scholar] [CrossRef]
- Catrysse, M.; Puers, R.; Hertleer, C.; Van Langenhove, L.; Van Egmond, H.; Matthys, D. Towards the integration of textile sensors in a wireless monitoring suit. Sens. Actuators A Phys. 2004, 114, 302–311. [Google Scholar] [CrossRef]
- Henry, S.; McAllister, D.V.; Allen, M.G.; Prausnitz, M.R. Microfabricated microneedles: A novel approach to transdermal drug delivery. J. Pharm. Sci. 1998, 87, 922–925. [Google Scholar] [CrossRef] [PubMed]
- Stankovic, J.A.; Cao, Q.; Doan, T.; Fang, L.; He, Z.; Kiran, R.; Lin, S.; Son, S.; Stoleru, R.; Wood, A. Wireless sensor networks for in-home healthcare: Potential and challenges. In Proceedings of the High Confidence Medical Device Software and Systems (HCMDSS) Workshop, Arlington, VA, USA, 16–17 November 2014. [Google Scholar]
- Ren, L.; Xu, S.; Gao, J.; Lin, Z.; Chen, Z.; Liu, B.; Liang, L.; Jiang, L. Fabrication of flexible microneedle array electrodes for wearable bio-signal recording. Sensors 2018, 18, 1191. [Google Scholar] [CrossRef] [Green Version]
- Li, C.G.; Lee, C.Y.; Lee, K.; Jung, H. An optimized hollow microneedle for minimally invasive blood extraction. Biomed. Microdevices 2013, 15, 17–25. [Google Scholar] [CrossRef]
- Rogers, J.A.; Someya, T.; Huang, Y. Materials and mechanics for stretchable electronics. Science 2010, 327, 1603–1607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, S.M.; Park, J.K.; Sul, O.J.; Cho, B.J. Determination of work function of graphene under a metal electrode and its role in contact resistance. Nano Lett. 2012, 12, 3887–3892. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, M.S.; Jeon, S.; Kim, M.; Kim, S.; Kim, K.; Bien, F.; Hong, S.Y.; Park, J.U. Highly Transparent and Stretchable Field-Effect Transistor Sensors Using Graphene–Nanowire Hybrid Nanostructures. Adv. Mater. 2015, 27, 3292–3297. [Google Scholar] [CrossRef] [PubMed]
- Steinhoff, G.; Baur, B.; Wrobel, G.; Ingebrandt, S.; Offenhäusser, A.; Dadgar, A.; Krost, A.; Stutzmann, M.; Eickhoff, M. Recording of cell action potentials with Al Ga N/Ga N field-effect transistors. Appl. Phys. Lett. 2005, 86, 033901. [Google Scholar] [CrossRef] [Green Version]
- Shao, Y.; Wang, J.; Wu, H.; Liu, J.; Aksay, I.A.; Lin, Y. Graphene based electrochemical sensors and biosensors: A review. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2010, 22, 1027–1036. [Google Scholar] [CrossRef]
- Kuzum, D.; Takano, H.; Shim, E.; Reed, J.C.; Juul, H.; Richardson, A.G.; De Vries, J.; Bink, H.; Dichter, M.A.; Lucas, T.H. Transparent and flexible low noise graphene electrodes for simultaneous electrophysiology and neuroimaging. Nat. Commun. 2014, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Park, D.-W.; Brodnick, S.K.; Ness, J.P.; Atry, F.; Krugner-Higby, L.; Sandberg, A.; Mikael, S.; Richner, T.J.; Novello, J.; Kim, H. Fabrication and utility of a transparent graphene neural electrode array for electrophysiology, in vivo imaging, and optogenetics. Nat. Protoc. 2016, 11, 2201–2222. [Google Scholar] [CrossRef] [PubMed]
- Araki, T.; Uemura, T.; Yoshimoto, S.; Takemoto, A.; Noda, Y.; Izumi, S.; Sekitani, T. Wireless monitoring using a stretchable and transparent sensor sheet containing metal nanowires. Adv. Mater. 2020, 32, 1902684. [Google Scholar]
- Choi, S.; Han, S.I.; Jung, D.; Hwang, H.J.; Lim, C.; Bae, S.; Park, O.K.; Tschabrunn, C.M.; Lee, M.; Bae, S.Y. Highly conductive, stretchable and biocompatible Ag–Au core–sheath nanowire composite for wearable and implantable bioelectronics. Nat. Nanotechnol. 2018, 13, 1048–1056. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.-W.; Ghidelli, M.; An, H.S.; Jang, J.; Li Bassi, A.; Lee, S.-Y.; Park, J.-U. Integration of Transparent Supercapacitors and Electrodes Using Nanostructured Metallic Glass Films for Wirelessly Rechargeable, Skin Heat Patches. Nano Lett. 2020, 20, 4872–4881. [Google Scholar] [CrossRef]
- Kim, J.; Kim, M.; Lee, M.-S.; Kim, K.; Ji, S.; Kim, Y.-T.; Park, J.; Na, K.; Bae, K.-H.; Kim, H.K. Wearable smart sensor systems integrated on soft contact lenses for wireless ocular diagnostics. Nat. Commun. 2017, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Li, X.; Cheng, H.; Liu, Z.; Zhao, L.; Yang, T.; Dai, Z.; Cheng, Z.; Shi, E.; Yang, L. Graphene reinforced carbon nanotube networks for wearable strain sensors. Adv. Funct. Mater. 2016, 26, 2078–2084. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, Y.; Qiao, M.; Ai, L.; Sun, K.; Mi, Q.; Zang, S.; Zuo, Y.; Yuan, X.; Wang, Q. Human motion monitoring in sports using wearable graphene-coated fiber sensors. Sens. Actuators A Phys. 2018, 274, 132–140. [Google Scholar] [CrossRef]
- Jang, J.; Hyun, B.G.; Ji, S.; Cho, E.; An, B.W.; Cheong, W.H.; Park, J.-U. Rapid production of large-area, transparent and stretchable electrodes using metal nanofibers as wirelessly operated wearable heaters. Npg Asia Mater. 2017, 9, e432. [Google Scholar] [CrossRef] [Green Version]
- Määttänen, A.; Vanamo, U.; Ihalainen, P.; Pulkkinen, P.; Tenhu, H.; Bobacka, J.; Peltonen, J. A low-cost paper-based inkjet-printed platform for electrochemical analyses. Sens. Actuators B Chem. 2013, 177, 153–162. [Google Scholar] [CrossRef]
- Bihar, E.; Roberts, T.; Ismailova, E.; Saadaoui, M.; Isik, M.; Sanchez-Sanchez, A.; Mecerreyes, D.; Hervé, T.; De Graaf, J.B.; Malliaras, G.G. Fully printed electrodes on stretchable textiles for long-term electrophysiology. Adv. Mater. Technol. 2017, 2, 1600251. [Google Scholar] [CrossRef]
- Yeo, W.H.; Kim, Y.S.; Lee, J.; Ameen, A.; Shi, L.; Li, M.; Wang, S.; Ma, R.; Jin, S.H.; Kang, Z. Multifunctional epidermal electronics printed directly onto the skin. Adv. Mater. 2013, 25, 2773–2778. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Lu, N.; Ma, R.; Kim, Y.-S.; Kim, R.-H.; Wang, S.; Wu, J.; Won, S.M.; Tao, H.; Islam, A. Epidermal electronics. Science 2011, 333, 838–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipomi, D.J.; Vosgueritchian, M.; Tee, B.C.; Hellstrom, S.L.; Lee, J.A.; Fox, C.H.; Bao, Z. Skin-like pressure and strain sensors based on transparent elastic films of carbon nanotubes. Nat. Nanotechnol. 2011, 6, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Segev-Bar, M.; Haick, H. Flexible sensors based on nanoparticles. ACS Nano 2013, 7, 8366–8378. [Google Scholar] [CrossRef]
- Unno, Y.; Affolder, A.; Allport, P.; Bates, R.; Betancourt, C.; Bohm, J.; Brown, H.; Buttar, C.; Carter, J.; Casse, G. Development of n-on-p silicon sensors for very high radiation environments. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometersdetectors Assoc. Equip. 2011, 636, S24–S30. [Google Scholar] [CrossRef]
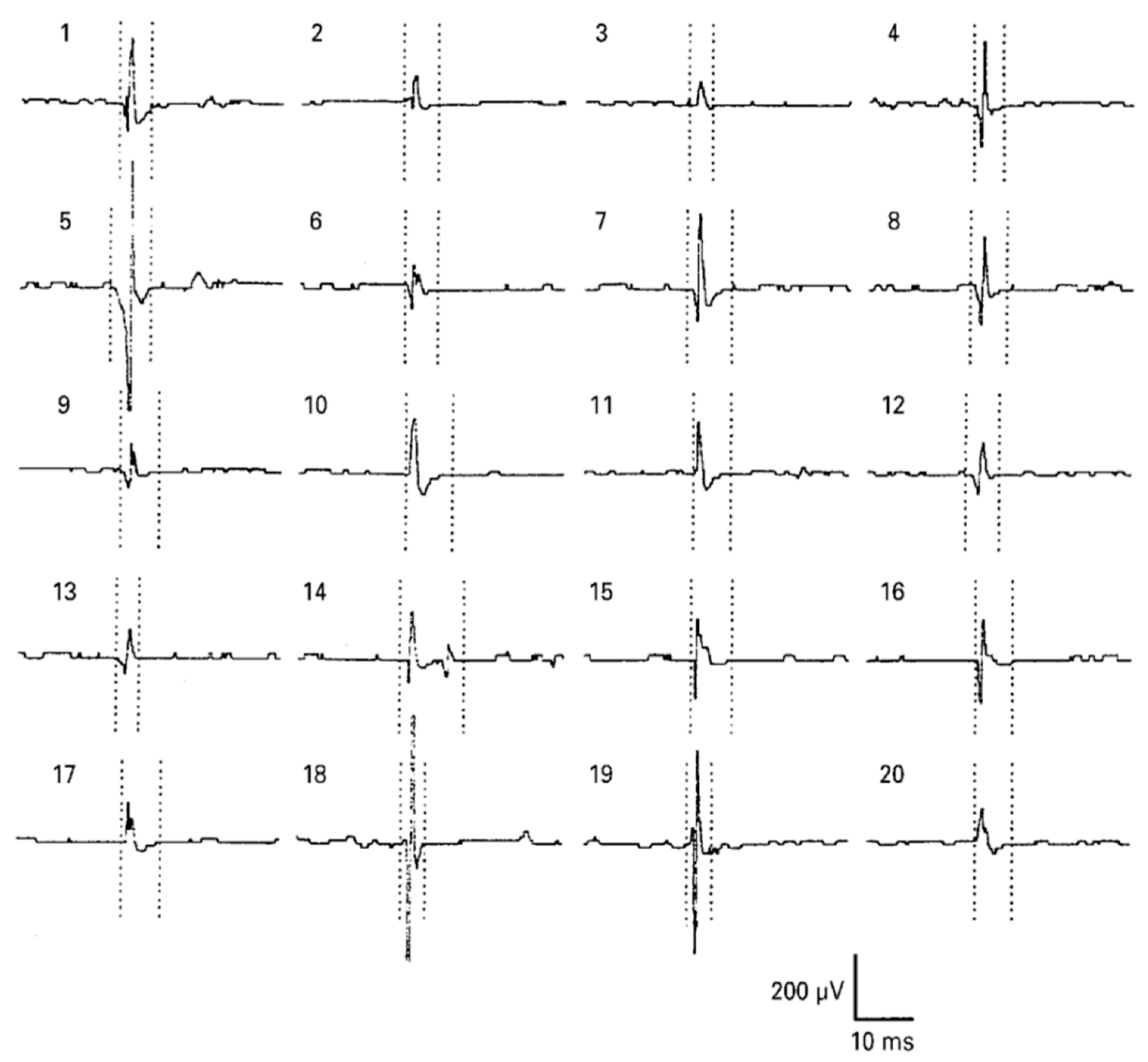
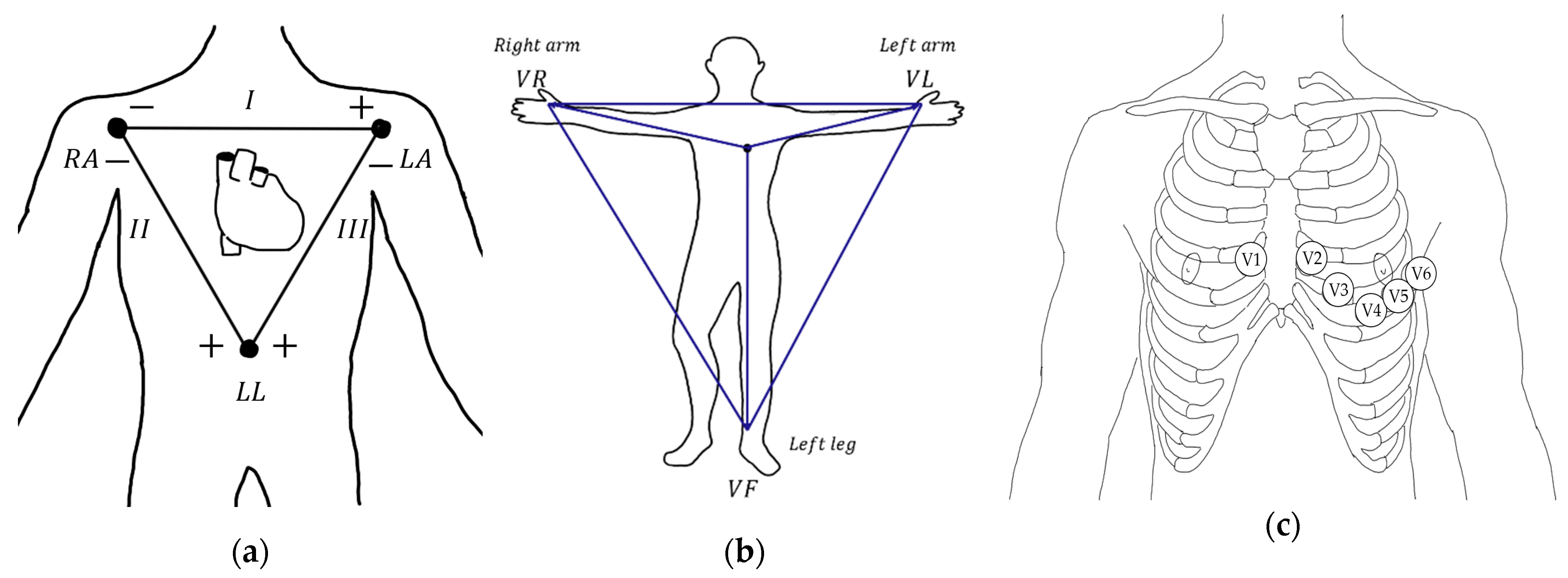
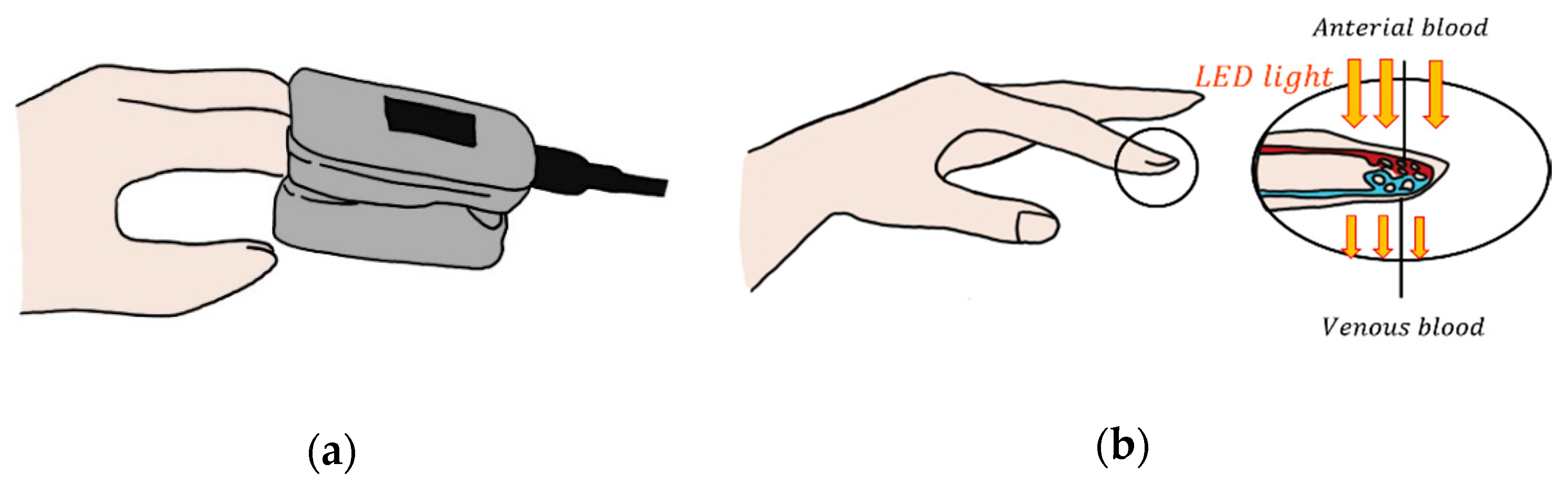
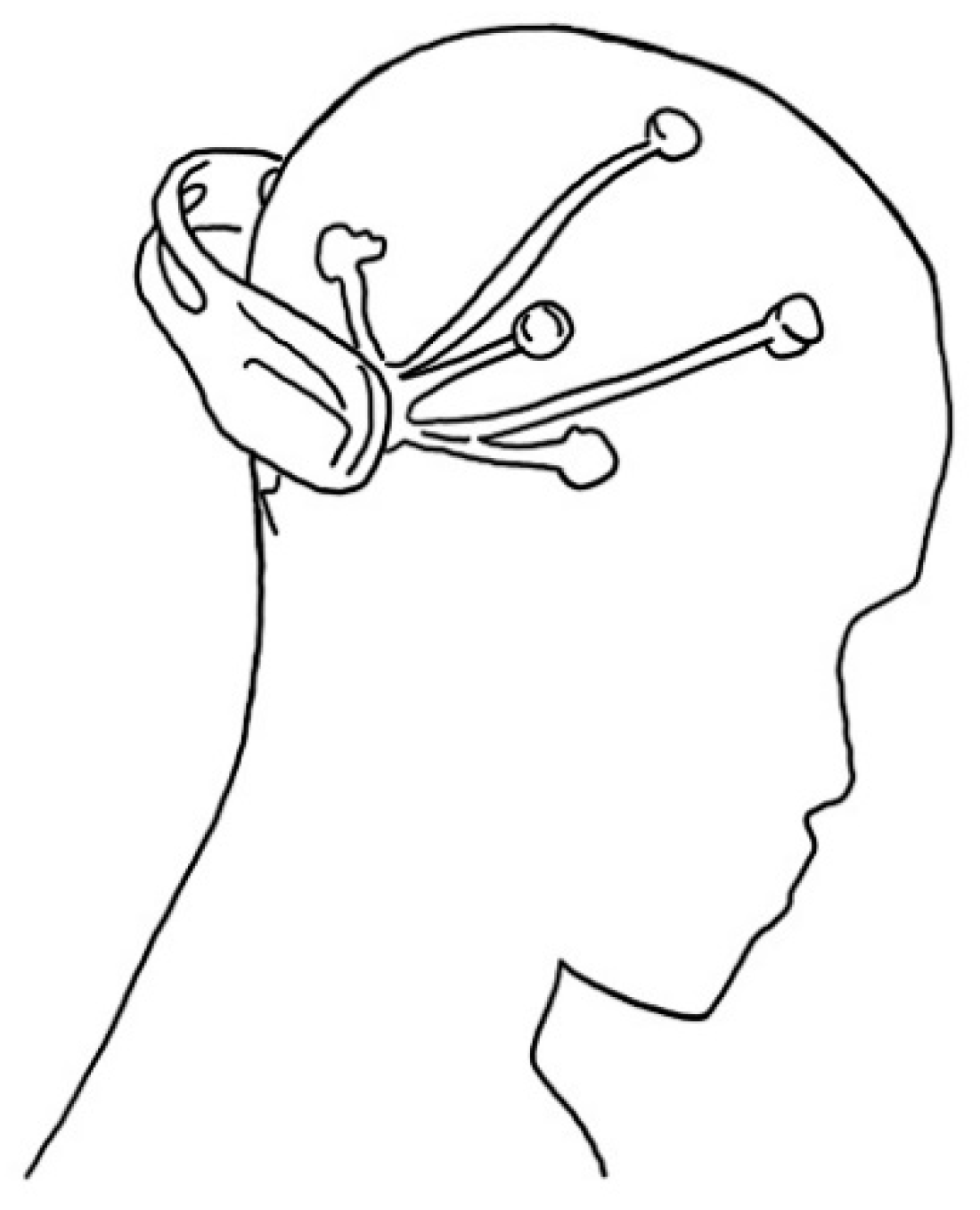
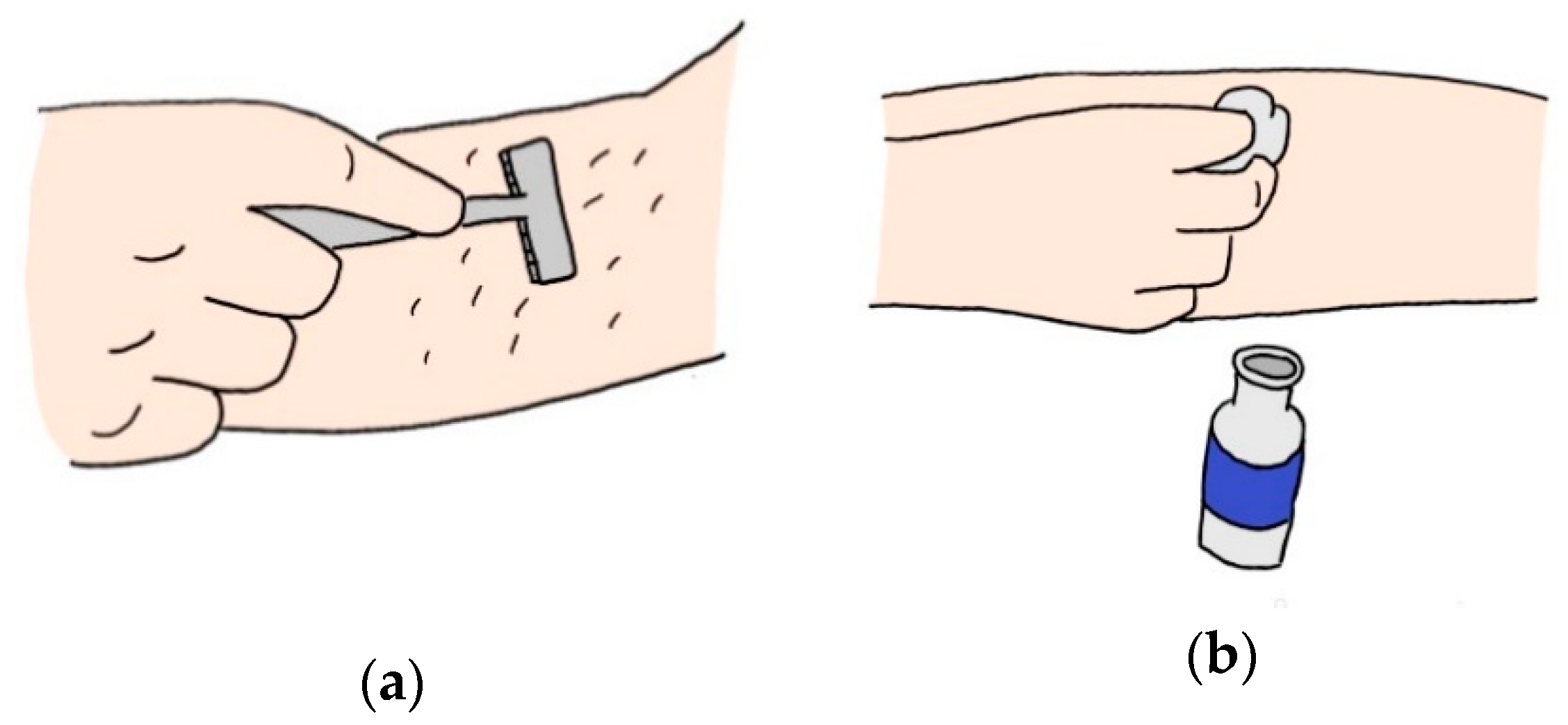
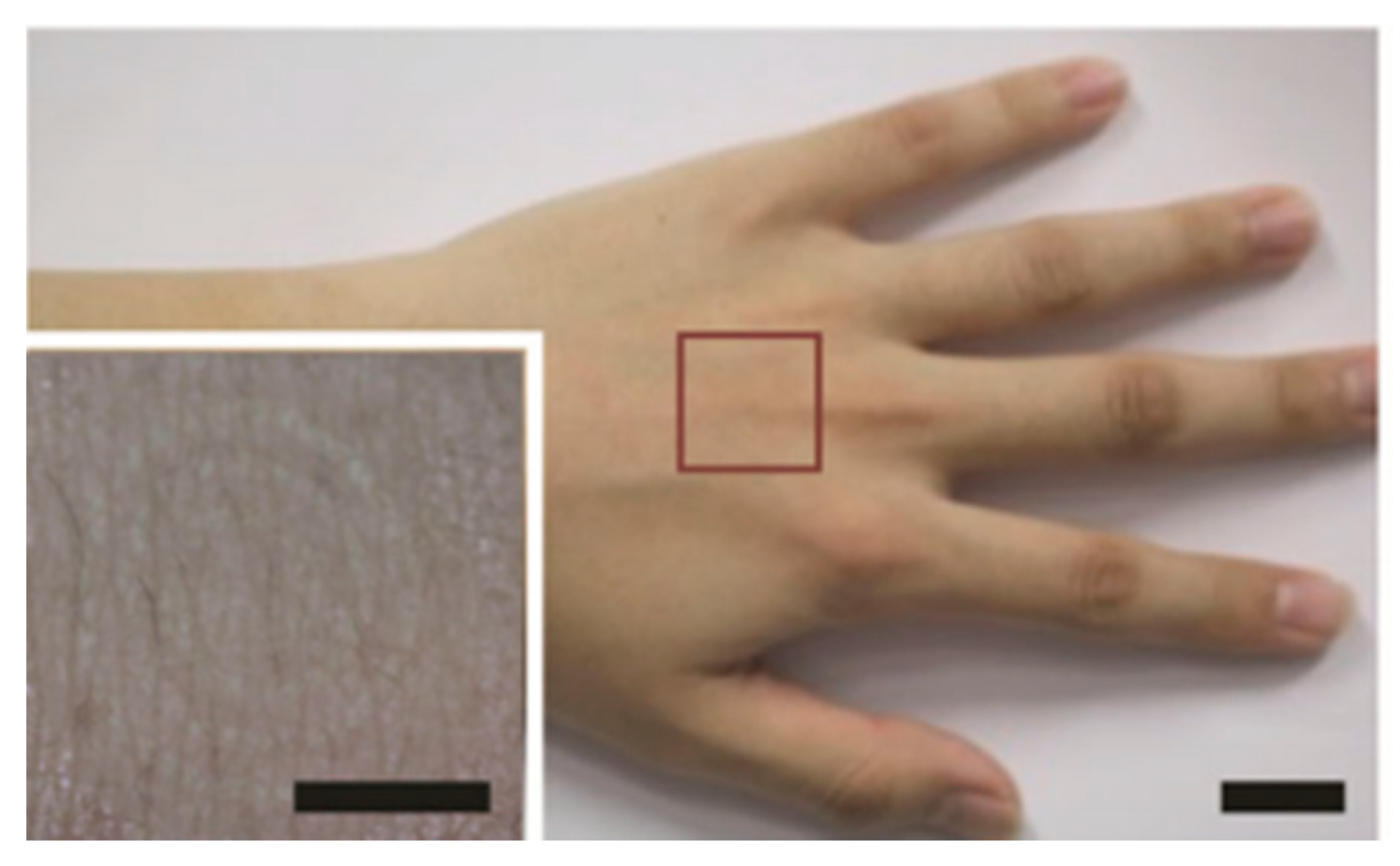
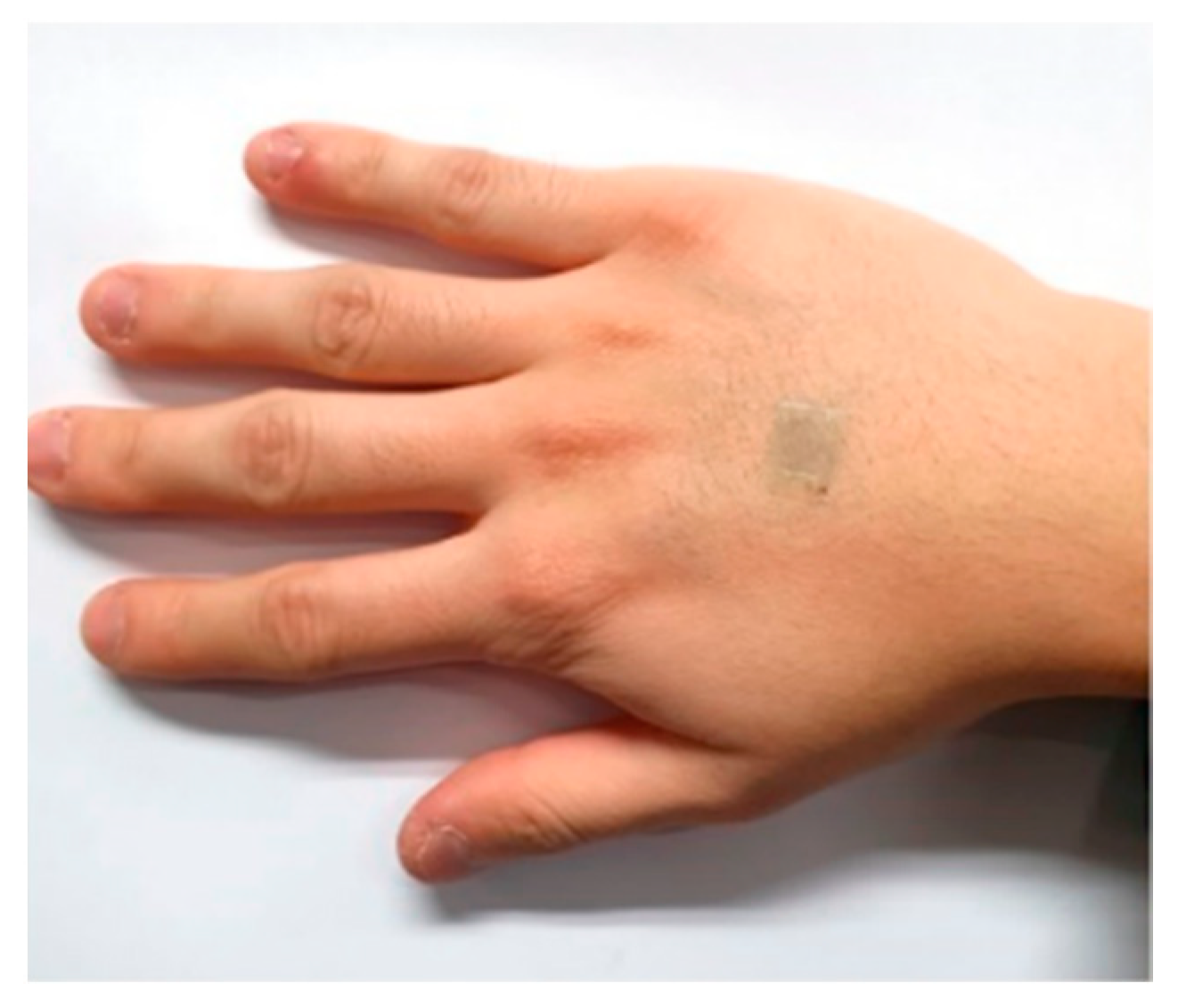
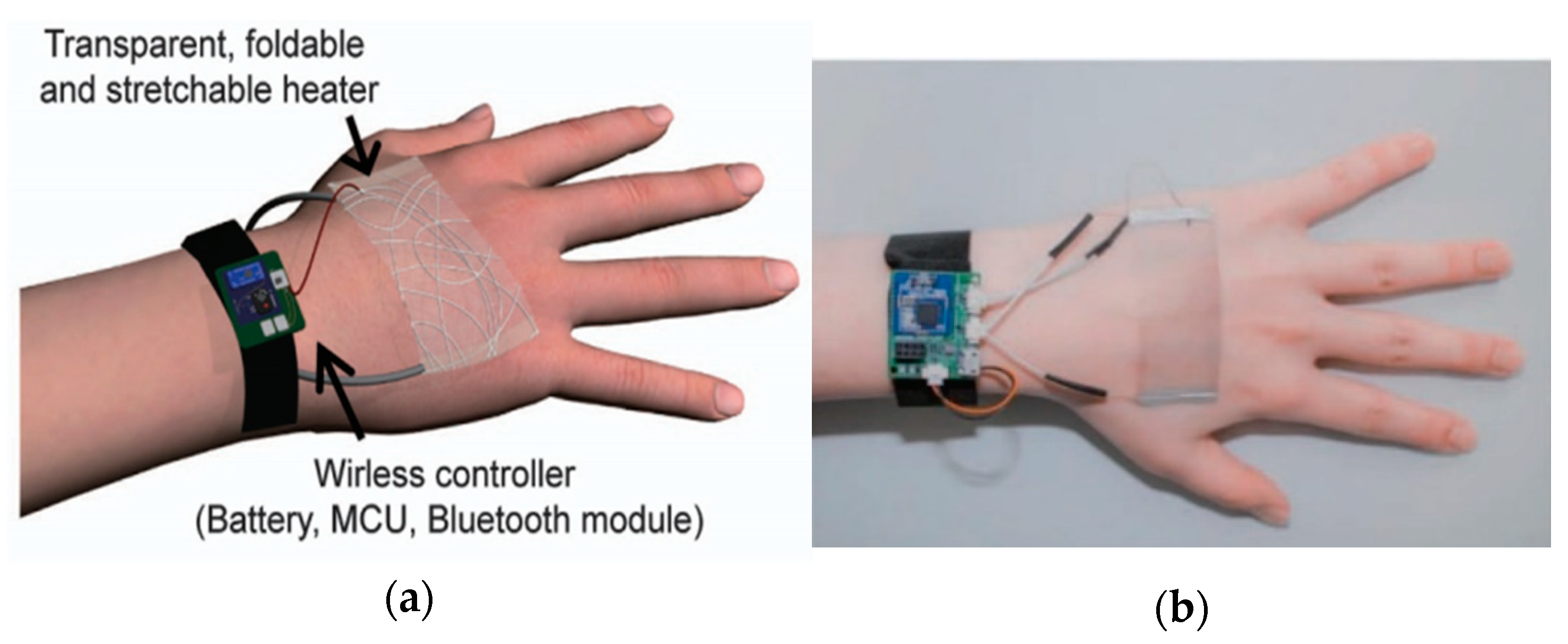
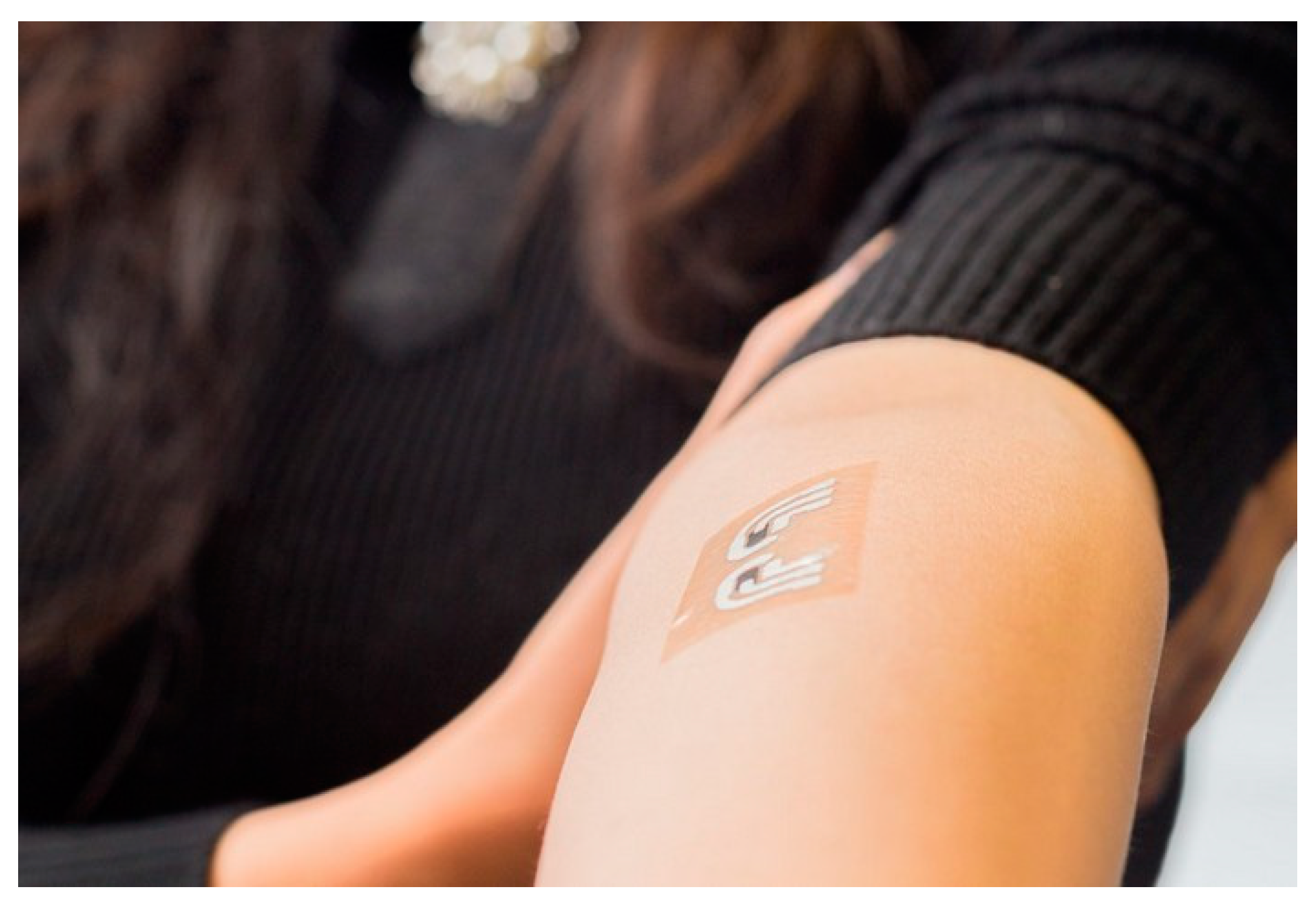
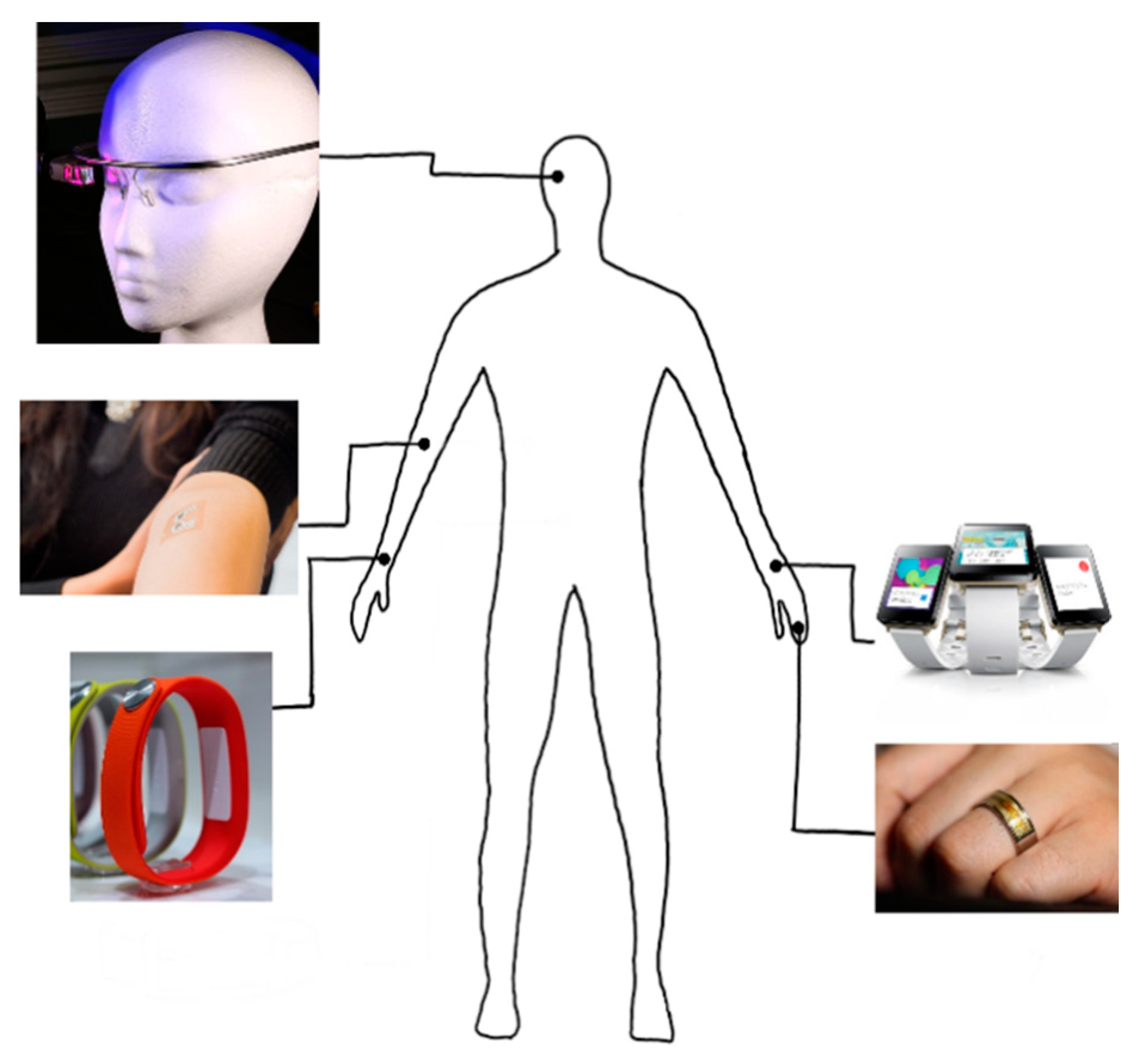
| Ref | Purpose | Electrode Location | Subjects | Experimental Method | Parameters Used | Conclusion |
|---|---|---|---|---|---|---|
| [20] 2000 | Investigation of EMG variable (MNF, RMS) for valid indicators of muscular fatigue | VL, RF, VM | 11 males, 10 females | Repetitive maximum isokinetic knee extensions | RMS, MNF, MDF, torque, knee joint position | MNF is a good criterion validity. |
| [21] 1999 | Investigation reliability of sEMG | VL, RF | 9 males, 9 females | Isometric knee extension | RMS, MDF, torque | MVC measurement is best suited for clinical applications from rectus femoris muscle. |
| [22] 1999 | Correlation of EMG fatigue data in the lower back to the subject’s assessment of fatigue | L1, L5 | 25 males, 25 females | Sørensen test | MDF, endurance time, Borg scale | The Borg scale correlated with endurance time and EMG median and mean power frequency slopes. |
| [23] 1982 | Examination of the changes in frequency and amplitude of sEMG | Adductor pollicis, handgrip muscles, biceps, quadriceps | 6 males | MVC, fatiguing contraction (25,40,70% MVC) | RMS, center frequency | The center frequency of sEMG appears to be a good noninvasive index of muscle fatigue. |
| [24] 1979 | Study of quantitative changes in the EMG pattern muscle fiber-type distribution | VL | 11 males | MV knee extensions | Integrated EMG, MNF | MNF decreases in FT-type muscles. |
| [25] 1986 | Determination of the effects of motor unit recruitment and firing frequency on the surface EMG power spectra during sustained MVC and 50% MVC of the bicep brachii muscle | Bicep brachii | 12 males | 50% MVC | RMS, MNF | Increasing RMS EMG amplitude and decreasing MPF could better represent the MU activity during fatigue. |
| [26] 1994 | Examination of the relationship between EMG manifestation of fatigue and endurance time during isometric contraction of the back extensors to fatigue | Erector spine at the levels of the 10th thoracic and 3rd lumbar vertebrae | 21 males, 208 females | Sørensen test | MF, endurance time | MFgrad is a suitable technique for monitoring back muscle fatigue. |
| Ref | Purpose | Electrode Location | Subject | Experimental Method | Parameters Used | Conclusion |
|---|---|---|---|---|---|---|
| [31] 1993 | Investigation of EMG median frequency of calf muscles during an exhausting treadmill exercise | Right soleus, gastrocnemius medialis, gastrocnemius lateralis | 7 males, 2 females | Uphill treadmill run till the moment of exhaustion | Heartrate, ECG, median frequency, | Immediately after the run, isometric median power frequency declined. |
| [32] 2007 | Determination if a difference existed in the rate of fatigue of select shoulder muscles during isometric shoulder elevation and if the measured rate of fatigue was consistent from day to day | Upper trapezius, middle deltoid, serratus anterior, lower trapezius muscles | 7 males, 9 females | 60% of their maximal voluntary isometric contraction force (MVIC) | MPF | Middle deltoid appears to fatigue faster than the other shoulder muscles tested at the selected level of shoulder elevation. |
| [33] 2009 | Determination of the difference in fatigue between athletes and non-athletes | VL, VM, RF | 11 males | Maximum versus forced repetition knee extension | Blood lactate, load in forced repetition, integrated EMG | Strength athletes produced neural fatigue in high-intensity resistance exercise. |
| [34] 1998 | EMG assessment of back muscle function during cyclical lifting | Mind-belly of the longissimus thoracis, iliocostalis lumborum, multifidus muscles at L1, L2, L5 | 3 males, 1 female | Dynamic and static lifting | instantaneous median frequency (Choi–Williams) | During dynamic contractions, instantaneous median frequency behavior is nonlinear and more complex than static contraction. |
| [35] (2005) | Evaluation of handgrip forces using sEMG of forearm muscles | 6 forearm muscles | 8 males | Isometric gripping tasks | Grip force, normalized EMG | For standardized grips, valid predictions of grip based on EMG were produced. |
| [36] (2001) | Evaluation of the potential health effects with respect to the low back of an office chair | L3, T10 | 3 females, 7 males | Simulated office work on a chair | Exposure variance analysis | Trunk kinematics and erector spinae EMG were strongly affected by the task performed but not by chair type. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, D.; Cha, J.M.; Park, K. Next-Generation Wearable Biosensors Developed with Flexible Bio-Chips. Micromachines 2021, 12, 64. https://doi.org/10.3390/mi12010064
Nam D, Cha JM, Park K. Next-Generation Wearable Biosensors Developed with Flexible Bio-Chips. Micromachines. 2021; 12(1):64. https://doi.org/10.3390/mi12010064
Chicago/Turabian StyleNam, Dahyun, Jae Min Cha, and Kiwon Park. 2021. "Next-Generation Wearable Biosensors Developed with Flexible Bio-Chips" Micromachines 12, no. 1: 64. https://doi.org/10.3390/mi12010064






