Advances in Label-Free Detections for Nanofluidic Analytical Devices
Abstract
:1. Introduction
2. Optical Detection
2.1. Diffraction-Based Detection
2.2. Scattering-Based Detection
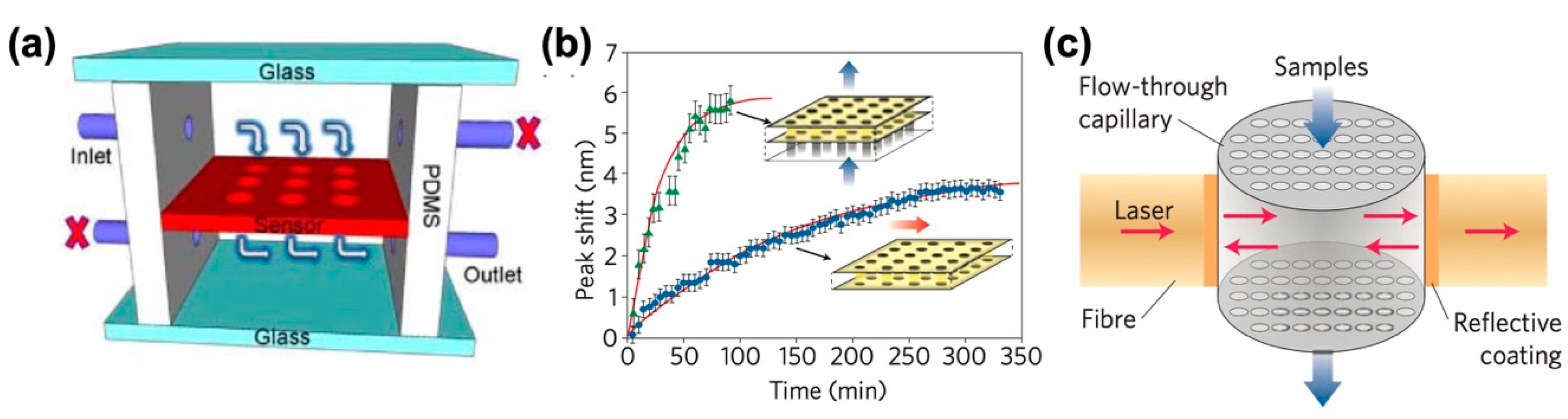
2.3. Plasmonic and Photonic Structure-Based Detection
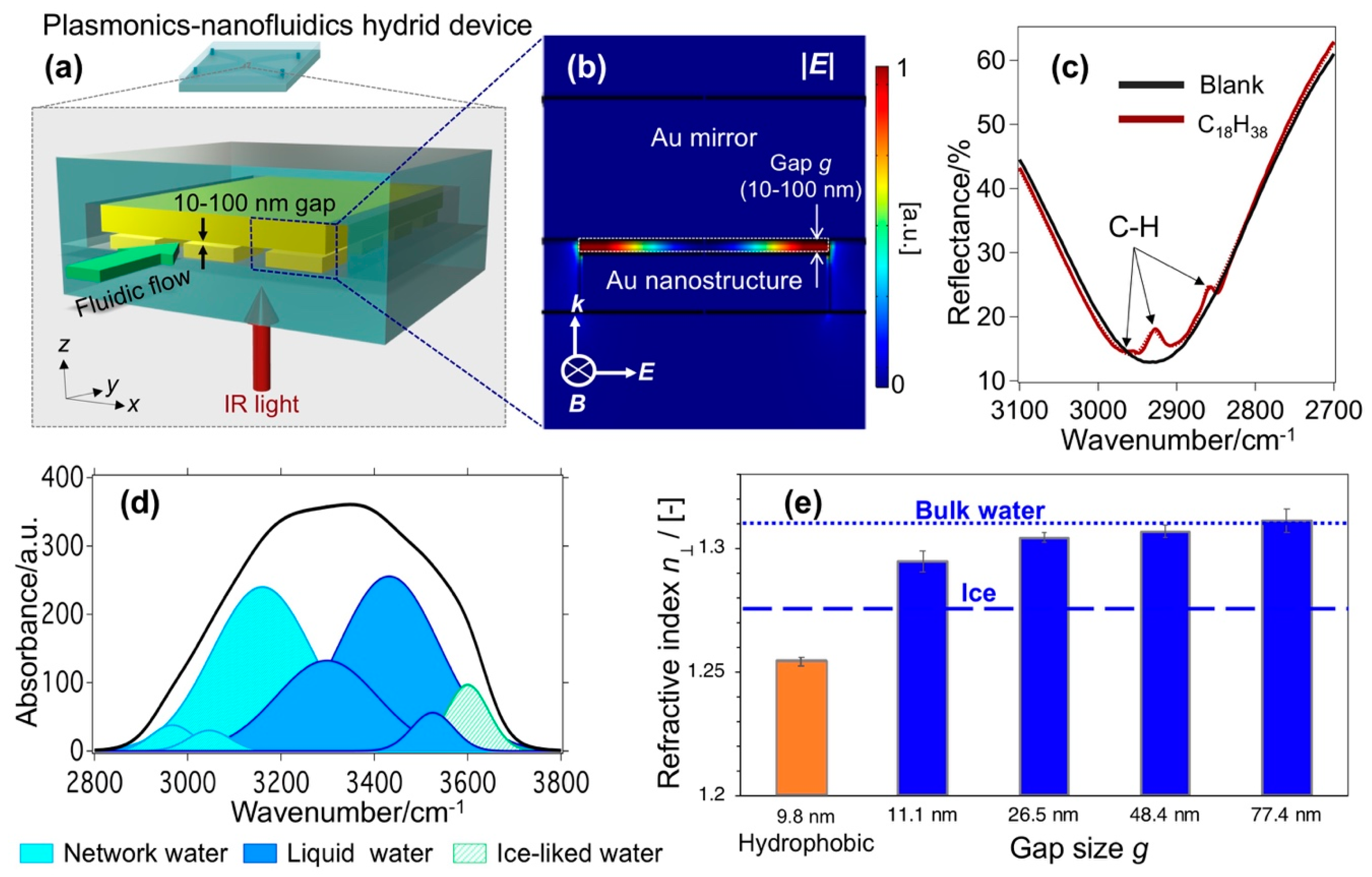
2.3.1. Refractive Index Sensing
2.3.2. Vibrational Spectroscopy
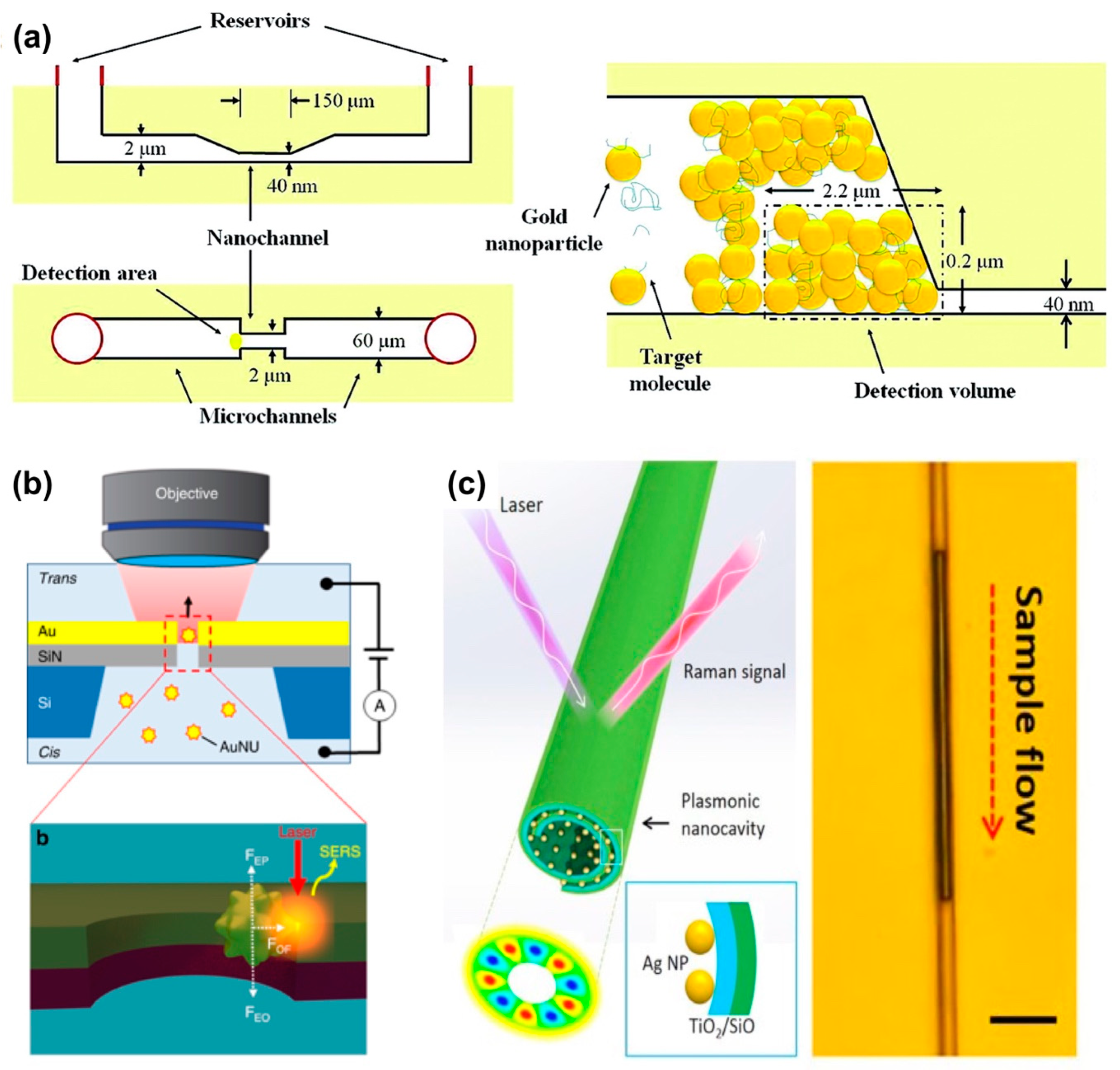
Surface-Enhanced Raman Spectroscopy (SERS)
Surface-Enhanced Infrared Absorption Spectroscopy (SEIRA)
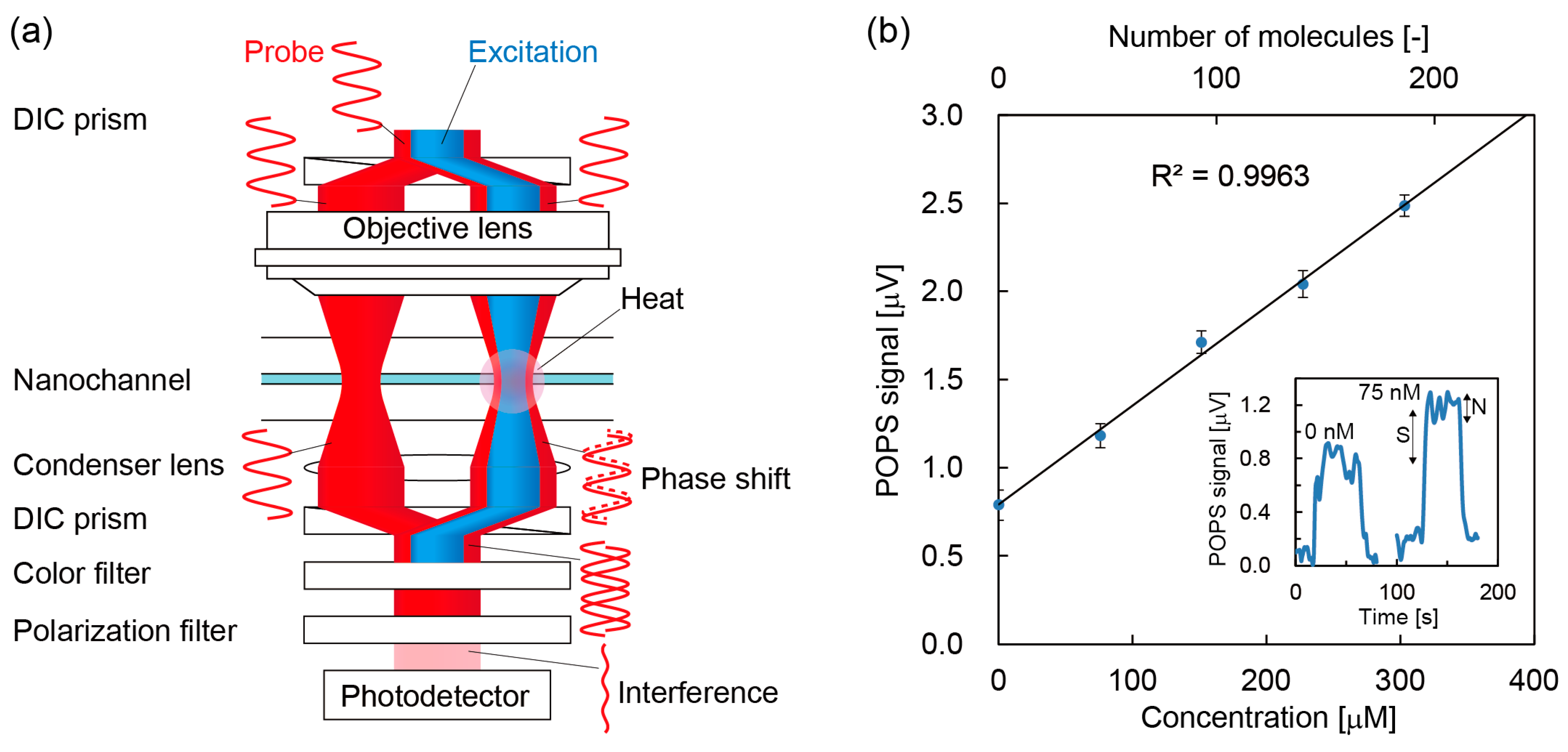
2.4. Photothermal Detection
3. Electrical/Electrochemical Detection
3.1. Detection Based on Conductivity Measurement
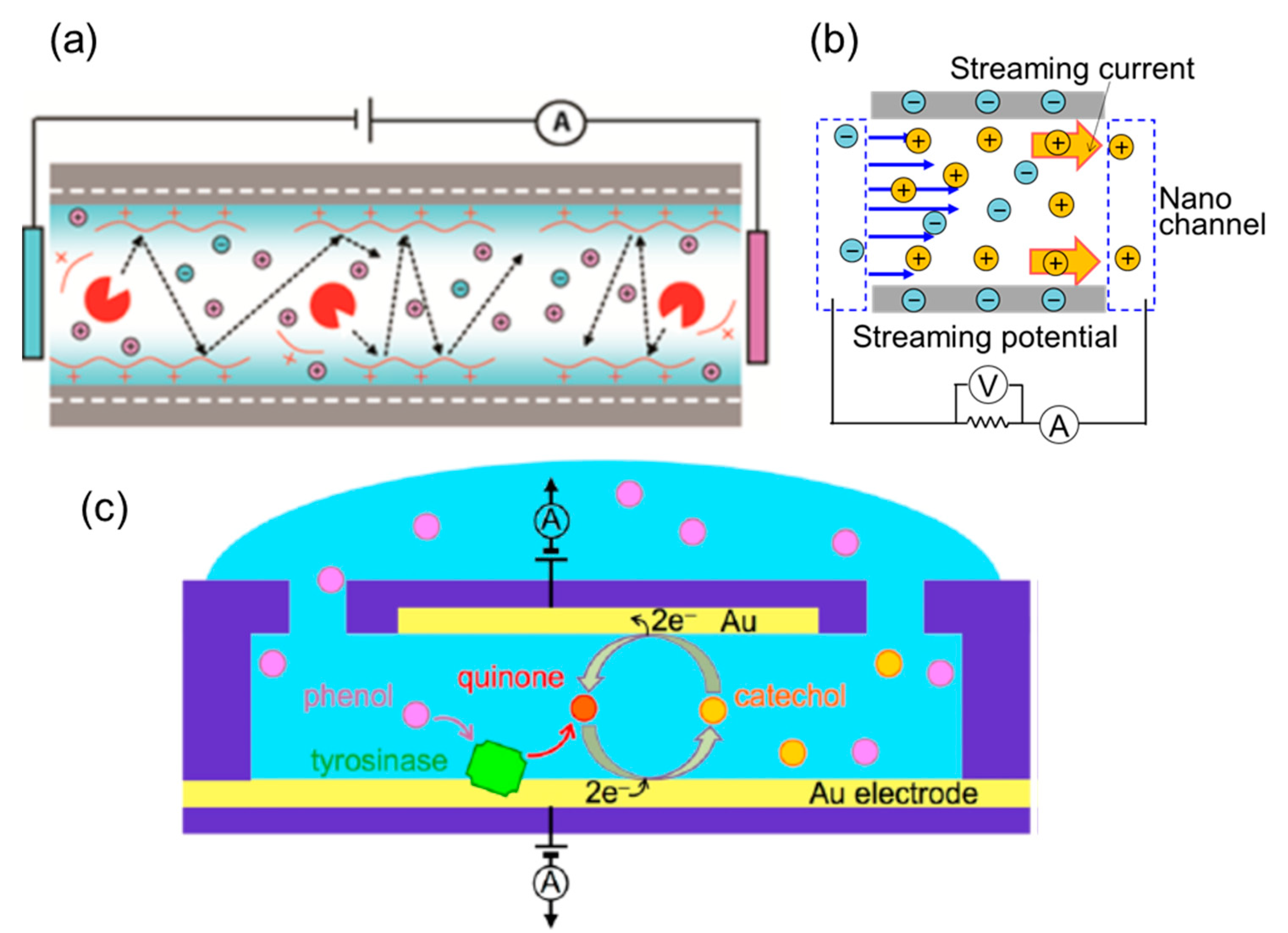
3.2. Detection Based on Electrokinetic Phenomena
3.3. Electrochemical Detection
4. Outlook
4.1. Exploring Fundamental Phenomena in Nanofluidics
4.2. Single Cell Analysis and Single-Cell Omics
4.3. Fully Integrated Diagnosis and Sensing Systems
Author Contributions
Funding
Conflicts of Interest
References
- Shang, L.; Cheng, Y.; Zhao, Y. Emerging Droplet Microfluidics. Chem. Rev. 2017, 117, 7964–8040. [Google Scholar] [CrossRef]
- Elvira, K.S.; Solvas, X.C.I.; Wootton, R.C.R.; de Mello, A.J. The past, present and potential for microfluidic reactor technology in chemical synthesis. Nat. Chem. 2013, 5, 905–915. [Google Scholar] [CrossRef]
- Zhang, B.; Korolj, A.; Lai, B.F.L.; Radisic, M. Advances in organ-on-a-chip engineering. Nat. Rev. Mater. 2018, 3, 257–278. [Google Scholar] [CrossRef]
- Morikawa, K.; Tsukahara, T. Investigation of Unique Protonic and Hydrodynamic Behavior of Aqueous Solutions Confined in Extended Nanospaces. Isr. J. Chem. 2014, 54, 1564–1572. [Google Scholar] [CrossRef]
- Schoch, R.B.; Han, J.; Renaud, P. Transport phenomena in nanofluidics. Rev. Mod. Phys. 2008, 80, 839–883. [Google Scholar] [CrossRef] [Green Version]
- Reisner, W.; Morton, K.J.; Riehn, R.; Wang, Y.M.; Yu, Z.; Rosen, M.; Sturm, J.C.; Chou, S.Y.; Frey, E.; Austin, R.H. Statics and dynamics of single DNA molecules confined in nanochannels. Phys. Rev. Lett. 2005, 94, 196101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reisner, W.; Larsen, N.B.; Silahtaroglu, A.; Kristensen, A.; Tommerup, N.; Tegenfeldt, J.O.; Flyvbjerg, H. Single-molecule denaturation mapping of DNA in nanofluidic channels. Proc. Natl. Acad. Sci. USA 2010, 107, 13294–13299. [Google Scholar] [CrossRef] [Green Version]
- Lam, E.T.; Hastie, A.; Lin, C.; Ehrlich, D.; Das, S.K.; Austin, M.D.; Deshpande, P.; Cao, H.; Nagarajan, N.; Xiao, M.; et al. Genome mapping on nanochannel arrays for structural variation analysis and sequence assembly. Nat. Biotechnol. 2012, 30, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Cipriany, B.R.; Zhao, R.; Murphy, P.J.; Levy, S.L.; Tan, C.P.; Craighead, H.G.; Soloway, P.D. Single Molecule Epigenetic Analysis in a Nanofluidic Channel. Anal. Chem. 2010, 82, 2480–2487. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Craighead, H.G. Separation of long DNA molecules in a microfabricated entropic trap array. Science 2000, 288, 1026–1029. [Google Scholar] [CrossRef]
- Kaji, N.; Tezuka, Y.; Takamura, Y.; Ueda, M.; Nishimoto, T.; Nakanishi, H.; Horiike, Y.; Baba, Y. Separation of long DNA molecules by quartz nanopillar chips under a direct current electric field. Anal. Chem. 2004, 76, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, R.; Mawatari, K.; Kitamori, T. Highly efficient and ultra-small volume separation by pressure-driven liquid chromatography in extended nanochannels. Small 2012, 8, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Stevens, A.L.; Han, J.Y. Million-fold preconcentration of proteins and peptides by nanofluidic filter. Anal. Chem. 2005, 77, 4293–4299. [Google Scholar] [CrossRef]
- Fontana, M.; Fijen, C.; Lemay, S.G.; Mathwig, K.; Hohlbein, J. High-throughput, non-equilibrium studies of single biomolecules using glass-made nanofluidic devices. Lab Chip 2019, 19, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Tsukahara, T.; Mawatari, K.; Kitamori, T. Integrated extended-nano chemical systems on a chip. Chem. Soc. Rev. 2010, 39, 1000–1013. [Google Scholar] [CrossRef]
- Segerink, L.I.; Eijkel, J.C.T. Nanofluidics in point of care applications. Lab Chip 2014, 14, 3201–3205. [Google Scholar] [CrossRef] [Green Version]
- Abgrall, P.; Nguyen, N.T. Nanofluidic Devices and Their Applications. Anal. Chem. 2008, 80, 2326–2341. [Google Scholar] [CrossRef]
- Piruska, A.; Gong, M.; Sweedler, J.V.; Bohn, P.W. Nanofluidics in chemical analysis. Chem. Soc. Rev. 2010, 39, 1060–1072. [Google Scholar] [CrossRef]
- Zhou, K.; Perry, J.M.; Jacobson, S.C. Transport and Sensing in Nanofluidic Devices. Annu. Rev. Anal. Chem. 2011, 4, 321–341. [Google Scholar] [CrossRef]
- Yu, H.; Peng, Y.; Yang, Y.; Li, Z. Plasmon-enhanced light–matter interactions and applications. NPJ Comput. Mater. 2019, 5, 45. [Google Scholar] [CrossRef]
- Dumond, J.J.; Low, H.Y.; Rodriguez, I. Isolated, sealed nanofluidic channels formed by combinatorial-mould nanoimprint lithography. Nanotechnology 2006, 17, 1975–1980. [Google Scholar] [CrossRef]
- Yasui, T.; Ogawa, K.; Kaji, N.; Nilsson, M.; Ajiri, T.; Tokeshi, M.; Horiike, Y.; Baba, Y. Label-free detection of real-time DNA amplification using a nanofluidic diffraction grating. Sci. Rep. 2016, 6, 31642. [Google Scholar] [CrossRef] [PubMed]
- Purr, F.; Bassu, M.; Lowe, R.D.; Thürmann, B.; Dietzel, A.; Burg, T.P. Asymmetric nanofluidic grating detector for differential refractive index measurement and biosensing. Lab Chip 2017, 17, 4265–4272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purr, F.; Eckardt, M.; Kieserling, J.; Gronwald, P.; Burg, T.P.; Dietzel, A. Robust smartphone assisted biosensing based on asymmetric nanofluidic grating interferometry. Sensors 2019, 19, 2065. [Google Scholar] [CrossRef] [Green Version]
- Enoki, S.; Iino, R.; Morone, N.; Kaihatsu, K.; Sakakihara, S.; Kato, N.; Noji, H. Label-free single-particle imaging of the influenza virus by objective-type total internal reflection dark-field microscopy. PLoS ONE 2012, 7, e49208. [Google Scholar] [CrossRef]
- Piliarik, M.; Sandoghdar, V. Direct optical sensing of single unlabelled proteins and super-resolution imaging of their binding sites. Nat. Commun. 2014, 5, 4495. [Google Scholar] [CrossRef]
- Mitra, A.; Deutsch, B.; Ignatovich, F.; Dykes, C.; Novotny, L. Nano-optofluidic detection of single viruses and nanoparticles. ACS Nano 2010, 4, 1305–1312. [Google Scholar] [CrossRef] [Green Version]
- Faez, S.; Lahini, Y.; Weidlich, S.; Garmann, R.F.; Wondraczek, K.; Zeisberger, M.; Schmidt, M.A.; Orrit, M.; Manoharan, V.N. Fast, label-free tracking of single viruses and weakly scattering nanoparticles in a nanofluidic optical fiber. ACS Nano 2015, 9, 12349–12357. [Google Scholar] [CrossRef]
- Fan, X.; White, I.M. Optofluidic microsystems for chemical and biological analysis. Nat. Photon. 2011, 5, 591–597. [Google Scholar] [CrossRef]
- Psaltis, D.; Quake, S.R.; Yang, C. Developing optofluidic technology through the fusion of microfluidics and optics. Nature 2006, 442, 381–386. [Google Scholar] [CrossRef]
- Monat, C.; Domachuk, P.; Eggleton, B.J. Integrated optofluidics: A new river of light. Nat. Photon. 2007, 1, 106–114. [Google Scholar] [CrossRef]
- Fainman, Y.; Psaltis, D.; Yang, C. Optofluidics: Fundamentals, Devices, and Applications; McGraw-Hill: New York, NY, USA, 2010. [Google Scholar]
- Testa, G.; Huang, Y.; Sarro, P.M.; Zeni, L.; Bernini, R. Integrated optofluidic ring resonator. Appl. Phys. Lett. 2010, 97, 131110. [Google Scholar] [CrossRef] [Green Version]
- Shopova, S.I.; White, I.M.; Sun, Y.; Zhu, H.; Fan, X.; Frye-Mason, G.; Thompson, A.; Ja, S. On-column micro gas chromatography detection with capillary-based optical ring resonators. Anal. Chem. 2008, 80, 2232–2238. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Schonbrun, E.; Crozier, K. Optical manipulation with planar silicon microring resonators. Nano Lett. 2010, 10, 2408–2411. [Google Scholar] [CrossRef]
- Soltani, M.; Lin, J.; Forties, R.A.; Inman, J.T.; Saraf, S.N.; Fulbright, R.M.; Lipson, M.; Wang, M.D. Nanophotonic trapping for precise manipulation of biomolecular arrays. Nat. Nanotechnol. 2014, 9, 448–452. [Google Scholar] [CrossRef]
- Huang, M.; Yanik, A.A.; Chang, T.; Altug, H. Sub-wavelength nanofluidics in photonic crystal sensors. Opt. Express 2009, 17, 24224–24233. [Google Scholar] [CrossRef] [Green Version]
- Eftekhari, F.; Escobedo, C.; Ferreira, J.; Duan, X.; Girotto, E.M.; Brolo, A.G.; Gordon, R.; Sinton, D. Nanoholes as nanochannels: Flow-through plasmonic sensing. Anal. Chem. 2009, 81, 4308–4311. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cruz, J.; Nair, S.; Manjarrez-Hernandez, A.; Gavilanes-Parra, S.; Ascanio, G.; Escobedo, C. Cost-effective flow-through nanohole array-based biosensing platform for the label-free detection of uropathogenic E. coli in real time. Biosens. Bioelectron. 2018, 106, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Escobedo, C.; Brolo, A.G.; Gordon, R.; Sinton, D. Flow-through vs flow-over: Analysis of transport and binding in nanohole array plasmonic biosensors. Anal. Chem. 2010, 82, 10015–10020. [Google Scholar] [CrossRef] [PubMed]
- Nakao, T.; Kazoe, Y.; Morikawa, K.; Lin, L.; Mawatari, K.; Kitamori, T. Femtoliter volumetric pipette and flask utilizing nanofluidics. Analyst 2020, 145, 2669–2675. [Google Scholar] [CrossRef]
- Guo, Y.; Li, H.; Reddy, K.; Shelar, H.; Nittoor, V.; Fan, X. Optofluidic Fabry-Pérot cavity biosensor with integrated flow-through micro-/nanochannels. Appl. Phys. Lett. 2011, 98, 041104. [Google Scholar] [CrossRef] [Green Version]
- Artar, A.; Yanik, A.; Altug, H. Fabry- Pérot nanocavities in multilayered plasmonic crystals for enhanced biosensing. Appl. Phys. Lett. 2009, 95, 051105. [Google Scholar] [CrossRef] [Green Version]
- Aroca, R. Surface Enhanced Vibrational Spectroscopy; Wiley & Sons, Ltd.: Chichester, UK, 2006. [Google Scholar]
- Kneipp, K.; Wang, Y.; Kneipp, H.; Perelman, L.T.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Single molecule detection using surface-enhanced raman scattering (SERS). Phys. Rev. Lett. 1997, 78, 1667–1670. [Google Scholar] [CrossRef] [Green Version]
- Adato, R.; Altug, H. In-situ ultra-sensitive infrared absorption spectroscopy of biomolecule interactions in real time with plasmonic nanoantennas. Nat. Commun. 2013, 4, 2154. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Yang, X.; Zhang, C.; Cerjan, B.; Zhou, L.; Tseng, M.L.; Zhang, Y.; Alabastri, A.; Nordlander, P.; Halas, N.J. Nanogapped Au antennas for ultrasensitive surface-enhanced infrared absorption spectroscopy. Nano Lett. 2017, 17, 5768–5774. [Google Scholar] [CrossRef]
- Kumar, S.; Cherukulappurath, S.; Johnson, T.W.; Oh, S. Millimeter-sized suspended plasmonic nanohole arrays for surface-tension-driven flow-through SERS. Chem. Mater. 2014, 26, 6523–6530. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Du, X.; Cui, Y.; Zhang, X.; Ge, Q.; Dong, J.; Zhao, X. Vertical flow assay for inflammatory biomarkers based on nanofluidic channel array and SERS nanotags. Small 2020, 2002801. [Google Scholar] [CrossRef]
- Zhao, X.; Xue, J.; Mu, Z.; Huang, Y.; Lu, M.; Gu, Z. Gold nanoparticle incorporated inverse opal photonic crystal capillaries for optofluidic surface enhanced Raman spectroscopy. Biosens. Bioelectron. 2015, 72, 268–274. [Google Scholar] [CrossRef]
- Chou, I.; Benford, M.; Beier, H.T.; Coté, G.L.; Wang, M.; Jing, N.; Kameoka, J.; Good, T.A. Nanofluidic biosensing for β-amyloid detection using surface enhanced raman spectroscopy. Nano Lett. 2008, 8, 1729–1735. [Google Scholar] [CrossRef] [Green Version]
- Choi, I.; Huh, Y.S.; Erickson, D. Size-selective concentration and label-free characterization of protein aggregates using a Raman active nanofluidic device. Lab Chip 2011, 11, 632–638. [Google Scholar] [CrossRef]
- Takeshita, T.; Suekuni, K.; Aiba, K.; Sugano, K.; Isono, Y. Surface-enhanced Raman spectroscopy analysis device with gold nanoparticle arranged nanochannel. Electron. Commun. Jpn. 2017, 100, 33–41. [Google Scholar] [CrossRef]
- Chen, C.; Li, Y.; Kerman, S.; Neutens, P.; Willems, K.; Cornelissen, S.; Lagae, L.; Stakenborg, T.; Dorpe, P.V. High spatial resolution nanoslit SERS for single-molecule nucleobase sensing. Nat. Commun. 2018, 9, 1733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Mousavi, M.Z.; Zhao, Y.; Hubarevich, A.; Omeis, F.; Giovannini, G.; Schütte, M.; Garoli, D.; Angelis, F.D. SERS discrimination of single DNA bases in single oligonucleotides by electro-plasmonic trapping. Nat. Commun. 2019, 10, 1733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Li, J.; Tang, S.; Fang, Y.; Wang, J.; Huang, G.; Liu, R.; Zheng, L.; Cui, X.; Mei, Y. Whispering-gallery nanocavity plasmon-enhanced Raman spectroscopy. Sci. Rep. 2015, 5, 15012. [Google Scholar] [CrossRef] [Green Version]
- Yoo, D.; Mohr, D.A.; Vidal-Codina, F.; John-Herpin, A.; Jo, M.; Kim, S.; Matson, J.; Caldwell, J.D.; Jeon, H.; Nguyen, N.; et al. High-Contrast Infrared Absorption Spectroscopy via Mass-Produced Coaxial Zero-Mode Resonators with Sub-10 nm Gaps. Nano Lett. 2018, 18, 1930–1936. [Google Scholar] [CrossRef] [Green Version]
- Rodrigo, D.; Tittl, A.; Ait-Bouziad, N.; John-Herpin, A.; Limaj, O.; Kelly, C.; Yoo, D.; Wittenberg, N.J.; Oh, S.; Lashuel, H.A.; et al. Resolving molecule-specific information in dynamic lipid membrane processes with multi-resonant infrared metasurfaces. Nat. Commun. 2018, 9, 2160. [Google Scholar] [CrossRef] [Green Version]
- Bomers, M.; Charlot, B.; Barho, F.; Chanuel, A.; Mezy, A.; Cerutti, L.; Gonzalez-Posada, F.; Taliercio, T. Microfluidic surface-enhanced infrared spectroscopy with semiconductor plasmonics for the fingerprint region. React. Chem. Eng. 2020, 5, 124–135. [Google Scholar] [CrossRef]
- Le, H.H.T.; Tanaka, T. Plasmonics-Nanofluidics Hydrid Metamaterial: An Ultrasensitive Platform for Infrared Absorption Spectroscopy and Quantitative Measurement of Molecules. ACS Nano 2017, 11, 9780–9788. [Google Scholar] [CrossRef]
- Le, H.H.T.; Morita, A.; Mawatari, K.; Kitamori, T.; Tanaka, T. Metamaterials-Enhanced Infrared Spectroscopic Study of Nanoconfined Molecules by Plasmonics-Nanofluidics Hydrid Device. ACS Photonics 2018, 5, 3179–3188. [Google Scholar] [CrossRef]
- Le, H.H.T.; Morita, A.; Tanaka, T. Refractive index of nanoconfined water reveals its anomalous physical properties. Nanoscale Horiz. 2020, 5, 1016–1024. [Google Scholar] [CrossRef]
- Dovichi, N.J.; Harris, J.M. Laser induced thermal lens effect for calorimetric trace analysis. Anal. Chem. 1979, 51, 728–731. [Google Scholar] [CrossRef]
- Tokeshi, M.; Uchida, M.; Hibara, A.; Sawada, T.; Kitamori, T. Determination of subyoctomole amounts of nonfluorescent molecules using a thermal lens microscope: subsingle-molecule determination. Anal. Chem. 2001, 73, 2112–2116. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Mawatari, K.; Kitamori, T. Sensitive determination of concentration of nonfluorescent species in an extended-nano channel by differential interference contrast thermal lens microscope. Anal. Chem. 2010, 82, 7479–7484. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Takeda, S.; Mawatari, K.; Kitamori, T. Ultrasensitive detection of nonlabelled bovine serum albumin using photothermal optical phase shift detection with UV excitation. Analyst 2020, 145, 2580–2585. [Google Scholar] [CrossRef]
- Le, H.H.T.; Mawatari, K.; Shimizu, H.; Kitamori, T. Detection of zeptomole quantities of nonfluorescent molecules in a 101 nm nanochannel by thermal lens microscopy. Analyst 2014, 139, 2721–2725. [Google Scholar] [CrossRef]
- Tsuyama, Y.; Mawatari, K. Nonfluorescent molecule detection in 102 nm nanofluidic channels by photothermal optical diffraction. Anal. Chem. 2019, 91, 9741–9746. [Google Scholar] [CrossRef]
- Zhao, P.; Zhao, Y.; Bao, H.; Ho, H.L.; Jin, W.; Fan, S.; Gao, S.; Wang, Y.; Wang, P. Mode-phase-difference photothermal spectroscopy for gas detection with an anti-resonant hollow-core optical fiber. Nat. Commun. 2020, 11, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Dekker, C. Solid-state nanopores. Nat. Nanotechnol. 2007, 2, 209–216. [Google Scholar] [CrossRef]
- Howorka, S.; Siwy, Z. Nanopore analytics: Sensing of single molecules. Chem. Soc. Rev. 2009, 38, 2360–2384. [Google Scholar] [CrossRef]
- Siwy, Z.S.; Howorka, S. Engineered voltage-responsive nanopores. Chem. Soc. Rev. 2010, 39, 1115–1132. [Google Scholar] [CrossRef]
- Mulero, R.; Prabhu, A.S.; Freedman, K.J.; Kim, M.J. Nanopore-Based Devices for Bioanalytical Applications. J. Assoc. Lab. Autom. 2010, 15, 243–252. [Google Scholar] [CrossRef]
- Hou, X.; Guo, W.; Jiang, L. Biomimetic smart nanopores and nanochannels. Chem. Soc. Rev. 2011, 40, 2385–2401. [Google Scholar] [CrossRef] [PubMed]
- Miles, B.N.; Ivanov, A.P.; Wilson, K.A.; Dogan, F.; Japrung, D.; Edel, J.B. Single molecule sensing with solid-state nanopores: Novel materials, methods, and applications. Chem. Soc. Rev. 2013, 42, 15–28. [Google Scholar] [CrossRef]
- Shi, W.; Friedman, A.K.; Baker, L.A. Nanopore Sensing. Anal. Chem. 2017, 89, 157–188. [Google Scholar] [CrossRef] [Green Version]
- Waduge, P.; Hu, R.; Bandarkar, P.; Yamazaki, H.; Cressiot, B.; Zhao, Q.; Whitford, P.C.; Wanunu, M. Nanopore-Based Measurements of Protein Size, Fluctuations, and Conformational Changes. ACS Nano 2017, 11, 5706–5716. [Google Scholar] [CrossRef]
- Lin, X.; Ivanov, A.P.; Edel, J.B. Selective single molecule nanopore sensing of proteins using DNA aptamer-functionalised gold nanoparticles. Chem. Sci. 2017, 8, 3905–3912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohshiro, T.; Tsutsui, M.; Yokota, K.; Taniguchi, M. Quantitative analysis of DNA with single-molecule sequencing. Sci. Rep. 2018, 8, 8517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heerema, S.J.; Vicarelli, L.; Pud, S.; Schouten, R.N.; Zandbergen, H.W.; Dekker, C. Probing DNA Translocations with Inplane Current Signals in a Graphene Nanoribbon with a Nanopore. ACS Nano 2018, 12, 2623–2633. [Google Scholar] [CrossRef] [Green Version]
- Giamblanco, N.; Coglitore, D.; Janot, J.M.; Coulon, P.E.; Charlot, B.; Balme, S. Detection of protein aggregate morphology through single antifouling nanopore. Sens. Actuat. B Chem. 2018, 260, 736–745. [Google Scholar] [CrossRef]
- Stein, D.; Kruithof, M.; Dekker, C. Surface-Charge-Governed Ion Transport in Nanofluidic Channels. Phys. Rev. Lett. 2004, 93, 035901. [Google Scholar] [CrossRef] [Green Version]
- Hatsuki, R.; Yujiro, F.; Yamamoto, T. Direct measurement of electric double layer in a nanochannel by electrical impedance spectroscopy. Microfluid. Nanofluid. 2013, 14, 983–988. [Google Scholar] [CrossRef]
- Lee, C.; Joly, L.; Siria, A.; Biance, A.; Fulcrand, R.; Bocquet, L. Large Apparent Electric Size of Solid-State Nanopores Due to Spatially Extended Surface Conduction. Nano Lett. 2012, 12, 4037–4044. [Google Scholar] [CrossRef] [PubMed]
- Karnik, R.; Castelino, K.; Fan, R.; Yang, P.; Majumdar, A. Effects of Biological Reactions and Modifications on Conductance of Nanofluidic Channels. Nano Lett. 2005, 5, 1638–1642. [Google Scholar] [CrossRef]
- Schoch, R.B.; Cheow, L.F.; Han, J. Electrical Detection of Fast Reaction Kinetics in Nanochannels with an Induced Flow. Nano Lett. 2007, 7, 3895–3900. [Google Scholar] [CrossRef] [Green Version]
- Durand, N.F.Y.; Renaud, P. Label-free determination of protein–surface interaction kinetics by ionic conductance inside a nanochannel. Lab Chip 2009, 9, 319–324. [Google Scholar] [CrossRef]
- Hsueh, H.T.; Lin, C.T. An incremental double-layer capacitance of a planar nano gap and its application in cardiac-troponin T detection. Biosens. Bioelectron. 2016, 79, 636–643. [Google Scholar] [CrossRef]
- Liao, T.; Li, X.; Tong, Q.; Zou, K.; Zhang, H.; Tang, L.; Sun, Z.; Zhang, G. Ultrasensitive Detection of MicroRNAs with Morpholino-Functionalized Nanochannel Biosensor. Anal. Chem. 2017, 89, 5511–5518. [Google Scholar] [CrossRef]
- Duan, C.; Alibakhshi, M.A.; Kim, D.K.; Brown, C.M.; Craik, C.S.; Majumdar, A. Label-Free Electrical Detection of Enzymatic Reactions in Nanochannels. ACS Nano 2016, 10, 7476–7484. [Google Scholar] [CrossRef]
- Kim, S.J.; Wang, Y.; Lee, J.H.; Jang, H.; Han, J. Concentration Polarization and Nonlinear Electrokinetic Flow near Nanofluidic Channel. Phys. Rev. Lett. 2007, 99, 044501. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.; Yeh, C.; Yang, R. Ion concentration polarization near microchannel–nanochannel interfaces: Effect of pH value. Electrophoresis 2012, 33, 758–764. [Google Scholar] [CrossRef]
- Huang, K.; Yang, R. A nanochannel-based concentrator utilizing the concentration polarization effect. Electrophoresis 2008, 29, 4862–4870. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.; Bu, Y.; Yobas, L. Conductance Interplay in Ion Concentration Polarization across 1D Nanochannels: Microchannel Surface Shunt and Nanochannel Conductance. Anal. Chem. 2020, 92, 1252–1259. [Google Scholar] [CrossRef] [PubMed]
- Karnik, R.; Duan, C.; Castelino, K.; Daiguji, H.; Majumdar, A. Rectification of Ionic Current in a Nanofluidic Diode. Nano Lett. 2007, 7, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Yang, F.; Li, L.; Song, Y.; Jiang, L.; Zhu, D. A Biomimetic Asymmetric Responsive Single Nanochannel. J. Am. Chem. Soc. 2010, 132, 11736–11742. [Google Scholar] [CrossRef]
- Perry, J.M.; Zhou, K.; Harms, Z.D.; Jacobson, S.C. Ion Transport in Nanofluidic Funnels. ACS Nano 2010, 4, 3897–3902. [Google Scholar] [CrossRef]
- Guan, W.; Fan, R.; Reed, M.A. Field-effect reconfigurable nanofluidic ionic diodes. Nat. Commun. 2011, 2, 1–8. [Google Scholar] [CrossRef]
- Eberle, P.; Höller, C.; Müller, P.; Suomalainen, M.; Greber, U.F.; Eghlidi, H.; Poulikakos, D. Single entity resolution valving of nanoscopic species in liquids. Nat. Nanotechnol. 2018, 13, 578–582. [Google Scholar] [CrossRef]
- Lin, L.; Mawatari, K.; Morikawa, K.; Kitamori, T. Living Single Cell Analysis Platform Utilizing Microchannel, Single Cell Chamber, and Extended-nano Channel. Anal. Sci. 2016, 32, 75–78. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; Mawatari, K.; Morikawa, K.; Pihosh, Y.; Yoshizaki, A.; Kitamori, T. Micro/extended-nano sampling interface from a living single cell. Analyst 2017, 142, 1689–1696. [Google Scholar] [CrossRef]
- Peng, R.; Li, D. Detection and sizing of nanoparticles and DNA on PDMS nanofluidic chips based on differential resistive pulse sensing. Nanoscale 2017, 9, 5964–5974. [Google Scholar] [CrossRef]
- Harms, Z.D.; Mogensen, K.B.; Nunes, P.S.; Zhou, K.; Hildenbrand, B.W.; Mitra, I.; Tan, Z.; Zlotnick, A.; Kutter, J.P.; Jacobson, S.C. Nanofluidic Devices with Two Pores in Series for Resistive-Pulse Sensing of Single Virus Capsids. Anal. Chem. 2011, 83, 9573–9578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondylis, P.; Schlicksup, C.J.; Brunk, N.E.; Zhou, J.; Zlotnick, A.; Jacobson, S.C. Competition between Normative and Drug-Induced Virus Self-Assembly Observed with Single-Particle Methods. J. Am. Chem. Soc. 2019, 141, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Yasaki, H.; Shimada, T.; Yasui, T.; Yanagida, T.; Kaji, N.; Kanai, M.; Nagashima, K.; Kawai, T.; Baba, Y. Robust Ionic Current Sensor for Bacterial Cell Size Detection. ACS Sens. 2018, 3, 574–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pennathur, S.; Santiago, J.G. Electrokinetic Transport in Nanochannels. 2. Experiments. Anal. Chem. 2005, 77, 6782–6789. [Google Scholar] [CrossRef]
- Haywood, D.G.; Harms, Z.D.; Jacobson, S.C. Electroosmotic Flow in Nanofluidic Channels. Anal. Chem. 2014, 86, 11174–11180. [Google Scholar] [CrossRef] [Green Version]
- Peng, R.; Li, D. Electroosmotic flow in single PDMS nanochannels. Nanoscale 2016, 8, 12237–12246. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Li, D. Electroosmotic flow velocity in DNA modified nanochannels. J. Colloid Interface Sci. 2019, 553, 31–39. [Google Scholar] [CrossRef]
- Van der Heyden, F.H.J.; Stein, D.; Dekker, C. Streaming currents in a single nanofluidic channel. Phys. Rev. Lett. 2005, 95, 116104. [Google Scholar] [CrossRef] [Green Version]
- Van der Heyden, F.H.J.; Stein, D.; Besteman, K.; Lemay, S.G.; Dekker, C. Charge inversion at high ionic strength studied by streaming currents. Phys. Rev. Lett. 2006, 96, 224502. [Google Scholar] [CrossRef] [Green Version]
- Van der Heyden, F.H.J.; Bonthuis, D.J.; Stein, D.; Meyer, C.; Dekker, C. Electrokinetic Energy Conversion Efficiency in Nanofluidic Channels. Nano Lett. 2006, 6, 2232–2237. [Google Scholar] [CrossRef]
- Morikawa, K.; Mawatari, K.; Kato, M.; Tsukahara, T.; Kitamori, T. Streaming potential/current measurement system for investigation of liquids confined in extended-nanospace. Lab Chip 2010, 10, 871–875. [Google Scholar] [CrossRef]
- Morikawa, K.; Mawatari, K.; Kazoe, Y.; Tsukahara, T.; Kitamori, T. Shift of isoelectric point in extended nanospace investigated by streaming current measurement. Appl. Phys. Lett. 2011, 99, 123115. [Google Scholar] [CrossRef]
- Morikawa, K.; Kazoe, Y.; Mawatari, K.; Tsukahara, T.; Kitamori, T. Dielectric Constant of Liquids Confined in the Extended Nanospace Measured by a Streaming Potential Method. Anal. Chem. 2015, 87, 1475–1479. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xu, B. An Integrated Glass Nanofluidic Device Enabling In-situ Electrokinetic Probing of Water Confined in a Single Nanochannel under Pressure-Driven Flow Conditions. Small 2015, 11, 6165–6171. [Google Scholar] [CrossRef]
- Siria, A.; Poncharal, P.; Biance, A.; Fulcrand, R.; Blase, X.; Purcell, S.T.; Bocquet, L. Giant osmotic energy conversion measured in a single transmembrane boron nitride nanotube. Nature 2013, 494, 455–458. [Google Scholar] [CrossRef]
- Zevenbergen, M.A.G.; Singh, P.S.; Goluch, E.D.; Wolfrum, B.L.; Lemay, S.G. Stochastic Sensing of Single Molecules in a Nanofluidic Electrochemical Device. Nano Lett. 2011, 11, 2881–2886. [Google Scholar] [CrossRef]
- Kang, S.; Nieuwenhuis, A.F.; Mathwig, K.; Mampallil, D.; Lemay, S.G. Electrochemical Single-Molecule Detection in Aqueous Solution Using Self-Aligned Nanogap Transducers. ACS Nano 2013, 7, 10931–10937. [Google Scholar] [CrossRef] [PubMed]
- Rassaei, L.; Mathwig, K.; Kang, S.; Heering, H.A.; Lemay, S.G. Integrated Biodetection in a Nanofluidic Device. ACS Nano 2014, 8, 8278–8284. [Google Scholar] [CrossRef]
- Mathwig, K.; Mampallil, D.; Kang, S.; Lemay, S.G. Electrical cross-correlation spectroscopy: Measuring picoliter-per-minute flows in nanochannels. Phys. Rev. Lett. 2012, 109, 118302. [Google Scholar] [CrossRef] [Green Version]
- Sanghavi, B.J.; Varhue, W.; Rohani, A.; Liao, K.; Bazydlo, L.A.L.; Chou, C.; Swami, N.S. Ultrafast immunoassays by coupling dielectrophoretic biomarker enrichment in nanoslit channel with electrochemical detection on graphene. Lab Chip 2015, 15, 4563–4570. [Google Scholar] [CrossRef]
- Ren, Y.; Stein, D. Slip-enhanced electrokinetic energy conversion in nanofluidic channels. Nanotechnology 2008, 19, 195707. [Google Scholar] [CrossRef] [PubMed]
- Kuang, C.; Wang, G. A novel far-field nanoscopic velocimetry for nanofluidics. Lab Chip 2010, 10, 240–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazoe, Y.; Iseki, K.; Mawatari, K.; Kitamori, T. Evanescent Wave-Based Particle Tracking Velocimetry for Nanochannel Flows. Anal. Chem. 2013, 85, 10780–10786. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Zhang, Y.; Liu, J. Advancing single-cell proteomics and metabolomics with microfluidic technologies. Analyst 2019, 144, 846–858. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Fan, L.; Zhu, R.; Sun, D. Microfluidic Single-Cell Manipulation and Analysis: Methods and Applications. Micromachines 2019, 10, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, T.; Ko, S.H.; Hamel, J.P.; Han, J. Continuous Online Protein Quality Monitoring during Perfusion Culture Production Using an Integrated Micro/Nanofluidic System. Anal. Chem. 2020, 92, 5267–5275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.; Yin, B.; Michel, J. On-chip light sources for silicon photonics. Light Sci. Appl. 2015, 4, e358. [Google Scholar] [CrossRef]
| Targets | Liquid | Ion | Small Molecules | Large Molecules (DNA, Proteins, Peptides, etc.) | Nano-Particle | Virus | Bacteria | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Methods | ||||||||||||
| Optical | Diffraction | [22,23,24] | ||||||||||
| Scattering | [27] | [28] | ||||||||||
| Plasmonics | RI | [37] | [40] | [38,39,40,42] | [39] | |||||||
| SERS | [48,54,55] | |||||||||||
| [50,53] | [56] | [49,51,52] | ||||||||||
| SEIRA | [61,62] | [60] | ||||||||||
| Photothermal | [65,67,68] | [66] | ||||||||||
| Electrical | Conductivity | [84,96] | [77,78,79,80,81,89] | [103,104] | [105] | |||||||
| [82,83,91,92,93,94,95] | [85,86,87,88,90,99] | |||||||||||
| [97,98,100,101] | [102] | |||||||||||
| Electrokinetic | [108,109,110,111,112,113,114,115,116] | [117] | ||||||||||
| Electrochemical | [118,119,120,121] | [122] | ||||||||||
 —Nanotube.
—Nanotube.  —Nanopore.
—Nanopore.  —Nanochannel.
—Nanochannel.© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, T.H.H.; Shimizu, H.; Morikawa, K. Advances in Label-Free Detections for Nanofluidic Analytical Devices. Micromachines 2020, 11, 885. https://doi.org/10.3390/mi11100885
Le THH, Shimizu H, Morikawa K. Advances in Label-Free Detections for Nanofluidic Analytical Devices. Micromachines. 2020; 11(10):885. https://doi.org/10.3390/mi11100885
Chicago/Turabian StyleLe, Thu Hac Huong, Hisashi Shimizu, and Kyojiro Morikawa. 2020. "Advances in Label-Free Detections for Nanofluidic Analytical Devices" Micromachines 11, no. 10: 885. https://doi.org/10.3390/mi11100885
APA StyleLe, T. H. H., Shimizu, H., & Morikawa, K. (2020). Advances in Label-Free Detections for Nanofluidic Analytical Devices. Micromachines, 11(10), 885. https://doi.org/10.3390/mi11100885





