Advances in Magnetoresistive Biosensors
Abstract
1. Introduction
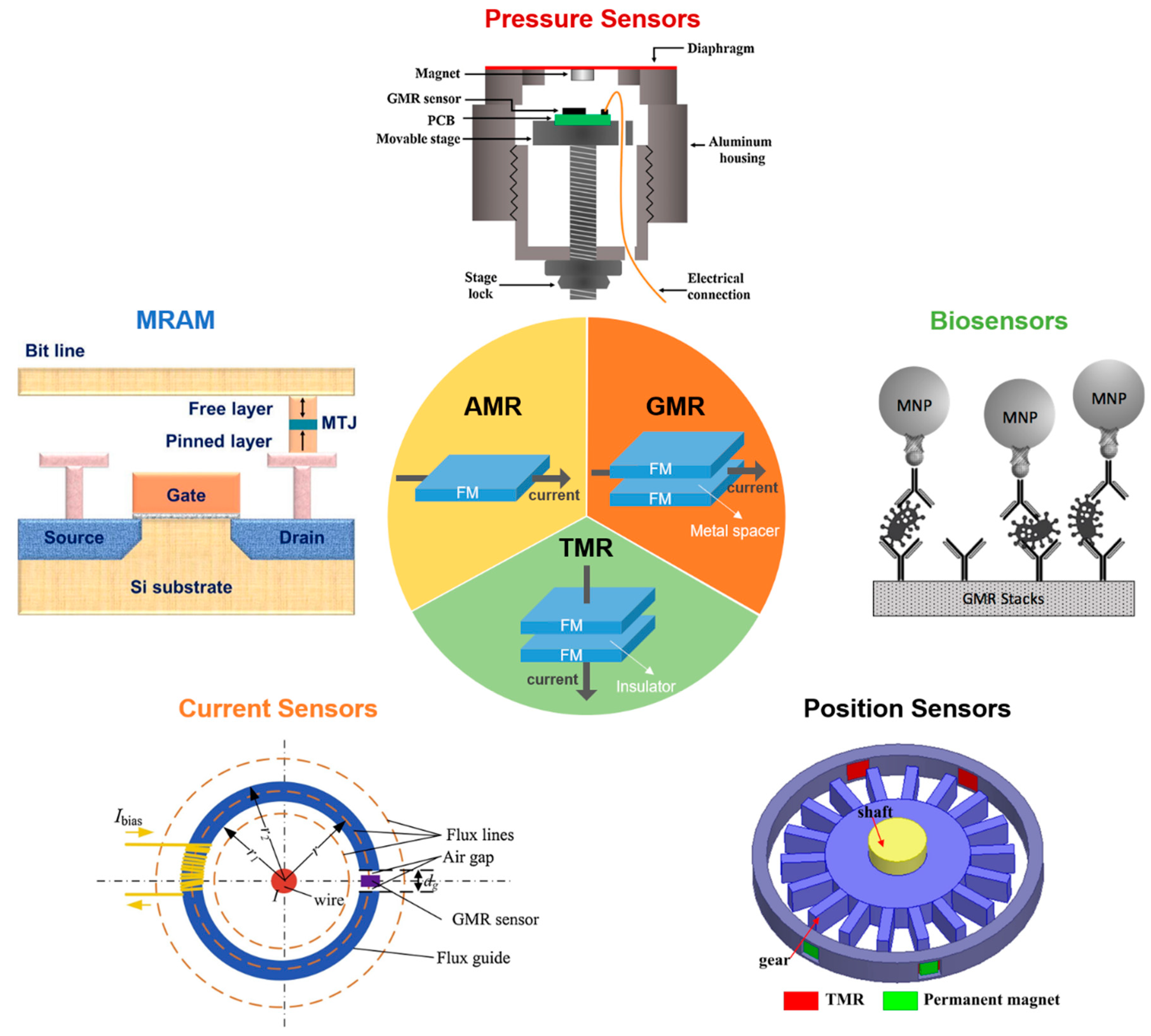
2. Magnetoresistive (MR) Devices
2.1. Anisotropic Magnetoresistance (AMR)
2.2. Giant Magnetoresistance (GMR)
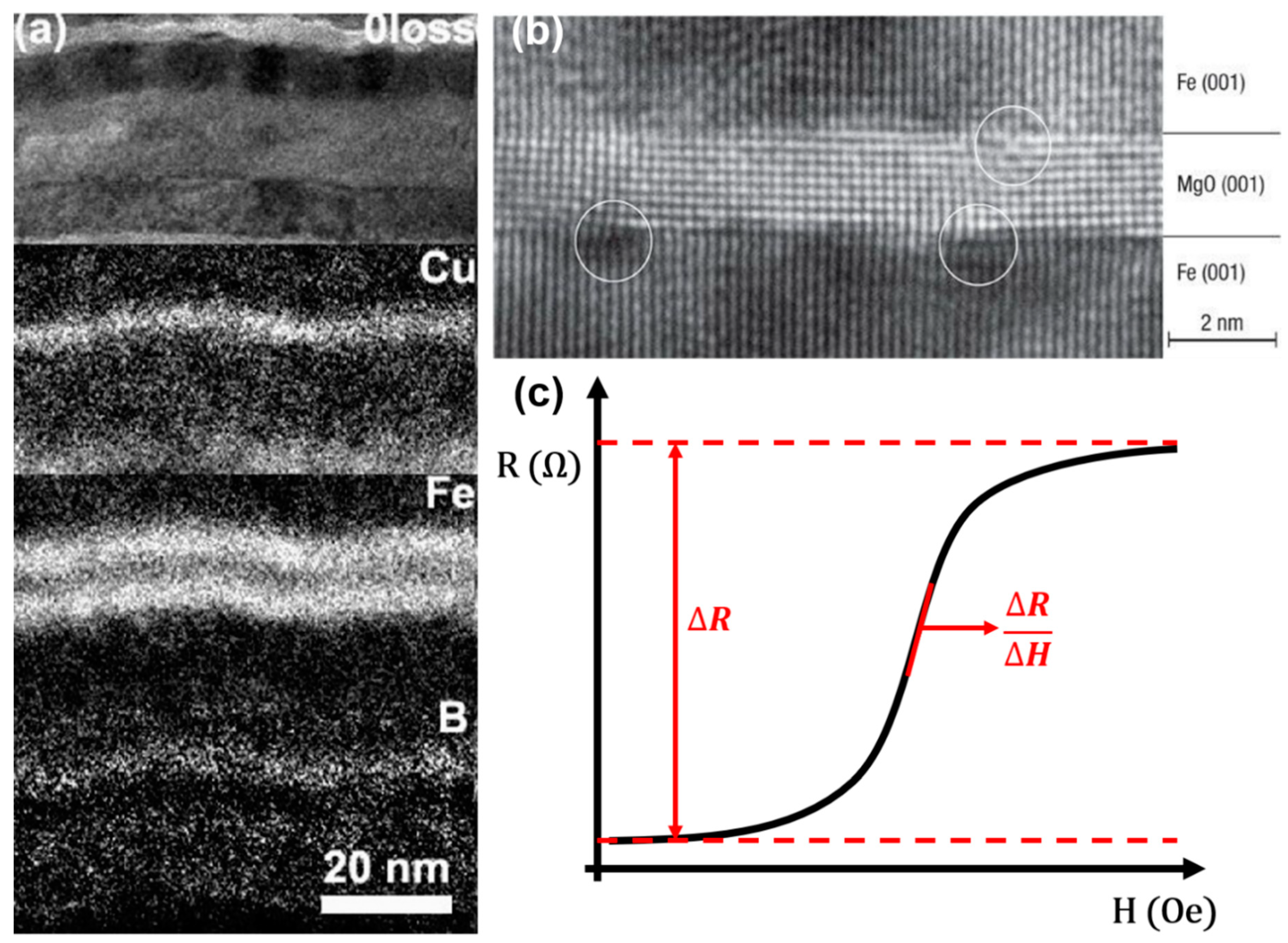
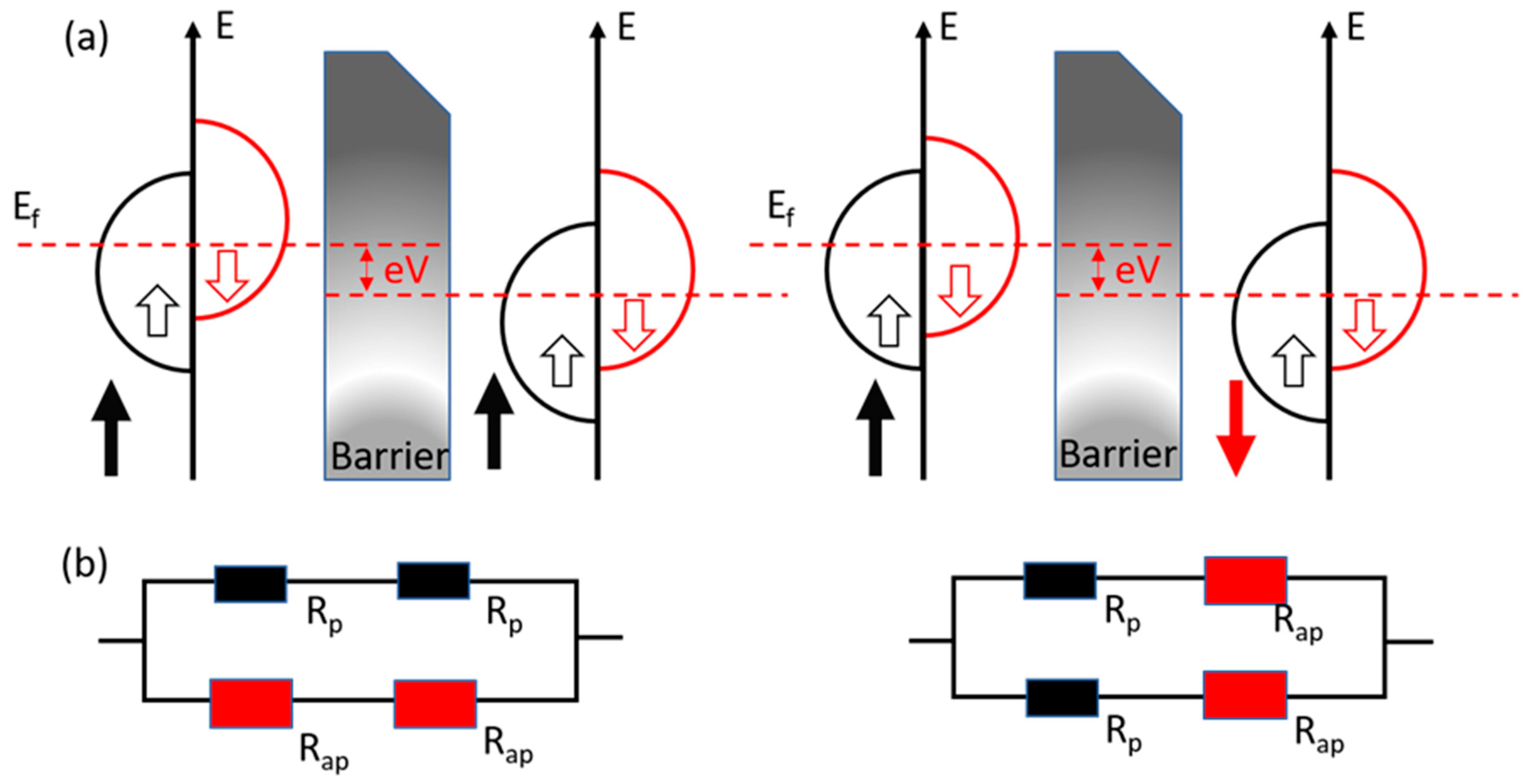
2.3. Tunneling Magnetoresistance (TMR)
3. Magnetoresistive Biosensors: From Fabrication to Surface Modification
3.1. Fabrication
3.1.1. Design of Sensing Elements
3.1.2. Design of Passivation Layer
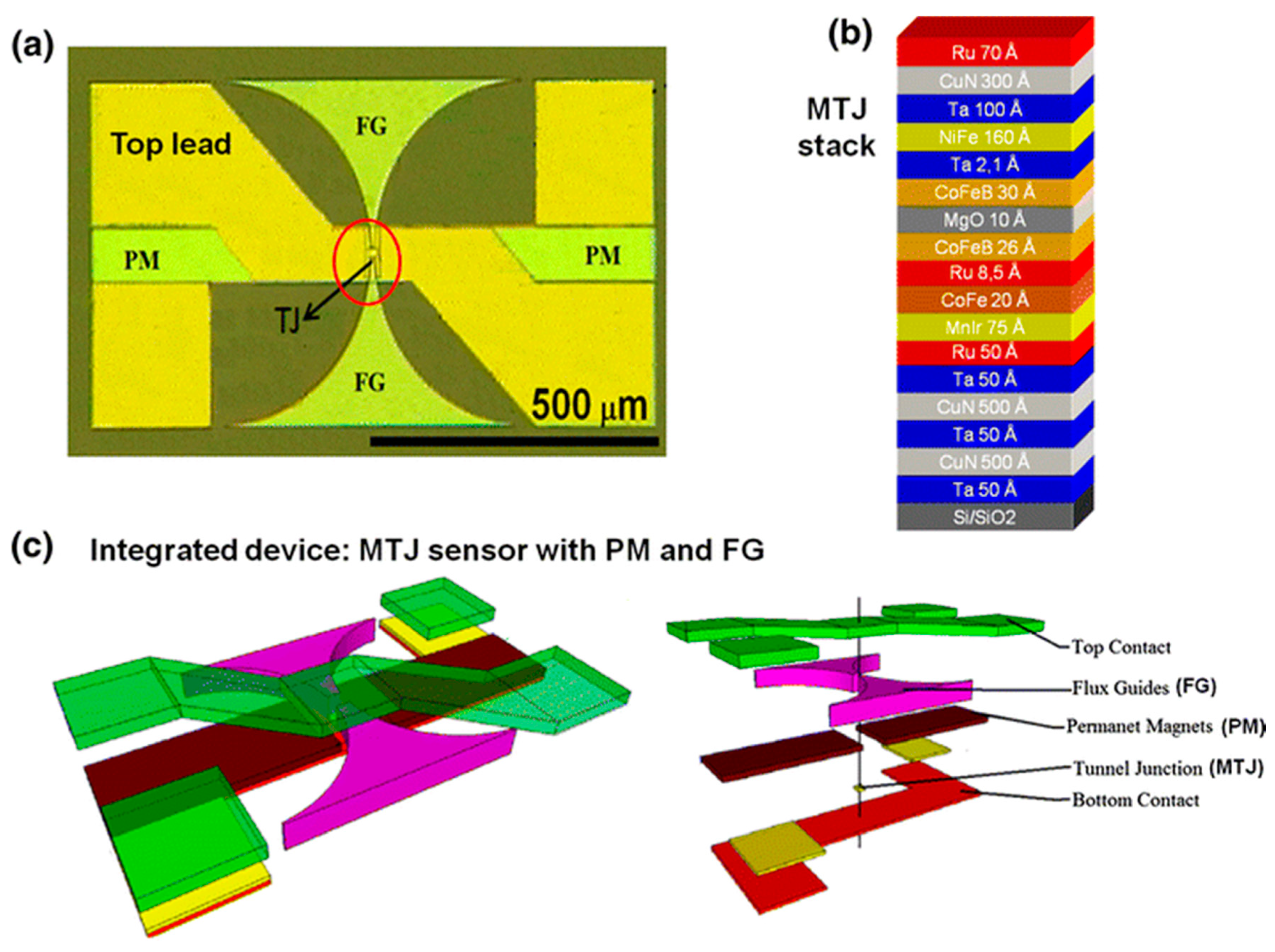
3.1.3. Integration with Magnetic Flux Concentrator (MFC)
3.1.4. Integration with Microfluidic Channels
3.2. Device Surface Chemical Modification
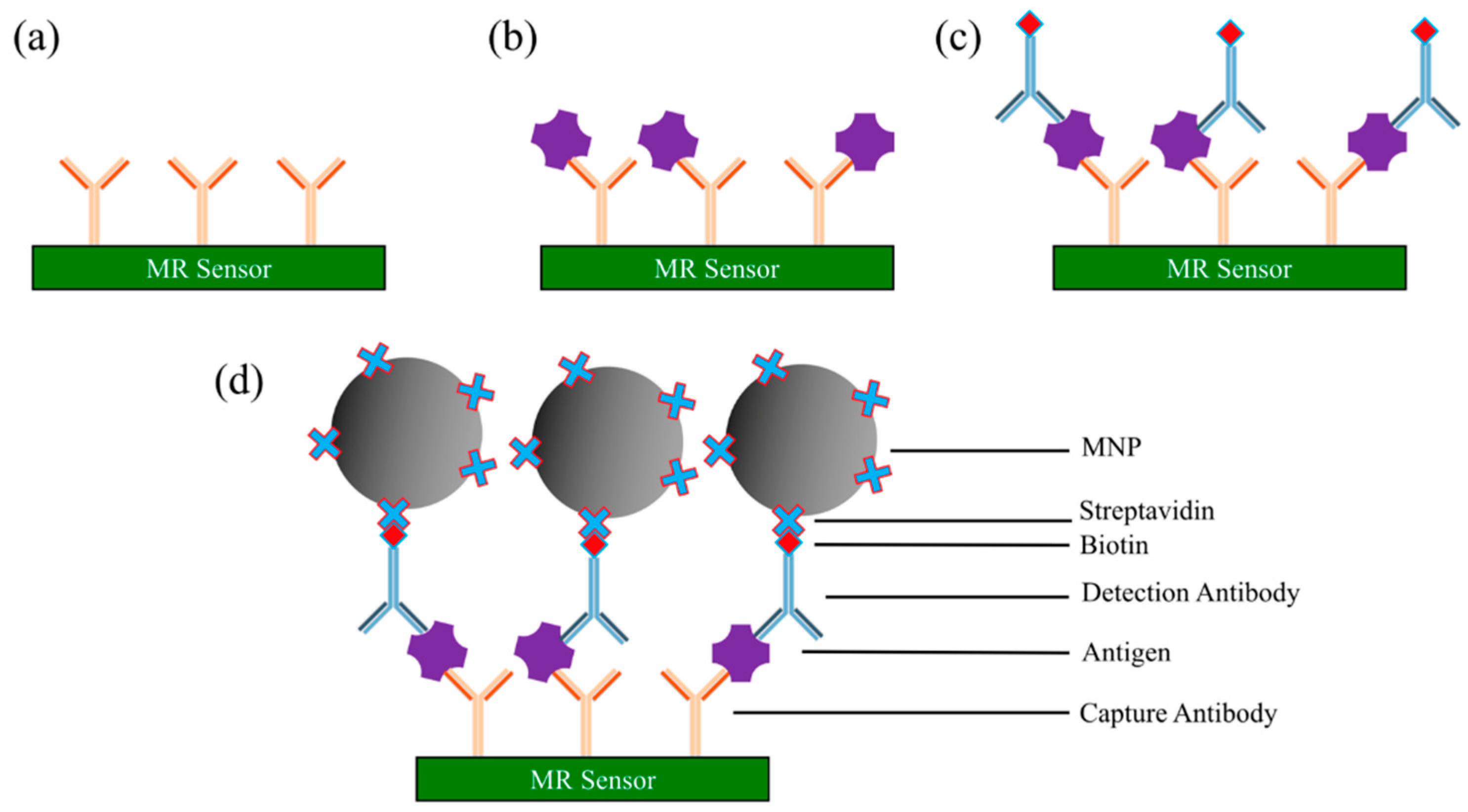
3.2.1. Sandwich Assay
- Immobilize capture antibodies onto sensor surface (e.g., AMR, GMR, and TMR sensor surface, etc.). Wash away unbound capture antibodies.
- The surface is then coated with blocking buffer (i.e., bovine serum albumin (BSA)) to block the nonspecific binding sites. Wash away blockers.
- Add a biological sample of antigens to the surface. The antigen is captured and immobilized onto the surface through specific binding to capture antibodies. Wash away unbound chemicals (including unbound antigens).
- Biotinylated detection antibodies (usually diluted in blocking buffer) are added to the surface for binding to the antigens. Wash away unbound biotinylated detection antibodies. After this step, only the capture antibody-antigen-biotinylated detection antibody complexes are left on the surface.
- Streptavidin-coated MNPs are added to the surface. The streptavidin binds specifically to the biotin from the detection antibody.
- An externally applied magnetic field, combined with the magnetic properties of MR sensors, is applied to convert the magnetic stray field from MNP into an electrical signal. The resistance or voltage or magnetoresistance of the MR sensor is measured to determine the presence and quantity of antigens.
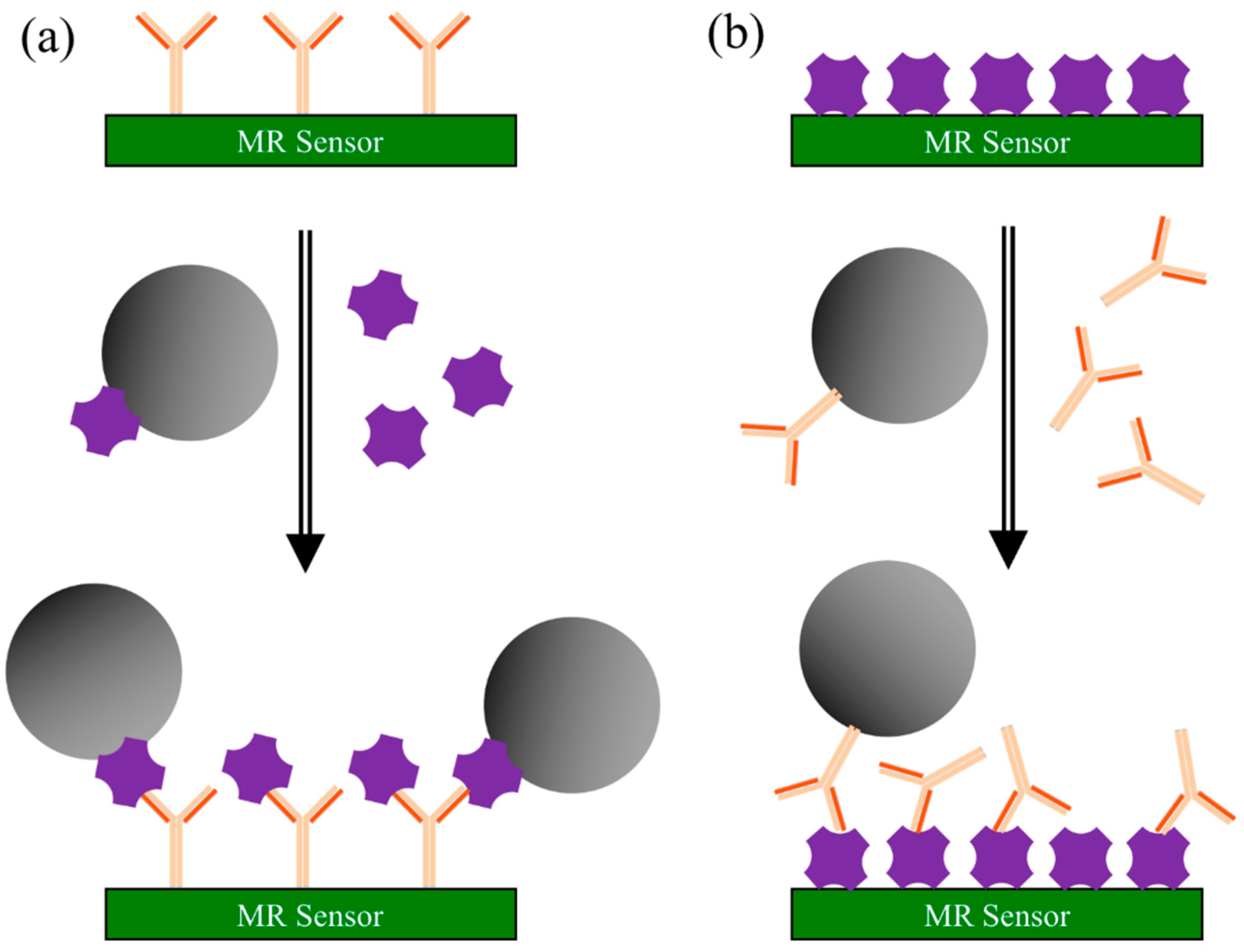
3.2.2. Competitive Binding Assay
- Capture antibodies are immobilized on the MR sensor surface. The surface is coated with blocking buffer to block the nonspecific binding sites.
- Add both MNP-linked antigens (labeled antigens) and antigen-containing sample (unlabeled antigens) to the sensor surface. Cumulative competition occurs between the two antigens for the antibody binding sites.
- Wash away unbound antigens. The MR sensor converts the number of MNPs into an electrical/magnetic signal. The signal strength of MR sensor determines the presence and quantity of target analytes.
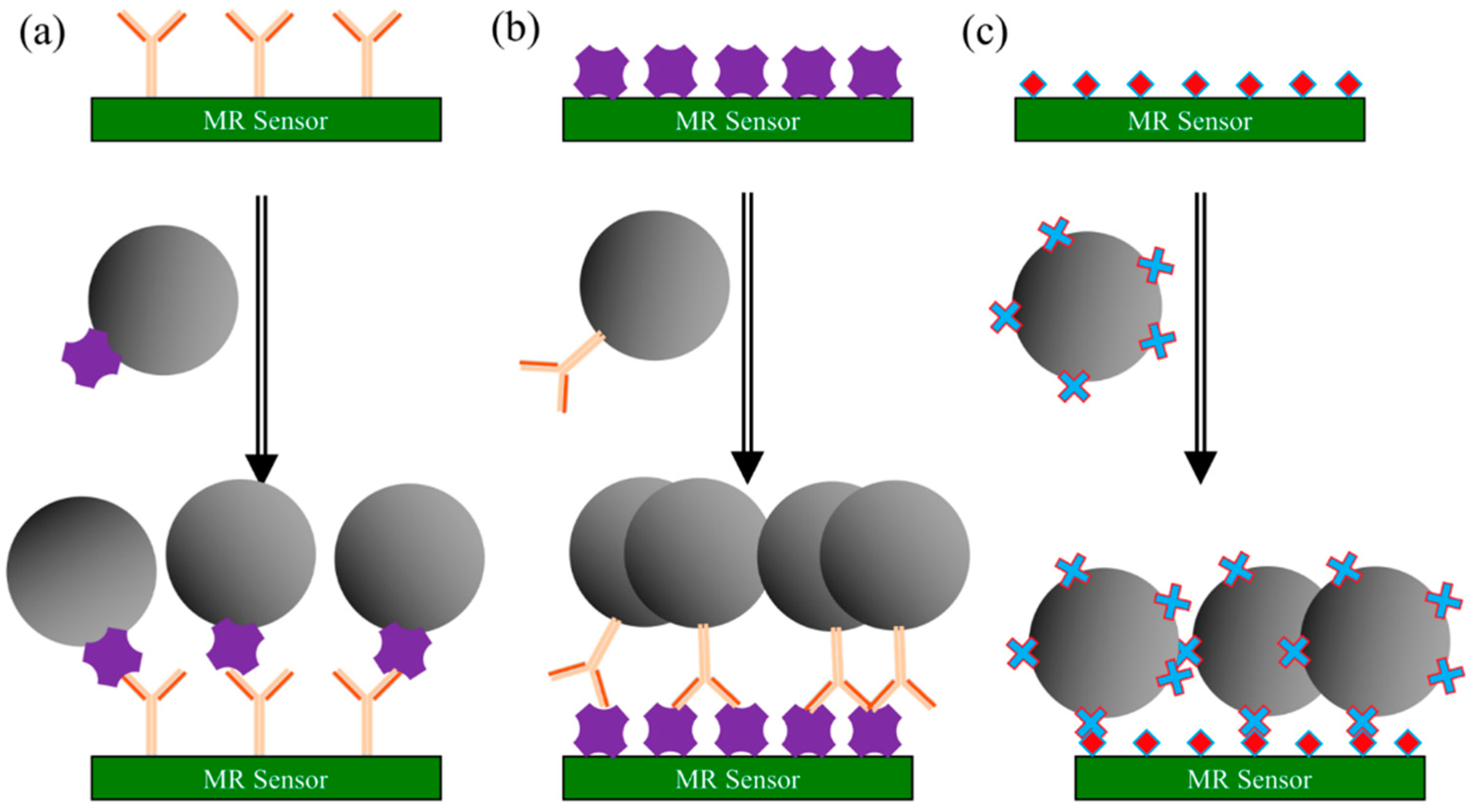
3.2.3. Direct Assay
- The testing sample containing target antigens (or antibodies) is added to the MR sensor surface, where it is given time to adhere to the sensor surface. Unbound antigens (or antibodies) are washed away followed by the blocking buffer to block the nonspecific binding sites.
- MNP-linked antibodies (or MNP-linked antigens) are added, which binds specifically to the testing antigens (or antibodies) that immobilized on the sensor surface.
- Wash away unbound complexes. The MR sensor converts the number of MNPs into electrical/magnetic signals. In this case, a stronger signal indicates a higher concentration of the target antigens (or antibodies) from the testing sample.
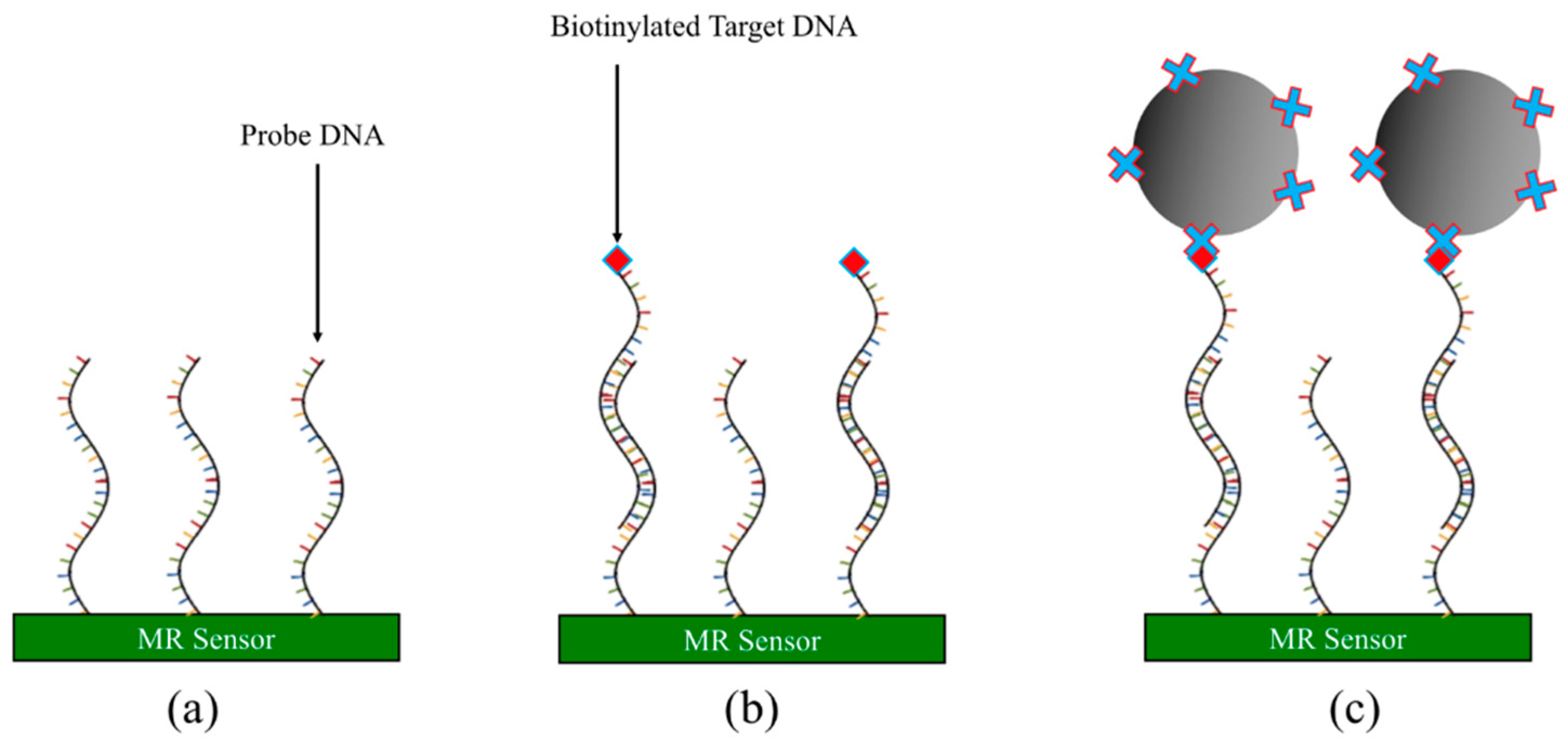
3.2.4. DNA-Based Assay
- Immobilize probe DNA onto MR sensor surface. Wash away unbound probe DNA.
- Apply testing sample containing biotinylated target DNA strands to the MR sensor surface and incubate to allow the complementary target DNA to hybridize with the probe DNA.
- Wash away unbound target DNAs and noncomplementary DNAs.
- Streptavidin-coated MNPs are added to the MR sensor surface and captured by the remaining target DNAs through the biotin-streptavidin conjugation. The MR sensor converts the number of MNPs into electrical/magnetic signals.
4. Magnetoresistive Biosensors for Immunoassay Applications
4.1. AMR-Based Biosensors
4.2. GMR-Based Biosensors
4.3. TMR-Based Biosensors
5. Magnetoresistive Biosensors for Genotyping Applications
5.1. AMR-Based Sensors for Genotyping
5.2. GMR-Based Sensors for Genotyping
5.3. TMR-Based Sensors for Genotyping
6. MR Biosensors for Brain Mapping
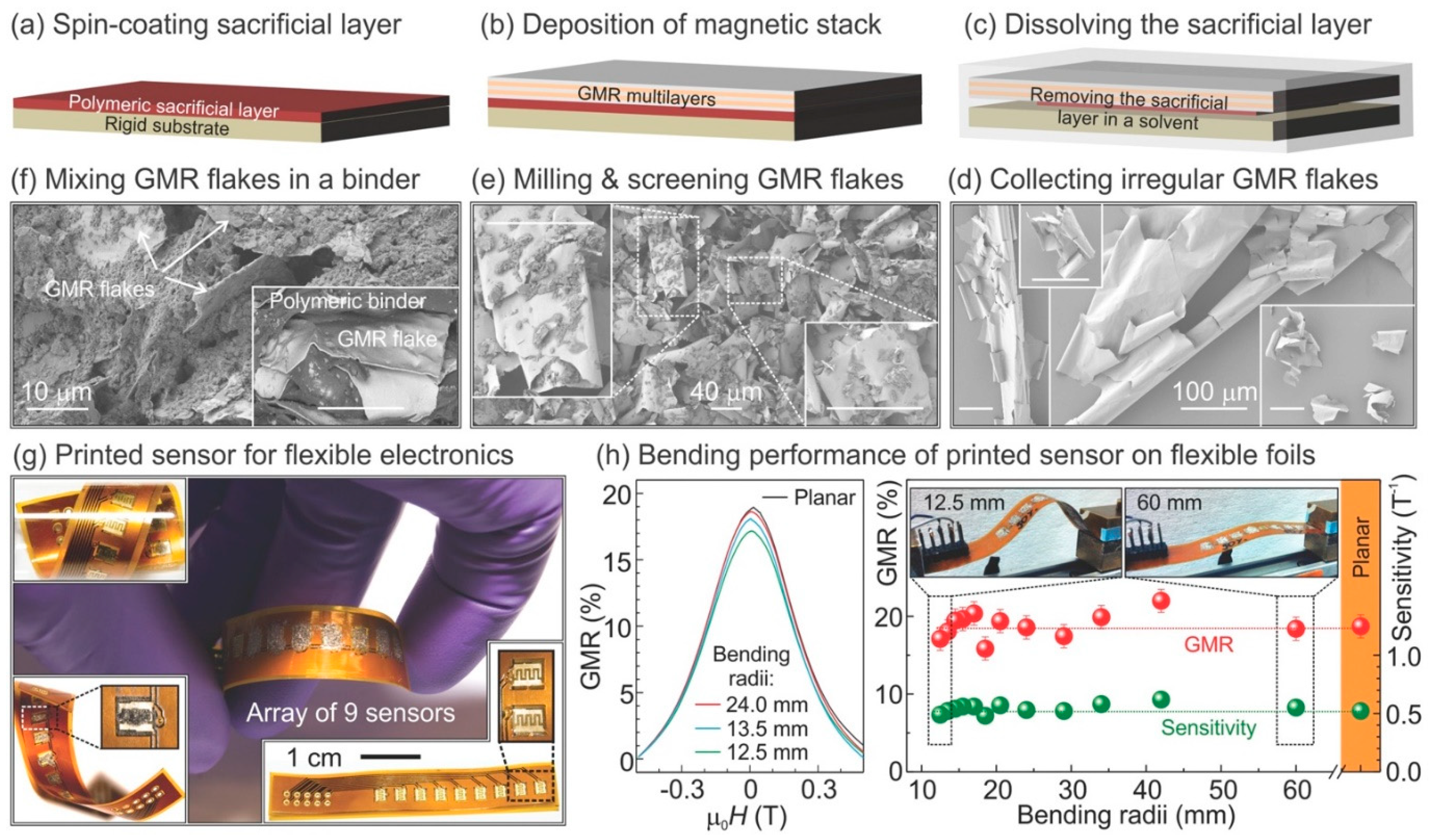
7. Flexible Magnetoresistive Biosensors
7.1. Printable MR Devices
7.2. Flexible MTJs
8. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tsang, C.H.; Fontana, R.E.; Lin, T.; Heim, D.E.; Gurney, B.A.; Williams, M.L. Design, fabrication, and performance of spin-valve read heads for magnetic recording applications. IBM J. Res. Dev. 1998, 42, 103–116. [Google Scholar] [CrossRef]
- Makarov, A.; Windbacher, T.; Sverdlov, V.; Selberherr, S. CMOS-compatible spintronic devices: A review. Semicond. Sci. Technol. 2016, 31, 11. [Google Scholar] [CrossRef]
- Borole, U.P.; Subramaniam, S.; Kulkarni, I.R.; Saravanan, P.; Barshilia, H.C.; Chowdhury, P. Highly sensitive giant magnetoresistance (GMR) based ultra low differential pressure sensor. Sens. Actuators A-Phys. 2018, 280, 125–131. [Google Scholar] [CrossRef]
- Park, J.J.; Reddy, K.S.M.; Stadler, B.; Flatau, A. Magnetostrictive Fe-Ga/Cu nanowires array with GMR sensor for sensing applied pressure. IEEE Sens. J. 2017, 17, 2015–2020. [Google Scholar] [CrossRef]
- Ouyang, Y.; Wang, Z.X.; Zhao, G.; Hu, J.; Ji, S.J.; He, P.N.; Wang, S.X. Current sensors based on GMR effect for smart grid applications. Sens. Actuators A-Phys. 2019, 294, 8–16. [Google Scholar] [CrossRef]
- Chen, Y.F.; Huang, Q.; Khawaja, A.H. An interference-rejection strategy for measurement of small current under strong interference with magnetic sensor array. IEEE Sens. J. 2019, 19, 692–700. [Google Scholar] [CrossRef]
- Huang, C.C.; Lin, C.L.; Kao, J.J.; Chang, J.J.; Sheu, G.J. Vehicle parking guidance for wireless charge using GMR sensors. IEEE Trans. Veh. Technol. 2018, 67, 6882–6894. [Google Scholar] [CrossRef]
- Bhaskarrao, N.K.; Sreekantan, A.C.; Dutta, P.K. Analysis of a linearizing direct digitizer with phase-error compensation for TMR angular position sensor. IEEE Trans. Instrum. Meas. 2018, 67, 1795–1803. [Google Scholar] [CrossRef]
- Wang, S.X.; Wu, Z.Y.; Peng, D.L.; Li, W.S.; Zheng, Y. Embedded position estimation using tunnel magnetoresistance sensors for permanent magnet linear synchronous motor systems. Measurement 2019, 147, 106860. [Google Scholar] [CrossRef]
- Giraud, M.; Delapierre, F.D.; Wijkhuisen, A.; Bonville, P.; Thevenin, M.; Cannies, G.; Plaisance, M.; Paul, E.; Ezan, E.; Simon, S.; et al. Evaluation of in-flow magnetoresistive chip cell-counter as a diagnostic tool. Biosensors 2019, 9, 105. [Google Scholar] [CrossRef]
- Denmark, D.J.; Bustos-Perez, X.; Swain, A.; Phan, M.H.; Mohapatra, S.; Mohapatra, S.S. Readiness of magnetic nanobiosensors for point-of-care commercialization. J. Electron. Mater. 2019, 48, 4749–4761. [Google Scholar] [CrossRef]
- Mu, X.H.; Liu, H.F.; Tong, Z.Y.; Du, B.; Liu, S.; Liu, B.; Liu, Z.W.; Gao, C.; Wang, J.; Dong, H. A new rapid detection method for ricin based on tunneling magnetoresistance biosensor. Sens. Actuators B-Chem. 2019, 284, 638–649. [Google Scholar] [CrossRef]
- Baselt, D.R.; Lee, G.U.; Natesan, M.; Metzger, S.W.; Sheehan, P.E.; Colton, R.J. A biosensor based on magnetoresistance technology. Biosens. Bioelectron. 1998, 13, 731–739. [Google Scholar] [CrossRef]
- Wimberger-Friedl, R.; Nellissen, T.; Weekamp, W.; van Delft, J.; Ansems, W.; Prins, M.; Megens, M.; Dittmer, W.; de Witz, C.; van Iersel, B. Packaging of silicon sensors for microfluidic bio-analytical applications. J. Micromec. Microeng. 2009, 19, 7. [Google Scholar] [CrossRef]
- Graham, D.L.; Ferreira, H.A.; Freitas, P.P. Magnetoresistive-based biosensors and biochips. Trends Biotechnol. 2004, 22, 455–462. [Google Scholar] [CrossRef]
- Peng, Z.C.; Ling, Z.G.; Tondra, M.; Liu, C.G.; Zhang, M.; Lian, K.; Goettert, J.; Hormes, J. CMOS compatible integration of three-dimensional microfluidic systems based on low-temperature transfer of SU-8 films. J. Microelectromec. Syst. 2006, 15, 708–716. [Google Scholar] [CrossRef]
- Srinivasan, B.; Li, Y.P.; Jing, Y.; Xu, Y.H.; Yao, X.F.; Xing, C.G.; Wang, J.P. A detection system based on giant magnetoresistive sensors and high-moment magnetic nanoparticles demonstrates zeptomole sensitivity: Potential for personalized medicine. Angew. Chem. Int. Ed. 2009, 48, 2764–2767. [Google Scholar] [CrossRef]
- Klein, T.; Wang, W.; Yu, L.N.; Wu, K.; Boylan, K.L.M.; Vogel, R.I.; Skubitz, A.P.N.; Wang, J.P. Development of a multiplexed giant magnetoresistive biosensor array prototype to quantify ovarian cancer biomarkers. Biosens. Bioelectron. 2019, 126, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Pannetier-Lecoeur, M.; Parkkonen, L.; Sergeeva-Chollet, N.; Polovy, H.; Fermon, C.; Fowley, C. Magnetocardiography with sensors based on giant magnetoresistance. Appl. Phys. Lett. 2011, 98, 153705. [Google Scholar] [CrossRef]
- Choi, J.; Gani, A.W.; Bechstein, D.J.B.; Lee, J.R.; Utz, P.J.; Wang, S.X. Portable, one-step, and rapid GMR biosensor platform with smartphone interface. Biosens. Bioelectron. 2016, 85, 1–7. [Google Scholar] [CrossRef]
- Su, D.Q.; Wu, K.; Krishna, V.D.; Klein, T.; Liu, J.M.; Feng, Y.L.; Perez, A.M.; Cheeran, M.C.J.; Wang, J.P. Detection of influenza a virus in swine nasal swab samples with a wash-free magnetic bioassay and a handheld giant magnetoresistance sensing system. Front. Microbiol. 2019, 10, 1077. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, S.; Sbiaa, R.; Hirohata, A.; Ohno, H.; Fukami, S.; Piramanayagam, S.N. Spintronics based random access memory: A review. Mater. Today 2017, 20, 530–548. [Google Scholar] [CrossRef]
- Wang, S.X.; Wu, Z.Y.; Peng, D.L.; Li, W.S.; Chen, S.; Liu, S.Y. An angle displacement sensor using a simple gear. Sens. Actuators A-Phys. 2018, 270, 245–251. [Google Scholar] [CrossRef]
- Thomson, W., XIX. On the electro-dynamic qualities of metals:—Effects of magnetization on the electric conductivity of nickel and of iron. Proc. R. Soc. Lond. 1857, 8, 546–550. [Google Scholar]
- McGuire, T.; Potter, R. Anisotropic magnetoresistance in ferromagnetic 3d alloys. IEEE Trans. Magn. 1975, 11, 1018–1038. [Google Scholar] [CrossRef]
- Fert, A.; Campbell, I.A. Electrical-resistivity of ferromagnetic nickel and iron based alloys. J. Phys. F-Met. Phys. 1976, 6, 849–871. [Google Scholar] [CrossRef]
- Malozemoff, A. Anisotropic magnetoresistance of amorphous and concentrated polycrystalline iron alloys. Phys. Rev. B 1985, 32, 6080. [Google Scholar] [CrossRef]
- Snoek, J. The weiss-heisenberg theory of ferro-magnetism and a new rule concerning magnetostriction and magneto-resistance. Nature 1949, 163, 837. [Google Scholar] [CrossRef]
- Mott, N.F. The resistance and thermoelectric properties of the transition metals. Pro. R. Soc. Lond. Ser. A Math. Phys. Sci. 1936, 156, 368–382. [Google Scholar]
- Baibich, M.N.; Broto, J.M.; Fert, A.; Van Dau, F.N.; Petroff, F.; Etienne, P.; Creuzet, G.; Friederich, A.; Chazelas, J. Giant magnetoresistance ofFe/(001)Cr magnetic superlattices. Phys. Rev. Lett. 1988, 61, 2472–2475. [Google Scholar] [CrossRef]
- Binasch, G.; Grünberg, P.; Saurenbach, F.; Zinn, W. Enhanced magnetoresistance in layered magnetic structures with antiferromagnetic interlayer exchange. Phys. Rev. B 1989, 39, 4828–4830. [Google Scholar] [CrossRef] [PubMed]
- Parkin, S.S.P.; More, N.; Roche, K.P. Oscillations in exchange coupling and magnetoresistance in metallic superlattice structures—Co/Ru, Co/Cr, and Fe/Cr. Phys. Rev. Lett. 1990, 64, 2304–2307. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.X.; Li, G. Advances in giant magnetoresistance biosensors with magnetic nanoparticle tags: Review and outlook. IEEE Trans. Magn. 2008, 44, 1687–1702. [Google Scholar] [CrossRef]
- vandenBerg, H.A.M.; Clemens, W.; Gieres, G.; Rupp, G.; Vieth, M.; Wecker, J.; Zoll, S. GMR angle detector with an artificial antiferromagnetic subsystem (AAF). J. Magn. Magn. Mater. 1997, 165, 524–528. [Google Scholar] [CrossRef]
- Parkin, S.; Jiang, X.; Kaiser, C.; Panchula, A.; Roche, K.; Samant, M. Magnetically engineered spintronic sensors and memory. Pro. IEEE 2003, 91, 661–680. [Google Scholar] [CrossRef]
- Tannous, C.; Comstock, R.L. Magnetic information-storage materials. In Springer Handbook of Electronic and Photonic Materials, 2nd ed.; Springer: Berlin, Germany, 2017; pp. 1185–1223. [Google Scholar]
- Gottwald, M.; Hehn, M.; Montaigne, F.; Lacour, D.; Lengaigne, G.; Suire, S.; Mangin, S. Magnetoresistive effects in perpendicularly magnetized Tb-Co alloy based thin films and spin valves. J. Appl. Phys. 2012, 111, 083904. [Google Scholar] [CrossRef]
- Xiao, J.Q.; Jiang, J.S.; Chien, C.L. Giant magnetoresistance in nonmultilayer magnetic systems. Phys. Rev. Lett. 1992, 68, 3749–3752. [Google Scholar] [CrossRef]
- Bernardi, J.; Hutten, A.; Thomas, G. Electron microscopy of giant magnetoresistive granular Au-Co alloys. J. Magn. Magn. Mater. 1996, 157, 153–155. [Google Scholar] [CrossRef]
- Hutten, A.; Bernardi, J.; Friedrichs, S.; Thomas, G.; Balcells, L. Microstructural influence on magnetic-properties and giant magnetoresistance of melt-spun gold-cobalt. Scr. Metall. Mater. 1995, 33, 1647–1666. [Google Scholar] [CrossRef]
- Garcia-Torres, J.; Valles, E.; Gomez, E. Giant magnetoresistance in electrodeposited Co-Ag granular films. Mater. Lett. 2011, 65, 1865–1867. [Google Scholar] [CrossRef]
- Kenane, S.; Voiron, J.; Benbrahim, N.; Chainet, E.; Robaut, F. Magnetic properties and giant magnetoresistance in electrodeposited Co-Ag granular films. J. Magn. Magn. Mater. 2006, 297, 99–106. [Google Scholar] [CrossRef]
- Odnodvorets, L.V.; Protsenko, I.Y.; Tkach, O.P.; Shabelnyk, Y.M.; Shumakova, N.I. Magnetoresistive effect in granular film alloys based on Ag and Fe or Co. J. Nano Electron. Phys. 2017, 9, 02021-1–02021-4. [Google Scholar] [CrossRef]
- De Toro, J.A.; Andres, J.P.; Gonzalez, J.A.; Goff, J.P.; Barbero, A.J.; Riveiro, J.M. Improved giant magnetoresistance in nanogranular Co/Ag: The role of interparticle RKKY interactions. Phys. Rev. B 2004, 70, 1–6. [Google Scholar] [CrossRef]
- Mitani, S.; Fujimori, H.; Ohnuma, S. Temperature dependence of tunnel-type GMR in insulating granular systems. J. Magn. Magn. Mater. 1998, 177, 919–920. [Google Scholar] [CrossRef]
- Kim, J.G.; Ha, J.G. Particle size distribution and GMR in Co46Al19O35 granular thin films by annealing. Mater. Chem. Phys. 2006, 96, 307–310. [Google Scholar] [CrossRef]
- Blythe, H.J.; Fedosyuk, V.M.; Kasyutich, O.I. Chemically deposited CuCo granular films: An alternative route to GMR. Mater. Lett. 1996, 26, 69–72. [Google Scholar] [CrossRef]
- Kumaraguru, S.; Pavulraj, R.; Vijayakumar, J.; Mohan, S. Electrodeposition of cobalt/silver multilayers from deep eutectic solvent and their giant magnetoresistance. J. Alloys Compd. 2017, 693, 1143–1149. [Google Scholar] [CrossRef]
- Weddemann, A.; Ennen, I.; Regtmeier, A.; Albon, C.; Wolff, A.; Eckstadt, K.; Mill, N.; Peter, M.K.H.; Mattay, J.; Plattner, C.; et al. Review and outlook: From single nanoparticles to self-assembled monolayers and granular GMR sensors. Beilstein J. Nanotechnol. 2010, 1, 75–93. [Google Scholar] [CrossRef]
- Julliere, M. Tunneling between ferromagnetic-films. Phys. Lett. A 1975, 54, 225–226. [Google Scholar] [CrossRef]
- You, C.Y.; Tian, N.; Goripati, H.S.; Furubayashi, T. Current-perpendicular-to-the-plane giant magnetoresistance of an all-metal spin valve structure with Co40Fe40B20 magnetic layer. Appl. Phys. Lett. 2010, 96, 142503. [Google Scholar] [CrossRef]
- Yuasa, S.; Nagahama, T.; Fukushima, A.; Suzuki, Y.; Ando, K. Giant room-temperature magnetoresistance in single-crystal Fe/MgO/Fe magnetic tunnel junctions. Nature Mater. 2004, 3, 868–871. [Google Scholar] [CrossRef] [PubMed]
- Nowak, E.R.; Weissman, M.B.; Parkin, S.S.P. Electrical noise in hysteretic ferromagnet-insulator-ferromagnet tunnel junctions. Appl. Phys. Lett. 1999, 74, 600–602. [Google Scholar] [CrossRef]
- Miyazaki, T.; Tezuka, N. Giant magnetic tunneling effect in Fe/Al2O3/Fe junction. J. Magn. Magn. Mater. 1995, 139, 231–234. [Google Scholar] [CrossRef]
- Moodera, J.S.; Kinder, L.R.; Wong, T.M.; Meservey, R. Large magnetoresistance at room-temperature in ferromagnetic thin-film tunnel-junctions. Phys. Rev. Lett. 1995, 74, 3273–3276. [Google Scholar] [CrossRef]
- Butler, W.H.; Zhang, X.G.; Schulthess, T.C.; MacLaren, J.M. Spin-dependent tunneling conductance of Fe vertical bar MgO vertical bar Fe sandwiches. Phys. Rev. B 2001, 63, 054416. [Google Scholar] [CrossRef]
- Parkin, S.S.P.; Kaiser, C.; Panchula, A.; Rice, P.M.; Hughes, B.; Samant, M.; Yang, S.H. Giant tunnelling magnetoresistance at room temperature with MgO tunnel barriers. Nat. Mater. 2004, 3, 862–867. [Google Scholar] [CrossRef]
- Klein, T.; Wang, Y.; Tu, L.; Yu, L.N.; Feng, Y.L.; Wang, W.; Wang, J.P. Comparative analysis of several GMR strip sensor configurations for biological applications. Sens. Actuators A-Phys. 2014, 216, 349–354. [Google Scholar] [CrossRef]
- Lee, J.R.; Sato, N.; Bechstein, D.J.B.; Osterfeld, S.J.; Wang, J.Y.; Gani, A.W.; Hall, D.A.; Wang, S.X. Experimental and theoretical investigation of the precise transduction mechanism in giant magnetoresistive biosensors. Sci. Rep. 2016, 6, 18692. [Google Scholar] [CrossRef]
- Li, G.X.; Sun, S.H.; Wang, S.X. Spin valve biosensors: Signal dependence on nanoparticle position. J. Appl. Phys. 2006, 99, 08P107. [Google Scholar] [CrossRef]
- Albisetti, E.; Petti, D.; Damin, F.; Cretich, M.; Torti, A.; Chiari, M.; Bertacco, R. Photolithographic bio-patterning of magnetic sensors for biomolecular recognition. Sens. Actuators B-Chem. 2014, 200, 39–46. [Google Scholar] [CrossRef]
- Osterfeld, S.J.; Yu, H.; Gaster, R.S.; Caramuta, S.; Xu, L.; Han, S.J.; Hall, D.A.; Wilson, R.J.; Sun, S.H.; White, R.L.; et al. Multiplex protein assays based on real-time magnetic nanotag sensing. Proc. Natl. Acad. Sci. USA 2008, 105, 20637–20640. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, G.; Lee, J.R.; Dahl, C.; Guldberg, P.; Dufva, M.; Wang, S.X.; Hansen, M.F. Simultaneous profiling of DNA mutation and methylation by melting analysis using magnetoresistive biosensor array. ACS Nano 2017, 11, 8864–8870. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Klein, T.; Krishna, V.D.; Su, D.Q.; Perez, A.M.; Wang, J.P. Portable GMR handheld platform for the detection of influenza a virus. ACS Sens. 2017, 2, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, Y.; Tu, L.; Klein, T.; Feng, Y.L.; Wang, J.P. Surface modification for protein and DNA immobilization onto GMR biosensor. IEEE Trans. Magn. 2013, 49, 296–299. [Google Scholar] [CrossRef]
- Martins, V.C.; Cardoso, F.A.; Germano, J.; Cardoso, S.; Sousa, L.; Piedade, M.; Freitas, P.P.; Fonseca, L.P. Femtomolar limit of detection with a magnetoresistive biochip. Biosens. Bioelectron. 2009, 24, 2690–2695. [Google Scholar] [CrossRef] [PubMed]
- Moretti, D.; DiFrancesco, M.L.; Sharma, P.P.; Dante, S.; Albisetti, E.; Monticelli, M.; Bertacco, R.; Petti, D.; Baldelli, P.; Benfenati, F. Biocompatibility of a magnetic tunnel junction sensor array for the detection of neuronal signals in culture. Front. Neurosci. 2018, 12, 909. [Google Scholar] [CrossRef]
- Pannetier-Lecoeur, M.; Fermon, C.; Dyvorne, H.; Jacquinot, J.F.; Polovy, H.; Walliang, A.L. Magnetoresistive-superconducting mixed sensors for biomagnetic applications. J. Magn. Magn. Mater. 2010, 322, 1647–1650. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, J.; Wu, K.; Wang, J.-P. Design and integration of magnetic flux concentrator with magnetic tunnel junction for weak magnetic field detection. J. Magn. Magn. Mater. 2019, 2019, 4475. [Google Scholar]
- Shen, H.M.; Hu, L.; Fu, X. Integrated giant magnetoresistance technology for approachable weak biomagnetic signal detections. Sensors 2018, 18, 148. [Google Scholar] [CrossRef]
- Cardoso, S.; Leitao, D.C.; Gameiro, L.; Cardoso, F.; Ferreira, R.; Paz, E.; Freitas, P.P. Magnetic tunnel junction sensors with pTesla sensitivity. Microsyst. Technol. 2014, 20, 793–802. [Google Scholar] [CrossRef]
- Leitao, D.C.; Coelho, P.; Borme, J.; Knudde, S.; Cardoso, S.; Freitas, P.P. Ultra-Compact 100 × 100 μm2 footprint hybrid device with spin-valve nanosensors. Sensors 2015, 15, 30311–30318. [Google Scholar] [CrossRef] [PubMed]
- Isiksacan, Z.; Guler, M.T.; Aydogdu, B.; Bilican, I.; Elbuken, C. Rapid fabrication of microfluidic PDMS devices from reusable PDMS molds using laser ablation. J. Micromech. Microeng. 2016, 26, 3. [Google Scholar] [CrossRef]
- Choudhury, S.; Dutta, S.; Chatterjee, S. Cost-effective template development for the microfluidic device. Micro Nano Lett. 2019, 14, 860–864. [Google Scholar] [CrossRef]
- Liu, D.D.; Tran, T. Microfluidic mixing using PDMS-based microporous structures. Micro. Nano. 2018, 22, 123. [Google Scholar] [CrossRef]
- Martins, V.C.; Germano, J.; Cardoso, F.A.; Loureiro, J.; Cardoso, S.; Sousa, L.; Piedade, M.; Fonseca, L.P.; Freitas, P.P. Challenges and trends in the development of a magnetoresistive biochip portable platform. J. Magn. Magn. Mater. 2010, 322, 1655–1663. [Google Scholar] [CrossRef]
- Tang, K.C.; Liao, E.; Ong, W.L.; Wong, J.D.S.; Agarwal, A.; Nagarajan, R.; Yobas, L. Evaluation of Bonding Between Oxygen Plasma Treated Polydimethyl Siloxane and Passivated Silicon. In Proceedings of the International MEMS Conference, Singapore, 9–12 May 2006; pp. 155–161. [Google Scholar]
- Zhang, B.; Meng, F.S.; Feng, J.G.; Wang, J.X.; Wu, Y.C.; Jiang, L. Manipulation of colloidal particles in three dimensions via microfluid engineering. Adv. Mater. 2018, 30, 1707291. [Google Scholar] [CrossRef]
- Ehresmann, A.; Koch, I.; Holzinger, D. Manipulation of superparamagnetic beads on patterned exchange-bias layer systems for biosensing applications. Sensors 2015, 15, 28854–28888. [Google Scholar] [CrossRef]
- Holzinger, D.; Ehresmann, A. Diffusion enhancement in a laminar flow liquid by near-surface transport of superparamagnetic bead rows. Microfluid. Nano 2015, 19, 395–402. [Google Scholar] [CrossRef]
- Donolato, M.; Vavassori, P.; Gobbi, M.; Deryabina, M.; Hansen, M.F.; Metlushko, V.; Ilic, B.; Cantoni, M.; Petti, D.; Brivio, S.; et al. On-chip manipulation of protein-coated magnetic beads via domain-wall conduits. Adv. Mater. 2010, 22, 2706–2710. [Google Scholar] [CrossRef]
- Rapoport, E.; Montana, D.; Beach, G.S.D. Integrated capture, transport, and magneto-mechanical resonant sensing of superparamagnetic microbeads using magnetic domain walls. Lab Chip 2012, 12, 4433–4440. [Google Scholar] [CrossRef]
- Monticelli, M.; Torti, A.; Cantoni, M.; Petti, D.; Albisetti, E.; Manzin, A.; Guerriero, E.; Sordan, R.; Gervasoni, G.; Carminati, M.; et al. On-Chip magnetic platform for single-particle manipulation with integrated electrical feedback. Small 2016, 12, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, Y.; Tu, L.; Feng, Y.; Klein, T.; Wang, J.-P. Magnetoresistive performance and comparison of supermagnetic nanoparticles on giant magnetoresistive sensor-based detection system. Sci. Rep. 2014, 4, 5716. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, W.; Yu, L.N.; Tu, L.; Feng, Y.L.; Klein, T.; Wang, J.P. Giant magnetoresistive-based biosensing probe station system for multiplex protein assays. Biosens. Bioelectron. 2015, 70, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Krishna, V.D.; Wu, K.; Perez, A.M.; Wang, J.P. Giant Magnetoresistance-based Biosensor for Detection of Influenza A Virus. Front. Microbiol. 2016, 7, 8. [Google Scholar] [CrossRef]
- Lee, J.-R.; Haddon, D.J.; Wand, H.E.; Price, J.V.; Diep, V.K.; Hall, D.A.; Petri, M.; Baechler, E.C.; Balboni, I.M.; Utz, P.J. Multiplex giant magnetoresistive biosensor microarrays identify interferon-associated autoantibodies in systemic lupus erythematosus. Sci. Rep. 2016, 6, 27623. [Google Scholar] [CrossRef]
- Cox, K.L.; Devanarayan, V.; Kriauciunas, A.; Manetta, J.; Montrose, C.; Sittampalam, S. Immunoassay methods. In Assay Guidance Manual; Sittampalam, G.S., Coussens, N.P., Nelson, H., Arkin, M., Auld, D., Austin, C., Eds.; Eli Lilly & Company: Washington, DC, USA, 2014. [Google Scholar]
- Srinivasan, B.; Li, Y.; Jing, Y.; Xing, C.; Slaton, J.; Wang, J.-P. A three-layer competition-based giant magnetoresistive assay for direct quantification of dndoglin from human urine. Anal. Chem. 2011, 83, 2996–3002. [Google Scholar] [CrossRef]
- Church, D.L.; Lloyd, T.; Larios, O.; Gregson, D.B. Evaluation of simplexa™ group a strep direct kit compared to hologic® group a streptococcal direct assay for detection of group a streptococcus (GAS) in throat swabs. J. Clin. Microbiol. 2018, 56, e01666-17. [Google Scholar] [CrossRef]
- Owen, L.J.; Wu, F.C.; Büttler, R.M.; Keevil, B.G. A direct assay for the routine measurement of testosterone, androstenedione, dihydrotestosterone and dehydroepiandrosterone by liquid chromatography tandem mass spectrometry. Ann. Clin. Biochem. 2016, 53, 580–587. [Google Scholar] [CrossRef]
- Binnicker, M.J.; Espy, M.J.; Irish, C.L. Rapid and direct detection of herpes simplex virus in cerebrospinal fluid by use of a commercial real-time PCR assay. J. Clin. Microbiol. 2014, 52, 4361–4362. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Y.; Tu, L.; Klein, T.; Feng, Y.; Li, Q.; Wang, J.-P. Magnetic detection of mercuric ion using giant magnetoresistance-based biosensing system. Anal. Chem. 2014, 86, 3712–3716. [Google Scholar] [CrossRef]
- Xu, L.; Yu, H.; Akhras, M.S.; Han, S.J.; Osterfeld, S.; White, R.L.; Pourmand, N.; Wang, S.X. Giant magnetoresistive biochip for DNA detection and HPV genotyping. Biosens. Bioelectron. 2008, 24, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, G.; Dufva, M.; Hansen, M.F. Two-dimensional salt and temperature DNA denaturation analysis using a magnetoresistive sensor. Lab Chip 2017, 17, 2256–2263. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, G.; Østerberg, F.W.; Henriksen, A.D.; Dufva, M.; Hansen, M.F. On-chip magnetic bead-based DNA melting curve analysis using a magnetoresistive sensor. J. Magn. Magn. Mater. 2015, 380, 215–220. [Google Scholar] [CrossRef]
- Miller, M.M.; Prinz, G.A.; Cheng, S.F.; Bounnak, S. Detection of a micron-sized magnetic sphere using a ring-shaped anisotropic magnetoresistance-based sensor: A model for a magnetoresistance-based biosensor. Appl. Phys. Lett. 2002, 81, 2211–2213. [Google Scholar] [CrossRef]
- Guo, Y.; Ouyang, Y.; Sato, N.; Ooi, C.C.; Wang, S.X. Exchange-biased anisotropic magnetoresistive field sensor. IEEE Sens. J. 2017, 17, 3309–3315. [Google Scholar] [CrossRef]
- Guo, Y.; Deng, Y.; Wang, S.X. Multilayer anisotropic magnetoresistive angle sensor. Sens. Actuators A-Phys. 2017, 263, 159–165. [Google Scholar] [CrossRef]
- Nazarov, A.V.; da Silva, F.C.S.; Pappas, D.P. Arrays of magnetoresistive sensors for nondestructive testing. J. Vac. Sci. Technol. A 2004, 22, 1375–1378. [Google Scholar] [CrossRef]
- Wang, Z.G.; Wang, X.J.; Li, M.H.; Gao, Y.; Hu, Z.Q.; Nan, T.X.; Liang, X.F.; Chen, H.H.; Yang, J.; Cash, S.; et al. Highly sensitive flexible magnetic sensor based on anisotropic magnetoresistance effect. Adv. Mater. 2016, 28, 9370–9377. [Google Scholar] [CrossRef]
- Weiss, R.; Mattheis, R.; Reiss, G. Advanced giant magnetoresistance technology for measurement applications. Meas. Sci. Technol. 2013, 24, 17. [Google Scholar] [CrossRef]
- Zabel, H. Progress in spintronics. Superlattices Microstruct. 2009, 46, 541–553. [Google Scholar] [CrossRef]
- Graham, D.L.; Ferreira, H.A.; Freitas, P.P.; Cabral, J.M.S. High sensitivity detection of molecular recognition using magnetically labelled biomolecules and magnetoresistive sensors. Biosens. Bioelectron. 2003, 18, 483–488. [Google Scholar] [CrossRef]
- Sun, X.C.; Zhi, S.T.; Lei, C.; Zhou, Y. Investigation of contactless detection using a giant magnetoresistance sensor for detecting prostate specific antigen. Biomed. Microdevices 2016, 18, 60. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kakkar, V. DARPin based GMR Biosensor for the detection of ESAT-6 Tuberculosis Protein. Tuberculosis 2019, 118, 101852. [Google Scholar] [CrossRef]
- Shirzadfar, H.; Nadi, M.; Kourtiche, D.; Yamada, S.; Shahabi, P. Characterization of a needle-type giant magnetoresistance sensor for detection of escherichia coli’s magnetic marker. Int. J. Smart Sens. Intell. Syst. 2015, 8, 220–234. [Google Scholar] [CrossRef]
- Gao, Y.; Huo, W.S.; Zhang, L.; Lian, J.; Tao, W.; Song, C.; Tang, J.P.; Shi, S.; Gao, Y.H. Multiplex measurement of twelve tumor markers using a GMR multi-biomarker immunoassay biosensor. Biosens. Bioelectron. 2019, 123, 204–210. [Google Scholar] [CrossRef]
- Yang, N.; Tao, L.; Ping, Z.P.; Xiaoqiang, C.; Xuefeng, H.; Wei, Z. An early cancer diagnosis platform based on micro-magnetic sensor array demonstrates ultra-high sensitivity. J. Nanomed. Nanotechnol. 2016, 7, 1000344. [Google Scholar] [CrossRef]
- Su, D.Q.; Wu, K.; Wang, J.P. Large-area GMR bio-sensors based on reverse nucleation switching mechanism. J. Magn. Magn. Mater. 2019, 473, 484–489. [Google Scholar] [CrossRef]
- Feng, Y.L.; Liu, J.M.; Klein, T.; Wu, K.; Wang, J.P. Localized detection of reversal nucleation generated by high moment magnetic nanoparticles using a large-area magnetic sensor. J. Appl. Phys. 2017, 122, 123901. [Google Scholar] [CrossRef]
- Bai, J.M.; Wang, J.P. High-magnetic-moment core-shell-type FeCo-Au/Ag nanoparticles. Appl. Phys. Lett. 2005, 87, 152502. [Google Scholar] [CrossRef]
- Liu, J.; Wu, K.; Wang, J.-P. Magnetic properties of cubic FeCo nanoparticles with anisotropic long chain structure. AIP Adv. 2016, 6, 056126. [Google Scholar] [CrossRef]
- Lei, Z.Q.; Li, L.; Li, G.J.; Leung, C.W.; Shi, J.; Wong, C.M.; Lo, K.C.; Chan, W.K.; Mak, C.S.K.; Chan, S.B.; et al. Liver cancer immunoassay with magnetic nanoparticles and MgO-based magnetic tunnel junction sensors. J. Appl. Phys. 2012, 111, 07E505. [Google Scholar] [CrossRef]
- Freitas, P.P.; Ferreira, H.A.; Graham, D.L.; Clarke, L.A.; Amaral, M.D.; Martins, V.; Fonseca, L.; Cabral, J.S. Magnetoresistive DNA chips. In Magnetoelectronics; Elsevier: Amsterdam, The Netherlands, 2004; pp. 331–386. [Google Scholar]
- Jiang, Z.; Llandro, J.; Mitrelias, T.; Bland, J.A.C. An integrated microfluidic cell for detection, manipulation, and sorting of single micron-sized magnetic beads. J. Appl. Phys. 2006, 99, 08S105. [Google Scholar] [CrossRef]
- Kasatkin, S.I.; Vasil’eva, N.P.; Murav’ev, A.M. Biosensors based on the thin-film magnetoresistive sensors. Autom. Remote. Control. 2010, 71, 156–166. [Google Scholar] [CrossRef]
- Tamanaha, C.R.; Mulvaney, S.P.; Rife, J.C.; Whitman, L.J. Magnetic labeling, detection, and system integration. Biosens. Bioelectron. 2008, 24, 1–13. [Google Scholar] [CrossRef] [PubMed]
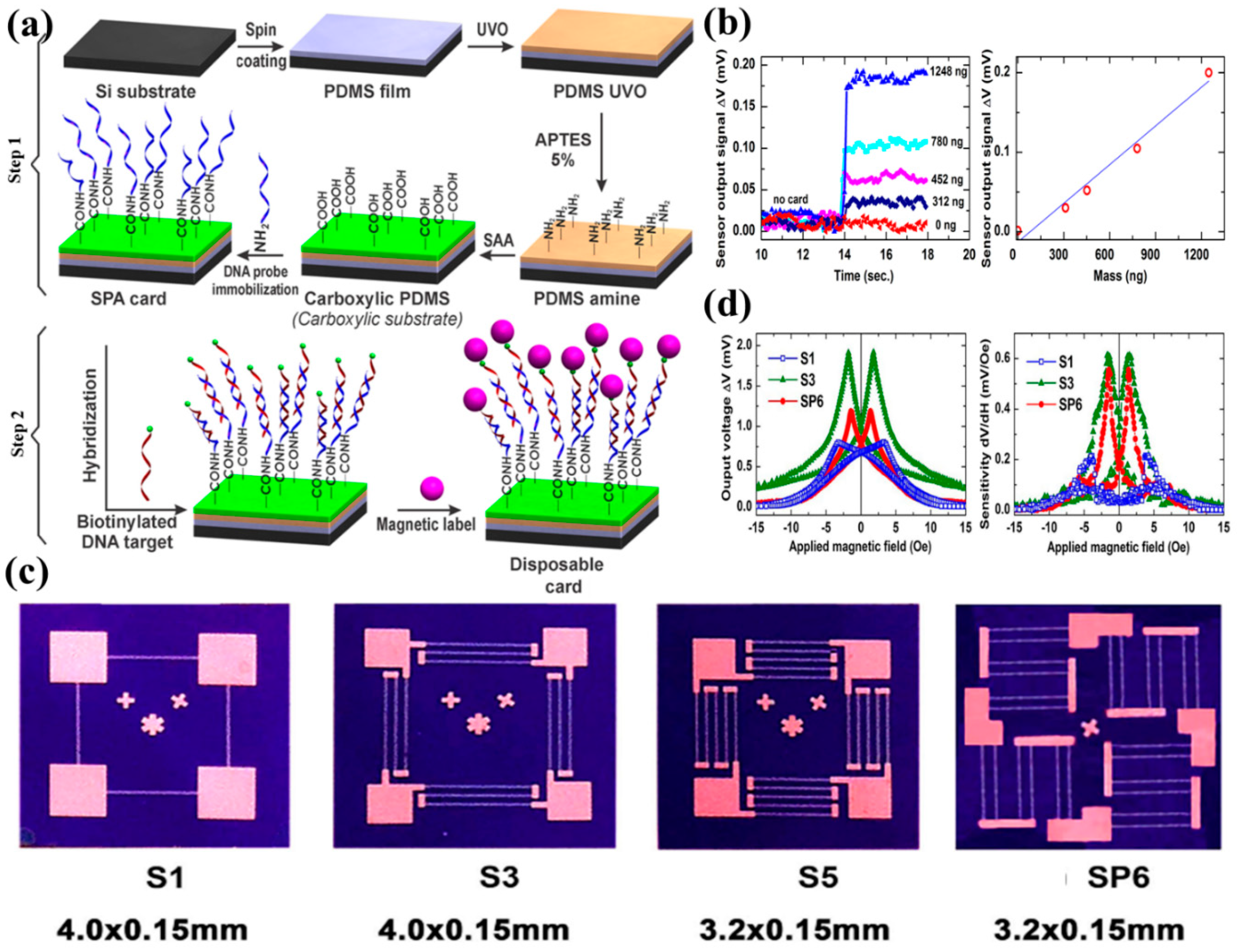
- Hien, L.T.; Quynh, L.K.; Huyen, V.T.; Tu, B.D.; Hien, N.T.; Phuong, D.M.; Nhung, P.H.; Giang, D.T.H.; Duc, N.H. DNA-magnetic bead detection using disposable cards and the anisotropic magnetoresistive sensor. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 045006. [Google Scholar] [CrossRef]
- Quynh, L.K.; Tu, B.D.; Anh, C.V.; Duc, N.H.; Phung, A.T.; Dung, T.T.; Giang, D.T.H. Design optimization of an anisotropic magnetoresistance sensor for detection of magnetic nanoparticles. J. Electron. Mater. 2019, 48, 997–1004. [Google Scholar] [CrossRef]
- Schotter, J.; Kamp, P.B.; Becker, A.; Puhler, A.; Reiss, G.; Bruckl, H. Comparison of a prototype magnetoresistive biosensor to standard fluorescent DNA detection. Biosens. Bioelectron. 2004, 19, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Koets, M.; van der Wijk, T.; van Eemeren, J.; van Amerongen, A.; Prins, M.W.J. Rapid DNA multi-analyte immunoassay on a magneto-resistance biosensor. Biosens. Bioelectron. 2009, 24, 1893–1898. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, G.; Lee, J.R.; Guldberg, P.; Dufva, M.; Wang, S.X.; Hansen, M.F. Denaturation strategies for detection of double stranded PCR products on GMR magnetic biosensor array. Biosens. Bioelectron. 2017, 93, 155–160. [Google Scholar] [CrossRef]
- Zhi, X.; Deng, M.; Yang, H.; Gao, G.; Wang, K.; Fu, H.L.; Zhang, Y.X.; Chen, D.; Cui, D.X. A novel HBV genotypes detecting system combined with microfluidic chip, loop-mediated isothermal amplification and GMR sensors. Biosens. Bioelectron. 2014, 54, 372–377. [Google Scholar] [CrossRef]
- Ravi, N.; Rizzi, G.; Chang, S.E.; Cheung, P.; Utz, P.J.; Wang, S.X. Quantification of cDNA on GMR biosensor array towards point-of-care gene expression analysis. Biosens. Bioelectron. 2019, 130, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Grancharov, S.G.; Zeng, H.; Sun, S.H.; Wang, S.X.; O’Brien, S.; Murray, C.B.; Kirtley, J.R.; Held, G.A. Bio-functionalization of monodisperse magnetic nanoparticles and their use as biomolecular labels in a magnetic tunnel junction based sensor. J. Phys. Chem. B 2005, 109, 13030–13035. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Schrag, B.D.; Carter, M.J.; Xiao, G. Quantitative detection of DNA labeled with magnetic nanoparticles using arrays of MgO-based magnetic tunnel junction sensors. Appl. Phys. Lett. 2008, 93, 033903. [Google Scholar] [CrossRef]
- Sharma, P.P.; Albisetti, E.; Massetti, M.; Scolari, M.; La Torre, C.; Monticelli, M.; Leone, M.; Damin, F.; Gervasoni, G.; Ferrari, G.; et al. Integrated platform for detecting pathogenic DNA via magnetic tunneling junction-based biosensors. Sens. Actuators B-Chem. 2017, 242, 280–287. [Google Scholar] [CrossRef]
- Shen, W.F.; Schrag, B.D.; Carter, M.J.; Xie, J.; Xu, C.J.; Sun, S.H.; Xiao, G. Detection of DNA labeled with magnetic nanoparticles using MgO-based magnetic tunnel junction sensors. J. Appl. Phys. 2008, 103, 3. [Google Scholar] [CrossRef]
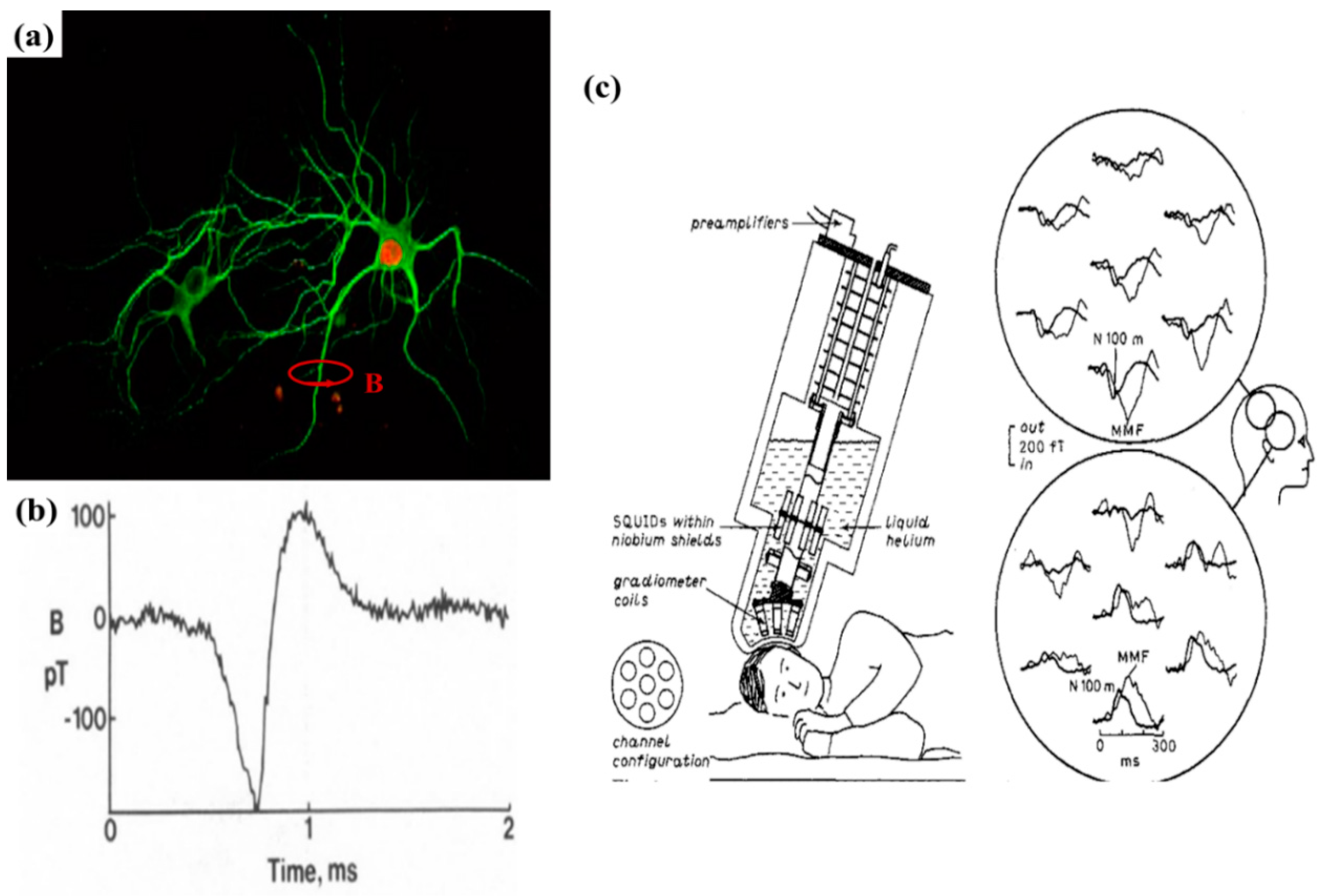
- Roth, B.J.; Wikswo, J.P., Jr. The magnetic field of a single axon. A comparison of theory and experiment. Biophys. J. 1985, 48, 93–109. [Google Scholar] [CrossRef]
- Knuutila, J.E.T.; Ahonen, A.I.; Hamalainen, M.S.; Kajola, M.J.; Laine, P.P.; Lounasmaa, O.V.; Parkkonen, L.T.; Simola, J.T.A.; Tesche, C.D. A 122-channel whole-cortex SQUID system for measuring the brain’s magnetic fields. IEEE Trans. Magn. 1993, 29, 3315–3320. [Google Scholar] [CrossRef]
- Lounasmaa, O.V.; Hari, R.; Joutsiniemi, S.L.; Hämäläinen, M. Multi-SQUID recordings of human cerebral magnetic fields may give information about memory processes. EPL (Europhys. Lett.) 1989, 9, 603. [Google Scholar] [CrossRef]
- Maynard, E.M.; Nordhausen, C.T.; Normann, R.A. The Utah intracortical electrode array: A recording structure for potential brain-computer interfaces. Electroencephalogr. Clin. Neurophysiol. 1997, 102, 228–239. [Google Scholar] [CrossRef]
- Hoogerwerf, A.C.; Wise, K.D. A three-dimensional microelectrode array for chronic neural recording. IEEE Trans. Biomed. Eng. 1994, 41, 1136–1146. [Google Scholar] [CrossRef]
- Malmivuo, J.A.; Suihko, V.E. Effect of skull resistivity on the spatial resolutions of EEG and MEG. IEEE Trans. Biomed. Eng. 2004, 51, 1276–1280. [Google Scholar] [CrossRef] [PubMed]
- Brookes, M.J.; Woolrich, M.W.; Price, D. An Introduction to MEG connectivity measurements. In Magnetoencephalography: From Signals to Dynamic Cortical Networks; Springer: Cham, Switzerland, 2019; pp. 433–470. [Google Scholar]
- Burnos, S.; Fedele, T.; Schmid, O.; Krayenbühl, N.; Sarnthein, J. Detectability of the somatosensory evoked high frequency oscillation (HFO) co-recorded by scalp EEG and ECoG under propofol. NeuroImage Clin. 2016, 10, 318–325. [Google Scholar] [CrossRef] [PubMed]
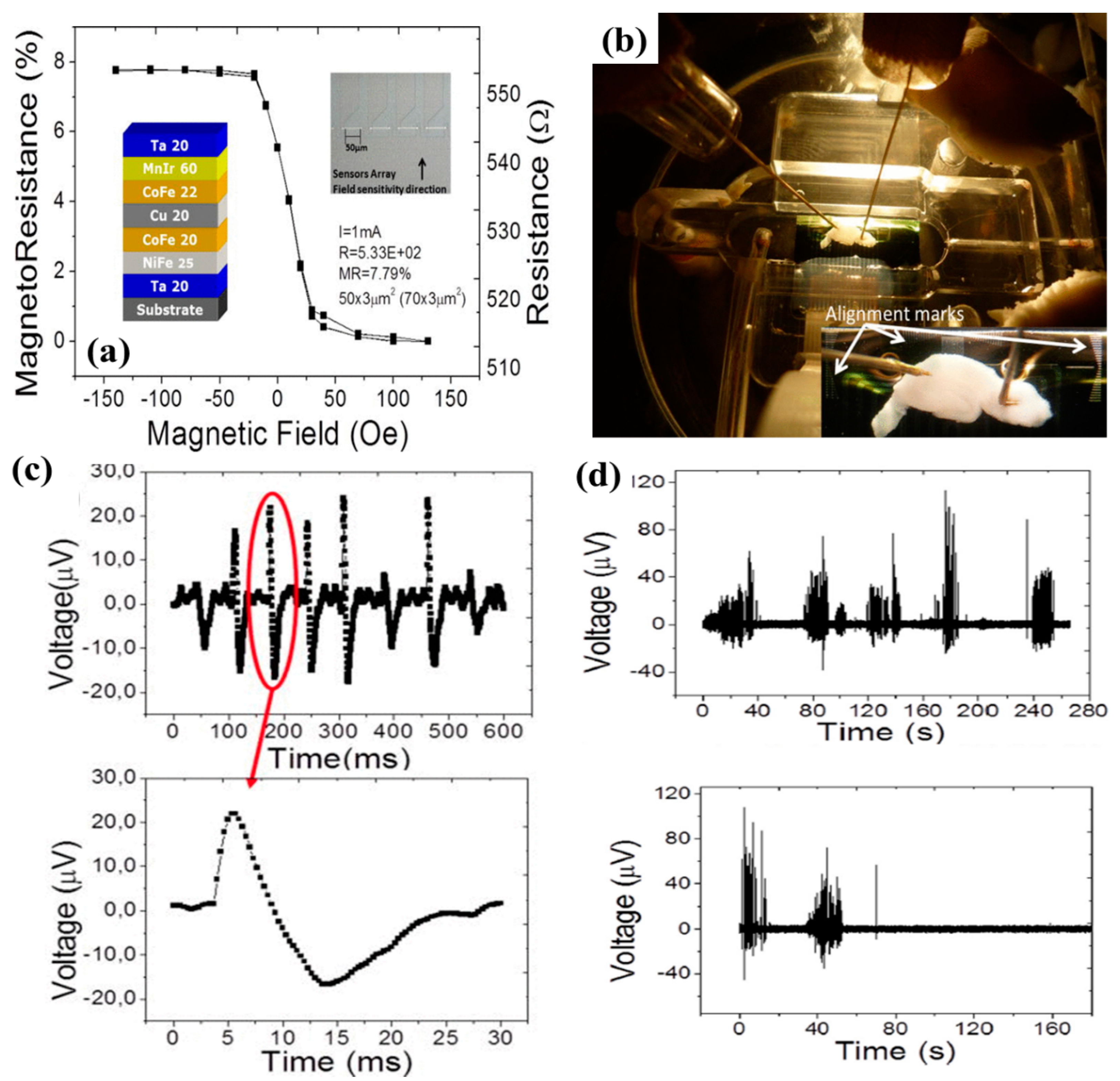
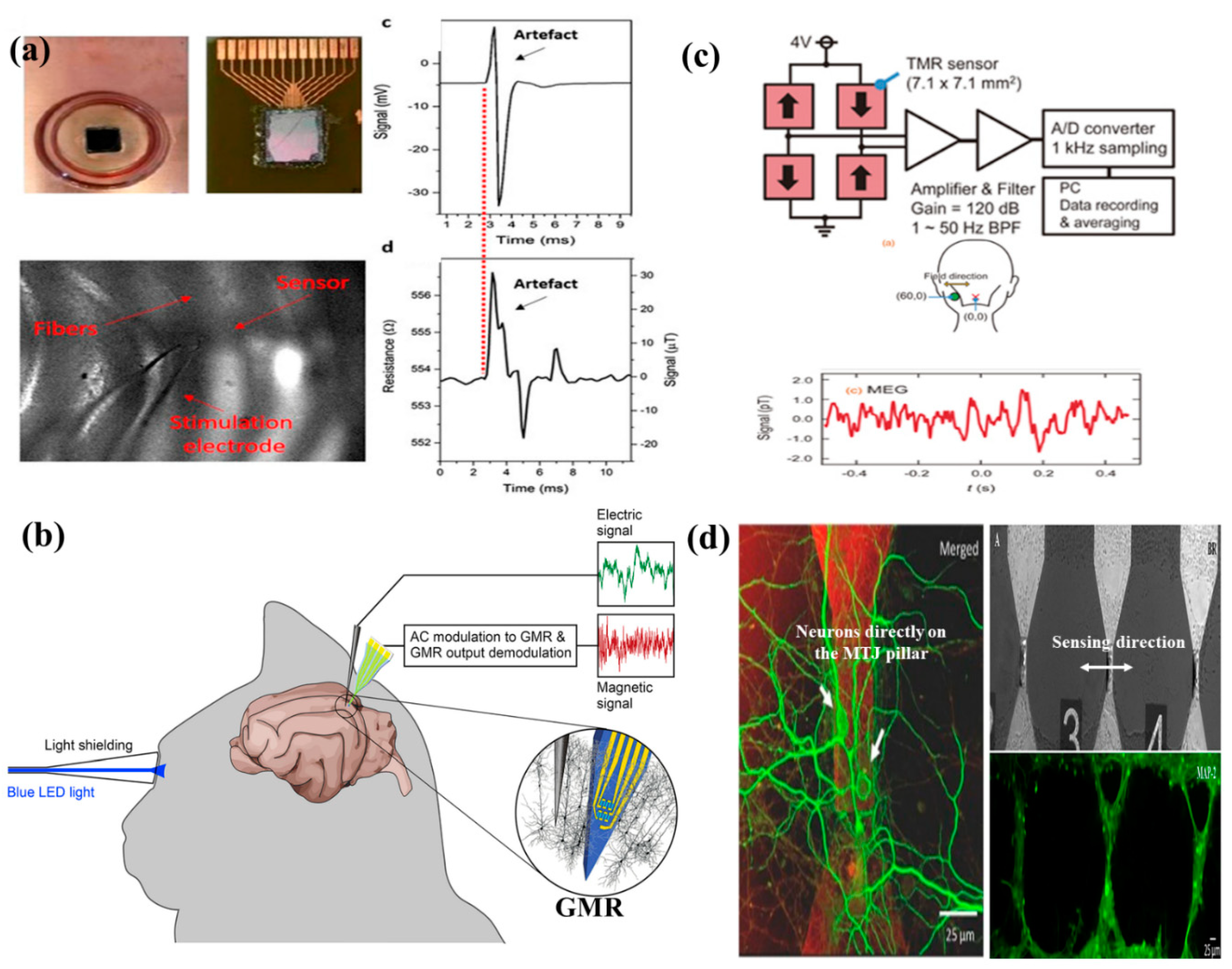
- Amaral, J.; Cardoso, S.; Freitas, P.P.; Sebastião, A.M. Toward a system to measure action potential on mice brain slices with local magnetoresistive probes. J. Appl. Phys. 2011, 109, 07B308. [Google Scholar] [CrossRef]
- Amaral, J.; Gaspar, J.; Pinto, V.; Costa, T.; Sousa, N.; Cardoso, S.; Freitas, P. Measuring brain activity with magnetoresistive sensors integrated in micromachined probe needles. Appl. Phys. A 2013, 111, 407–412. [Google Scholar] [CrossRef]
- Caruso, L.; Wunderle, T.; Lewis, C.M.; Valadeiro, J.; Trauchessec, V.; Rosillo, J.T.; Amaral, J.P.; Ni, J.; Jendritza, P.; Fermon, C. In vivo magnetic recording of neuronal activity. Neuron 2017, 95, 1283–1291. [Google Scholar] [CrossRef]
- Sharma, P.P.; Gervasoni, G.; Albisetti, E.; D’Ercoli, F.; Monticelli, M.; Moretti, D.; Forte, N.; Rocchi, A.; Ferrari, G.; Baldelli, P. Towards a magnetoresistive platform for neural signal recording. AIP Adv. 2017, 7, 056706. [Google Scholar] [CrossRef]
- Fujiwara, K.; Oogane, M.; Kanno, A.; Imada, M.; Jono, J.; Terauchi, T.; Okuno, T.; Aritomi, Y.; Morikawa, M.; Tsuchida, M. Magnetocardiography and magnetoencephalography measurements at room temperature using tunnel magneto-resistance sensors. Appl. Phys. Exp. 2018, 11, 023001. [Google Scholar] [CrossRef]
- Barrows, C.M.; McCabe, M.P.; Chen, H.; Swann, J.W.; Weston, M.C. PTEN Loss increases the connectivity of fast synaptic motifs and functional connectivity in a developing hippocampal network. J. Neurosci. 2017, 37, 8595–8611. [Google Scholar] [CrossRef]
- Hooge, F.N. 1/f noise is no surface effect. Phys. Lett. A 1969, 29, 139–140. [Google Scholar] [CrossRef]
- Melzer, M.; Karnaushenko, D.; Makarov, D.; Baraban, L.; Calvimontes, A.; Monch, I.; Kaltofen, R.; Mei, Y.F.; Schmidt, O.G. Elastic magnetic sensor with isotropic sensitivity for in-flow detection of magnetic objects. RSC Adv. 2012, 2, 2284–2288. [Google Scholar] [CrossRef]
- Melzer, M.; Karnaushenko, D.; Lin, G.G.; Baunack, S.; Makarov, D.; Schmidt, O.G. Direct transfer of magnetic sensor devices to elastomeric supports for stretchable electronics. Adv. Mater. 2015, 27, 1333–1338. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Lau, Y.C.; Coey, J.M.D.; Li, M.; Wang, J.P. High performance Mgo-barrier magnetic tunnel junctions for flexible and wearable spintronic applications. Sci. Rep. 2017, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Makarov, D.; Melzer, M.; Karnaushenko, D.; Schmidt, O.G. Shapeable magnetoelectronics. Appl. Phys. Rev. 2016, 3, 011101. [Google Scholar] [CrossRef]
- Cebers, A. Flexible magnetic filaments. Curr. Opin. Colloid Interface Sci. 2005, 10, 167–175. [Google Scholar] [CrossRef]
- Karnaushenko, D.; Makarov, D.; Yan, C.L.; Streubel, R.; Schmidt, O.G. Printable giant magnetoresistive devices. Adv. Mater. 2012, 24, 4518–4522. [Google Scholar] [CrossRef]
- Karnaushenko, D.; Makarov, D.; Stober, M.; Karnaushenko, D.D.; Baunack, S.; Schmidt, O.G. High-Performance magnetic sensorics for printable and flexible electronics. Adv. Mater. 2015, 27, 880–885. [Google Scholar] [CrossRef]
- Barraud, C.; Deranlot, C.; Seneor, P.; Mattana, R.; Dlubak, B.; Fusil, S.; Bouzehouane, K.; Deneuve, D.; Petroff, F.; Fert, A. Magnetoresistance in magnetic tunnel junctions grown on flexible organic substrates. Appl. Phys. Lett. 2010, 96, 072502. [Google Scholar] [CrossRef]
- Bedoya-Pinto, A.; Donolato, M.; Gobbi, M.; Hueso, L.E.; Vavassori, P. Flexible spintronic devices on Kapton. Appl. Phys. Lett. 2014, 104, 5. [Google Scholar] [CrossRef]
- Loong, L.M.; Lee, W.; Qiu, X.P.; Yang, P.; Kawai, H.; Saeys, M.; Ahn, J.H.; Yang, H. Flexible MgO Barrier Magnetic Tunnel Junctions. Adv. Mater. 2016, 28, 4983–4990. [Google Scholar] [CrossRef]
- Zhang, Y.U.; He, G.Y.; Zhang, X.X.; Xiao, G. Magnetotransport and electronic noise in superparamagnetic magnetic tunnel junctions. Appl. Phys. Lett. 2019, 115, 022402. [Google Scholar] [CrossRef]
- Chen, J.Y.; Feng, J.F.; Coey, J.M.D. Tunable linear magnetoresistance in MgO magnetic tunnel junction sensors using two pinned CoFeB electrodes. Appl. Phys. Lett. 2012, 100, 142407. [Google Scholar] [CrossRef]
- Franco, F.; Cardoso, S.; Freitas, P.P. Reconfigurable spintronics wheatstone bridge sensors with offset voltage compensation at wafer level. IEEE Trans. Magn. 2019, 55, 4400705. [Google Scholar] [CrossRef]
- Ouyang, Y.; He, J.L.; Hu, J.; Zhao, G.; Wang, Z.X.; Wang, S.X. Contactless current sensors based on magnetic tunnel junction for smart grid applications. IEEE Trans. Magn. 2015, 51, 4004904. [Google Scholar] [CrossRef]
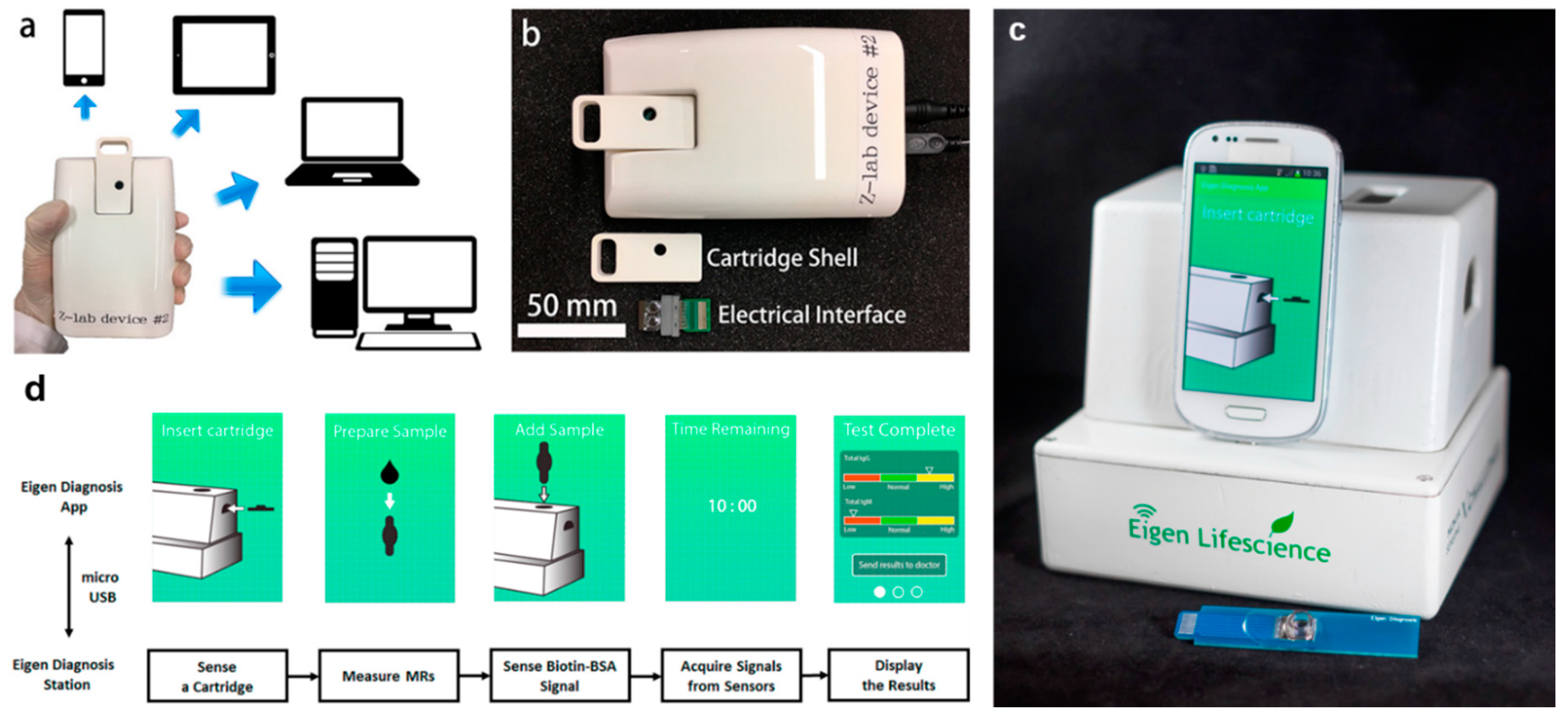
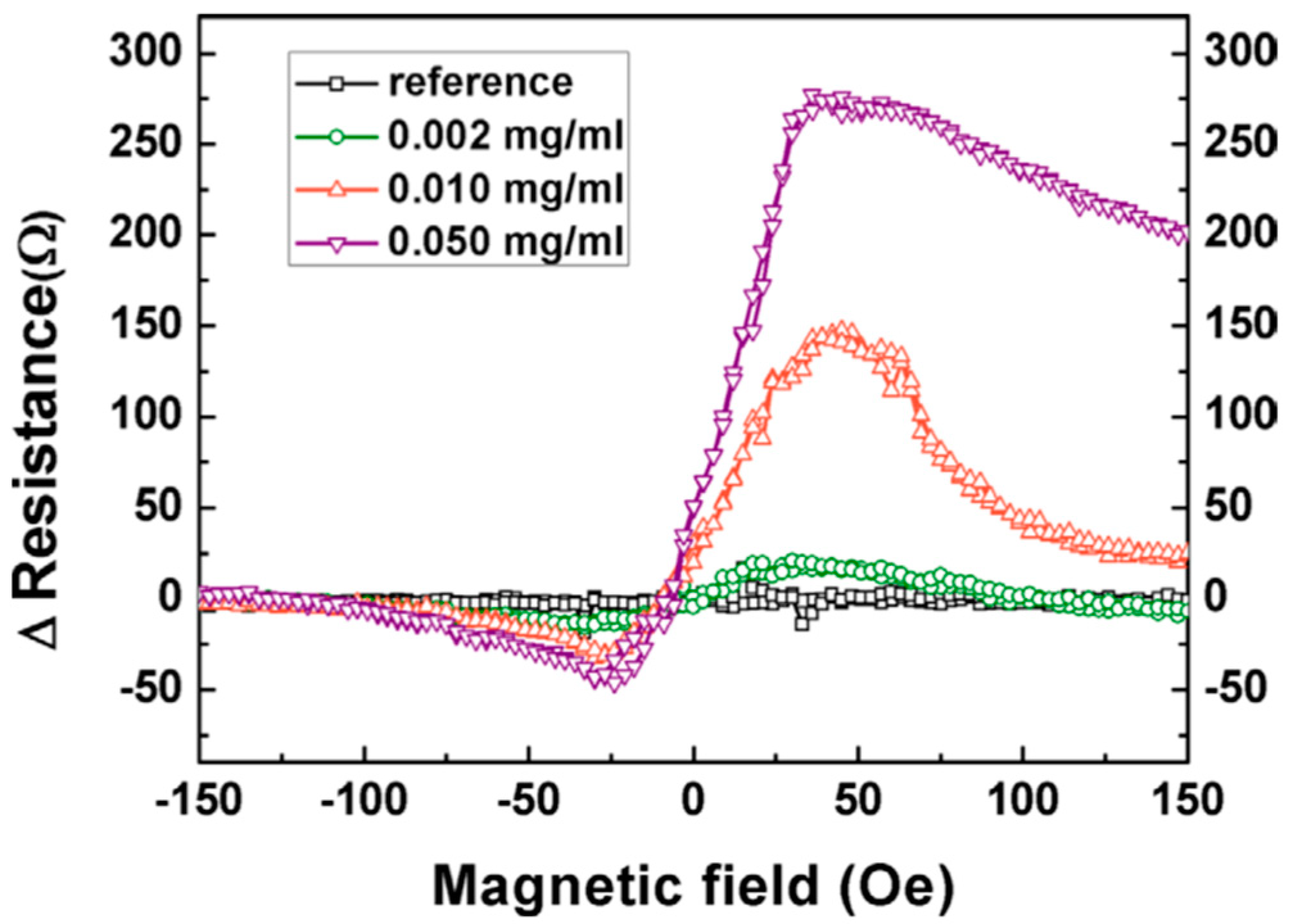
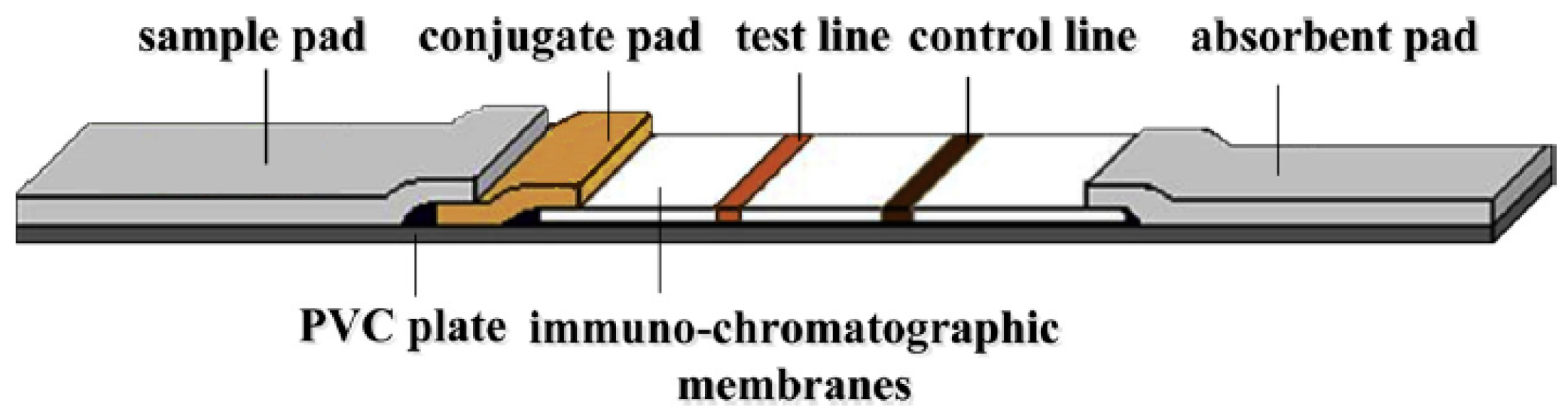
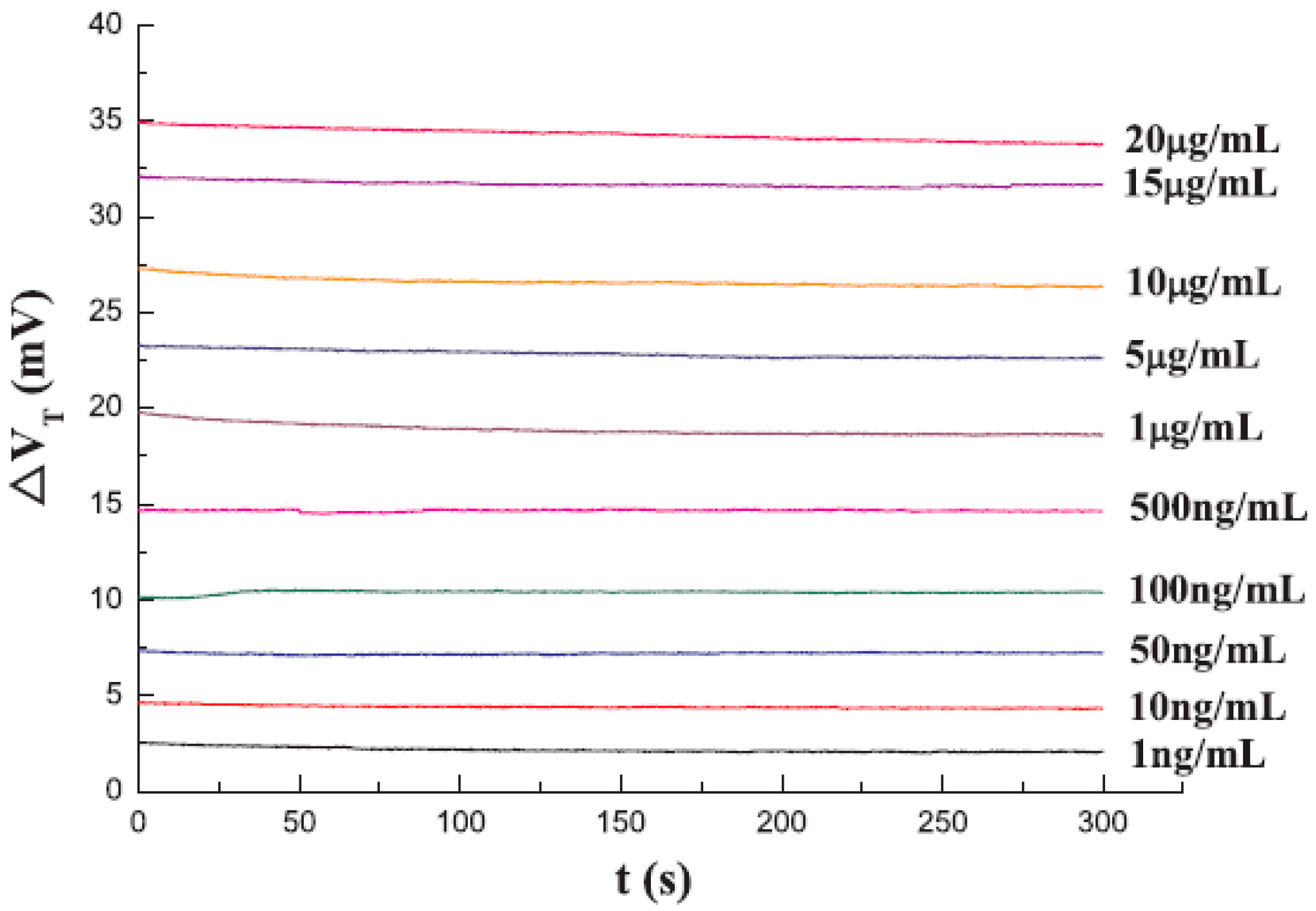
| MR Sensor | Magnetic Label | Assay Time | Target Analyte | Limit of Detection (LoD) | Matrices | References |
|---|---|---|---|---|---|---|
| AMR | 50 nm Fe3O4 | NA | Fe3O4 MNP | 0.56 µemu | NA | [120] |
| AMR | 1 μm Fe3O4 | 3 h | ssDNA | 4.5 pM | HCl, EDTA, NaCl buffer | [119] |
| AMR | 4.3 μm NiFe | NA | NiFe Microbead | Single particle | NA | [97] |
| GMR | 50 nm Fe3O4 | NA | ssDNA | 0.1 pM | Saline sodium citrate | [125] |
| GMR | 50 nm Fe3O4 | 1 h | Influenza A virus | 0.3 nM | Swine Nasal Swab Sample | [21] |
| GMR | 50 nm Fe3O4 | 2 h | Ovarian cancer biomarkers | 7.4 pg/mL | PBS | [18] |
| GMR | 50 nm Fe3O4 | 3 min | LamB gene of E-Coli | 4 pM | Borate buffer | [122] |
| GMR | 0.35 μm Fe3O4 | 12 h | ssDNA | 10 ng/μL | 35% formamide solution | [121] |
| TMR | 16 and 50 nm Fe3O4 | 2 h | ssDNA | 2.5 μM | PBS | [129] |
| TMR | 20 nm Fe3O4 | NA | AFP antigens | 0.002 mg/mL | PBS | [114] |
| TMR | 16 nm Fe3O4 | NA | ssDNA | 100 nM | DI water | [127] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, D.; Wu, K.; Saha, R.; Peng, C.; Wang, J.-P. Advances in Magnetoresistive Biosensors. Micromachines 2020, 11, 34. https://doi.org/10.3390/mi11010034
Su D, Wu K, Saha R, Peng C, Wang J-P. Advances in Magnetoresistive Biosensors. Micromachines. 2020; 11(1):34. https://doi.org/10.3390/mi11010034
Chicago/Turabian StyleSu, Diqing, Kai Wu, Renata Saha, Chaoyi Peng, and Jian-Ping Wang. 2020. "Advances in Magnetoresistive Biosensors" Micromachines 11, no. 1: 34. https://doi.org/10.3390/mi11010034
APA StyleSu, D., Wu, K., Saha, R., Peng, C., & Wang, J.-P. (2020). Advances in Magnetoresistive Biosensors. Micromachines, 11(1), 34. https://doi.org/10.3390/mi11010034






