Sorting of Particles Using Inertial Focusing and Laminar Vortex Technology: A Review
Abstract
1. Introduction
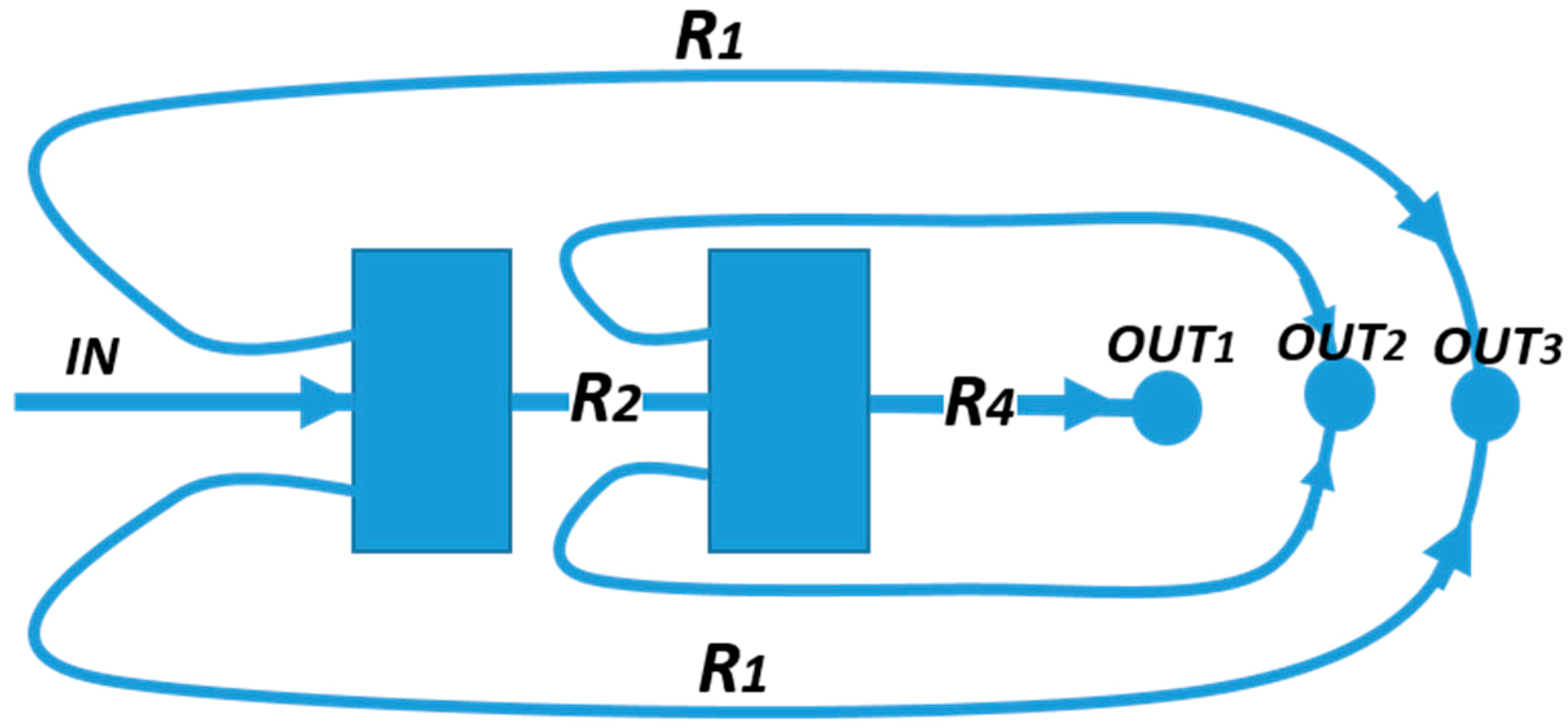
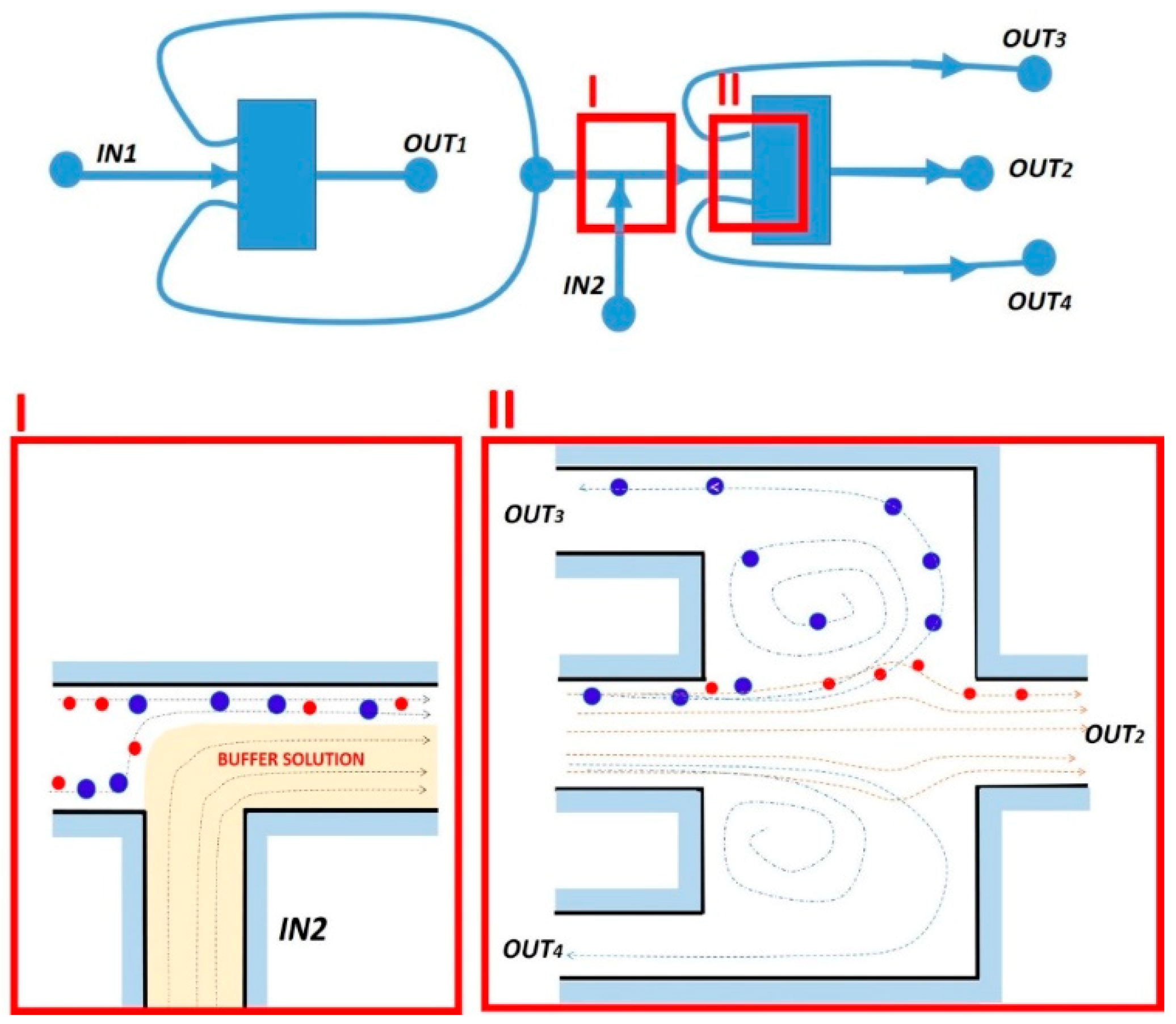
2. Inertial Focusing and Laminar Vortex Technology
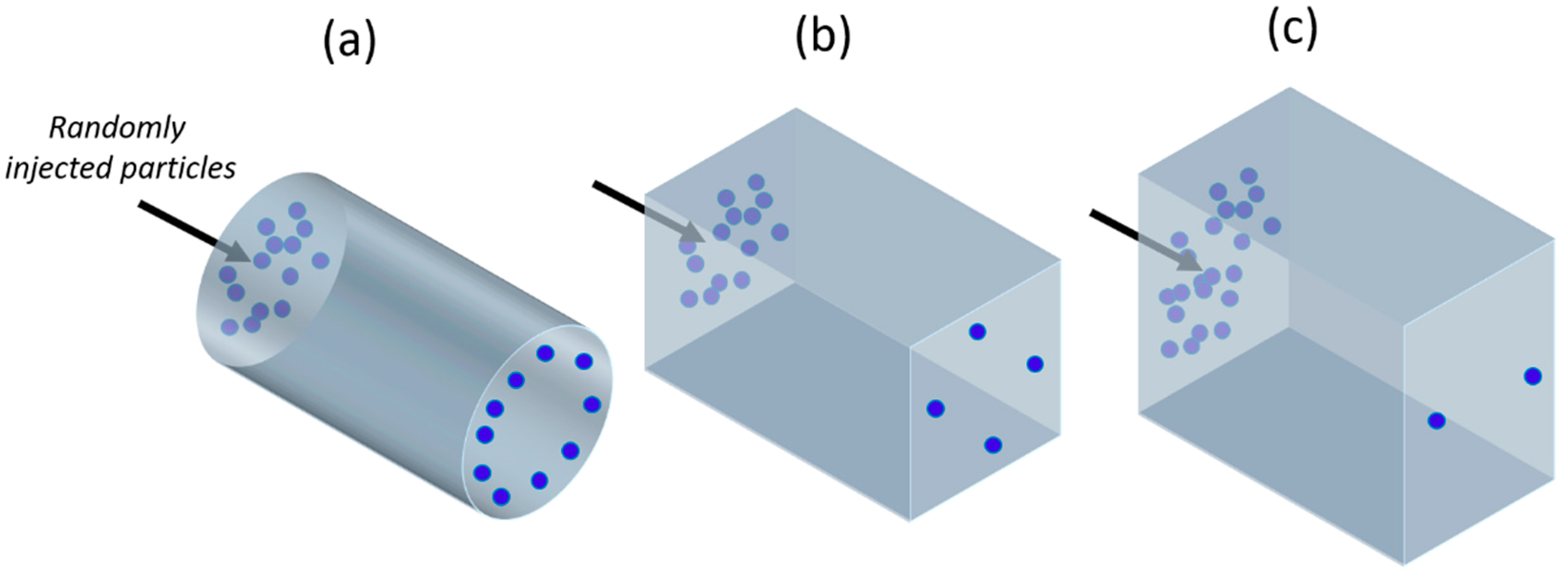
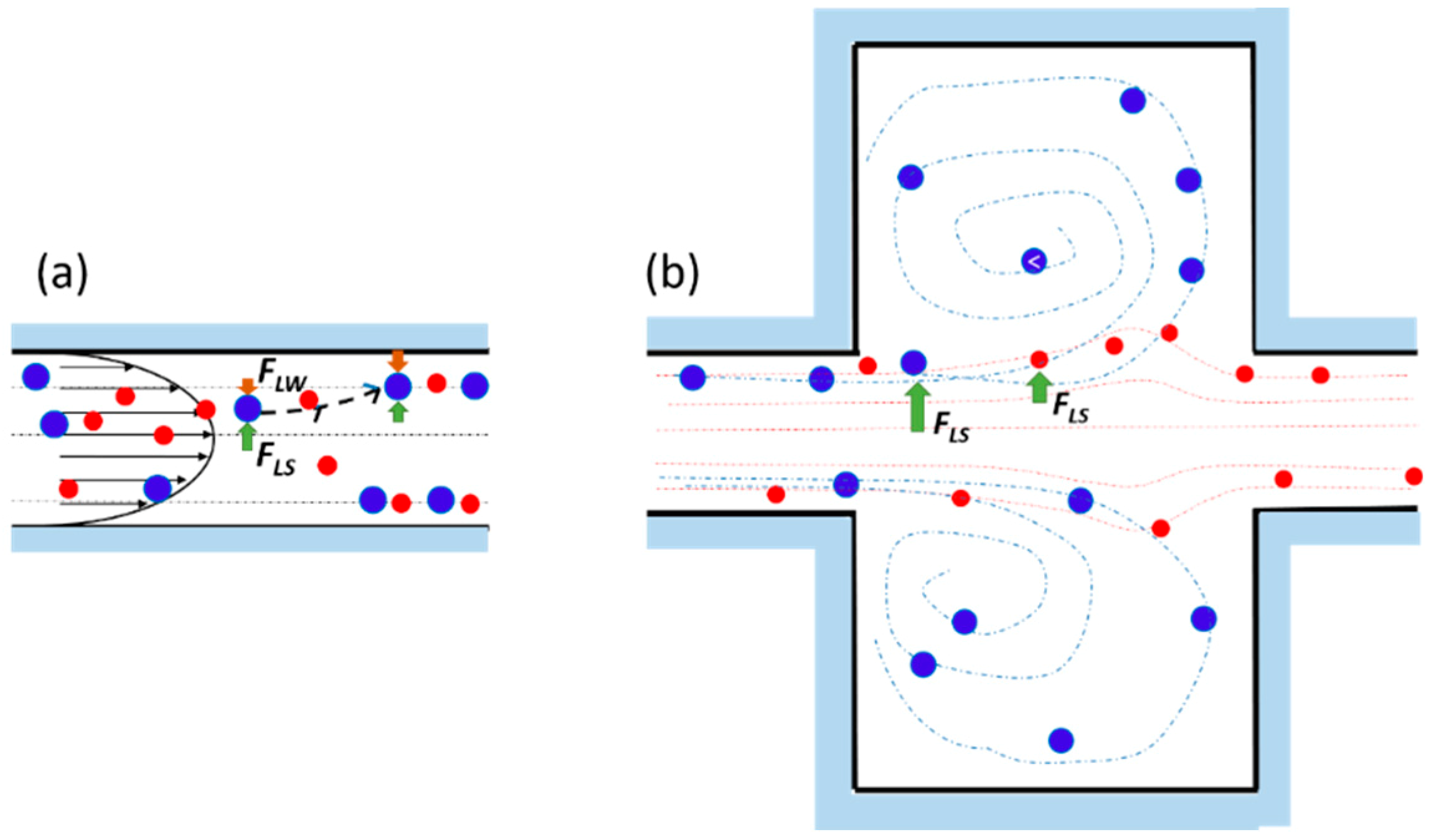
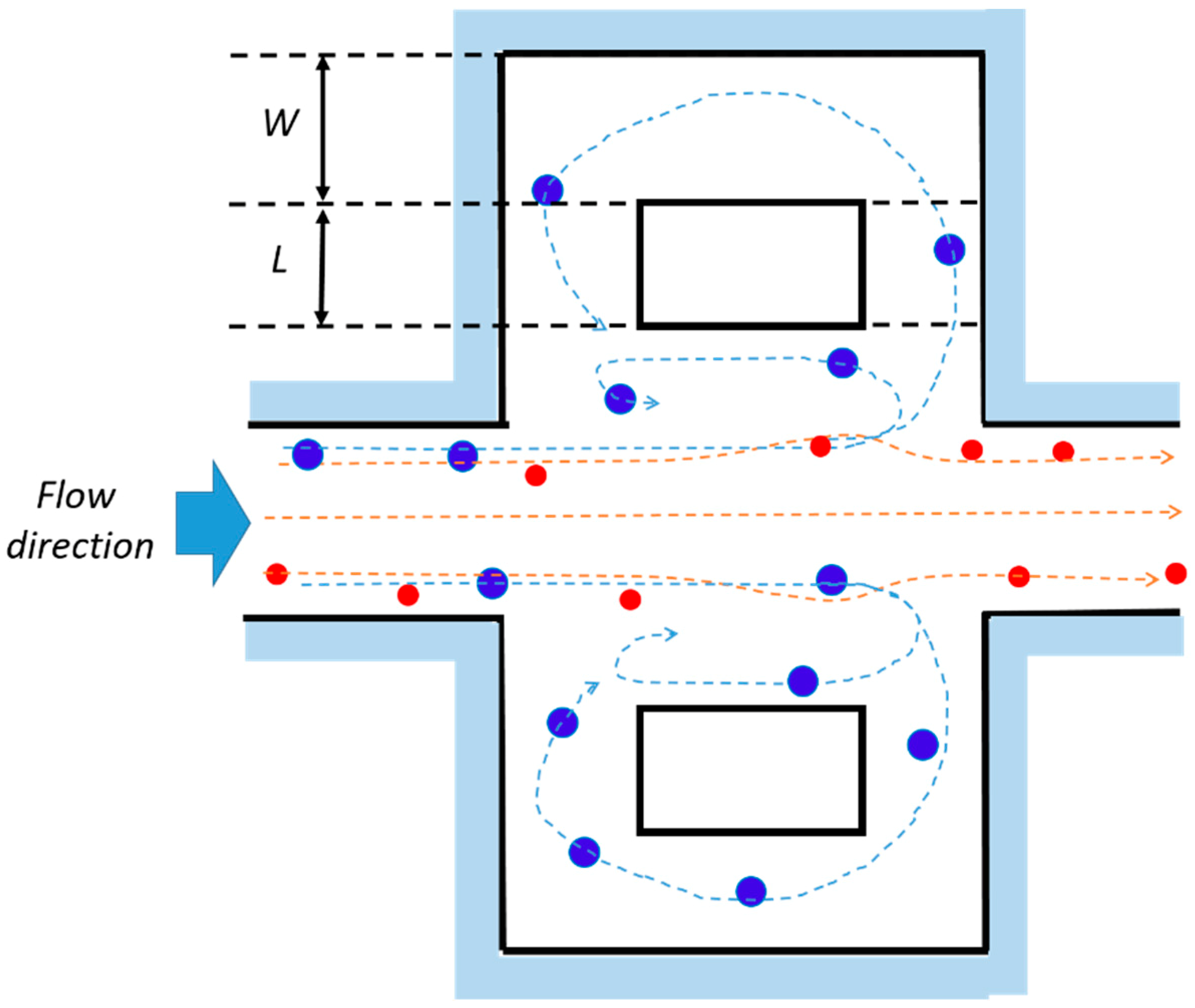
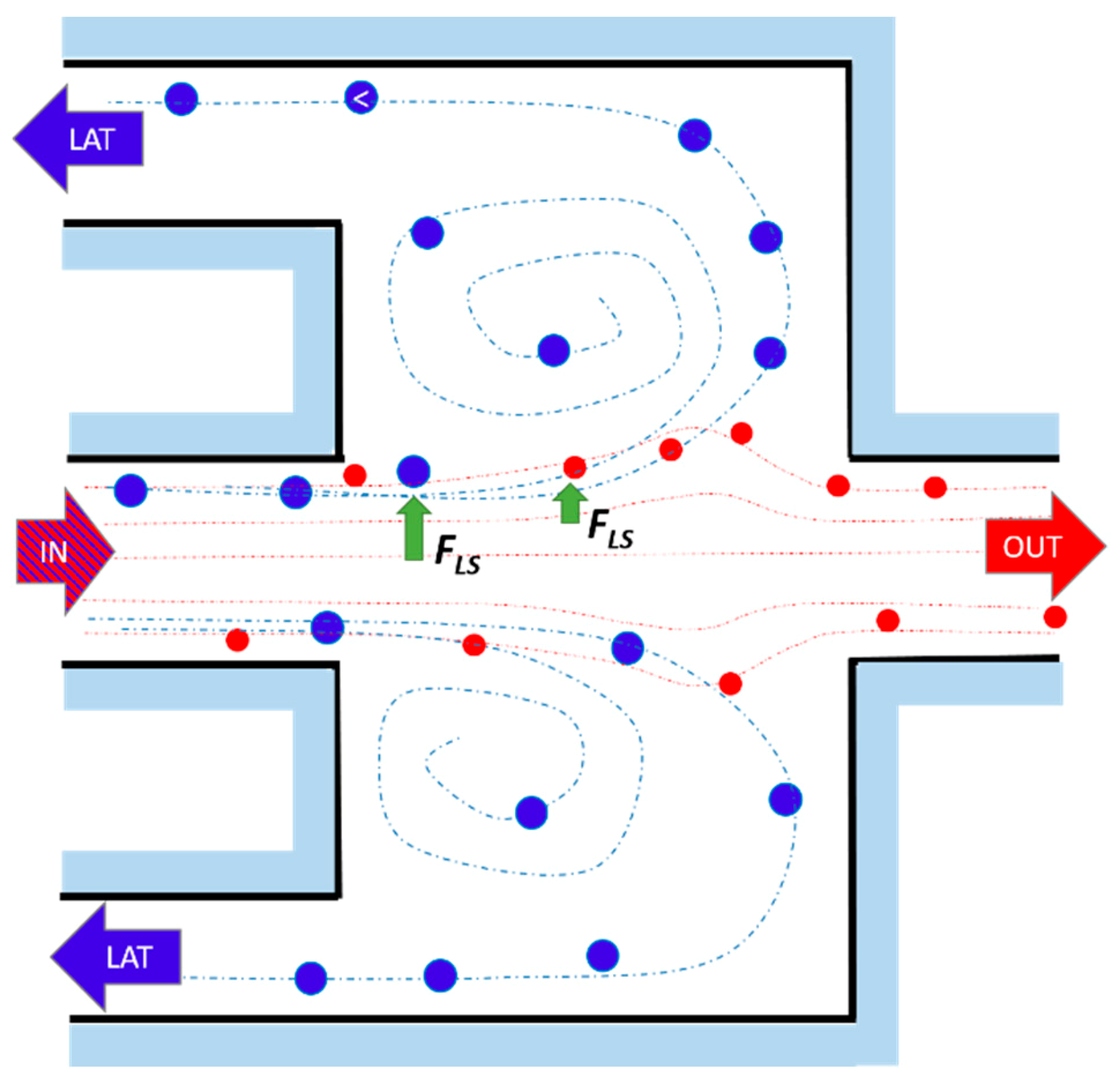
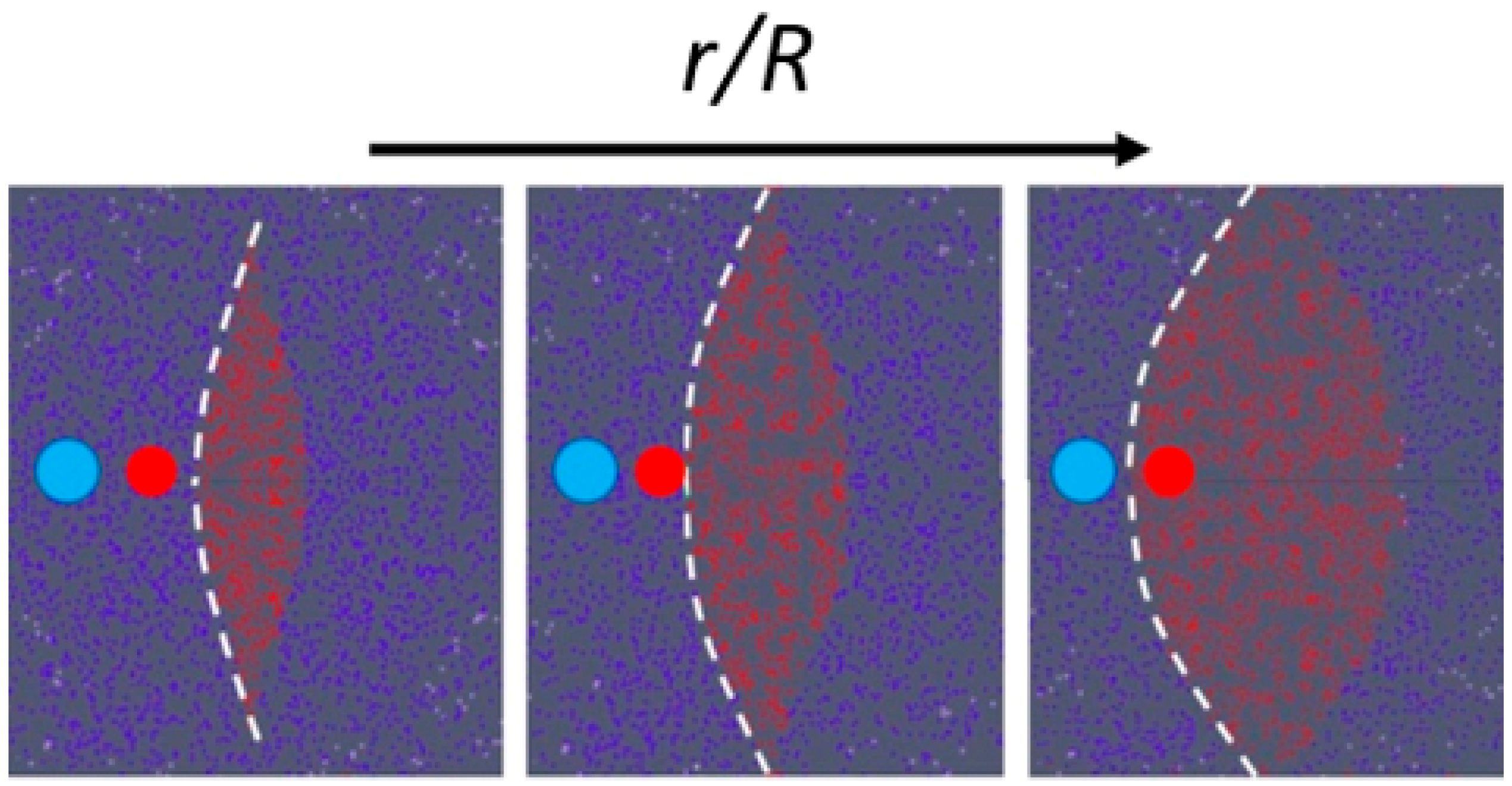
2.1. Working Principle
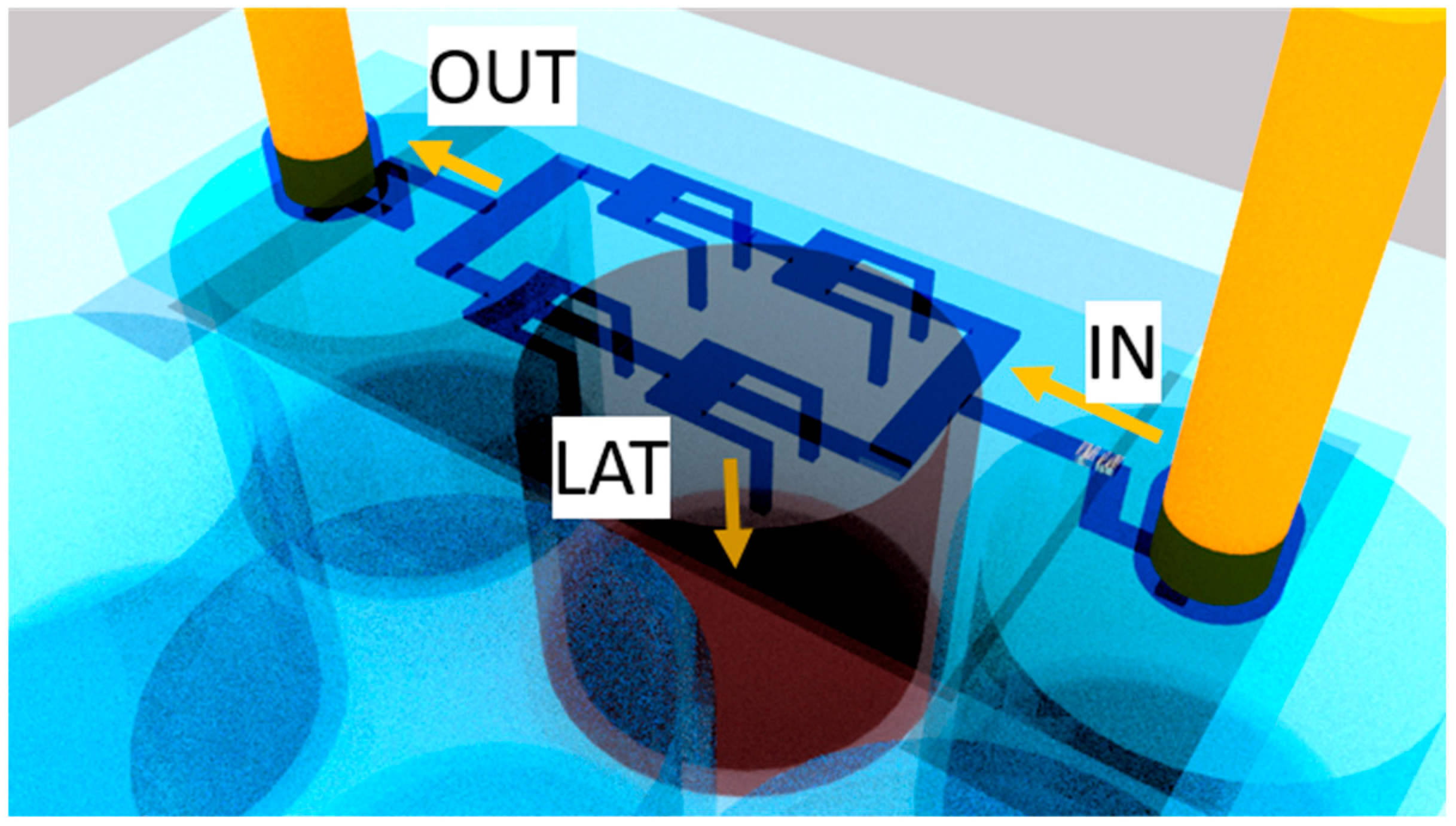
2.2. Fabrication and Testing Methods
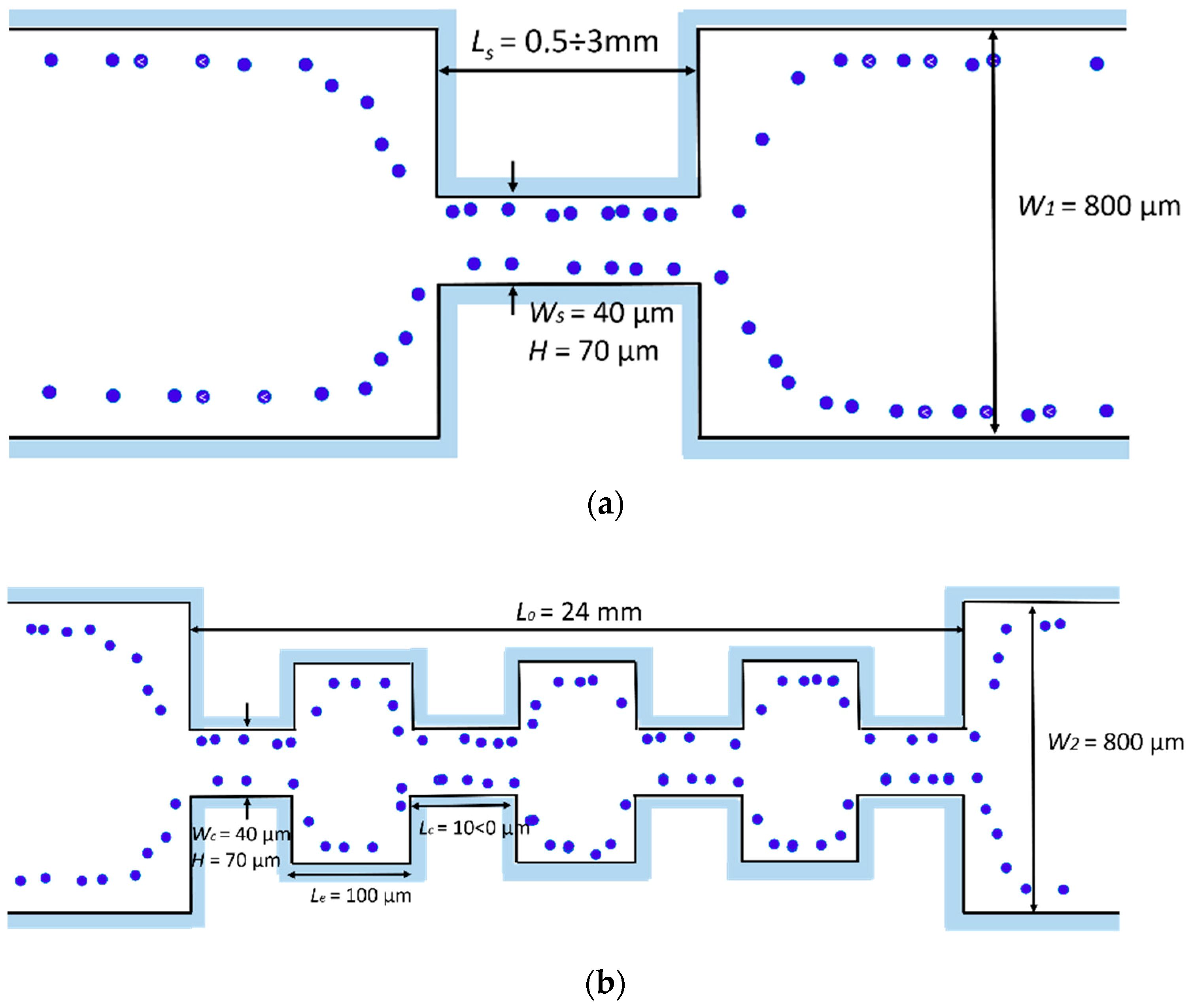
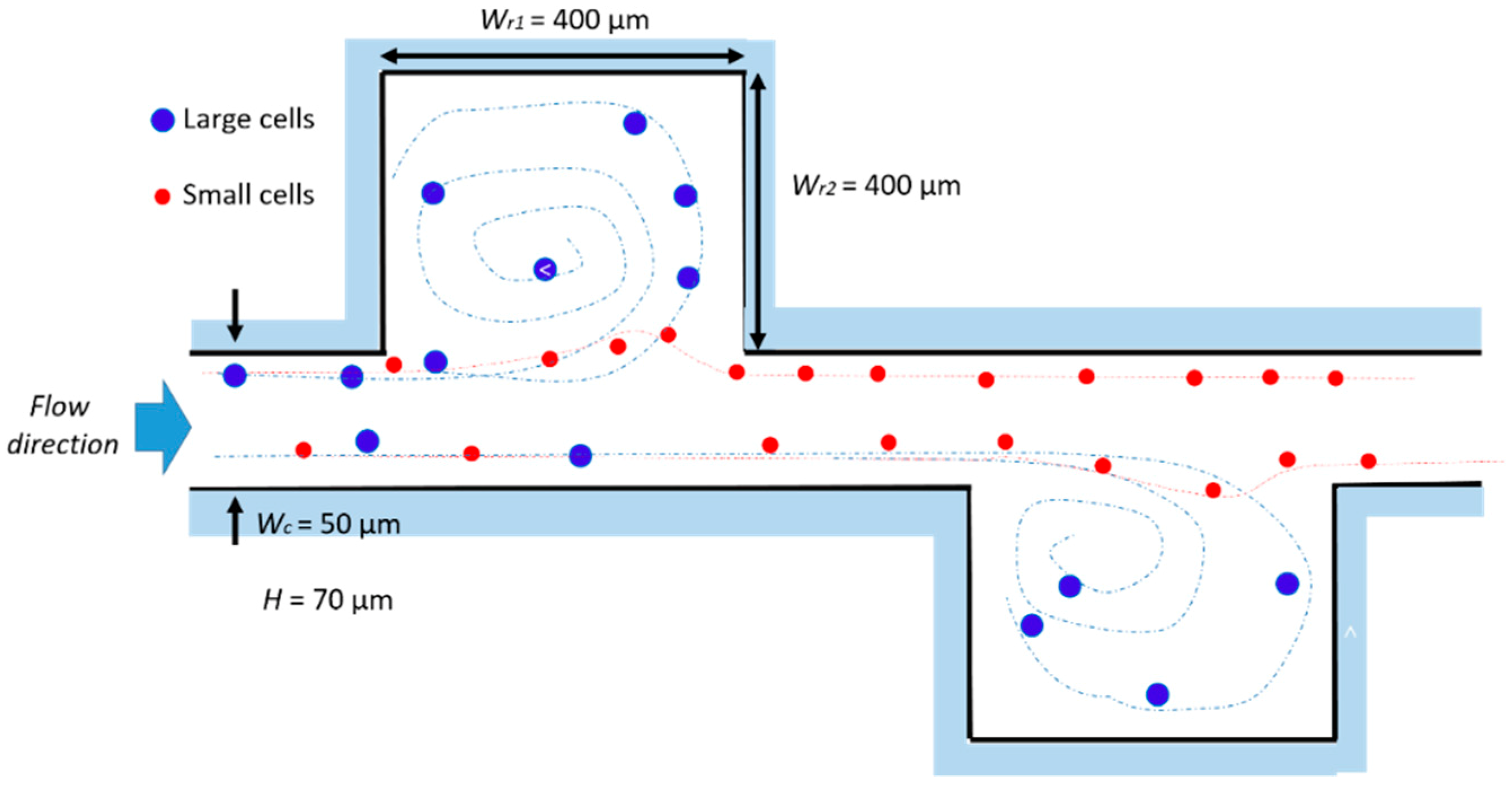
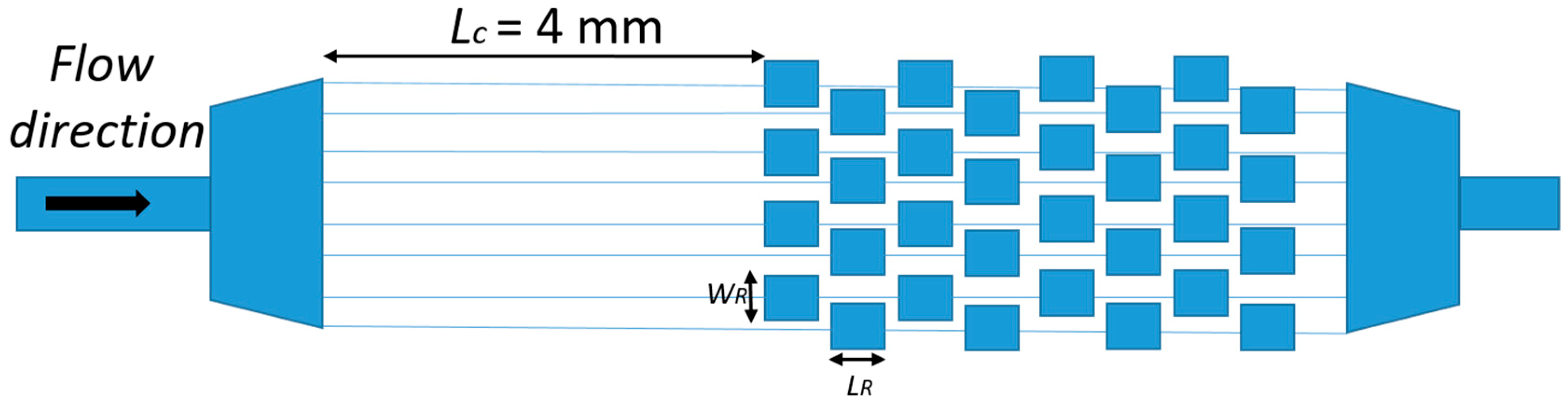
2.3. Evolution of the Devices Design
3. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, L.M.; Rosano, J.M.; Wang, Y.; Klarmann, G.J.; Garson, C.J.; Prabhakarpandian, B.; Pant, K.; Alvarez, L.M.; Lai, E. Label-free mesenchymal stem cell enrichment from bone marrow samples by inertial micro-fluidics. Anal. Methods 2018, 10, 713–721. [Google Scholar] [CrossRef]
- Wylot, B.; Konarzewska, K.; Bugajski, L.; Piwocka, K.; Zawadzka, M. Isolation of vascular endothelial cells from intact and injured murine brain cortex-technical issues and pitfalls in FACS analysis of the nervous tissue. Cytom. Part A 2015, 87, 908–920. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.W.; Péault, B.; Huard, J. Regenerative translation of human blood-vessel-derived MSC precursors. Stem Cells Int. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xue, K.; Zhang, X.; Qi, L.; Zhou, J.; Liu, K. Isolation, identification, and comparison of cartilage stem progenitor/cells from auricular cartilage and perichondrium. Am. J. Transl. Res. 2016, 8, 732–741. [Google Scholar] [PubMed]
- Xu, H.; Dong, B.; Xu, S.; Xu, S.; Sun, X.; Sun, J.; Yang, Y.; Xu, L.; Bai, X.; Zhang, S.; et al. High purity micro-fluidic sorting and in situ inactivation of circulating tumor cells based on multifunctional magnetic composites. Biomaterials 2017, 138, 69–79. [Google Scholar] [CrossRef]
- Jeanbart, L.; Swartz, M.A. Engineering opportunities in cancer immunotherapy. Proc. Natl. Acad. Sci. USA 2015, 112, 14467–14472. [Google Scholar] [CrossRef] [PubMed]
- Haverty, P.M.; Lin, E.; Tan, J.; Yu, Y.; Lam, B.; Lianoglou, S.; Neve, R.M.; Martin, S.; Settleman, J.; Yauch, R.L.; et al. Reproducible pharmacogenomic profiling of cancer cell line panels. Nature 2016, 533, 333–337. [Google Scholar] [CrossRef]
- Di Meo, A.; Bartlett, J.; Cheng, Y.; Pasic, M.D.; Yousef, G.M. Liquid biopsy: A step forward towards precision medicine in urologic malignancies. Mol. Cancer 2017, 16, 158. [Google Scholar] [CrossRef]
- Varillas, J.I.; Zhang, J.; Chen, K.; Barnes, I.I.; Liu, C.; George, T.J.; Fan, Z.H. Micro-fluidic isolation of circulating tumor cells and cancer stem-like cells from patients with pancreatic ductal adenocarcinoma. Theranostics 2019, 9, 1417–1425. [Google Scholar] [CrossRef]
- Kim, T.H.; Yoon, H.J.; Fouladdel, S.; Wang, Y.; Kozminsky, M.; Burness, M.L.; Paoletti, C.; Zhao, L.; Azizi, E.; Wicha, M.S. Characterizing circulating tumor cells isolated from metastatic breast cancer patients using graphene oxide based micro-fluidic assay. Adv. Biosyst. 2019, 3, 1–10. [Google Scholar] [CrossRef]
- Kim, S.H.; Ito, H.; Kozuka, M.; Takagi, H.; Hirai, M.; Fujii, T. Cancer marker-free enrichment and direct mutation detection in rare cancer cells by combining multi-property isolation and micro-fluidic concentration. Lab Chip 2019, 19, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Tseng, P.; di Carlo, D. Micro-fluidic Cell Sorting and Separation Technology. In Microtechnology for Cell Manipulation and Sorting; Lee, W., Tseng, P., di Carlo, D., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–14. [Google Scholar]
- Chu, W.; Tan, Y.; Wang, P.; Xu, J.; Li, W.; Qi, J.; Cheng, Y. Centimeter-height 3d printing with femtosecond laser two-photon polymerization. Adv. Mater. Technol. 2018, 3, 1–6. [Google Scholar] [CrossRef]
- Li, M.; Muñoz, H.E.; Goda, K.; di Carlo, D. Shape-based separation of microalga Euglena gracilis using inertial micro-fluidics. Sci. Rep. 2017, 7, 1–8. [Google Scholar]
- Iv, C.W.S.; Reyes, C.D.; López, G.P. Micro-fluidic cell sorting: A review of the advances in the separation of cells from debulking to rare cell isolation. Lab Chip 2015, 15, 1230–1249. [Google Scholar]
- Olm, F.; Urbansky, A.; Dykes, J.H.; Laurell, T.; Scheding, S. Label-free neuroblastoma cell separation from hematopoietic progenitor cell products using acoustophoresis—Towards cell processing of complex biological samples. Sci. Rep. 2019, 9, 8777. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Huang, H.; Stratton, Z.; Huang, Y.; Huang, T.J. Continuous particle separation in a micro-fluidic channel via standing surface acoustic waves (SSAW). Lab Chip 2009, 9, 3354–3359. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, M.; Ren, L.; Liu, J.; Whitley, P.H.; Wang, L.; Huang, T.J. High-throughput acoustic separation of platelets from whole blood. Lab Chip 2016, 16, 3466–3472. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Lim, H.; Kim, C.; Kang, J.Y.; Shin, S. Density-dependent separation of encapsulated cells in a micro-fluidic channel by using a standing surface acoustic wave. Biomicro Fluid. 2012, 6, 1–10. [Google Scholar]
- Gupta, S.; Feke, D.L.; Manas-Zloczower, I. Fractionation of mixed particulate solids according to compressibility using ultrasonic standing wave fields. Chem. Eng. Sci. 1995, 50, 3275–3284. [Google Scholar] [CrossRef]
- Li, P.; Mao, Z.; Peng, Z.; Zhou, L.; Chen, Y.; Huang, P.-H.; Truica, C.I.; Drabick, J.J.; El-Deiry, W.S.; Dao, M.; et al. Acoustic separation of circulating tumor cells. Proc. Natl. Acad. Sci. USA 2015, 112, 4970–4975. [Google Scholar] [CrossRef]
- Rosano, J.M.; Garson, C.J.; Klarmann, G.J.; Perantoni, A.; Alvarez, L.M.; Lai, E.; Song, H.; Wang, Y.; Prabhakarpandian, B.; Pant, K. Continuous-flow sorting of stem cells and differentiation products based on dielectrophoresis. Lab Chip 2015, 15, 1320–1328. [Google Scholar]
- Lee, J.J.; Jeong, K.J.; Hashimoto, M.; Kwon, A.H.; Rwei, A.; Shankarappa, S.A.; Tsui, J.H.; Kohane, D.S. Synthetic ligand-coated magnetic nanoparticles for micro-fluidic bacterial separation from blood. Nano Lett. 2014, 14, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Huang, H.; Lim, H.; Lim, C.; Shin, S. Magnetic separation of malaria-infected red blood cells in various developmental stages. Anal. Chem. 2013, 85, 7316–7323. [Google Scholar] [CrossRef] [PubMed]
- Han, K.H.; Frazier, A.B. Continuous magnetophoretic separation of blood cells in microdevlce format. J. Appl. Phys. 2004, 96, 5797–5802. [Google Scholar] [CrossRef]
- Jung, J.; Han, K.-H. Lateral-driven continuous magnetophoretic separation of blood cells. Appl. Phys. Lett. 2008, 93, 223902. [Google Scholar] [CrossRef]
- Landenberger, B.; Höfemann, H.; Wadle, S.; Rohrbach, A. Micro-fluidic sorting of arbitrary cells with dynamic optical tweezers. Lab Chip 2012, 12, 3177–3183. [Google Scholar] [CrossRef]
- Yang, A.H.J.; Moore, S.D.; Schmidt, B.S.; Klug, M.; Lipson, M.; Erickson, D. Optical manipulation of nanoparticles and biomolecules in sub-wavelength slot waveguides. Nature 2009, 457, 71–75. [Google Scholar] [CrossRef]
- Ashkin, A.; Dziedzic, J.M.; Chu, S. Observation of a single-beam gradient-force optical trap for dielectric particles in air. Opt. Lett. 1986, 22, 288–290. [Google Scholar] [CrossRef]
- Kuga, T.; Torii, Y.; Shiokawa, N.; Hirano, T.; Shimizu, Y.; Sasada, H. Novel optical trap of atoms with a doughnut beam. Phys. Rev. Lett. 1997, 78, 4713–4716. [Google Scholar] [CrossRef]
- Li, X.; Chen, W.; Liu, G.; Lu, W.; Fu, J. Continuous-flow micro-fluidic blood cell sorting for unprocessed whole blood using surface-micromachined microfiltration membranes. Lab Chip 2014, 14, 2565–2575. [Google Scholar] [CrossRef]
- Kang, Y.-T.; Doh, I.; Byun, J.; Chang, H.J.; Cho, Y.-H. Label-free rapid viable enrichment of circulating tumor cell by photosensitive polymer-based microfilter device. Theranostics 2017, 7, 3179–3191. [Google Scholar] [CrossRef] [PubMed]
- Doh, I.; Yoo, H.-I.; Cho, Y.-H.; Lee, J.; Kim, H.K.; Kim, J. Viable capture and release of cancer cells in human whole blood. Appl. Phys. Lett. 2012, 101, 43701. [Google Scholar] [CrossRef]
- Alvankarian, J.; Bahadorimehr, A.; Majlis, B.Y. A pillar-based microfilter for isolation of white blood cells on elastomeric substrate. Biomicro Fluid. 2013, 7, 14102. [Google Scholar] [CrossRef] [PubMed]
- Wilding, P.; Kricka, L.J.; Cheng, J.; Hvichia, G.; Shoffner, M.A.; Fortina, P. Integrated cell isolation and polymerase chain reaction analysis using silicon microfilter chambers. Anal. Biochem. 1998, 257, 95–100. [Google Scholar] [CrossRef]
- McGrath, J.; Jimenez, M.; Bridle, H. Deterministic lateral displacement for particle separation: A review. Lab Chip 2014, 14, 4139–4158. [Google Scholar] [CrossRef] [PubMed]
- Au, S.H.; Edd, J.; Stoddard, A.E.; Wong, K.H.; Fachin, F.; Maheswaran, S.; Haber, D.A.; Stott, S.L.; Kapur, R.; Toner, M. Micro-fluidic isolation of circulating tumor cell clusters by size and asymmetry. Sci. Rep. 2017, 7, 2433. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.L.; Yan, Q.; Guo, J.; Zhao, H.; Zhao, L.; Zhe, J. Inertial particle focusing in micro-channels with gradually changing geometrical structures. J. Micromech. Microeng. 2017, 27, 2017. [Google Scholar] [CrossRef]
- Amini, H.; Lee, W.; di Carlo, D. Inertial micro-fluidic physics. Lab Chip 2014, 14, 2739–2761. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yan, S.; Yuan, D.; Alici, G.; Nguyen, N.T.; Warkiani, M.E.; Li, W. Fundamentals and applications of inertial micro-fluidics: A review. Lab Chip 2016, 16, 10–34. [Google Scholar] [CrossRef]
- Di Carlo, D. Inertial micro-fluidics. Lab Chip 2009, 9, 3038–3046. [Google Scholar] [CrossRef]
- Hur, S.C.; Henderson-Maclennan, N.K.; McCabe, E.R.B.; di Carlo, D. Deformability-based cell classification and enrichment using inertial micro-fluidics. Lab Chip 2011, 11, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Seo, K.W.; Lee, S.J. Lateral and cross-lateral focusing of spherical particles in a square micro-channel. Lab Chip 2011, 11, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, D.; Irimia, D.; Tompkins, R.G.; Toner, M. Continuous inertial focusing, ordering, and separation of particles in micro-channels. Proc. Natl. Acad. Sci. USA 2007, 104, 18892–18897. [Google Scholar] [CrossRef] [PubMed]
- Masaeli, M.; Sollier, E.; Amini, H.; Mao, W.; Camacho, K.; Doshi, N.; Mitragotri, S.; Alexeev, A.; Di Carlo, D. Continuous inertial focusing and separation of particles by shape. Phys. Rev. X 2012, 2, 031017. [Google Scholar] [CrossRef]
- Martel, J.M.; Toner, M. Inertial focusing in micro-fluidics. Annu. Rev. Biomed. Eng. 2014, 16, 371–396. [Google Scholar] [CrossRef] [PubMed]
- Hur, S.C.; Brinckerhoff, T.Z.; Walthers, C.M.; Dunn, J.C.; di Carlo, D. Label-free enrichment of adrenal cortical progenitor cells using inertial micro-fluidics. PLoS ONE 2012, 7, e46550. [Google Scholar] [CrossRef] [PubMed]
- Hur, S.C.; Tse, H.T.K.; Di Carlo, D. Sheathless inertial cell ordering for extreme throughput flow cytometry. Lab Chip 2010, 10, 274–280. [Google Scholar] [CrossRef]
- Mach, A.J.; di Carlo, D. Continuous scalable blood filtration device using inertial micro-fluidics. Biotechnol. Bioeng. 2010, 107, 302–311. [Google Scholar] [CrossRef]
- Zhou, J.; Giridhar, P.V.; Kasper, S.; Papautsky, I. Modulation of aspect ratio for complete separation in an inertial micro-fluidic channel. Lab Chip 2013, 13, 1919–1929. [Google Scholar] [CrossRef]
- Zhou, J.; Papautsky, I. Fundamentals of inertial focusing in micro-channels. Lab Chip 2013, 13, 1121–1132. [Google Scholar] [CrossRef]
- Kuntaegowdanahalli, S.S.; Bhagat, A.A.S.; Kumar, G.; Papautsky, I. Inertial micro-fluidics for continuous particle separation in spiral micro-channels. Lab Chip 2009, 20, 2973–2978. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.W.; Warkiani, M.E.; Khoo, B.L.; Li, Z.R.; Soo, R.A.; Tan, D.S.-W.; Lim, W.-T.; Han, J.; Bhagat, A.A.S.; Lim, C.T. Isolation and retrieval of circulating tumor cells using centrifugal forces. Sci. Rep. 2013, 3, 1259. [Google Scholar] [CrossRef]
- Nivedita, N.; Papautsky, I. Continuous separation of blood cells in spiral micro-fluidic devices. Biomicrofluidics 2013, 7, 54101. [Google Scholar] [CrossRef] [PubMed]
- Warkiani, M.E.; Khoo, B.L.; Wu, L.; Tay, A.K.P.; Bhagat, A.A.S.; Han, J.; Lim, C.T. Ultra-fast, label-free isolation of circulating tumor cells from blood using spiral micro-fluidics. Nat. Protoc. 2016, 11, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Wu, L.; Bhagat, A.A.; Li, Z.; Chen, P.C.; Chao, S.; Ong, C.J.; Han, J. Spiral microchannel with rectangular and trapezoidal cross-sections for size based particle separation. Sci. Rep. 2013, 3, 1475. [Google Scholar] [CrossRef] [PubMed]
- Warkiani, M.E.; Guan, G.; Luan, K.B.; Lee, W.C.; Bhagat, A.A.S.; Chaudhuri, P.K.; Tan, D.S.W.; Lim, W.T.; Lee, S.C.; Chen, P.C.; et al. Slanted spiral micro-fluidics for the ultra-fast, label-free isolation of circulating tumor cells. Lab Chip 2014, 14, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Petchakup, C.; Tay, H.M.; Li, K.H.H.; Hou, H.W. Spiral Inertial Micro-fluidics for Cell Separation and Biomedical Applications. In Applications of Micro-Fluidic Systems in Biology and Medicine; Tokeshi, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 99–150. [Google Scholar]
- Di Carlo, D.; Edd, J.F.; Humphry, K.J.; Stone, H.A.; Toner, M. particle segregation and dynamics in confined flows. Phys. Rev. Lett. 2009, 102, 094503. [Google Scholar] [CrossRef] [PubMed]
- Moffatt, H.K. Viscous and resistive eddies near a sharp corner. J. Fluid Mech. 1964, 18, 1–18. [Google Scholar] [CrossRef]
- Cherdron, W.; Durst, F.; Whitelaw, J.H. Asymmetric flows and instabilities in symmetric ducts with sudden expansions. J. Fluid Mech. 1978, 84, 13. [Google Scholar] [CrossRef]
- Hur, S.C.; Mach, A.J.; Di Carlo, D. High-throughput size-based rare cell enrichment using microscale vortices. Biomicrofluidics 2011, 5, 022206. [Google Scholar] [CrossRef]
- Sajeesh, P.; Sen, A.K. Particle separation and sorting in micro-fluidic devices: A review. Microfluid. Nanofluidics 2014, 17, 1–52. [Google Scholar] [CrossRef]
- Dalili, A.; Samiei, E.; Hoorfar, M. A review of sorting, separation and isolation of cells and microbeads for biomedical applications: Micro-fluidic approaches. Analyst 2019, 144, 87–113. [Google Scholar] [CrossRef] [PubMed]
- Segré, G.; Silberberg, A.; Segr, A.S.G. Radial particle displacements in poiseuille flow of suspensions. Nature 1961, 189, 209–210. [Google Scholar] [CrossRef]
- Segré, G.; Silberberg, A. Behaviour of macroscopic rigid spheres in Poiseuille flow. J. Fluid Mech. 1962, 14, 115. [Google Scholar] [CrossRef]
- Chun, B.; Ladd, A.J.C. Inertial migration of neutrally buoyant particles in a square duct: An investigation of multiple equilibrium positions. Phys. Fluids 2006, 18, 31704. [Google Scholar] [CrossRef]
- Liu, C.; Hu, G.; Jiang, X.; Sun, J. Inertial focusing of spherical particles in rectangular micro-channels over a wide range of Reynolds numbers. Lab Chip 2015, 15, 1168–1177. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, J.; Wu, C.; Nam, S.; di Carlo, D.; Lee, W. Inertial focusing in non-rectangular cross-section micro-channels and manipulation of accessible focusing positions. Lab Chip 2016, 16, 992–1001. [Google Scholar] [CrossRef]
- Tang, W.; Fan, N.; Yang, J.; Li, Z.; Zhu, L.; Jiang, D.; Shi, J.; Xiang, N. Elasto-inertial particle focusing in 3D-printed micro-channels with unconventional cross sections. Microfluid. Nanofluidics 2019, 23, 42. [Google Scholar] [CrossRef]
- Mashhadian, A.; Shamloo, A. Inertial micro-fluidics: A method for fast prediction of focusing pattern of particles in the cross section of the channel. Anal. Chim. Acta 2019, 1083, 137–149. [Google Scholar] [CrossRef]
- Chupin, L.; Cindea, N. Numerical Method for Inertial Migration of Particles in 3D Channels; To Cite This Version: Hal-01971999; CCSD: Las Vegas, VN, USA, 2019. [Google Scholar]
- Bayat, P.; Rezai, P. Microfluidic curved-channel centrifuge for solution exchange of target? Microparticles and their simultaneous separation from bacteria. Soft Matte. 2018, 14, 5356–5363. [Google Scholar] [CrossRef]
- Wang, L.; Dandy, D.S. High-throughput inertial focusing of micrometer- and sub-micrometer-sized particles separation. Adv. Sci. 2017, 4, 1700153. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, A.A.S.; Kuntaegowdanahalli, S.S.; Papautsky, I. Inertial micro-fluidics for continuous particle filtration and extraction. Microfluid. Nanofluidics 2009, 7, 217–226. [Google Scholar] [CrossRef]
- Saffman, P.G. The lift force on a small shpere in a slow shear flow. J. Fluid Mech. 1965, 22, 385–400. [Google Scholar] [CrossRef]
- Asmolov, E.S. The inertial lift on a spherical particle in a plane poiseuille flow at large channel Reynolds number. J. Fluid Mech. 1999, 381, 63–87. [Google Scholar] [CrossRef]
- Matas, J.P.; Morris, J.F.; Guazzelli, É. Lateral forces on a sphere solid/liquid dispersions in drilling and production fluides chargés en forage et production pétrolière. Oil Gas Sci. Technol. IFP 2004, 59, 59–70. [Google Scholar] [CrossRef]
- Moffatt, H.K. Viscous eddies near a sharp corner. Fluid Dyn. Trans. 2013, 217–224. [Google Scholar] [CrossRef]
- Kim, P.; Kwon, K.W.K.; Park, M.M.C.; Lee, S.H.S.; Kim, S.S.M.; Suh, K.Y.K. Soft lithography for micro-fluidics: A review. Biochip J. 2008, 2, 1–11. [Google Scholar]
- Park, S.; Song, S.H.; Jung, H.I. Continuous focusing of micro-particles using inertial lift force and vorticity via multi-orifice micro-fluidic channels. Lab Chip 2009, 9, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Duffy, D.C.; Mcdonald, J.C.; Schueller, O.J.A.; Whitesides, G.M. Rapid prototyping of micro-fluidic systems in poly (dimethylsiloxane). Anal. Chem. 1998, 70, 4974–4984. [Google Scholar] [CrossRef] [PubMed]
- Volpe, A.; Paiè, P.; Ancona, A.; Osellame, R. Polymeric fully inertial lab-on-a-chip with enhanced-throughput sorting capabilities. Microfluid. Nanofluidics 2019, 23, 37. [Google Scholar] [CrossRef]
- Sima, F.; Sugioka, K.; Vázquez, R.M.; Osellame, R.; Kelemen, L. Review article Three-dimensional femtosecond laser processing for lab-on-a-chip applications. Nanophotonics 2018, 7, 1–22. [Google Scholar] [CrossRef]
- Sollier, E.; Go, D.E.; Che, J.; Gossett, D.R.; O’Byrne, S.; Weaver, W.M.; Kummer, N.; Rettig, M.; Goldman, J.; Nickols, N.; et al. Size-selective collection of circulating tumor cells using Vortex technology. Lab Chip 2014, 14, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Sollier, E.; Murray, C.; Maoddi, P.; di Carlo, D. Rapid prototyping polymers for micro-fluidic devices and high pressure injections. Lab Chip 2011, 11, 3752–3765. [Google Scholar] [CrossRef] [PubMed]
- Paiè, P.; Che, J.; Di Carlo, D. Effect of reservoir geometry on vortex trapping of cancer cells. Microfluid. Nanofluidics 2017, 21, 1–11. [Google Scholar] [CrossRef]
- Wang, X.; Papautsky, I. Size-based micro-fluidic multimodal micro-particle sorter. Lab Chip 2015, 15, 1350–1359. [Google Scholar] [CrossRef]
- Volpe, A.; Paiè, P.; Ancona, A.; Osellame, R.; Lugarà, P.M.; Pascazio, G. A computational approach to the characterization of a micro-fluidic device for continuous size-based inertial sorting. J. Phys. D Appl. Phys. 2017, 50, 2017. [Google Scholar] [CrossRef]
- Wang, X.; Yang, X.; Papautsky, I. An integrated inertial micro-fluidic vortex sorter for tunable sorting and purification of cells. Technology 2016, 4, 88–97. [Google Scholar] [CrossRef]
- Bhagat, A.A.S.; Kuntaegowdanahalli, S.S.; Kaval, N.; Seliskar, C.J.; Papautsky, I. Inertial micro-fluidics for sheath-less high-throughput flow cytometry. Biomed. Microdevices 2010, 12, 187–195. [Google Scholar] [CrossRef]
- Nivedita, N.; Garg, N.; Lee, A.P.; Papautsky, I. A high throughput micro-fluidic platform for size-selective enrichment of cell populations in tissue and blood samples. Analyst 2017, 142, 2558–2569. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volpe, A.; Gaudiuso, C.; Ancona, A. Sorting of Particles Using Inertial Focusing and Laminar Vortex Technology: A Review. Micromachines 2019, 10, 594. https://doi.org/10.3390/mi10090594
Volpe A, Gaudiuso C, Ancona A. Sorting of Particles Using Inertial Focusing and Laminar Vortex Technology: A Review. Micromachines. 2019; 10(9):594. https://doi.org/10.3390/mi10090594
Chicago/Turabian StyleVolpe, Annalisa, Caterina Gaudiuso, and Antonio Ancona. 2019. "Sorting of Particles Using Inertial Focusing and Laminar Vortex Technology: A Review" Micromachines 10, no. 9: 594. https://doi.org/10.3390/mi10090594
APA StyleVolpe, A., Gaudiuso, C., & Ancona, A. (2019). Sorting of Particles Using Inertial Focusing and Laminar Vortex Technology: A Review. Micromachines, 10(9), 594. https://doi.org/10.3390/mi10090594






