1. Introduction
Owing to its superior performance over real-time nucleic acid amplification technology (qNAAT) in terms of accuracy, specificity, and reproducibility, digital nucleic acid amplification technology (dNAAT) is widely used in low-abundance nucleic acid quantification for the diagnosis of cancer, viruses, and bacterial infections [
1,
2]. For dNAAT-based nucleic acid detection, DNA samples are divided into thousands of microdroplets or microchambers, which are subsequently amplified at specific temperatures. DNA concentrations can be accurately measured through the combination of endpoint fluorescence detection and Poisson probability models [
3]. According to the temperatures required for nucleic acid amplification, dNAAT can be divided into digital polymerase chain reaction (dPCR) and digital isothermal amplification technology (dIAT) [
4]. As a branch of dIAT, digital loop-mediated isothermal amplification (digital LAMP) permits nucleic acid amplification under isothermal conditions, thus eliminating the need for the complex thermocycling procedures used in dPCR [
5,
6].
Studies on dNAAT have focused on sample segmentation using innovative microfluidic chips [
7,
8,
9]. Although the microfluidic chips for dNAAT have emerged endlessly, most dNAAT instrument systems still require more than three sets of auxiliary devices, including sample segmentation unit, heating unit and fluorescence detection unit. As an example, in commercial dNAAT systems based on microdroplets such as QX 200 (Bio-rad), DNA samples must be transferred between a microdroplet generator, a thermal cycler, a sealing instrument, and a flow fluorescence detection device [
2,
10]. In the QuantStudio 3D (Thermo Fisher Scientific) dNAAT system, DNA samples are dispersed into the microchambers of a silicon-based chip using a sample loading device, and incubated in a Peltier heater prior to fluorescence imaging [
11]. These systems perform to a high level, but rely on multiple auxiliary devices leading to complex operating procedure and high-power usage. The future of dNAAT development; therefore, focuses on portability, integration, and miniaturization [
12,
13,
14].
In the course of NAAT, a series of heating schemes based on Peltier [
15], near infrared (NIR) laser [
16], acoustic waves [
17], and other mechanisms were proposed and combined with fluorescence detection technology, thereby promoting the emergence of integrated qNAAT instruments including qPCR devices. Among these heating schemes, the NIR heating demonstrates the advantages of large heating area, fast speed, and easy integration with fluorescence detection unit. Since 2015, gold nanostructures with strong surface plasmon resonance effect have been gradually applied in the field of NAAT research. Due to its excellent photothermal efficiency, the introduction of gold nanostructures allowed photothermal NAAT to get rid of the reliance on costly laser equipment, and even realized LED-driven NAAT [
18]. Lee and colleagues employed a polymethylmethacrylate (PMMA) cavity covered with two thin gold nanofilms to evenly absorb light to heat the PCR mixture, and established a heating device based on a 3 W LED arrays [
19,
20]. Roche and coworkers introduced gold nanorods into the PCR reactions to achieve ultra-fast and real-time plasmonic qPCR under the illumination of a 2 W laser, which completed 30 thermal cycles in 54 s [
21]. Weizmann’s group extended their photothermal system based on gold bipyramids to isothermal nucleic acid amplification and restriction enzyme digestion [
22]. In the current development of qNAAT towards dNAAT, the introduction of LED-driven heating technology into dNAAT is of great value in promoting the integration and miniaturization of related instruments. However, these heating methods that performed well in qNAAT are not directly applicable to dNAAT, which is a complex technique that requires the integration of sample segmentation and heating. For example, the opaque gold films employed by Lee led to challenges for subsequent fluorescence detection, and the PMMA substrate is not the ideal material for dNAAT microfluidic chip due to its reliance on complex and expensive processing methods. If gold nanoparticles (AuNPs) are mixed into microchambers or microdroplets, it is difficult to ensure the uniform distribution of AuNPs. In addition, in Roche and Weizmann’s research, AuNPs have a certain inhibitory effect on DNA amplification. Although they have proposed some methods to reduce the inhibition effect of AuNPs for DNA amplification, it is not clear whether these methods can be applied to dNAAT reagent, which is a biochemical system with lower tolerance to external additives than qNAAT [
23]. To employ gold nanostructures for dNAAT heating, not only the heating performance itself, but also sample segmentation, chip manufacturing and subsequent optical detection should be taken into consideration systematically.
In this study, to overcome the limitations of these methods, we proposed an AuNPs-PDMS microfluidic chip that realizes microdroplet-based sample segmentation and surface plasmon resonance heating-based nucleic acid amplification. Due to its high performance for microstructure manufacturing and thermostability, PDMS has been used in microfluidic chips for microdroplet dNAAT (ddNAAT) and microchamber dNAAT (cdNAAT). The introduction of gold nanoparticles into the PDMS substrate imparts the PDMS with a compact and integrated heating function under illumination. The AuNPs-PDMS described herein were obtained through the mixing of silica-coated and dodecanol-modified AuNPs-ethanol with PDMS prepolymers and then evaporating the ethanol. The advantage of this strategy is that the content and morphology of the AuNPs can be independently regulated with no loss of manufacturing capability and bonding performance of PDMS. In addition, the AuNPs dispersed in the PDMS substrate will not inhibit nucleic acid amplification.
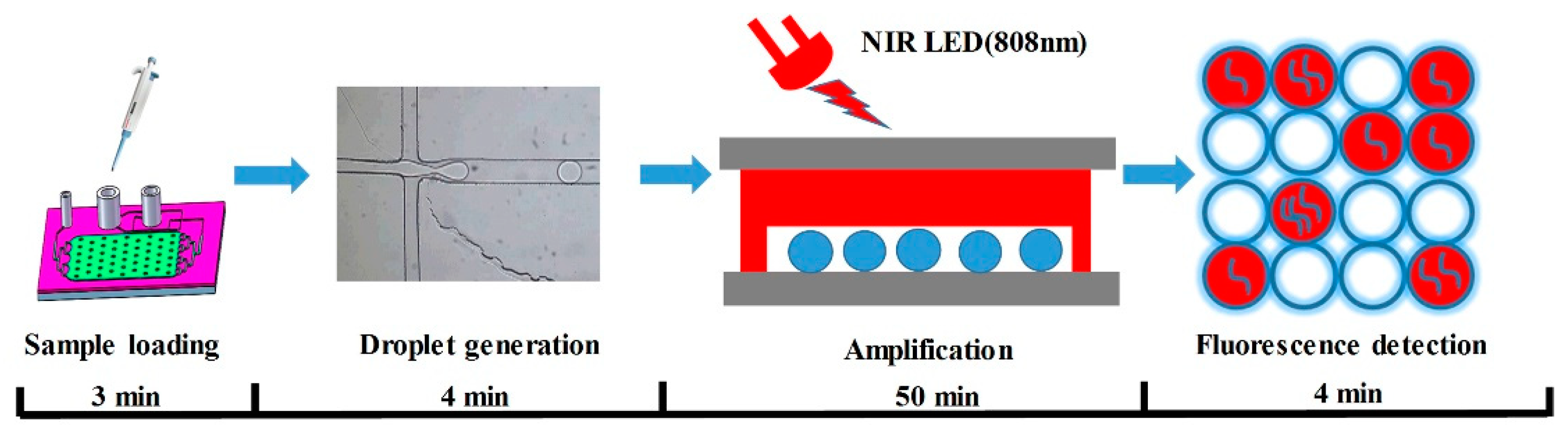
We designed and prepared patterned AuNPs-PDMS films based on an SU-8 mold, and bonded the AuNPs-PDMS film between a bottom and a top glass substrate to fabricate a “glass–PDMS–glass” sandwich digital LAMP chip. The digital LAMP chip integrates a cross microchannel for droplet generation and a microcavity for LAMP amplification and fluorescence detection. Driven by two syringe pumps, the digital LAMP mixture was evenly divided into multiple microdroplets and tiled in the AuNPs-PDMS microcavity. Under the illumination of a near-infrared LED (808 nm), the AuNPs-doped PDMS achieved uniform and stable heating, leading to the simultaneous amplification of target DNA in the droplets. In addition, we established an integrated device that combined NIR-LED heating and fluorescent detection. As a proof of concept, we evaluated the performance of the chip and integrated device with serial dilutions of hepatitis B virus (HBV) DNA, which demonstrated an accurate detection of low-abundance nucleic acids. To the best of our knowledge, this is the first study to achieve an absolute quantification of nucleic acids based on the photothermal effects of gold nanoparticle. The integrated device for NIR LED heating and fluorescence detection has the advantage of low costs, a compact size, and low energy consumption. This highlights the potential of the system to promote the development of dNAAT towards portability, integration, and miniaturization.
2. Experimental Design
2.1. Materials and Reagents
Hepatitis B Virus (HBV) DNA templates were obtained from Nucleic Acid Quantitative Assay Kits (P101, Tianlong, China) and their initial concentrations were confirmed by qPCR instrument (Gentier 96E, Tianlong, China). DNA template was stored at -20 °C prior to use. Digital LAMP primers were purchased from Sangon Biolotech Co., Ltd (Shanghai, China) according to HBV sequence. The LAMP primers were as follows:
Forward outer primer (F3): 5-TCCTCACAATACCGCAGAGT-3; backward outer primer (B3): 5-GCAGCAGGATGAAGAGGAAT-3; forward interior primer (FIP): 5-GTTGGGGACTGCGAATTTT-GGCTTTTTAGACTCGTGGTGGACTTCT3; reverse interior primer (BIP): 5-TCACTCACCAACC-TCCTGTCCTTTTTAAAACGCCGC-AGACACAT-3.
Oil phase reagent (HFE7500, 3M, containing 2% surfactant) were purchased from Bio-Rad (Hercules, USA). Fluorescent dye including calcein and manganese chloride were purchased from Sigma-Aldrich (St. Louis, MO, USA). Bst DNA polymerase, ThermoPol® buffer, betaine, and deoxyribonucleotide triphosphate (dNTP) were purchased from Sangon Biolotech (Shanghai, China). The components of the 20 μL digital LAMP reagent system used herein are shown in
Table 1. It should be noted that before adding the DNA template to the LAMP reagent, the HBV-DNA template needs to be quantified by standard qPCR method and then serially ten-fold diluted, so that the initial template concentration range in the LAMP system is 1 × 10
1 to 1 × 10
4 copies/μL.
PDMS prepolymer (SYLGARD 184 A) and curing agent (SYLGARD 184 B) were purchased from Dow Corning Inc (Midland, USA). All other chemicals were obtained commercially and used without purification.
2.2. Preparation of AuNPs and AuNPs–PDMS
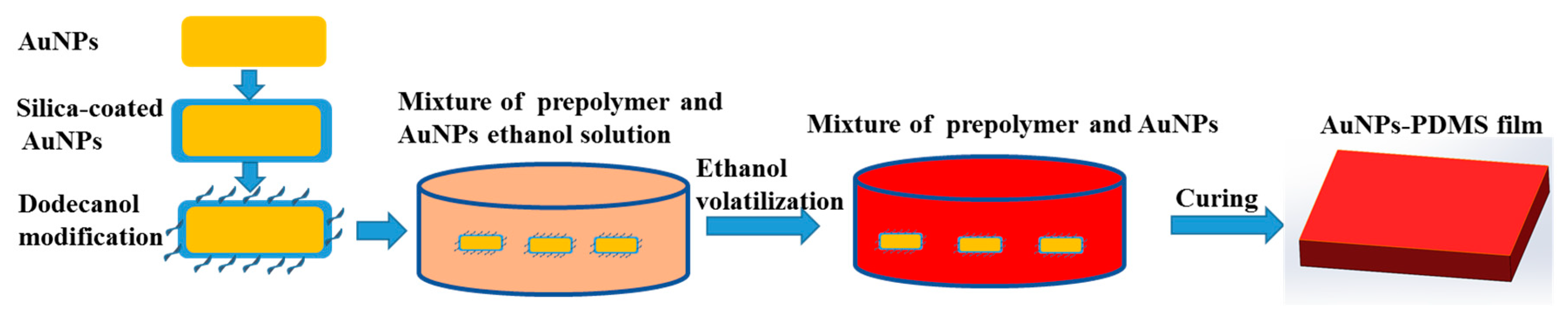
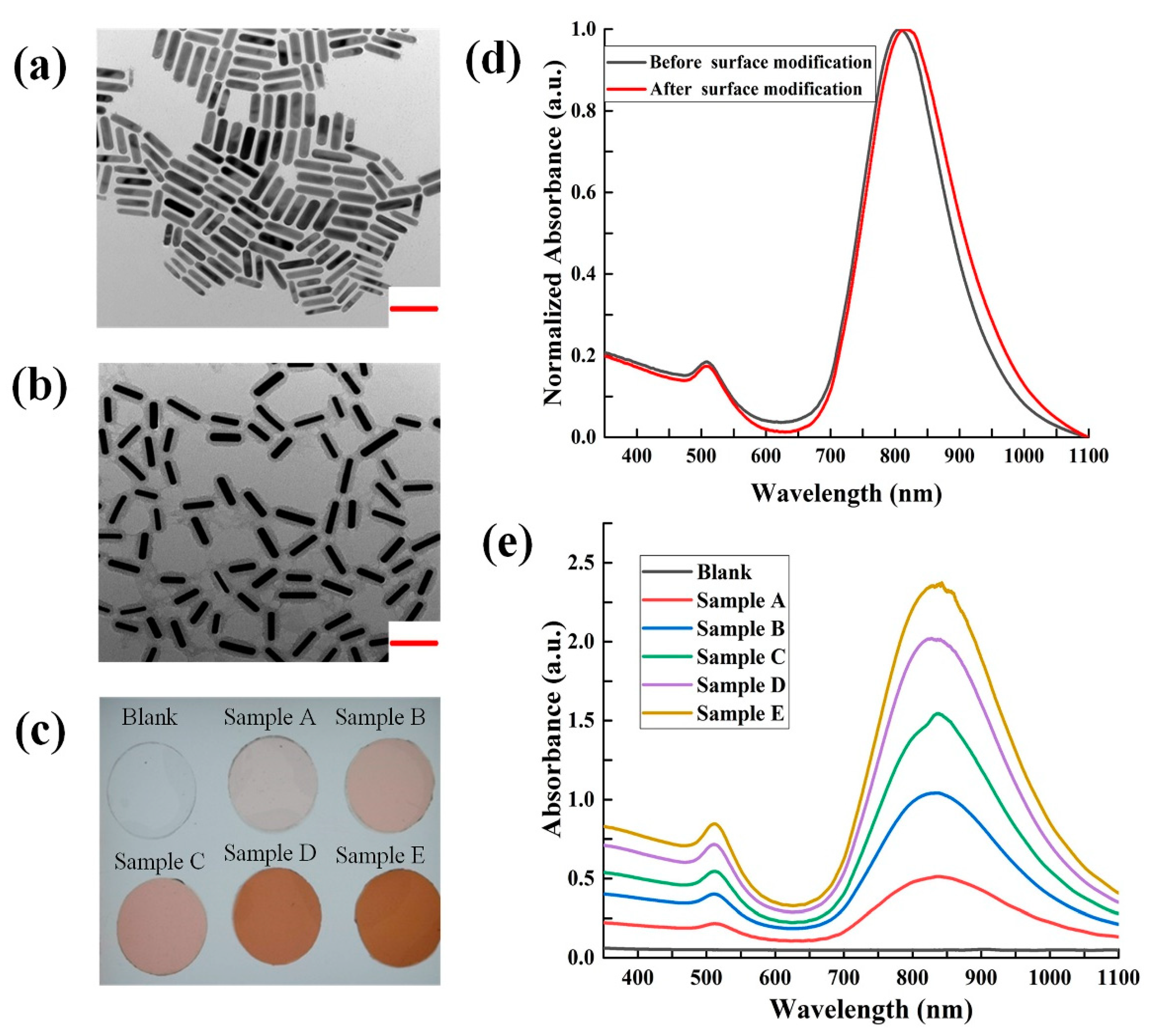
AuNPs prepared in aqueous reagent have a tendency to agglomerate in organic PDMS [
24]. Herein, we adopted a method of silica coating and dodecanol modification to make AuNPs dispersed in ethanol that are miscible with PDMS prepolymer, as shown in
Figure 1. As a class of AuNPs with tunable absorption peaks, gold nanorods were synthesized using the seed-mediated growth method [
25]. Briefly, seed solutions were prepared through the mixing of hexadecyl trimethyl ammonium bromide (CTAB) solution (10 mL, 0.1 M) and HAuCl
4 (0.085 mL, 0.028 M) with fresh NaBH
4 (0.07 mL, 0.1 M). For the growth of the gold nanorods, 0.3 mL seed solution was added to CTAB (12 mL, 0.1 M), sodium oleate (18 mL, 0.013 M), HAuCl
4 (0.5 mL, 0.028 M), HCl (1 mL, 0.1 M), AgNO
3 (0.32~0.37 mL, 0.01 M), and ascorbic acid (0.05 mL, 0.1 M). Following incubation at 30 °C for 24 h, the newly-produced AuNPs colloids were centrifuged at 10,000 rpm for 30 min, decanted, and resuspended in 30 mL of 1 mM CTAB to decrease free CTAB and sodium oleate levels. To enhance the stability of the gold nanorods at high temperatures, silica-coated gold nanoparticles (AuNPs@SiO
2) were synthesized using the Stober method. NaOH (0.1 M) was added dropwise to adjust the pH of AuNPs to 10.4~11.0. Next, 0.1 mL of tetraethyl orthosilicate (TEOS) was added for 1 h with shaking and left for 12 h for static growth. A layer of silica was successfully coated onto the surface of the nanorods which were then centrifuged at 10,000 rpm for 20 min and decanted. The AuNPs were mixed with dodecanol to a total volume of 30 mL. Next, 1 g of C
7H
8O
3S was added and after ultrasonic dispersion for 10 min, the solution was transferred to a high-temperature reactor and incubated at 70 °C for 3 h. The solution was then centrifuged at 11,000 rpm for 30 min, decanted, and resuspended in 30 mL of ethanol. Following ethanol washing and centrifugation, aqueous AuNPs were concentrated in 3 mL of ethanol. Of note, dodecanol modifications could reduce the hydrophilic hydroxyl groups on the silica shell, enhancing the lipophilicity and dispersion of the AuNPs in PDMS.
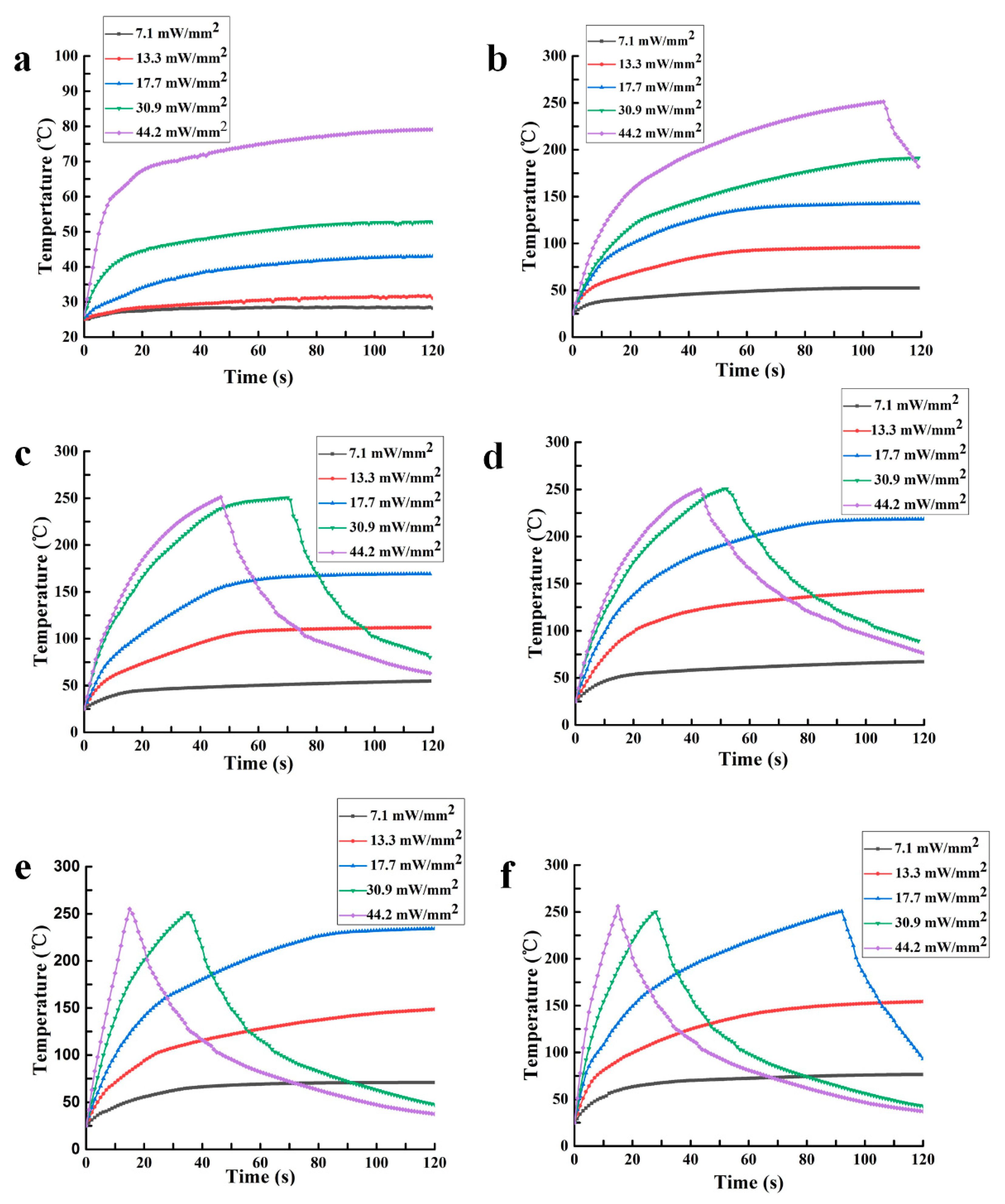
AuNPs-PDMS was prepared as shown in
Figure 1. The AuNPs-ethanol solution was added to PDMS prepolymers, stirred, and then heated in a 70 °C ventilated dryer for ≥ 2 h to fully evaporate the ethanol. AuNP-PDMS films were obtained after mixing the AuNPs-doped PDMS prepolymers with curing agent at a weight ratio of 10:1, and then curing at 90 °C for 1 h. To study the photothermal characteristics of AuNPs-PDMS film, we prepared 5 AuNPs-PDMS films at a range of AuNPs concentrations. For these 5 sample films, the volume of the concentrated AuNP solution incorporated into 3 g of PDMS before evaporating were 1, 2, 3, 4, and 5 mL, respectively. The relative mass of the AuNPs in the AuNPs-PDMS films were estimated as 0.031%, 0.062%, 0.093%, 0.124%, and 0.155%, respectively. It should be noted in advance that the AuNPs content for the digital LAMP chips was 0.093%.
2.3. Digital LAMP Chip Design and Fabrication
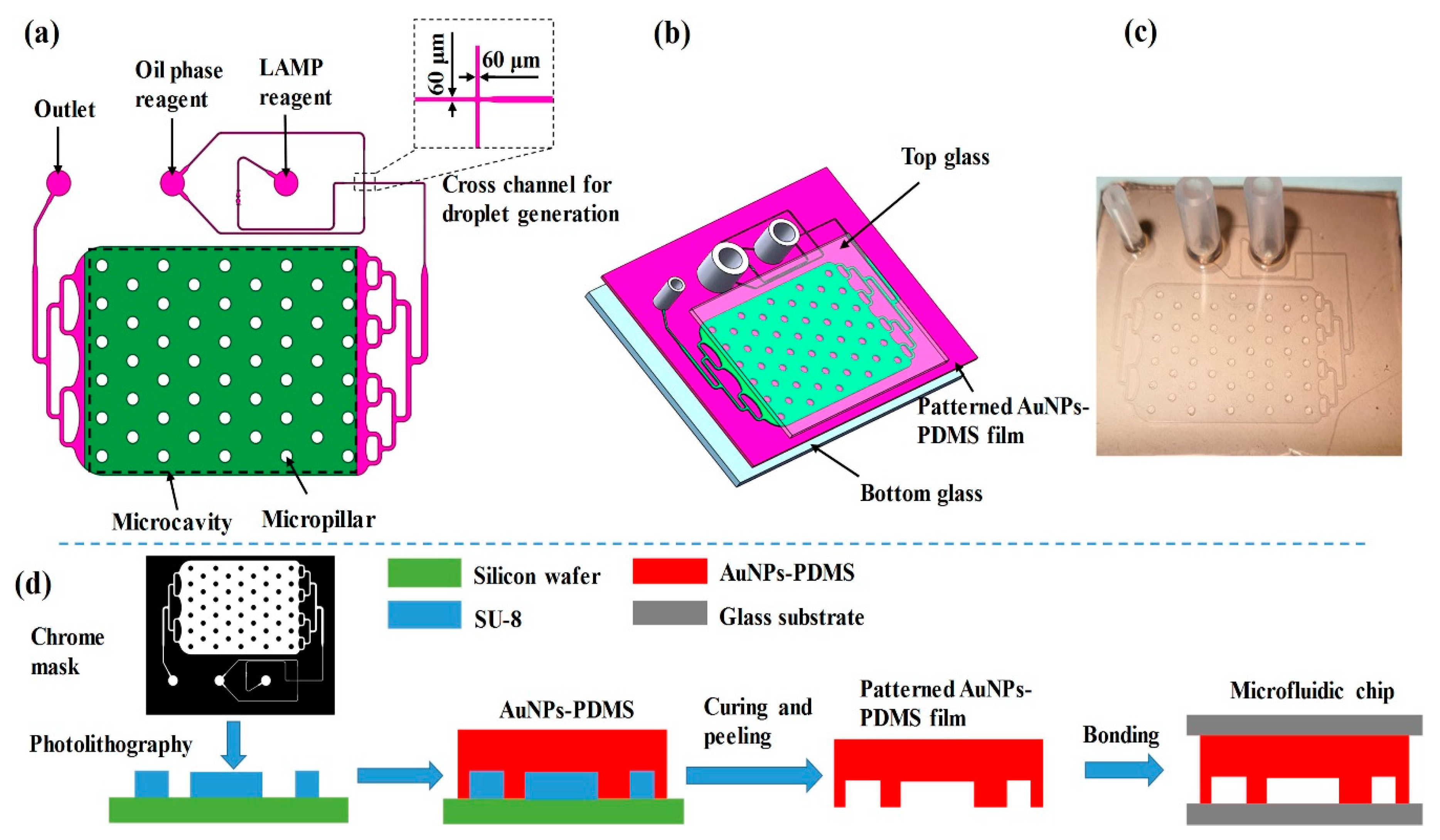
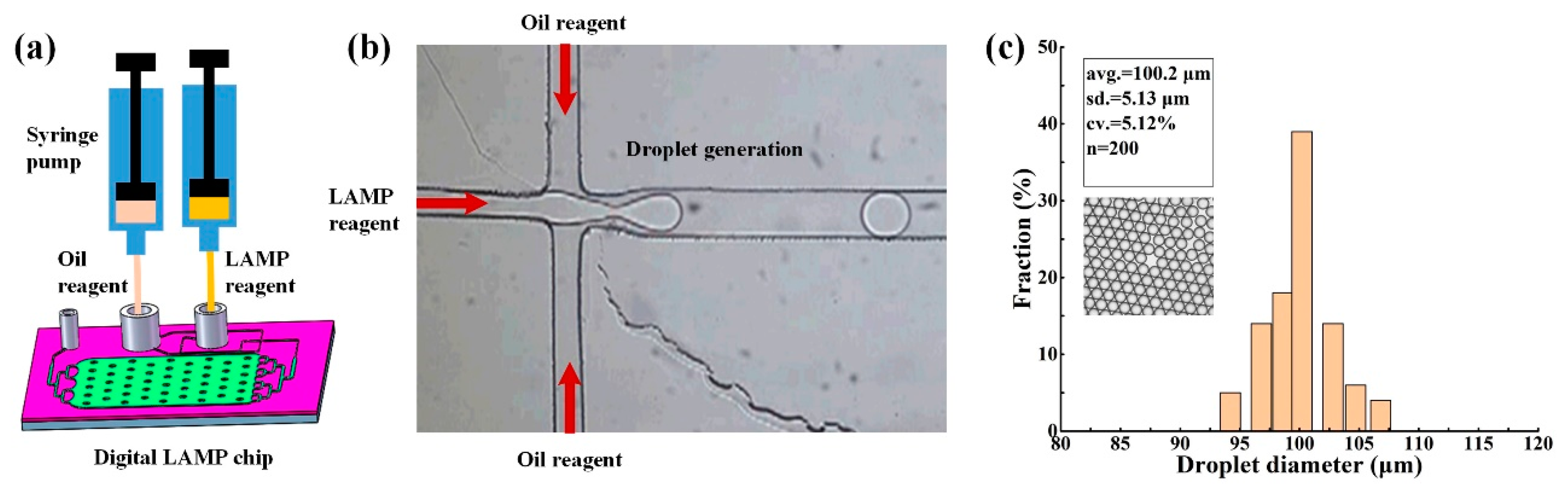
A schematic of the digital LAMP microfluidic chip is shown in
Figure 2 and was designed using Auto CAD. The chip consists of a cross microchannel for droplet generation and a microcavity for droplet tiling. Based on the principle of flow focusing, nucleic acid samples and LAMP reagents were divided and wrapped into multiple water-in-oil droplets. These water-in-oil droplets were transferred and tiled in the microcavity through a three-stage branch flow channel. The integrated chip has a small footprint with a length of 43 mm, a width of 32 mm, and a height of 8 mm. The width of droplet-generating microchannel is 60 μm (
Figure 2a), which is a dimension that can be easily prepared by PDMS. The microcavity has a length of 20 mm, a width of 15 mm, a height of 0.1 mm, and can collect and tile ~20,000 droplets with a diameter of ~100 μm. To avoid the collapse of the microcavity, 50 micro-pillars with diameters of 1 mm were designed.
The digital LAMP microfluidic chip was fabricated based on soft lithography processes and sandwich assembly. As shown in
Figure 2b,d, a layer of SU-8 2050 negative thick photoresist (PR, MicroChem Corp., Newton, MA, USA) with a thickness of 100 μm was first spun onto a silicon substrate and followed by a soft bake process. A standard lithography process with an exposure dose equal to 230 mJ/cm
2 was performed to copy the pattern of the chrome mask onto the SU-8 photoresist. The SU-8 development process was finished by immersing the exposed substrates into a developer solution (MicroChem Corp., Newton, MA, USA) and using ultrasonic agitation to obtain well-defined SU-8 structures. After treatment with octafluorocyclobutane (C
4F
8) for 3 min to facilitate demolding, the SU-8 mold was ready for the preparation of patterned PDMS film. Similar to the preparation of the AuNPs-PDMS film, the AuNPs-doped PDMS prepolymer (with 0.093% AuNPs) and curing reagent were mixed at a weight ratio of 10:1, respectively, and poured onto the SU-8 mold. Following degassing and heating in a vacuum desiccator at 90 °C for 1 h, the microchannel of the SU-8 mold was replicated on the AuNPs-PDMS film. The patterned AuNP-PDMS film had a thickness of 1.5 mm and was peeled off from the silicon wafer, installed with three joints, and bonded to a top and a bottom glass through oxygen plasma treatment. The sandwich assembly avoided the thermal evaporation of droplets. To ensure reliability, drops of pure PDMS were applied onto the edge of the microfluidic chip and heated for curing. After rinsing with fluorosilane and drying, the chips were ready for digital LAMP experiments (
Figure 2c).
2.4. Design and Establishment of Integrated Device for NIR Heating and Fluorescence Detection
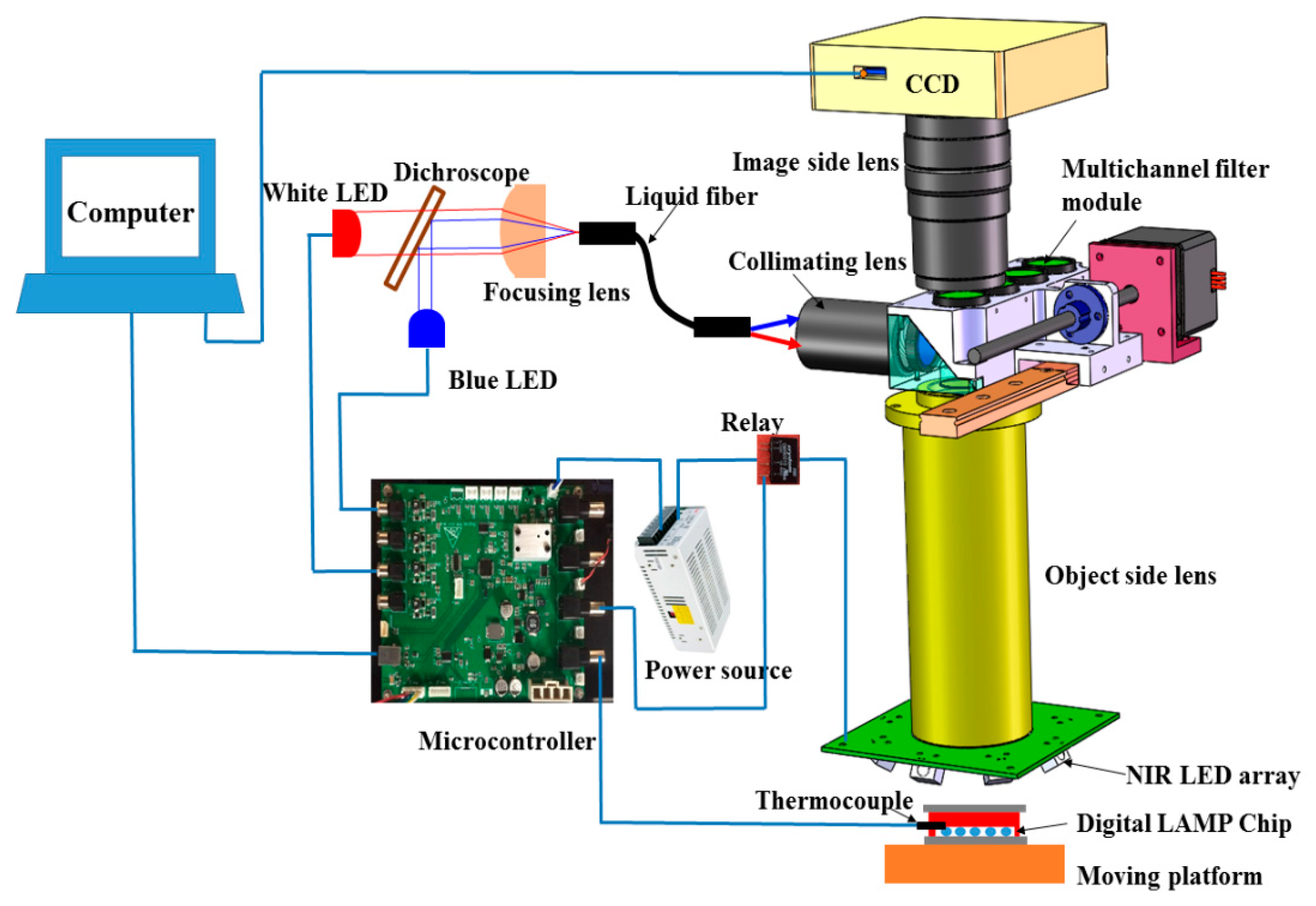
To integrate the heating and fluorescence detection unit for digital LAMP into a single device, we designed and fabricated an integrated prototype as shown in
Figure 3. The overall dimensions of the device were 210 mm in length, 150 mm in width, and 330 mm in height, which was more compact than the existing commercial instrument.
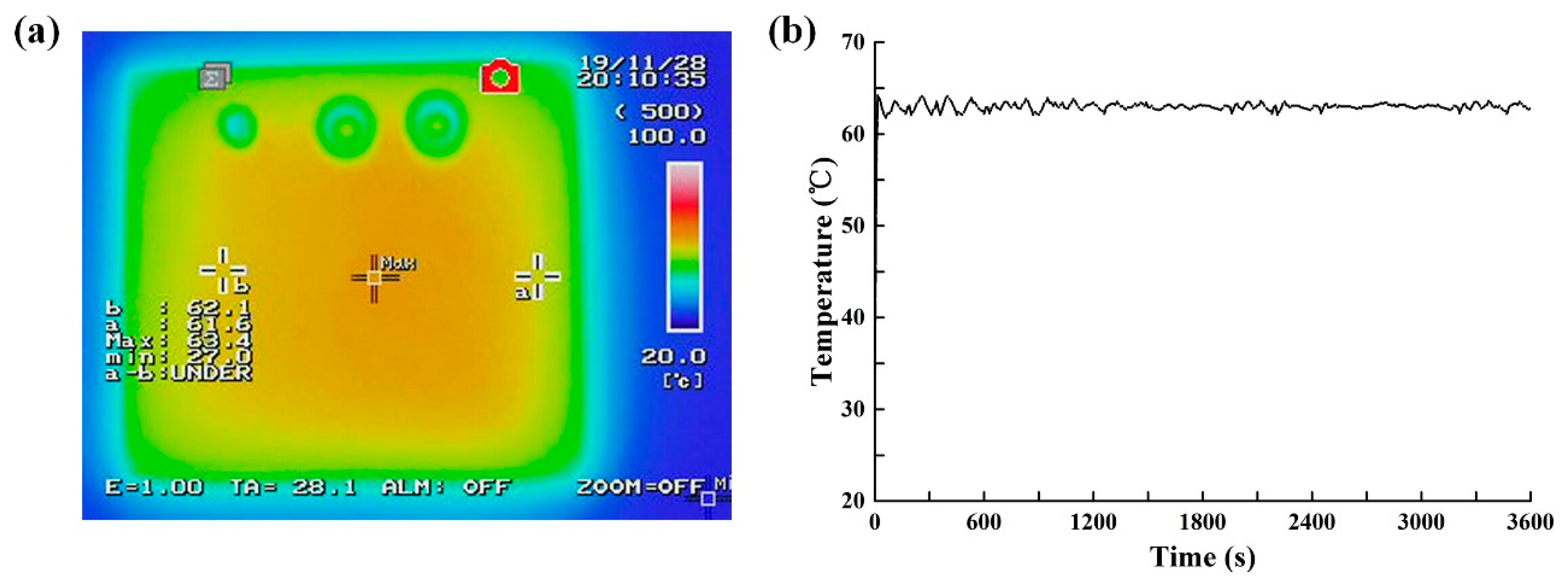
The NIR heating unit consisted of a circularly arranged NIR LED array (24 V, 12 W, peak wavelength at 808 nm, Vanch Photoelectric, Inc., Shanghai, China) to provide the NIR radiation for heating. Type-K thermocoupling (5SC-TT-K-40-36, Omega Engineering) was used for temperature monitoring, and a switching mode power supply (24 V, Weihua Electronics, Inc., Xi’an, China). The NIR-LED array has a small footprint with a length of 58 mm, a width of 58 mm, and a height of 22 mm. The distance between the chip and the object side lens is 27 mm. The circularly-arranged LED array consisted of 8 LEDs in series illuminated on the microcavity zone of the chip at an angle of incidence of 45°. The circularly arranged LED array made the NIR irradiation more uniform, and multiple LEDs ensured sufficient NIR heating power and large heating area. The temperature controlled system was implemented using a microcontroller based on ARM (STM32F103RET6). The NIR LED array was powered through a 24 V power supply controlled by a TTL (transistor transistor logic)-controlled relay (CMX60D10, Crydom Co., San Diego, CA, USA). The TTL line was actuated at 1000 Hz with the duty cycle controlled by the program built into the microcontroller. During thermal incubation, the microcontroller showed an output of 3.3 V to close the TTL-controlled relay to illuminate the NIR LED. Once the temperature from the thermocoupler exceeded 62.5 °C, the microcontroller led to an output of 0 V TTL and disconnected the power supply of the NIR LED, prompting the temperature of the digital LAMP reagent to return to ~62 °C. When the temperature dropped below 61.5 °C, the reverse operation was performed.
The fluorescence detection unit consisted of a CCD camera (C11440-50U, 6.97 × 5.23 mm, Hamamatsu, Japan), a customized object side lens (149 mm focal length), an image side lens (MVL100M1, 100 mm focal length, Thorlabs), a multichannel filter module, a blue LED (M470L3, 470nm, 3.3 V, 0.76 W, Thorlabs), and a white LED (MCWHL5, 0.38 W, 3.3V, Thorlabs). Light from the blue LED or white LED passed through a long-pass dichroscope (DMLP470R, 25 × 36 mm, Thorlabs), a focusing lens (ACL2520U-B, Thorlabs), a liquid optical fiber (16 mm diameter, NA 0.5, Chunhui, Inc., Nanjing, China), a collimating lens (65-553, Edmund), a multichannel filter module, and a customized object side lens and illuminated the microcavity zone. The filters and dichroic mirror mounted in the multichannel filter module were: FF01-495/28-25 (Semrock) for excitation, FF01-525/39-25 (Semrock) for fluorescence detection, and FF497-Di01-25×37 (Semrock) to split the beams of excited and omitted light. Using the drive of the linear motor, the multichannel filter module could switch from fluorescent detection to brightfield imaging. According to the focal length of the object-side and image-lens, the magnification of the optical system was 0.67, and the size of a single imaging zone was 10.4 × 7.8 mm. Since the size of micro cavity was 20 × 15 mm, four shots were required to obtain the complete images of all the droplets.
2.5. Image Analysis
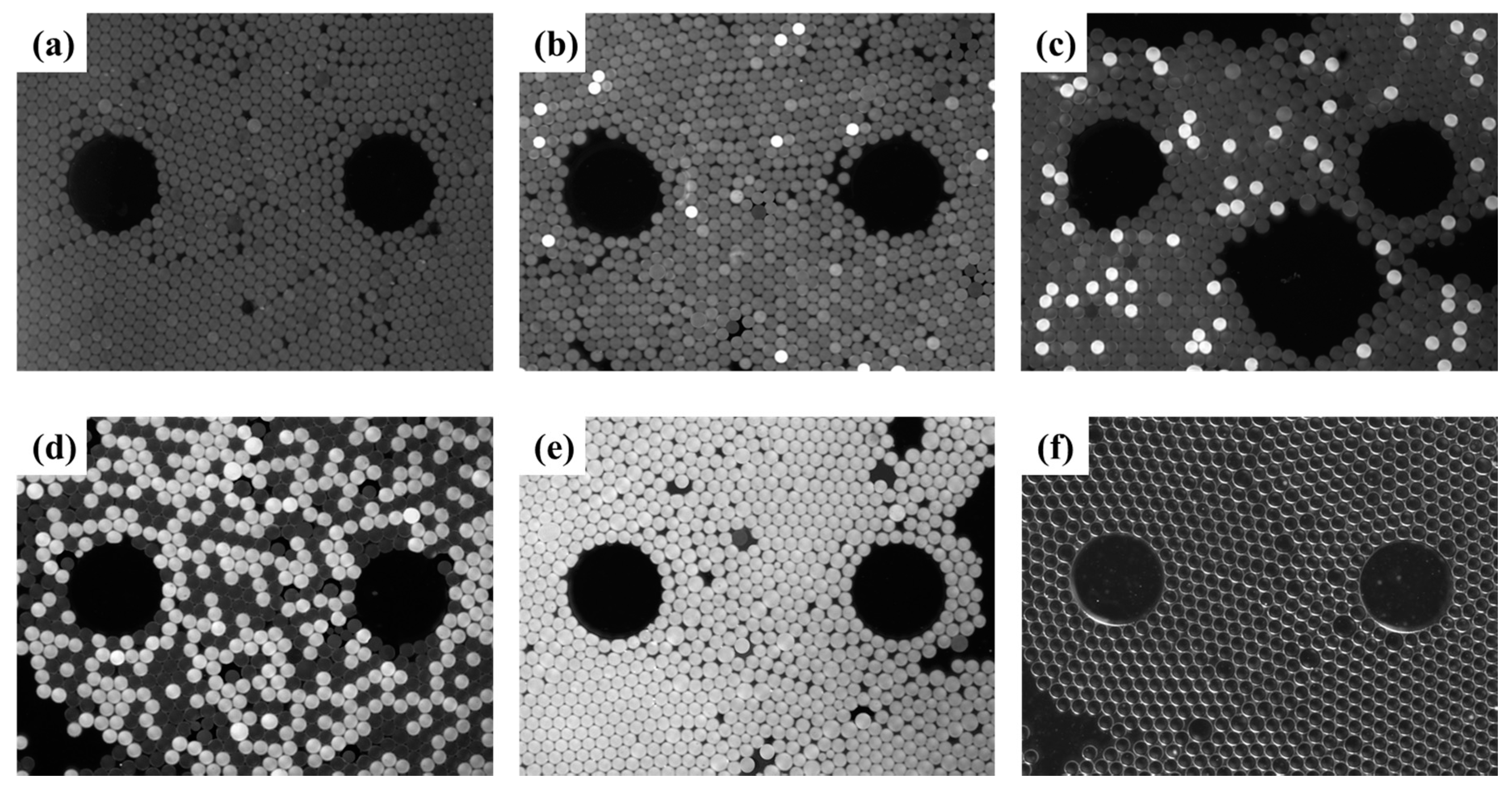
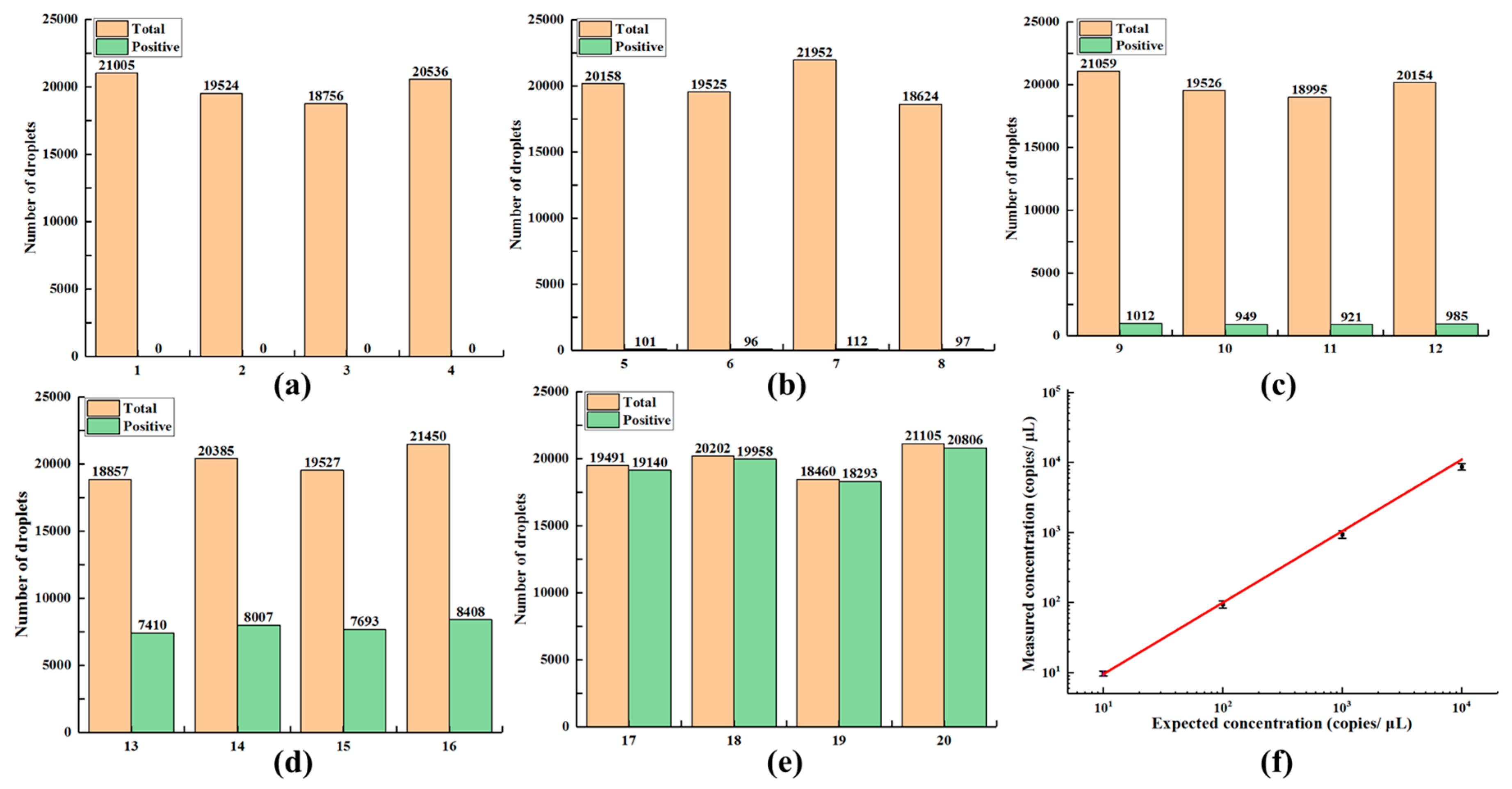
Under white LED, the CCD camera obtained bright-field images, which were analyzed to count the total number of droplets. Fluorescence images of the droplets were acquired under the action of blue LED and filter components, and were then used to distinguish whether the droplet was positive or negative for the target DNA. Image processing was performed using Image J and MATLAB. Briefly, according to the algorithms such as image filtering, local threshold processing, watershed-based image segmentation, and particle statistics [
26], both the number of total and positive droplets were individually counted. According to the Poisson probability model, the average DNA copy number were calculated using the following equation:
Where
Nt represents the total number of droplets in the digital LAMP chip,
Np represents the number of positive droplets,
Vd represents the droplet volume [
7].