Simulation of Picking Up Metal Microcomponents Based on Electrochemistry
Abstract
1. Introduction
2. Principles and Influencing Factors
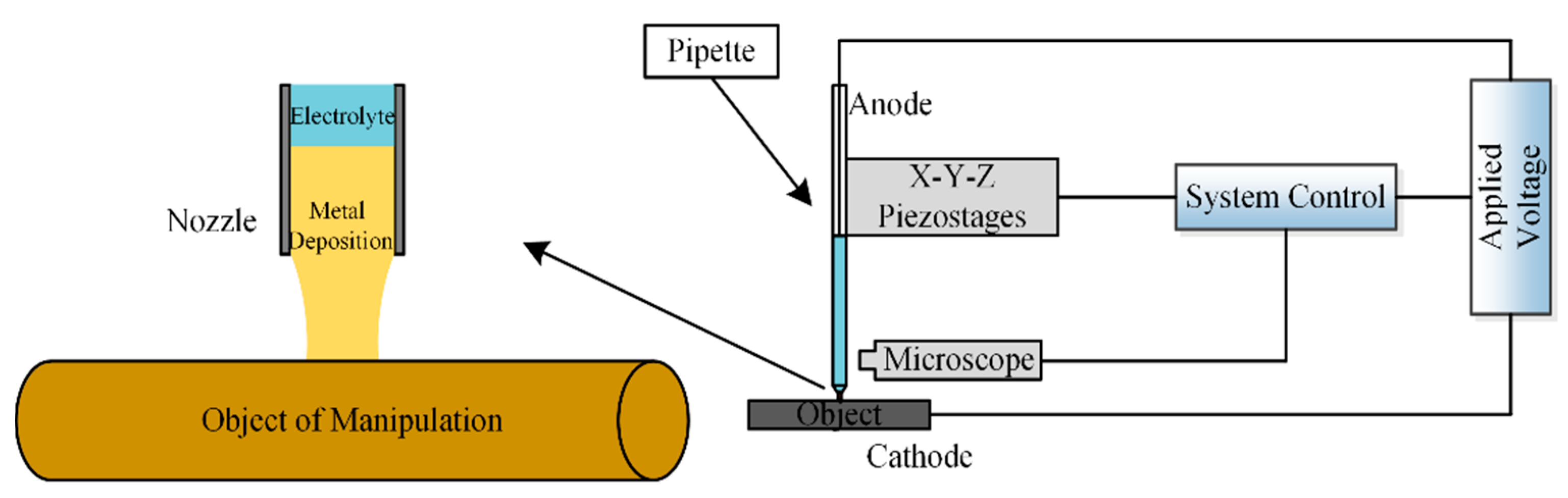
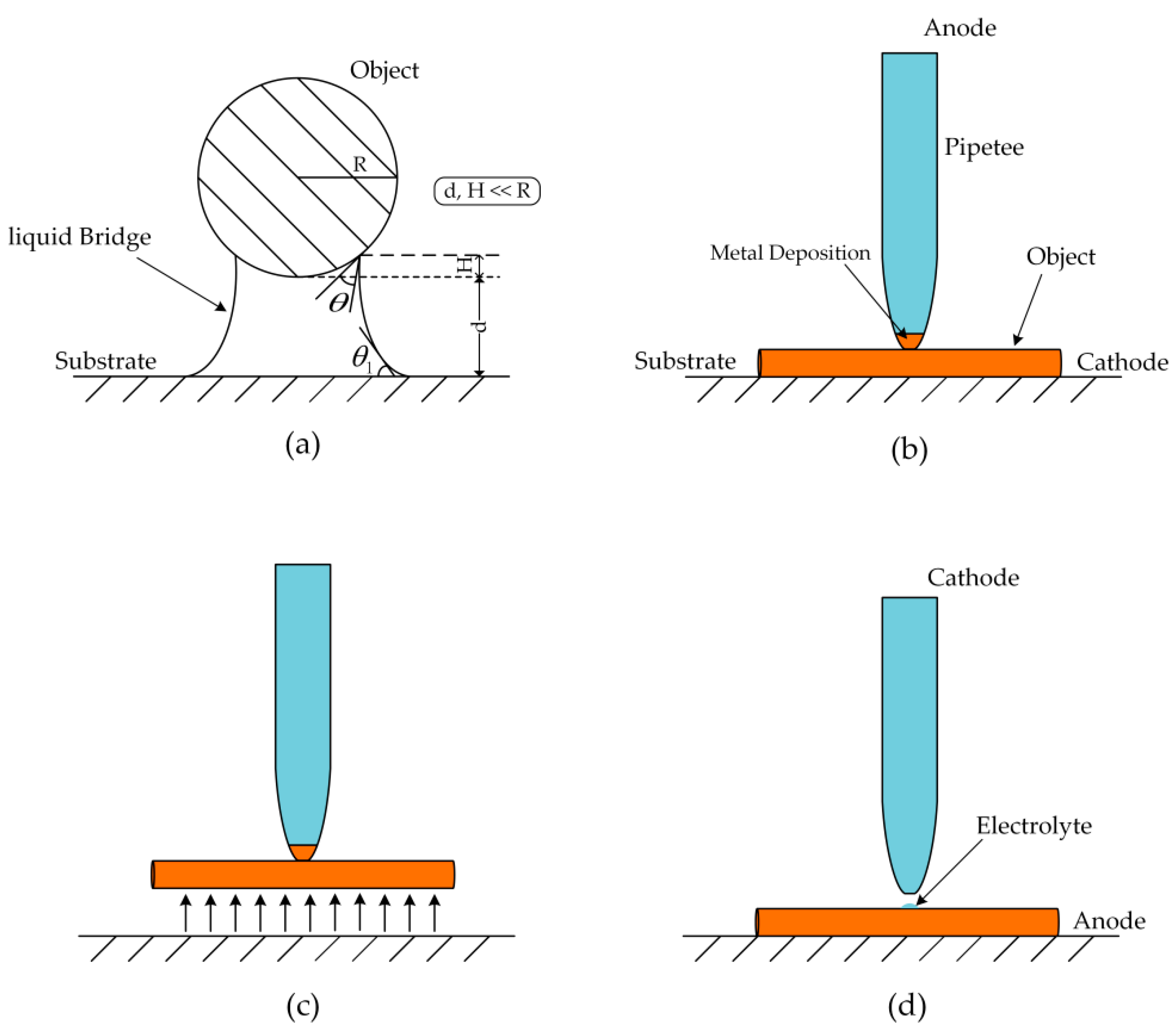
2.1. Basic Principle and Experimental Device
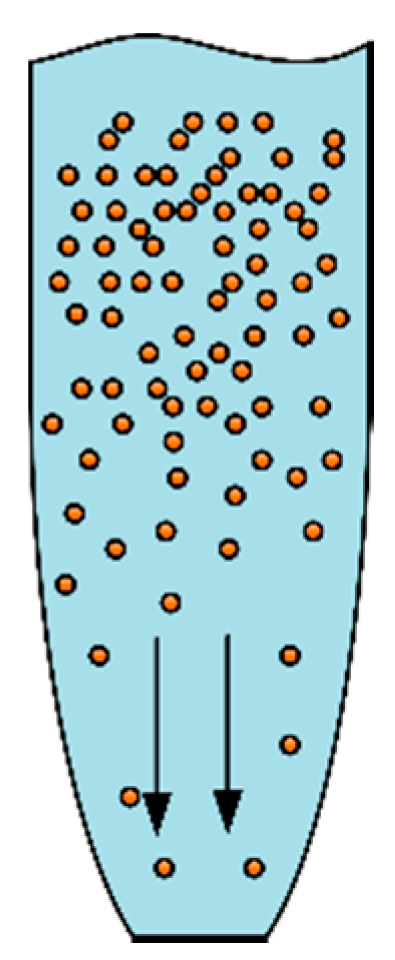
2.2. Effect of Ambient Relative Humidity on Pickup
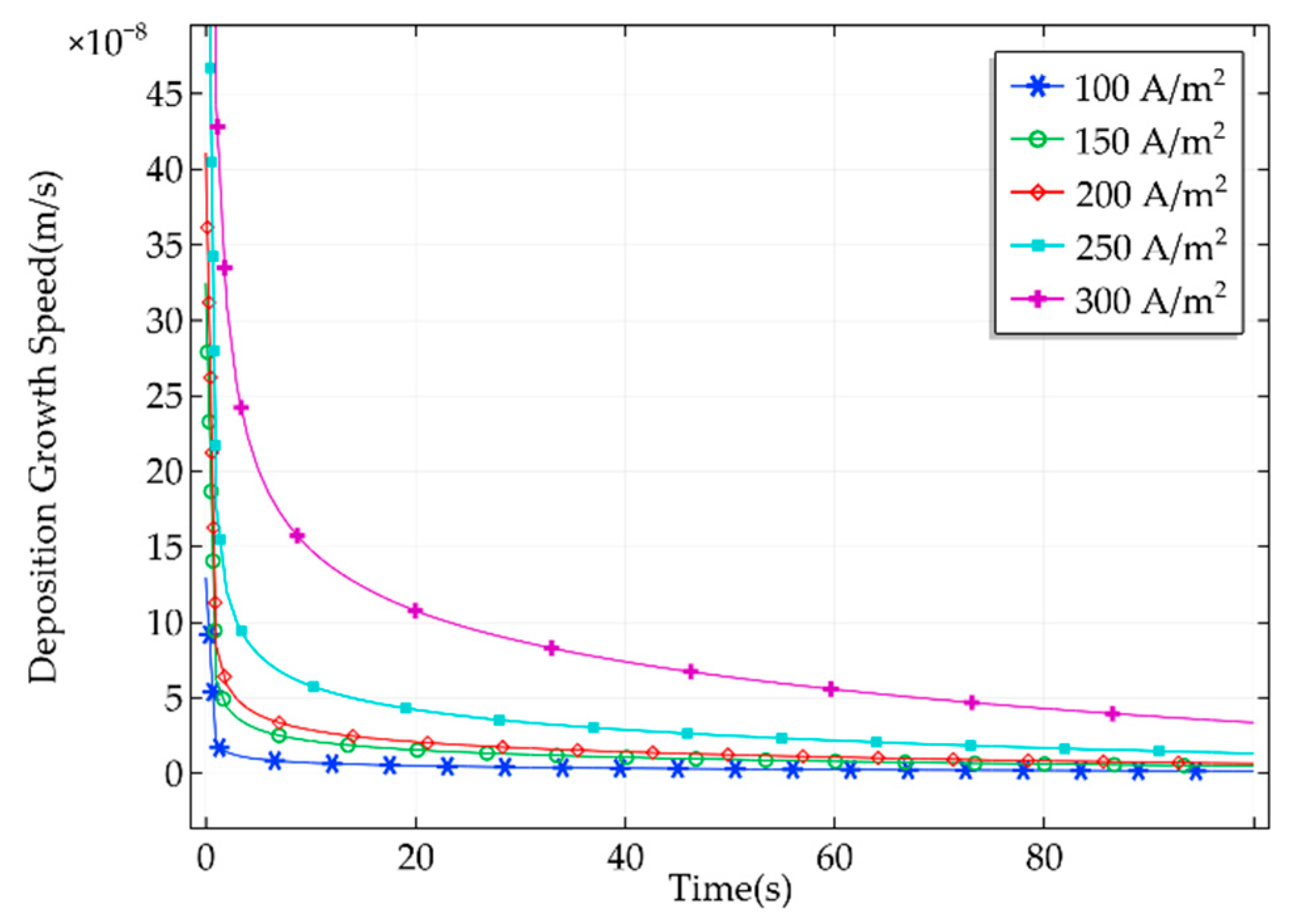
2.3. Effect of Current Density on Pickup
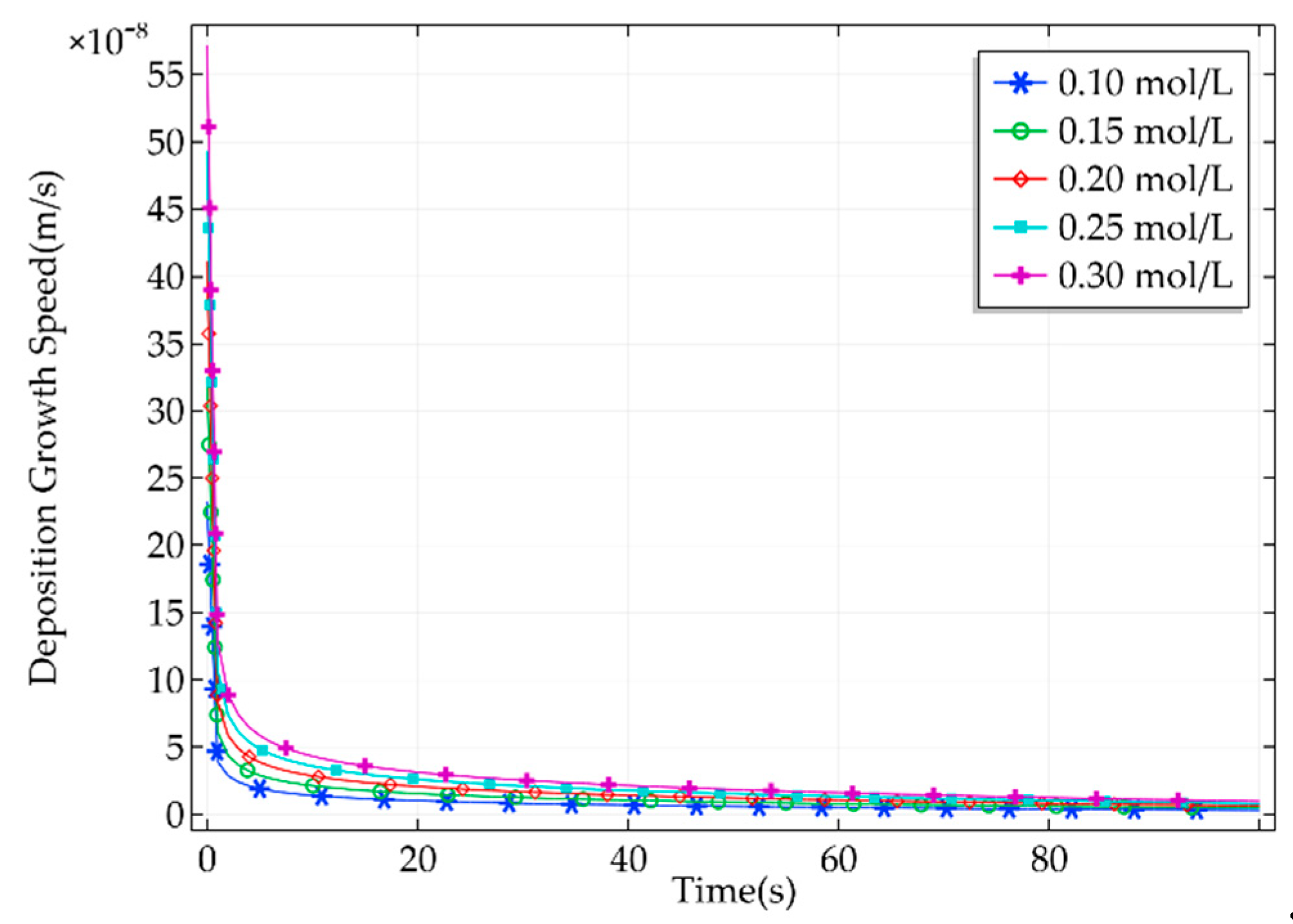
2.4. Effect of Electrolyte Concentration on Pickup
2.5. Effect of Force on Pickup
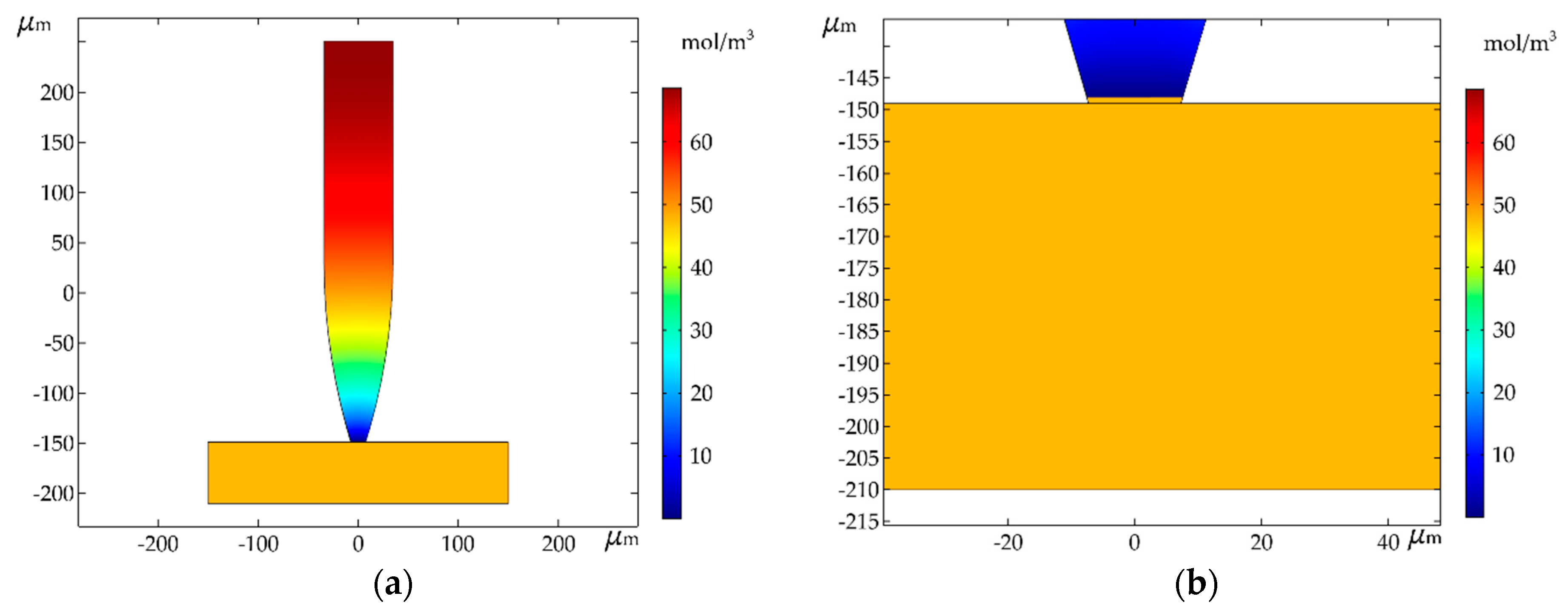
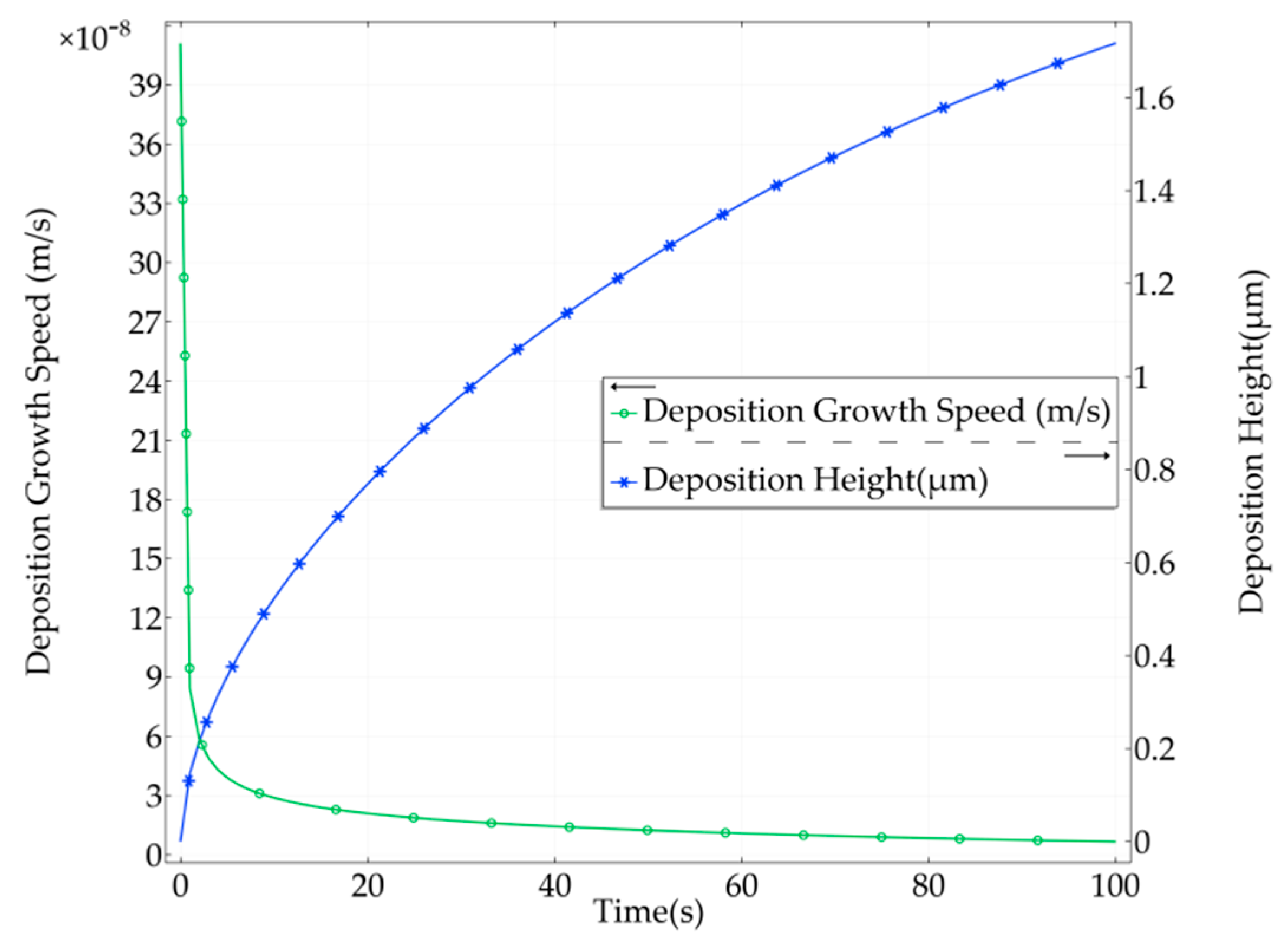
3. Simulation and Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dsouza, R.D.; Navin, K.P.; Theodoridis, T.; Sharma, P. Design, fabrication and testing of a 2 DOF compliant flexural microgripper. Microsyst. Technol. 2018, 24, 3867–3883. [Google Scholar] [CrossRef]
- Wu, L.; Dong, Z.C.; Li, F.Y.; Song, Y.L. Designing Laplace Pressure Pattern for micro-Droplet Manipulation. Langmuir 2018, 34, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Bharanidaran, R.; Ramesh, T. A modified post-processing technique to design a compliant based microgripper with a plunger using topological optimization. Int. J. Adv. Manuf. Technol. 2017, 93, 103–112. [Google Scholar] [CrossRef]
- Lofrotn, M.; Avci, E. Development of a Novel Modular Compliant Gripper for Manipulation of Micro Objects. Micromachines 2019, 10, 313. [Google Scholar] [CrossRef] [PubMed]
- Agnus, J.; Chaillet, N.; Clévy, C.; Dembélé, S.; Gauther, M.; Haddab, Y.; Laurent, G.; Lutz, P.; Piat, N.; Rabenorosoa, K.; et al. Robotic microassembly and micromanipulation at FEMTO-ST. J. Micro-Bio Robot. 2013, 8, 91–106. [Google Scholar] [CrossRef]
- Santochi, M.; Fantoni, G.; Fassi, I. Assembly of Microproducts: State of the Art and New Solutions. In AMST’05 Advanced Manufacturing Systems and Technology; Kuljanic, E., Ed.; Springer: Vienna, Austria, 2005; Volume 486, pp. 99–115. [Google Scholar]
- Almeida, A.; Andrews, G.; Jaiswal, D.; Hoshino, K. The Actuation Mechanism of 3D Printed Flexure-Based Robotic Microtweezers. Micromachines 2019, 10, 470. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.B.; Zhu, J.X. Design of a Monolithic Double-Slider Based Compliant Gripper with Large Displacement and Anti-Buckling Ability. Micromachines 2019, 10, 665. [Google Scholar] [CrossRef] [PubMed]
- Power, M.; Thompson, A.J.; Anastasova, S.; Yang, G.Z. A Monolithic Force-Sensitive 3D Microgripper Fabricated on the Tip of an Optical Fiber Using 2-Photon Polymerization. Small 2018, 14, 1703964. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.G.; Chen, T.; Sun, L.N.; Rong, W.B.; Shao, B.; Yang, Q. Active control of adhesion force for pick-and-place of micro objects with compound vibration in micromanipulation. In Proceedings of the 2010 IEEE International Conference on Automation Science and Engineering, Toronto, ON, Canada, 21–24 August 2010. [Google Scholar]
- Xu, Q.S. Robust Impedance Control of a Compliant Microgripper for High-Speed Position/Force Regulation. IEEE Trans. Ind. Electron. 2015, 62, 1201–1209. [Google Scholar] [CrossRef]
- Design News. Available online: https://www.designnews.com/content/ice-gripper-handles-micro-sized-components/103664446826851 (accessed on 26 December 2019).
- Gursky, B.; Butefisch, S.; Leester-Schadel, M.; Li, K.Q.; Matheis, B.; Dietzel, A. A Disposable Pneumatic Microgripper for Cell Manipulation with Image-Based Force Sensing. Micromachines 2019, 10, 707. [Google Scholar] [CrossRef] [PubMed]
- Cecchi, R.; Verotti, M.; Capata, R.; Dochshanov, A.; Broggiato, G.B.; Crescenzi, R.; Balucani, M.; Natali, S.; Razzano, G.; Lucchese, F.; et al. Development of Micro-Grippers for Tissue and Cell Manipulation with Direct Morphological Comparison. Micromachines 2015, 6, 1710–1728. [Google Scholar] [CrossRef]
- Alogla, A.F.; Amalou, F.; Balmer, C.; Scanlan, P.; Shu, W.; Reuben, R.L. Micro-tweezers: Design, fabrication, simulation and testing of a pneumatically actuated micro-gripper for micromanipulation and microtactile sensing. Sens. Actuator A Phys. 2015, 236, 394–404. [Google Scholar] [CrossRef]
- Kim, S.P.; Choe, H.C. Functional Elements Coatings on the Plasma Electrolytic Oxidation-Treated Ti-6AL-4V Alloy by Electrochemical Precipitation Method. J. Nanosci. Nanotechnol. 2019, 19, 4344–4349. [Google Scholar] [CrossRef] [PubMed]
- Sato, K. Quartz formation in natural environment with respect to diffusion-reaction of water molecules in nano-scale open spaces. Int. J. Environ. Eng. 2014, 6, 324–332. [Google Scholar] [CrossRef]
- Paran, Y.; Bendel, P.; Margalit, R.; Degani, H. Water diffusion in the different microenvironments of breast cancer. NMR Biomed. 2004, 17, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.Q.; Deng, G.H.; Lapp, J.; Rao, Y. Interfaces of Gas-Aerosol Particles: Relative Humidity and Salt Concentration Effects. J. Phys. Chem. A 2019, 123, 6304–6312. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.T.; Jiang, L.; Liu, J.W.; Deng, C.B.; Qian, L.M. Preliminary investigation of the effect of environmental humidity on the nanoscale mechanical removal of KDP crystal. Micro Nano Lett. 2019, 14, 538–543. [Google Scholar] [CrossRef]
- Murr, A.; Lackner, R. Analysis on the influence of grain size and grain layer thickness on the sorption kinetics of grained wood at low relative humidity with the use of water vapour sorption experiments. Wood Sci. Technol. 2018, 52, 753–776. [Google Scholar] [CrossRef]
- Ansaloni, L.; Mineli, M.; Baschetti, M.G.; Sarti, G.C. Effect of relative humidity and temperature on gas transport in Matrimie®: Experimental study and modeling. J. Membr. Sci. 2014, 471, 392–401. [Google Scholar] [CrossRef]
- Bisang, J.M.; Kreysa, G. Study of the effect of electrode resistance on current density distribution in cylindrical electrochemical reactors. J. Appl. Electrochem. 1988, 18, 422–430. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Xu, J.; Rong, W.; Yang, L. Simulation of Picking Up Metal Microcomponents Based on Electrochemistry. Micromachines 2020, 11, 33. https://doi.org/10.3390/mi11010033
Li D, Xu J, Rong W, Yang L. Simulation of Picking Up Metal Microcomponents Based on Electrochemistry. Micromachines. 2020; 11(1):33. https://doi.org/10.3390/mi11010033
Chicago/Turabian StyleLi, Dongjie, Jiyong Xu, Weibin Rong, and Liu Yang. 2020. "Simulation of Picking Up Metal Microcomponents Based on Electrochemistry" Micromachines 11, no. 1: 33. https://doi.org/10.3390/mi11010033
APA StyleLi, D., Xu, J., Rong, W., & Yang, L. (2020). Simulation of Picking Up Metal Microcomponents Based on Electrochemistry. Micromachines, 11(1), 33. https://doi.org/10.3390/mi11010033



