Assessment of Flow through Microchannels for Inertia-Based Sorting: Steps toward Microfluidic Medical Devices
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Fluid and Test Parameters
2.2. Test Setup
2.3. Failure Modes
2.4. Particle Analysis Methods
2.5. Data Analysis
3. Results
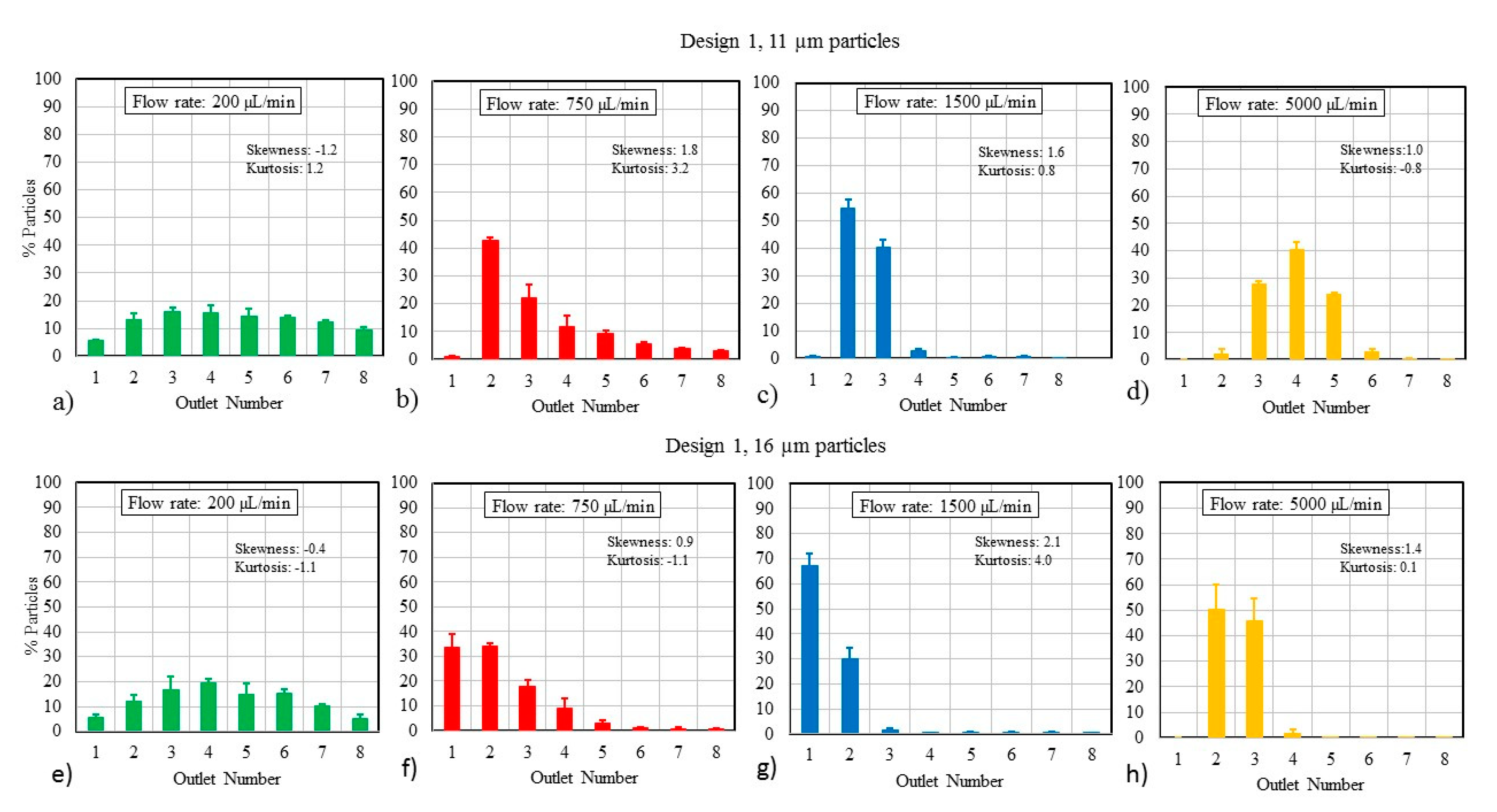
3.1. Particle Size and Flow Rate
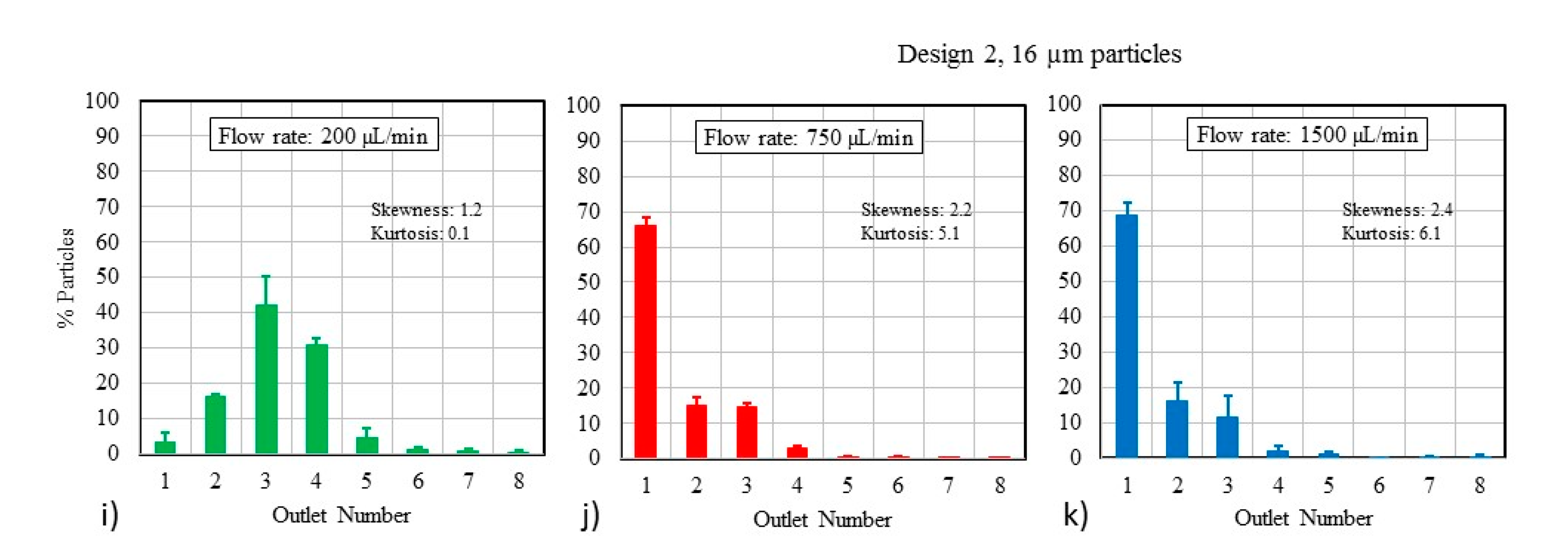
3.2. Particle Mixtures
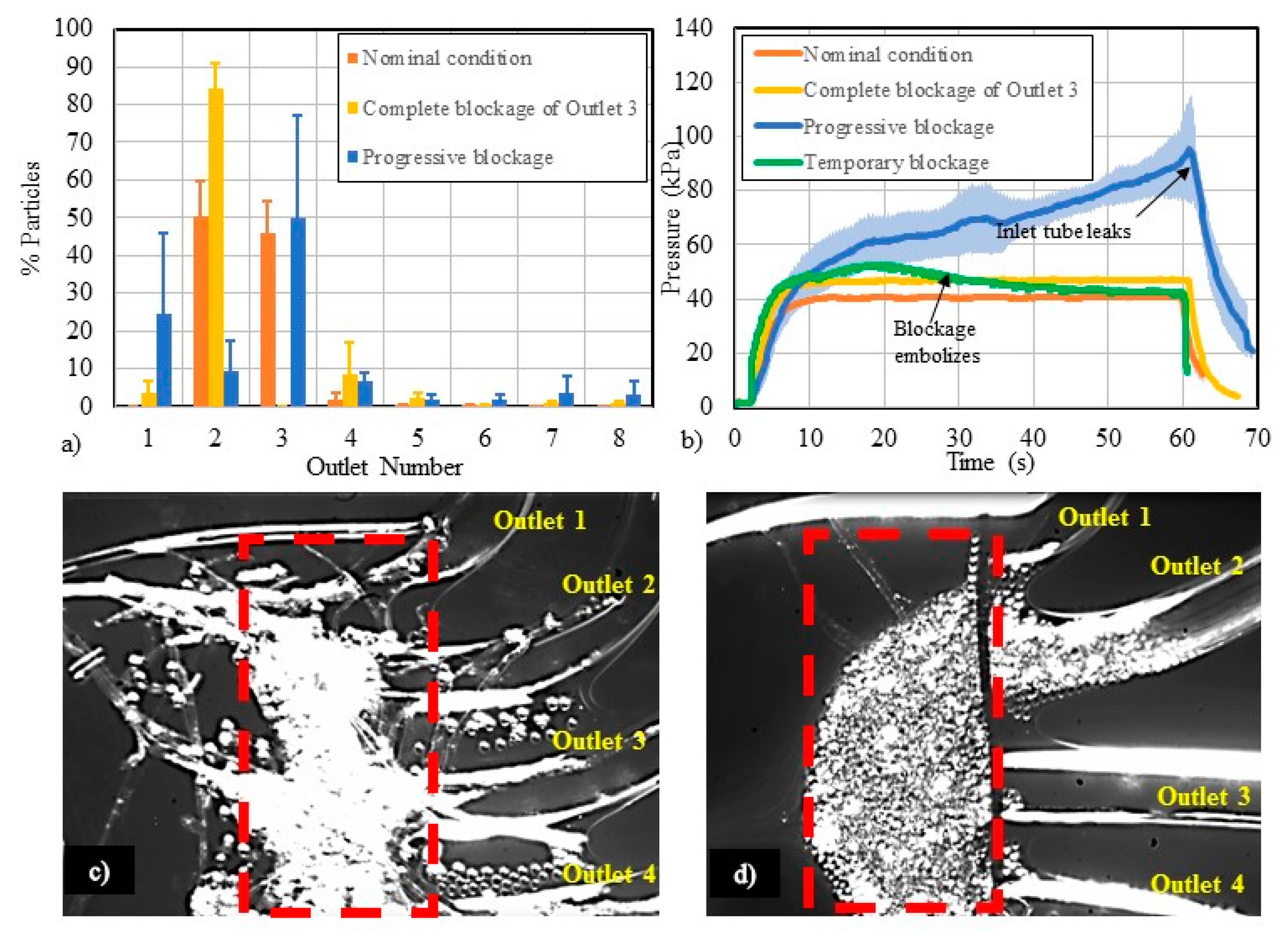
3.3. Flow-Related Failure Modes
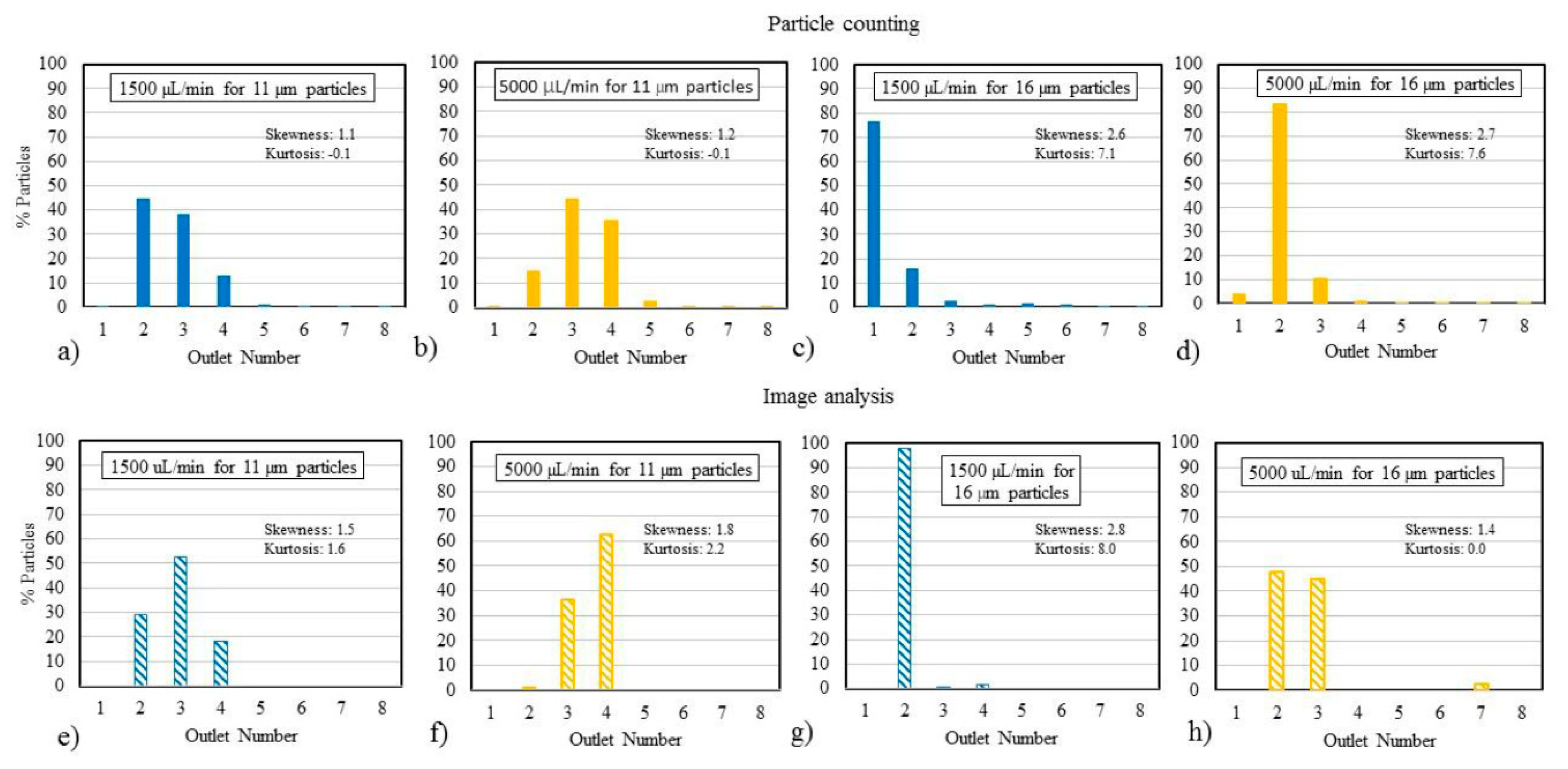
3.4. Study of Analysis Methods
4. Discussion
4.1. Particle Focusing Behavior
4.2. Effect of Using Particle Mixtures
4.3. Study of Flow-Related Failure Modes
4.4. Differences in Analysis Methods
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Ashraf, M.W.; Tayyaba, S.; Afzulpurkar, N. Micro Electromechanical Systems (MEMS) Based Microfluidic Devices for Biomedical Applications. Int. J. Mol. Sci. 2011, 12, 3648–3704. [Google Scholar] [CrossRef] [PubMed]
- Warkiani, M.E.; Wu, L.; Tay, A.K.; Han, J. Large-Volume Microfluidic Cell Sorting for Biomedical Applications. Annu. Rev. Biomed. Eng. 2015, 17, 1–34. [Google Scholar] [CrossRef]
- Li, P. Microfluidics For IVD: In Pursuit Of The Holy Grail. J. Bioeng. Biomed. Sci. 2012, 8, 2. [Google Scholar]
- FDA. Regulatory Information. Available online: https://www.fda.gov/regulatory-information/standards (accessed on 21 September 2020).
- Griffith, E.J.; Akella, S.; Goldberg, M.K. Performance Characterization of a Reconfigurable Planar-Array Digital Microfluidic System. IEEE Trans. Comput.-Aided Des. Integr. Circuits Syst. 2006, 25, 345–357. [Google Scholar] [CrossRef]
- Ma, R.; Kaler, K.V.; Backhouse, C.J. A Rapid Performance Assessment Method for Microfluidic Chips. In Proceedings of the 2004 International Conference on MEMS, NANO and Smart Systems (ICMENS’04), Banff, AB, Canada, 25–27 August 2004; pp. 680–686. [Google Scholar]
- Tao, X.; Chakrabarty, K. Parallel scan-like test and multiple-defect diagnosis for digital microfluidic biochips. IEEE Trans. Biomed. Circuits Syst. 2007, 1, 148–158. [Google Scholar]
- Kai, H.; Feiqiao, Y.; Tsung-Yi, H.; Chakrabarty, K. Testing of Flow-Based Microfluidic Biochips: Fault Modeling, Test Generation, and Experimental Demonstration. IEEE Trans. Comput.-Aided Des. Integr. Circuits Syst. 2014, 33, 1463–1475. [Google Scholar] [CrossRef]
- McRae, M.P.; Simmons, G.; McDevitt, J.T. Challenges and opportunities for translating medical microdevices: Insights from the programmable bio-nano-chip. Bioanalysis 2016, 8, 905–919. [Google Scholar] [CrossRef]
- Day, J. Introduction to In Vitro Diagnostic Device Regulatory Requirements. In Microfluidic Diagnostics; Humana Press: Totowa, NJ, USA, 2013; pp. 103–112. [Google Scholar]
- Tantra, R.; van Heeren, H. Product qualification: A barrier to point-of-care microfluidic-based diagnostics? Lab Chip 2013, 13, 2199–2201. [Google Scholar] [CrossRef]
- Reyes, D.R.; van Heeren, H. Proceedings of the First Workshop on Standards for Microfluidics. J. Res. Natl. Inst. Stand. Technol. 2019, 124, 124001. [Google Scholar] [CrossRef]
- Chin, C.D.; Vincent, L.; Sia, S.K. Commercialization of microfluidic point-of-care diagnostic devices. Lab Chip 2012, 12, 2118–2134. [Google Scholar] [CrossRef]
- Nivedita, N.; Ligrani, P.; Papautsky, I. Dean Flow Dynamics in Low-Aspect Ratio Spiral Microchannels. Sci. Rep. 2017, 7, 44072. [Google Scholar] [CrossRef] [PubMed]
- Kuntaegowdanahalli, S.S.; Bhagat, A.A.S.; Kumar, G.; Papautsky, I. Inertial microfluidics for continuous particle separation in spiral microchannels. Lab Chip 2009, 9, 2973–2980. [Google Scholar] [CrossRef] [PubMed]
- Martel, J.M.; Toner, M. Particle Focusing in Curved Microfluidic Channels. Sci. Rep. 2013, 3, 1–8. [Google Scholar] [CrossRef]
- Gou, Y.; Jia, Y.; Wang, P.; Sun, C. Progress of Inertial Microfluidics in Principle and Application. Sensors 2018, 18, 1762. [Google Scholar] [CrossRef]
- Jung, B.-J.; Kim, J.; Kim, J.-A.; Jang, H.; Seo, S.; Lee, W. PDMS-Parylene Hybrid, Flexible Microfluidics for Real-Time Modulation of 3D Helical Inertial Microfluidics. Micromachines 2018, 9, 255. [Google Scholar] [CrossRef]
- Al-Halhouli, A.; Al-Faqheri, W.; Alhamarneh, B.; Hecht, L.; Dietzel, A. Spiral Microchannels with Trapezoidal Cross Section Fabricated by Femtosecond Laser Ablation in Glass for the Inertial Separation of Microparticles. Micromachines 2018, 9, 171. [Google Scholar] [CrossRef]
- Liu, N.; Petchakup, C.; Tay, H.M.; Li, K.H.H.; Hou, H.W. Spiral Inertial Microfluidics for Cell Separation and Biomedical Applications. In Applications of Microfluidic Systems in Biology and Medicine; Volume Bioanalysis; Springer: New York, NY, USA, 2019; Volume 7, pp. 99–150. [Google Scholar]
- Nivedita, N.; Papautsky, I. Continuous separation of blood cells in spiral microfluidic devices. Biomicrofluidics 2013, 7, 54101. [Google Scholar] [CrossRef]
- Wu, L.; Guan, G.; Hou, H.W.; Bhagat, A.A.; Han, J. Separation of leukocytes from blood using spiral channel with trapezoid cross-section. Anal. Chem. 2012, 84, 9324–9331. [Google Scholar] [CrossRef]
- Xiang, N.; Ni, Z. High-throughput blood cell focusing and plasma isolation using spiral inertial microfluidic devices. Biomed. Microdevices 2015, 17, 110. [Google Scholar] [CrossRef]
- Xiang, N.; Yi, H.; Chen, K.; Sun, D.; Jiang, D.; Dai, Q.; Nia, Z. High-throughput inertial particle focusing in a curved microchannel: Insights into the flow-rate regulation mechanism and process model. Biomicrofluidics 2013, 7, 44116. [Google Scholar] [CrossRef]
- Hou, H.; Warkiani, M.E.; Khoo, B.L.; Li, Z.R.; Soo, R.A.; Tan, D.S.-W.; Lim, W.-T.; Han, J.; Bhagat, A.A.S.; Lim, C.T. Isolation and retrieval of circulating tumor cells using centrifugal forces. Sci. Rep. 2013, 3, 1259. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A. Size Dependant Separation of Multiple Particles in Spiral Microchannels. Master’s Thesis, School of Electronics and Computing Systems of the College of Engineering and Applied Science, University of Cincinnati, Cincinnati, OH, USA, 2011. [Google Scholar]
- Lefebvre, O.; Cao, H.H.; Francisco, M.C.; Woytasik, M.; Dufour-Gergam, E.; Ammar, M.; Martincic, E. Reusable Embedded Microcoils for Magnetic Nano-Beads Trapping in Microfluidics: Magnetic Simulation and Experiments. Micromachines 2020, 11, 257. [Google Scholar] [CrossRef] [PubMed]
- Sanjay, S.T.; Li, M.; Zhou, W.; Li, X.; Li, X. A reusable PMMA/paper hybrid plug-and-play microfluidic device for an ultrasensitive immunoassay with a wide dynamic range. Microsyst. Nanoeng. 2020, 6, 1–11. [Google Scholar] [CrossRef]
- Wen, X.; Ou, Y.-C.; Zarick, H.F.; Zhang, X.; Hmelo, A.B.; Victor, Q.J.; Paul, E.P.; Slocik, J.M.; Naik, R.R.; Bellan, L.M.; et al. PRADA: Portable Reusable Accurate Diagnostics with nanostar Antennas for multiplexed biomarker screening. Bioeng. Transl. Med. 2020. [Google Scholar] [CrossRef]
- Sun, H.; Chan, C.-W.; Wang, Y.; Yao, X.; Mu, X.; Lu, X.; Zhou, J.; Cai, Z.; Ren, K. Reliable and reusable whole polypropylene plastic microfluidic devices for a rapid, low-cost antimicrobial susceptibility test. Lab Chip 2019, 19, 2915–2924. [Google Scholar] [CrossRef] [PubMed]
- Han, J.-H.; Yoon, J.-Y. Reusable, polyethylene glycol-structured microfluidic channel for particle immunoassays. J. Biol. Eng. 2009, 3, 6. [Google Scholar] [CrossRef]
- Boecker, D.; Freeman, D.M. Method and Apparatus for a Multi-Use Body Fluid Sampling Device. U.S. Patent 7,198,606 B2, 3 April 2007. [Google Scholar]
- Xiang, N.; Chen, K.; Sun, D.; Wang, S.; Yi, H.; Ni, Z. Quantitative characterization of the focusing process and dynamic behavior of differently sized microparticles in a spiral microchannel. Microfluid. Nanofluid. 2012, 14, 89–99. [Google Scholar] [CrossRef]
- Johnston, I.D.; McDonnell, M.B.; Tan, C.K.L.; McCluskey, D.K.; Davies, M.J.; Tracey, M.C. Dean flow focusing and separation of small microspheres within a narrow size range. Microfluid. Nanofluid. 2014, 17, 509–518. [Google Scholar] [CrossRef]
- Guan, G.; Wu, L.; Bhagat, A.A.; Li, Z.; Chen, P.C.Y.; Chao, S.; Ong, C.H.; Han, J. Spiral microchannel with rectangular and trapezoidal cross-sections for size based particle separation. Sci. Rep. 2013, 3, 1. [Google Scholar] [CrossRef]
- Russom, A.; Gupta, A.K.; Nagrath, S.; Di Carlo, D.; Edd, J.F.; Toner, M. Differential inertial focusing of particles in curved low-aspect-ratio microchannels. New J. Phys. 2009, 11, 75025. [Google Scholar] [CrossRef]
- Nivedita, N.; Ligrani, P.; Papautsky, I. Evolution of secondary Dean vortices in Spiral. In Proceedings of the 17th International Conference on Miniaturized Systems for Chemistry and Life Sciences, Freiburg, Germany, 27–31 October 2013; pp. 27–31. [Google Scholar]
- Ostadfar, A. Chapter 1—Fluid Mechanics and Biofluids Principles. In Biofluid Mechanics: Principles and Applications; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- NIH. Analyze Menu-Image. Available online: https://imagej.nih.gov/ij/docs/menus/analyze.html (accessed on 21 September 2020).
- Bhagat, A.S.; Kuntaegowdanahalli, S.S.; Papautsky, I. Continuous particle separation in spiral microchannels using dean flows and differential migration. Lab Chip 2008, 8, 1906–1914. [Google Scholar] [CrossRef] [PubMed]
- Kuntaegowdanahalli, S.S.; Bhagat, A.A.S.; Papautsky, I. Continuous Multi-Particle Separation Using Deterministic Focusing in Spiral Microchannels. In International Solid-State Sensors, Actuators and Microsystems Conference; IEEE: Piscataway, NJ, USA, 2009; pp. 2139–2142. [Google Scholar]
- Schaap, A.; Dumon, J.; Toonder, J. Sorting algal cells by morphology in spiral microchannels using inertial microfluidics. Microfluid. Nanofluid. 2016, 20, 125. [Google Scholar] [CrossRef]
- Zhou, J.; Papautsky, I. Fundamentals of inertial focusing in microchannels. Lab Chip 2013, 13, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Deiss, F.; Liu, X.; Akbulut, O.; Whitesides, G.M. Integration of paper-based microfluidic devices with commercial electrochemical readers. Lab Chip 2010, 10, 3163–3169. [Google Scholar] [CrossRef] [PubMed]
- Van Heeren, H. Microfluidic Survey: Reliability of Microfluidics Based Devices and Components. Available online: http://mf-manufacturing.eu/wp-content/uploads/General-results-survey-microfluidic-reliability-21-3-2015.pdf (accessed on 3 August 2020).
- Lochovsky, C.; Yasotharan, S.; Gunther, A. Bubbles no more: In-plane trapping and removal of bubbles in microfluidic devices. Lab Chip 2012, 12, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Dressaire, E.; Sauret, A. Clogging of microfluidic systems. Soft Matter 2016, 13, 37–48. [Google Scholar] [CrossRef]
- Wyss, H.M.; Blair, D.L.; Morris, J.F.; Stone, H.A.; Weitz, D.A. Mechanism for clogging of microchannels. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2006, 74, 061402. [Google Scholar] [CrossRef]
- Moloudi, R.; Oh, S.; Yang, C.; Warkiani, M.E.; Naing, M.W. Inertial particle focusing dynamics in a trapezoidal straight microchannel: Application to particle filtration. Microfluid. Nanofluid. 2018, 22, 33. [Google Scholar] [CrossRef]
- Caffiyar, M.Y.; Lim, K.P.; Basha, I.H.K.; Hamid, N.H.; Cheong, S.C.; Ho, E.T.W. Label-Free, High-Throughput Assay of Human Dendritic Cells from Whole-Blood Samples with Microfluidic Inertial Separation Suitable for Resource-Limited Manufacturing. Micromachines 2020, 11, 514. [Google Scholar] [CrossRef]
- Gervais, T.; El-Ali, J.; Günther, A.; Jensen, K.F. Flow-induced deformation of shallow microfluidic channels. Lab Chip 2006, 6, 500–507. [Google Scholar] [CrossRef]
- Li, Z.; Mak, S.Y.; Sauret, A.; Shum, H.C. Syringe-pump-induced fluctuation in all-aqueous microfluidic system implications for flow rate accuracy. Lab Chip 2014, 14, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Jacobi, I.; Li, S.; Stone, H. Characterization of syringe-pump-driven induced pressure fluctuations in elastic microchannels. Lab Chip 2014, 15, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Wang, W.; Bai, L. Performance evaluation of the Z2 coulter counter for WBC and RBC counting. Int. J. Lab. Hematol. 2007, 5, 361–368. [Google Scholar] [CrossRef]
- Rivet, C.; Lee, H.; Hirsch, A.; Hamilton, S.; Lu, H. Microfluidics for medical diagnostics and biosensors. Chem. Eng. Sci. 2011, 66, 1490–1507. [Google Scholar] [CrossRef]



| Parameter | Equation | Design 1 | Design 2 |
|---|---|---|---|
| Aspect ratio (AR) | h/w; where is channel height and is channel width | 0.24 | 0.27 |
| Hydraulic diameter () | 193 µm | 126 µm | |
| Confinement ratio (δ) | ; where is particle diameter | 0.01 (for 2 µm particles) 0.13 (for 16 µm particles) | 0.02 (for 2 µm particles) 0.20 (for 16 µm particles) |
| Reynold’s number (Re) | ; where is fluid density, u is fluid velocity and µ is dynamic viscosity | 7 (at 200 µL/min) 168 (at 5000 µL/min) | 16 (at 200 µL/min) 123 (at 1500 µL/min) |
| Dean number (De) | ; where is radius of curvature | 1.2 (at 200 µL/min) 29.2 (at 5000 µL/min) | 2.8 (at 200 µL/min) 21.2 (at 1500 µL/min) |
| Test Parameter | Description |
|---|---|
| Test Fluid | Glycerin (18% by volume) in ultrapure deionized water, with Tween 20 and concentrated particle solution (fluid density: 1049 kg/m3, dynamic viscosity: 1.6 × 10−3 Pa·s) |
| Surrogate Particles | 2 µm, 6 µm, 11 µm, and 16 µm |
| Particle Concentration | 106–3 × 108 particles/mL (for 16 µm particles at a concentration of 1.6 × 106 particles/mL; for 11 µm particles ~ 3.3 × 106 particles/mL; for 6 µm particles ~ 9.2 × 107 particles/mL; for 2 µm particles ~ 2.7 × 108 particles/mL) |
| Flow Rates | 200, 750, 1500, and 5000 µL/min |
| Microchannel Material | Polymethyl methacrylate (PMMA) |
| Device Design 1 | 8 outlets, 4 rotations, 120 µm height, 500 µm width, 2 mm radius of curvature, 0.3 mm spacing between channels, and 56 mm channel length |
| Device Design 2 | 8 outlets, 4 rotations, 80 µm height, 300 µm width, 2 mm radius of curvature along the horizontal for the first loop, 0.3 mm spacing between channels, and 38 mm channel length |
| Test Parameter | Effect | Clinical Significance |
| Particle size ↑ | Lift force on particles ↑, particle focusing shift → inner wall | A wide range of cell sizes can reduce the focusing effect and spread the particle streams, thus decreasing test sensitivity. Larger particle sizes are more impacted by Dean vortices. Particle-to-particle interactions are more prevalent with larger particles. |
| Channel hydraulic diameter ↑ | Throughput ↑, channel pressure ↓, confinement ratio ↓ | While processing time can be expedited by increasing the cross-sectional area of the channel, the separation efficiency may suffer. Particle-wall interactions are reduced in larger channels. |
| Flow rate ↑ | Reynold’s number ↑, Dean number ↑, particle focusing shift → outer wall, particle focusing ↑ (except at highest flow rates) | Throughput increases at higher flow rates, as does pressure. However, leaks and fluctuations in flow can cause system failures. |
| Confinement ratio ↑ | Lift force ↑, particle focusing shift → inner wall | Device effectiveness is increased for higher confinement ratios, and the particle streamline becomes more impacted by microchannel geometry. |
| Number of repeat uses of the same microchannel ↑ | Uncertainty ↓ | Increased testing reduces the uncertainty (e.g., variability) and causes fewer false positives, thus improving the reliability of the clinical results. |
| Channel blockage ↑ | Channel pressure ↑, predicted particle focusing ↓ | Blockages can cause sample loss, impact test sensitivity, and result in false positive/negative readings. |
| Detection method sensitivity ↑ | Particle focusing ↑, reliability (i.e., repeatability and reproducibility) ↑ | Better particle separation and detection methods will improve reliability, test sensitivity, and device performance. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Natu, R.; Guha, S.; Dibaji, S.A.R.; Herbertson, L. Assessment of Flow through Microchannels for Inertia-Based Sorting: Steps toward Microfluidic Medical Devices. Micromachines 2020, 11, 886. https://doi.org/10.3390/mi11100886
Natu R, Guha S, Dibaji SAR, Herbertson L. Assessment of Flow through Microchannels for Inertia-Based Sorting: Steps toward Microfluidic Medical Devices. Micromachines. 2020; 11(10):886. https://doi.org/10.3390/mi11100886
Chicago/Turabian StyleNatu, Rucha, Suvajyoti Guha, Seyed Ahmad Reza Dibaji, and Luke Herbertson. 2020. "Assessment of Flow through Microchannels for Inertia-Based Sorting: Steps toward Microfluidic Medical Devices" Micromachines 11, no. 10: 886. https://doi.org/10.3390/mi11100886
APA StyleNatu, R., Guha, S., Dibaji, S. A. R., & Herbertson, L. (2020). Assessment of Flow through Microchannels for Inertia-Based Sorting: Steps toward Microfluidic Medical Devices. Micromachines, 11(10), 886. https://doi.org/10.3390/mi11100886




