A Micromachined Metal Oxide Composite Dual Gas Sensor System for Principal Component Analysis-Based Multi-Monitoring of Noxious Gas Mixtures
Abstract
1. Introduction
2. Materials and Methods
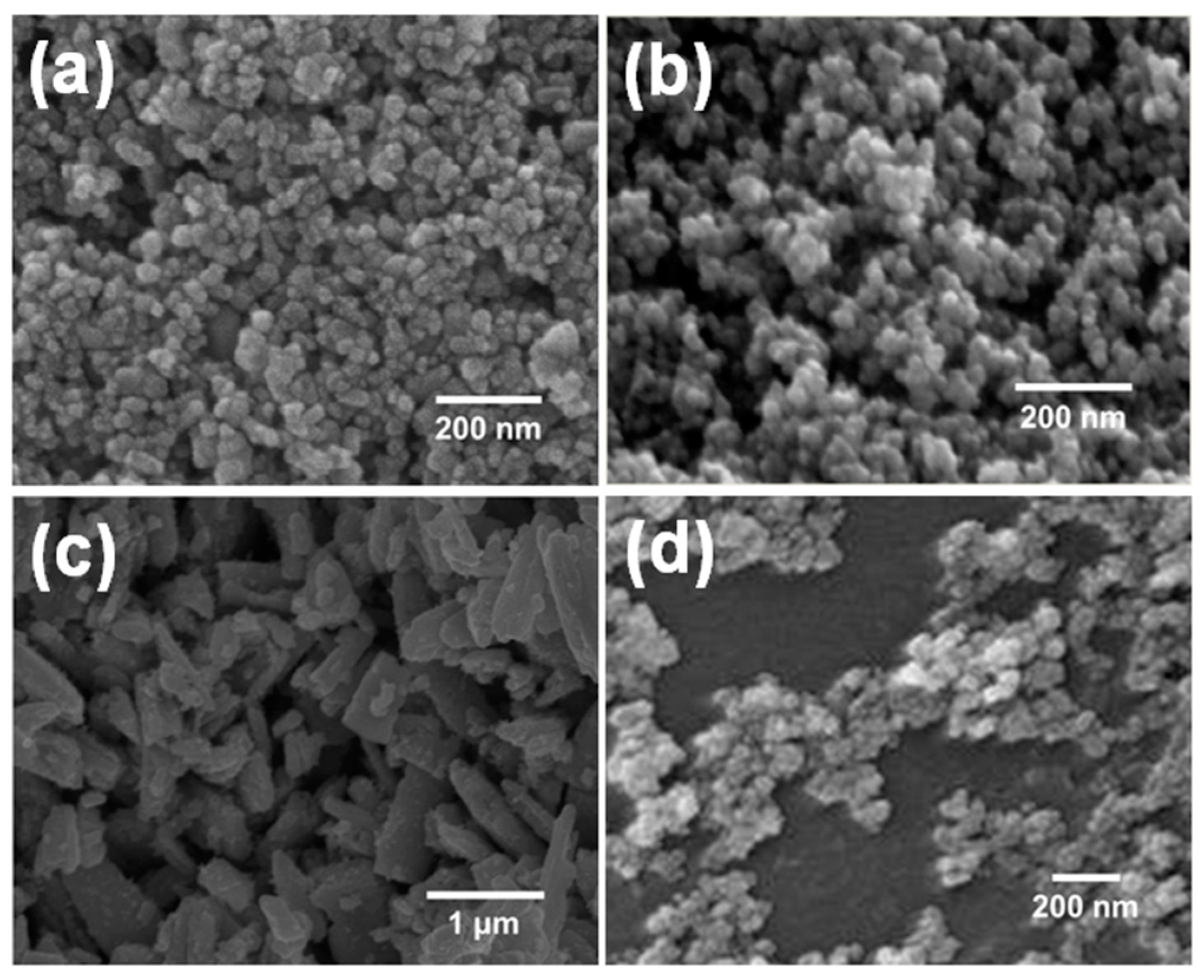
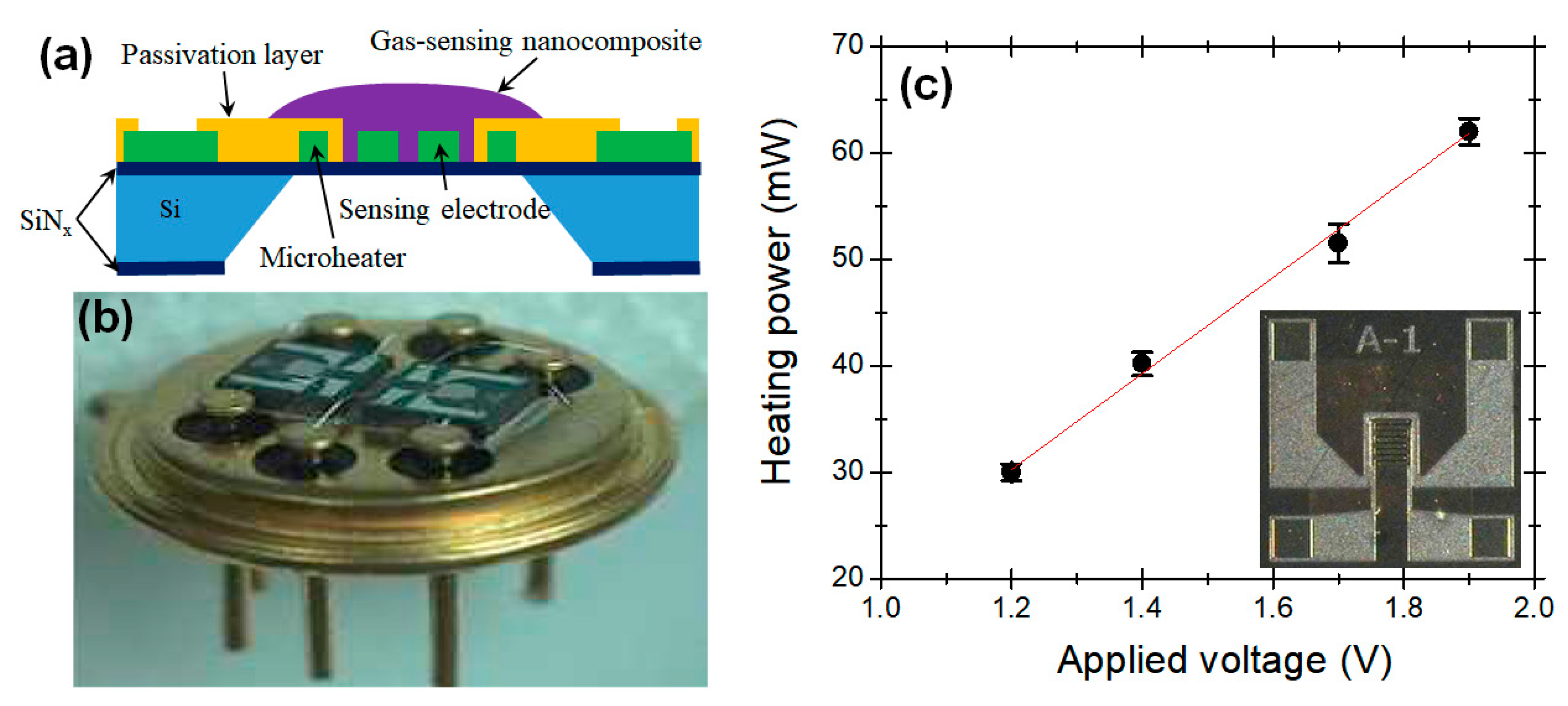
2.1. Fabrication of Metal Oxide Semiconductor (MOS) Sensor Modules
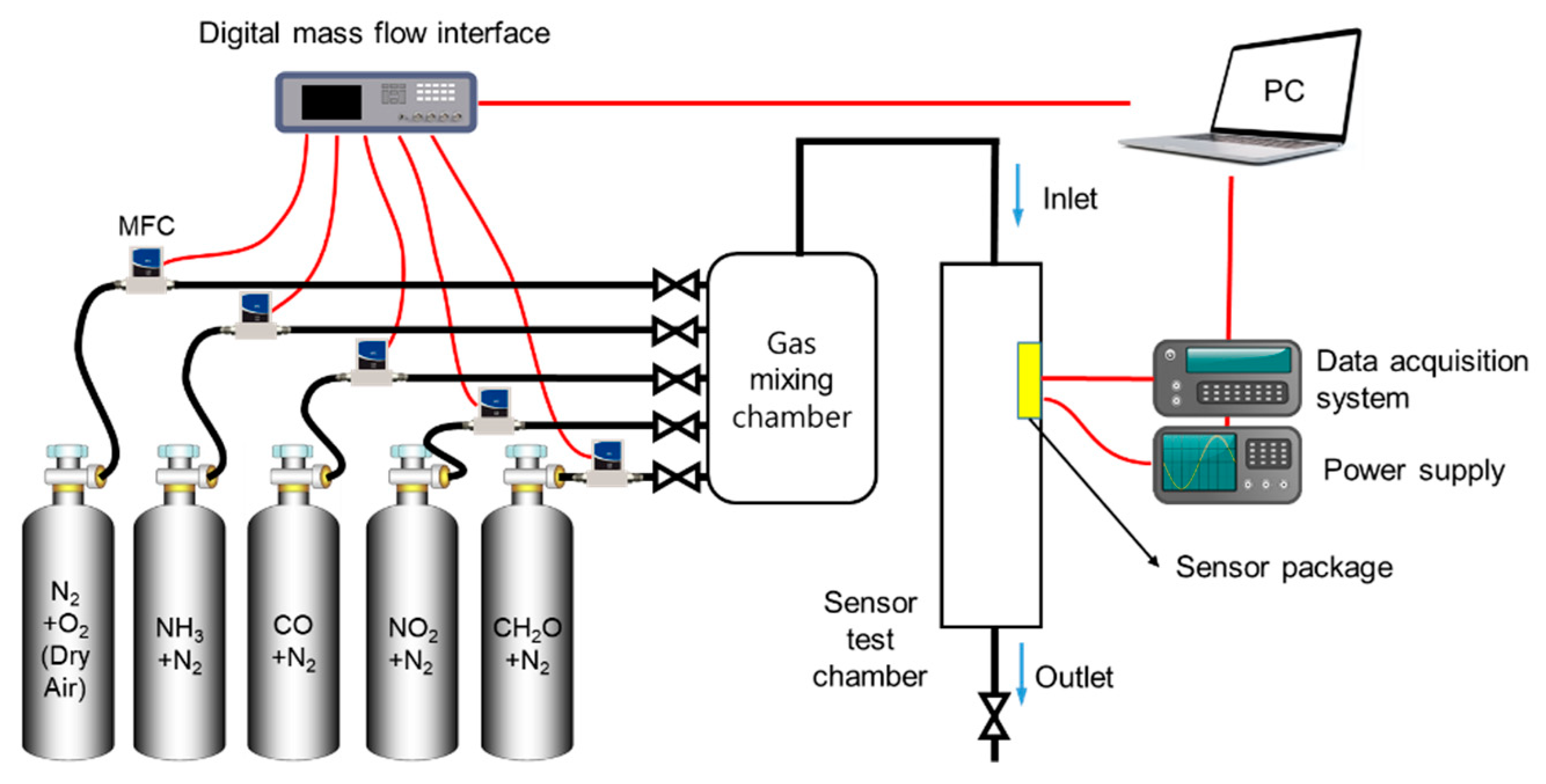
2.2. Measurements
3. Results
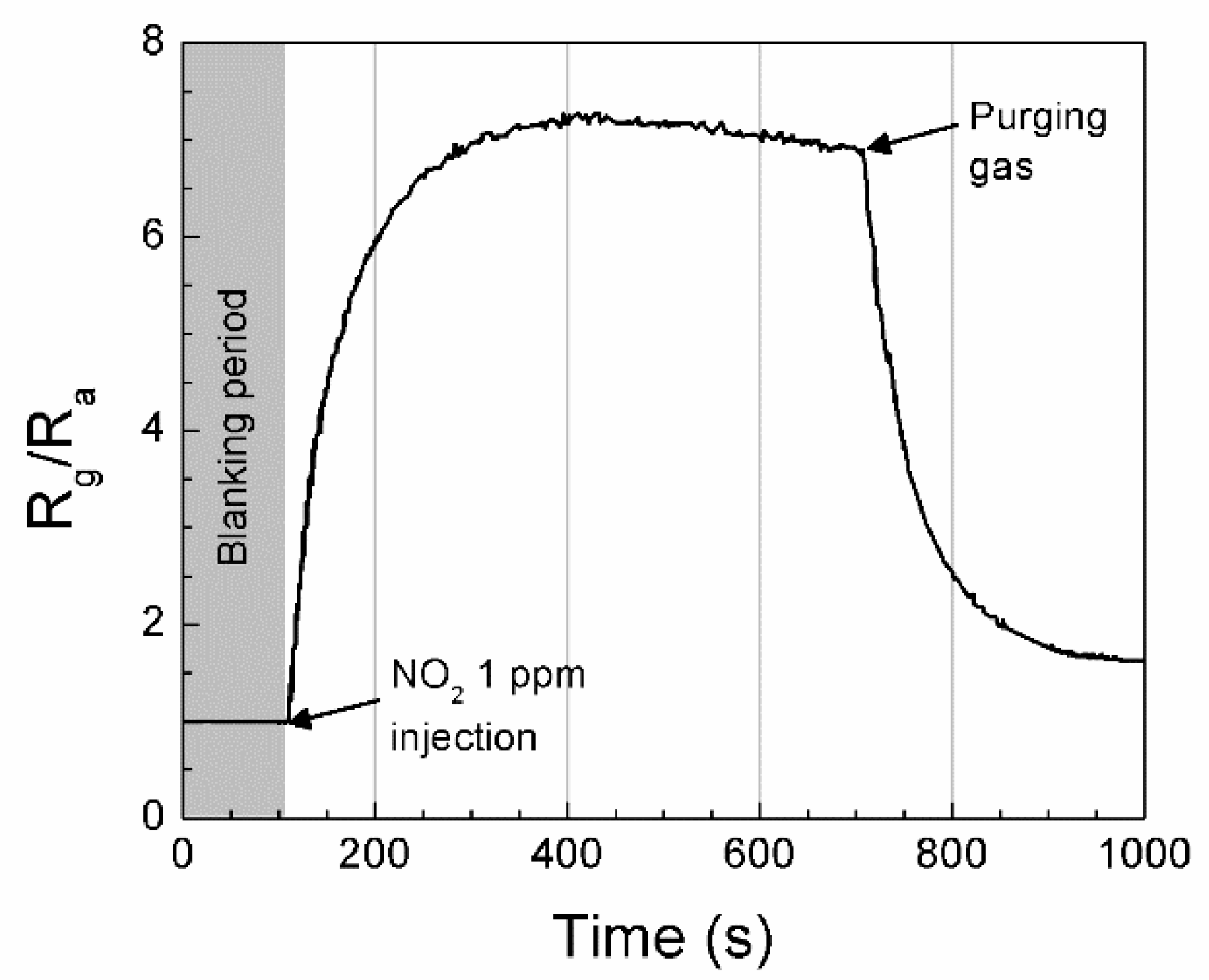
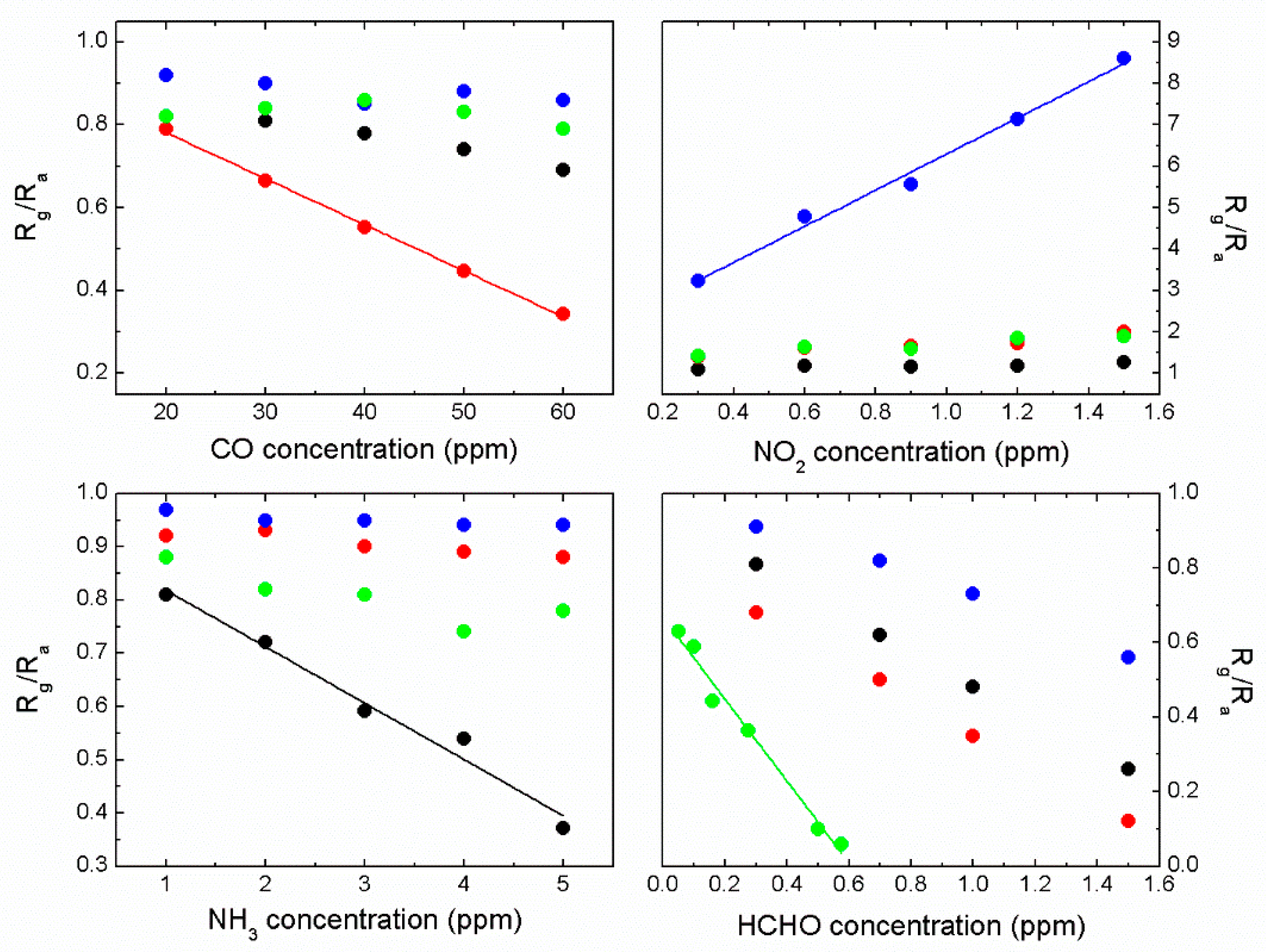
3.1. Relative Sensitivity of Single Sensor Module
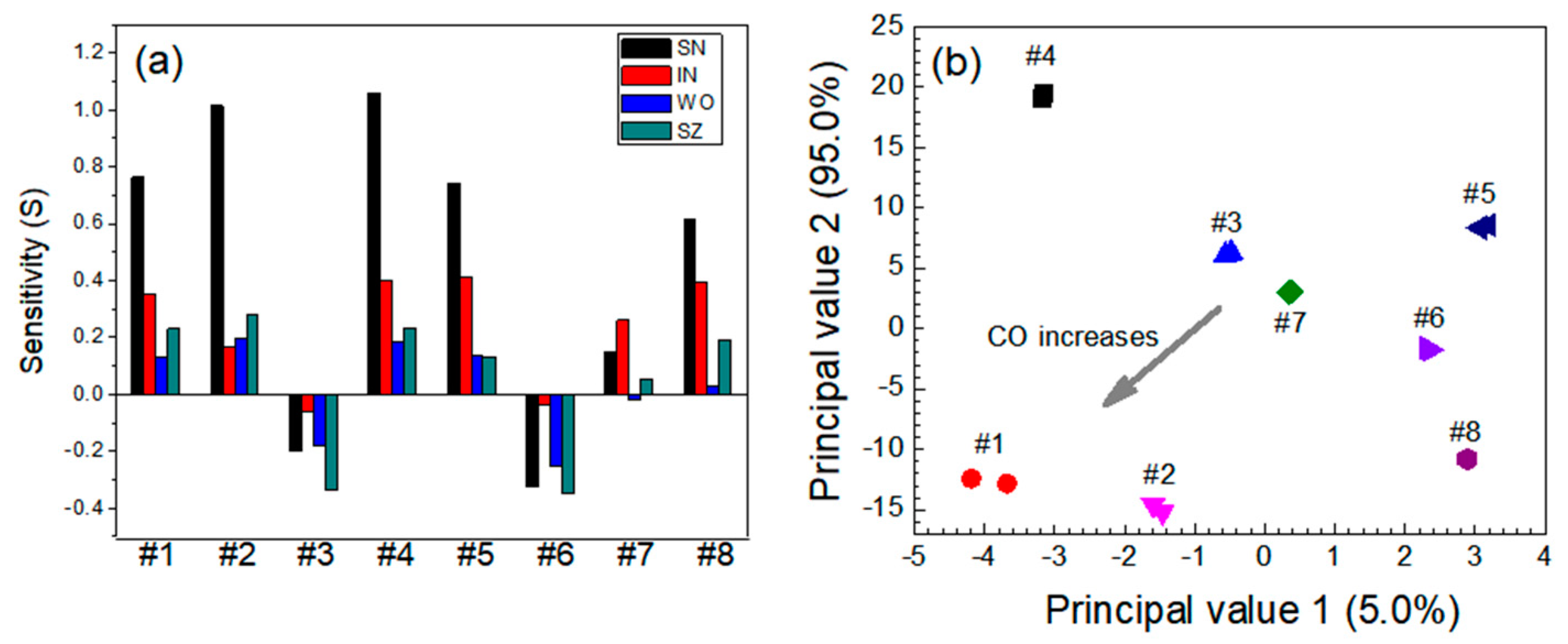
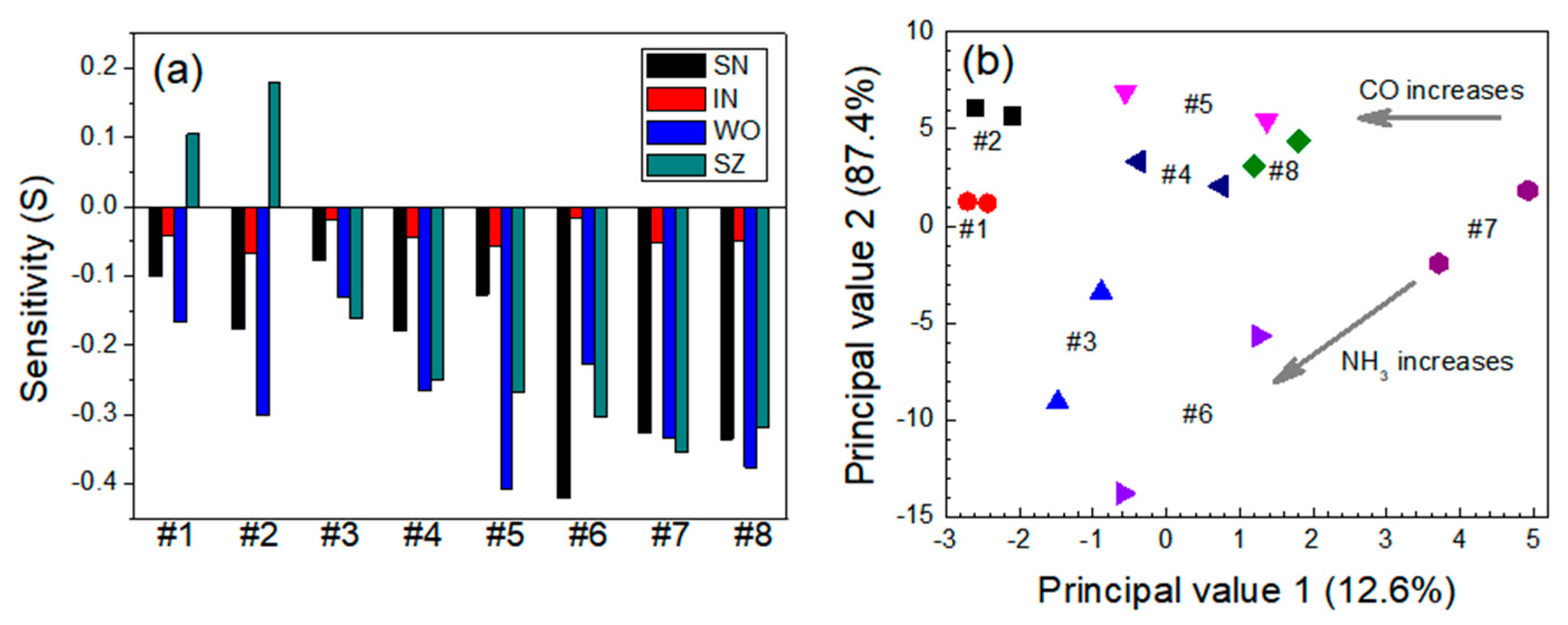
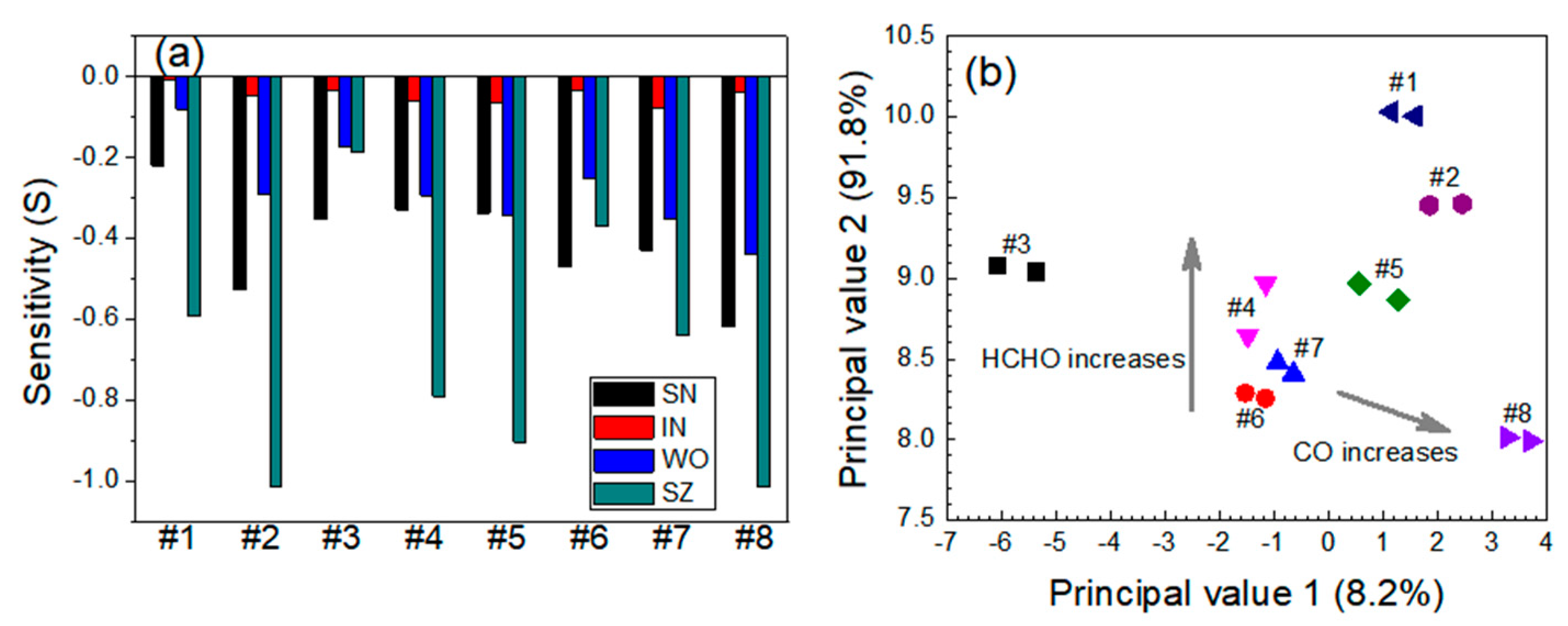
3.2. Quantitative Approach to Investigate Response Characteristics of Dual-Sensor Modules
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Barsan, N.; Koziej, D.; Weimar, U. Metal oxide-based gas sensor research: How to? Sens. Actuators B Chem. 2007, 121, 18–35. [Google Scholar] [CrossRef]
- Morrison, S.R. Semiconductor gas sensors. Sens. Actuators 1982, 2, 329–341. [Google Scholar] [CrossRef]
- Schierbaum, K.D.; Weimar, U.; Gopel, W. Comparison of ceramic, thick-film and thin-film chemical sensors based upon SnO2. Sens. Actuators B Chem. 1992, 7, 709–716. [Google Scholar] [CrossRef]
- Giuntini, J.C.; Granier, W.; Zanchetta, J.V.; Taha, A. Sol-Gel preparation and transport-properties of a tin oxide. J. Mater. Sci. Lett. 1990, 9, 1383–1388. [Google Scholar] [CrossRef]
- Liu, H.T.; Zhang, L.; Li, K.H.H.; Tan, O.K. Microhotplates for metal oxide semiconductor gas sensor applicationstowards the CMOS-MEMS monolithic approach. Micromachines 2018, 9, 557. [Google Scholar] [CrossRef]
- Korotcenkov, G. Gas response control through structural and chemical modification of metal oxide films: State of the art and approaches. Sens. Actuators B Chem. 2005, 107, 209–232. [Google Scholar] [CrossRef]
- Kiselev, I.; Sommer, M.; Mann, J.K.; Sysoev, V.V. Employment of electric potential to build a gas-selective response of metal oxide gas sensor array. IEEE Sens. J. 2010, 10, 849–855. [Google Scholar] [CrossRef]
- Barsan, N.; Weimar, U. Understanding the fundamental principles of metal oxide based gas sensors; the example of CO sensing with SnO2 sensors in the presence of humidity. J. Phys. Condens. Matter 2003, 15, R813. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B Adv. 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Wang, C.X.; Yin, L.W.; Zhang, L.Y.; Xiang, D.; Gao, R. Metal oxide gas sensors: Sensitivity and influencing factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef]
- Burgues, J.; Marco, S. Multivariate estimation of the limit of detection by orthogonal partial least squares in temperature-modulated MOX sensors. Anal. Chim. Acta 2018, 1019, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.P.; Reedy, B.J. Temperature modulation in semiconductor gas sensing. Sens. Actuators B Chem. 1999, 60, 35–42. [Google Scholar] [CrossRef]
- Arnold, C.; Harms, M.; Goschnick, J. Air quality monitoring and fire detection with the karlsruhe electronic micronose KAMINA. IEEE Sens. J. 2002, 2, 179–188. [Google Scholar] [CrossRef]
- Althainz, P.; Goschnick, J.; Ehrmann, S.; Ache, H.J. Multisensor microsystem for contaminants in air. Sens. Actuators B Chem. 1996, 33, 72–76. [Google Scholar] [CrossRef]
- Ivanov, P.; Llobet, E.; Vilanova, X.; Brezmes, J.; Hubalek, J.; Correig, X. Development of high sensitivity ethanol gas sensors based on Pt-doped SnO2 surfaces. Sens. Actuators B Chem. 2004, 99, 201–206. [Google Scholar] [CrossRef]
- Kim, B.J.; Lee, H.J.; Yoon, J.H.; Kim, J.S. Highly sensitive formaldehyde gas sensors based on SnO2-ZnO nanocomposites. Sens. Lett. 2012, 10, 1–7. [Google Scholar] [CrossRef]
- Choi, W.S.; Kim, B.J.; Lee, H.J.; Choi, J.W.; Kim, S.D.; Min, N.K. Study on the micro-heater geometry in In2O3 micro electro mechanical systems gas sensor platforms and effects on NO2 gas detecting performances. J. Nanosci. Nanotechnol. 2012, 12, 1170–1173. [Google Scholar] [CrossRef]
- Kim, B.J.; Kim, J.S. Gas sensing characteristics of MEMS gas sensor arrays in binary mixed-gas system. Mater. Chem. Phys. 2013, 138, 366–374. [Google Scholar] [CrossRef]
- Kim, S.D.; Kim, B.J.; Yoon, J.H.; Kim, J.S. Design, fabrication and characterization of a low-power gas sensor with high sensitivity to CO gas. J. Korean Phys. Soc. 2007, 51, 2069–2076. [Google Scholar] [CrossRef]
- Lee, H.J.; Yoon, J.H.; Kim, B.J.; Jang, H.D.; Kim, J.S. Gas sensing characteristics of Ru doped-WO3 micro gas sensors. Korean J. Met. Mater. 2011, 49, 395–399. [Google Scholar] [CrossRef]
- Hammond, J.; Marquis, B.; Michaels, R.; Oickle, B.; Segee, B.; Vetelino, J.; Bushway, A.; Camire, M.E.; Davis-Dentici, K. A semiconducting metal-oxide array for monitoring fish freshness. Sens. Actuators B Chem. 2002, 84, 113–122. [Google Scholar] [CrossRef]
- Neri, G. First fifty years of chemoresistive gas sensors. Chemosensors 2015, 3, 1–20. [Google Scholar] [CrossRef]
- Yamazoe, N.; Shimanoe, K. Basic approach to the transducer function of oxide semiconductor gas sensors. Sens. Actuators B Chem. 2011, 160, 1352–1362. [Google Scholar] [CrossRef]
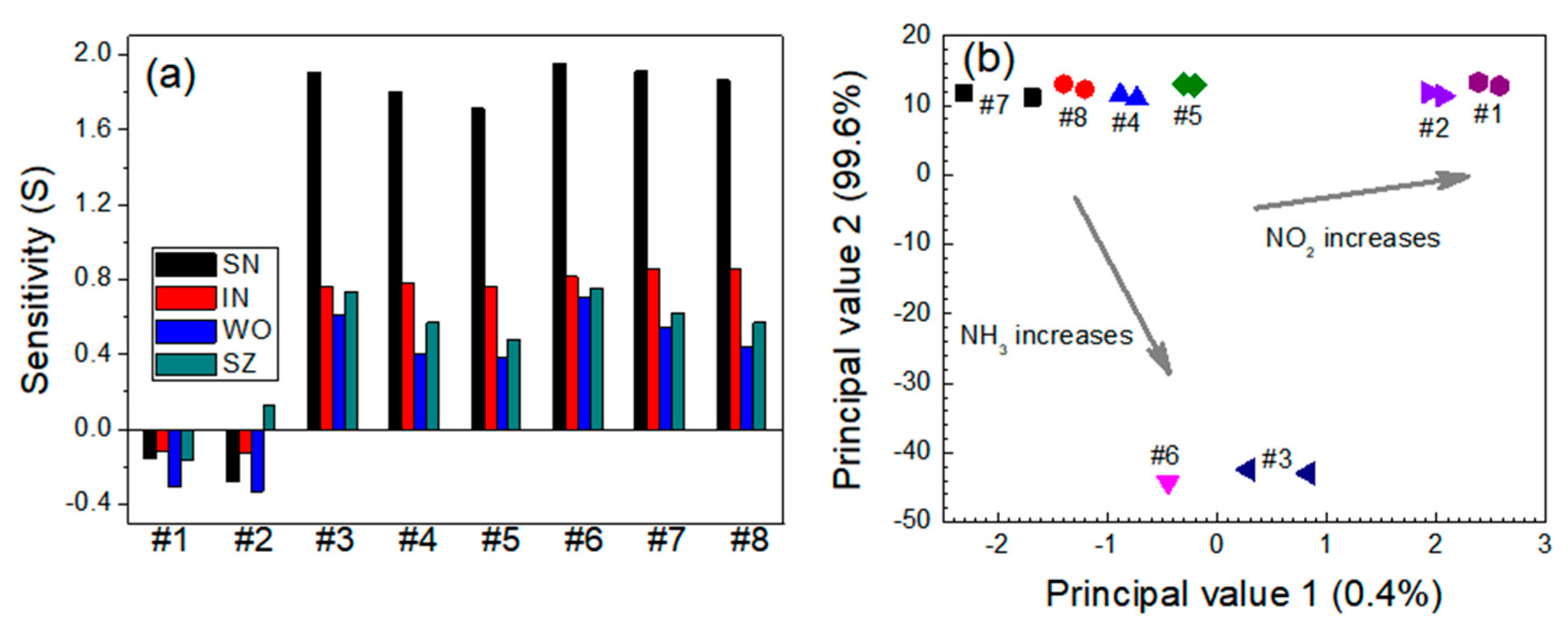
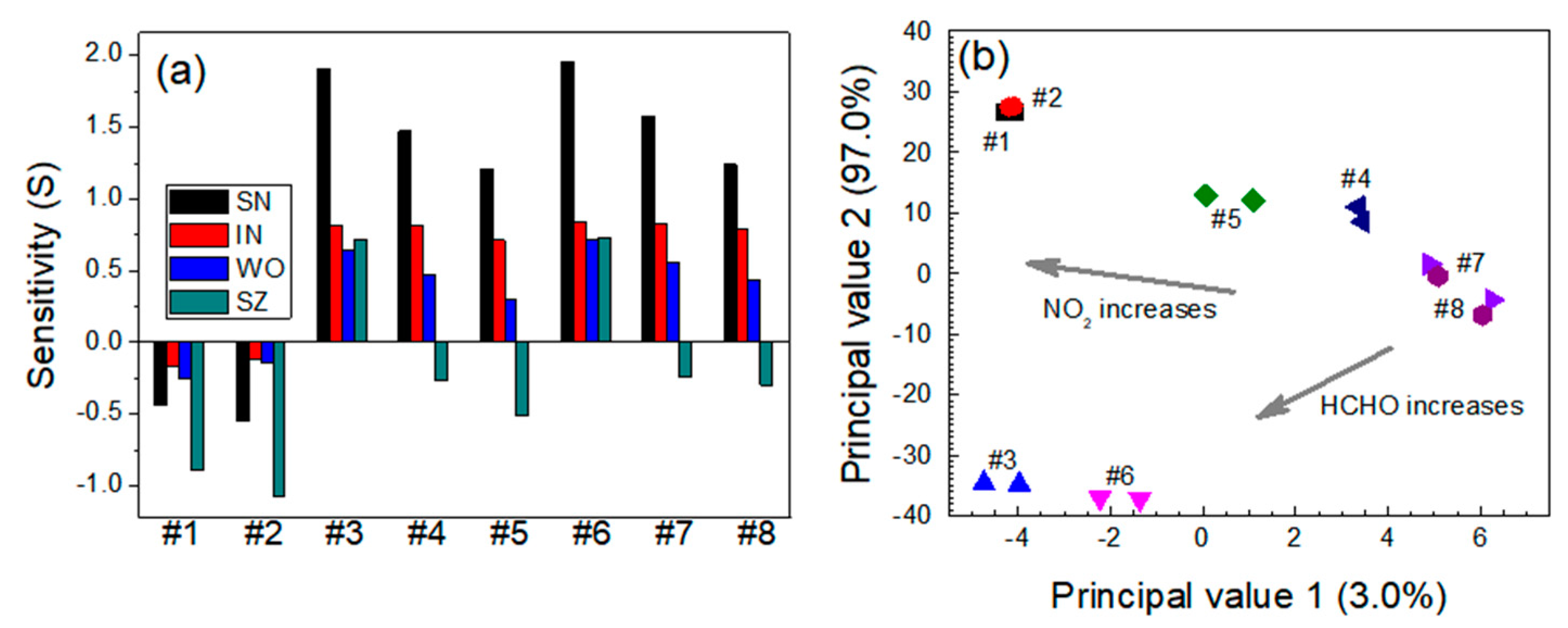
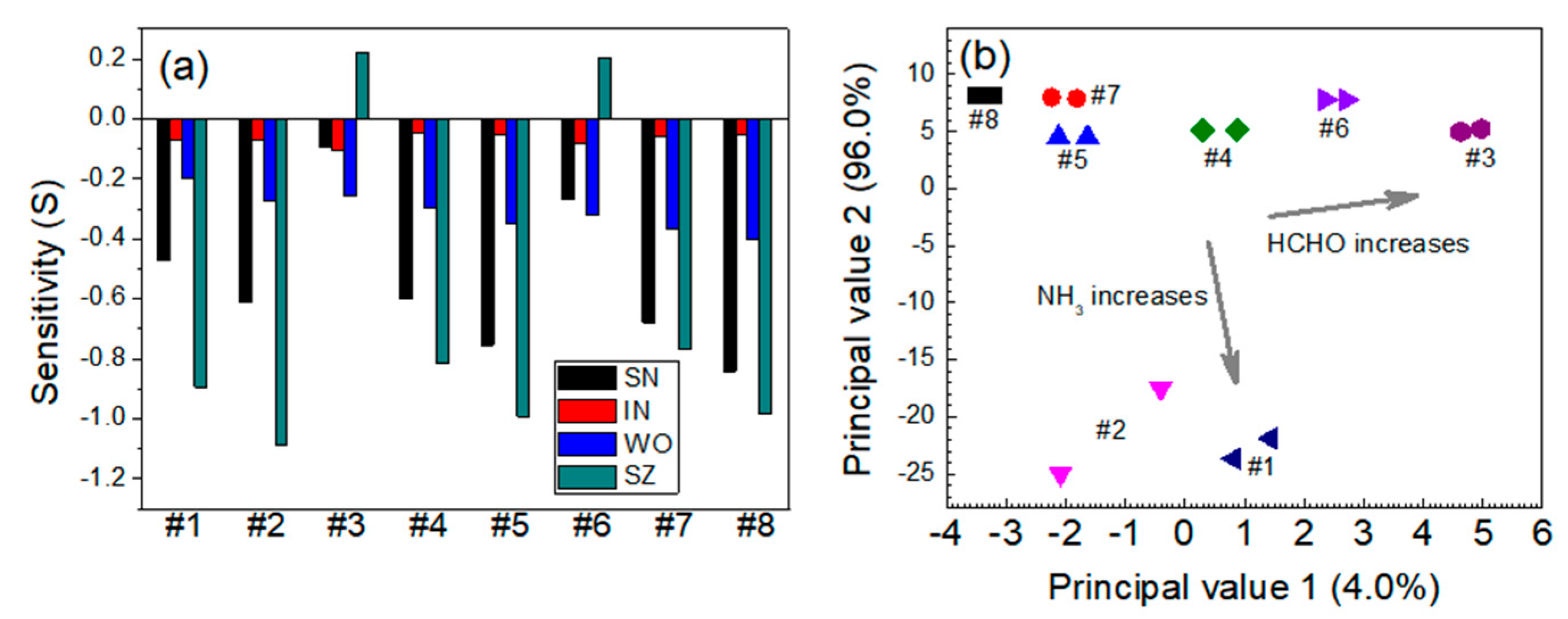
| Sensor | Gas Mixtures | |||||
|---|---|---|---|---|---|---|
| CO + NO2 | CO + NH3 | CO + HCHO | NO2 + NH3 | NO2 + HCHO | NH3 + HCHO | |
| SN | NO2 | CO | both | NO2 | NO2 | HCHO |
| IN | NO2 | None | none | NO2 | NO2 | none |
| WO | NO2 | CO | both | NO2 | NO2 | HCHO |
| SZ | NO2 | CO | HCHO | NO2 | HCHO | HCHO |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, I.-H.; Jin, J.-H.; Min, N.K. A Micromachined Metal Oxide Composite Dual Gas Sensor System for Principal Component Analysis-Based Multi-Monitoring of Noxious Gas Mixtures. Micromachines 2020, 11, 24. https://doi.org/10.3390/mi11010024
Yang I-H, Jin J-H, Min NK. A Micromachined Metal Oxide Composite Dual Gas Sensor System for Principal Component Analysis-Based Multi-Monitoring of Noxious Gas Mixtures. Micromachines. 2020; 11(1):24. https://doi.org/10.3390/mi11010024
Chicago/Turabian StyleYang, In-Hwan, Joon-Hyung Jin, and Nam Ki Min. 2020. "A Micromachined Metal Oxide Composite Dual Gas Sensor System for Principal Component Analysis-Based Multi-Monitoring of Noxious Gas Mixtures" Micromachines 11, no. 1: 24. https://doi.org/10.3390/mi11010024
APA StyleYang, I.-H., Jin, J.-H., & Min, N. K. (2020). A Micromachined Metal Oxide Composite Dual Gas Sensor System for Principal Component Analysis-Based Multi-Monitoring of Noxious Gas Mixtures. Micromachines, 11(1), 24. https://doi.org/10.3390/mi11010024





