Fabrication of MoO3 Nanowire-based Membrane Devices for the Selective Adsorption of Cationic Dyes from Aqueous Solutions with High Performance and Reusability
Abstract
1. Introduction
2. Experimental Section
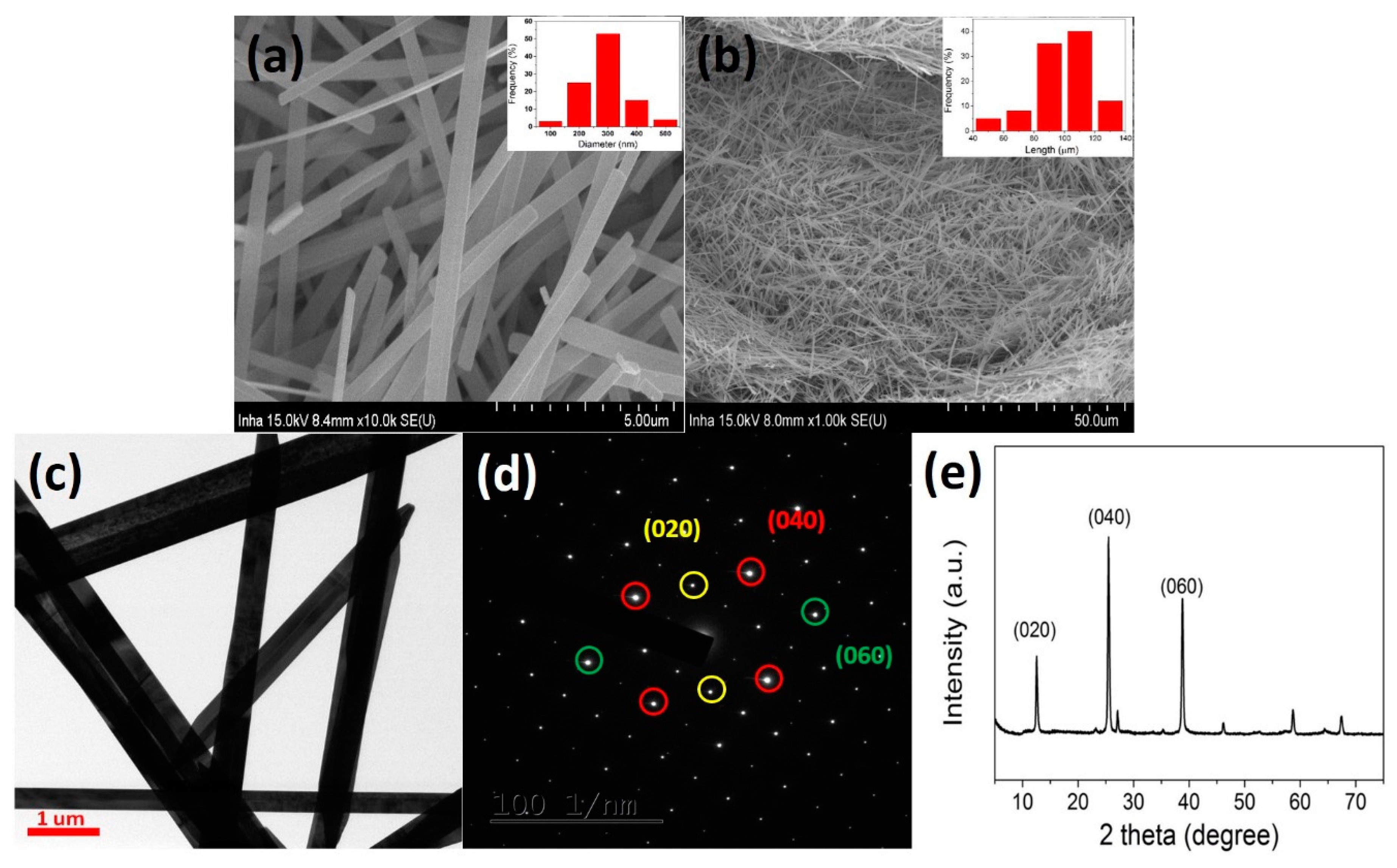
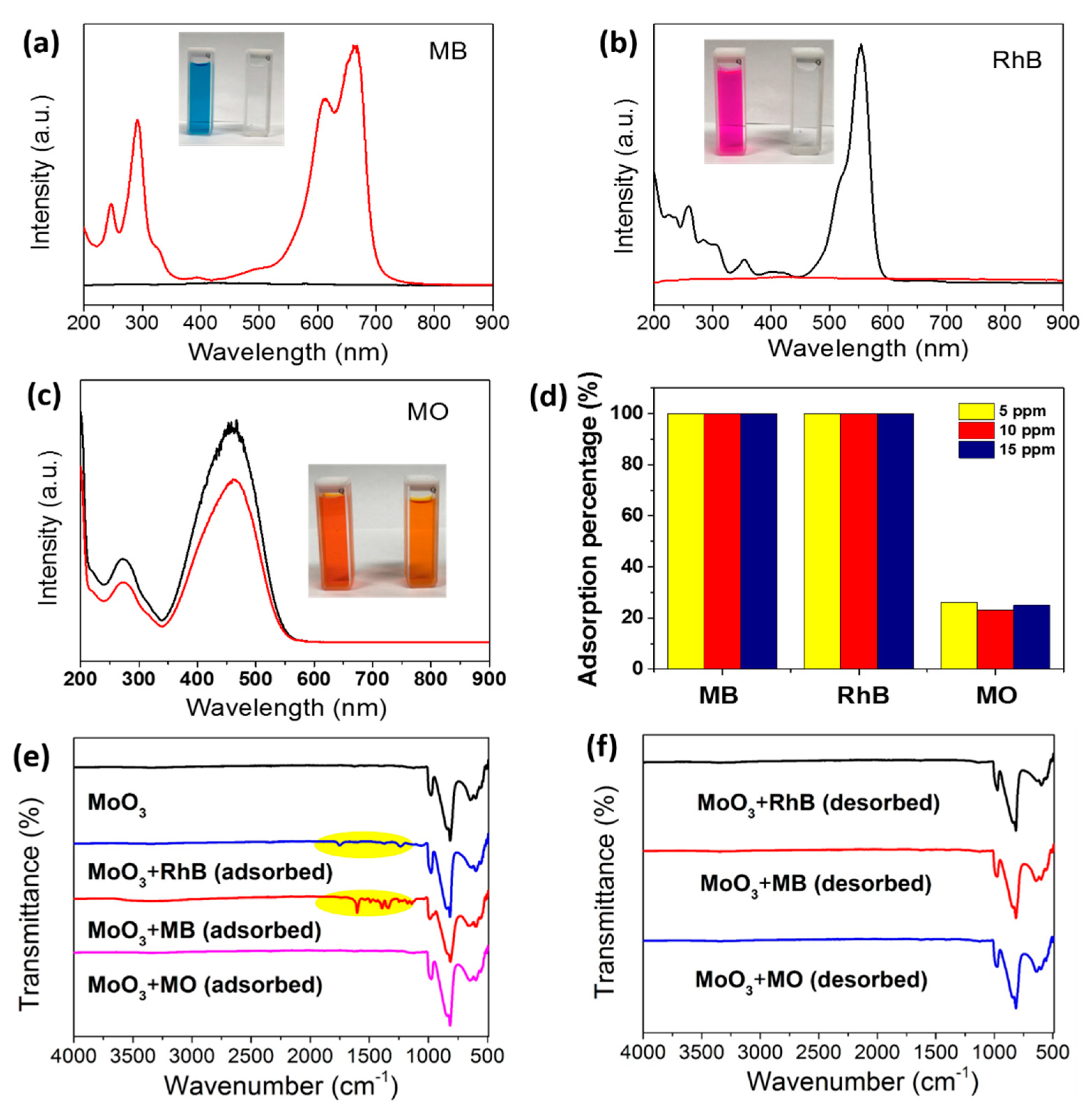
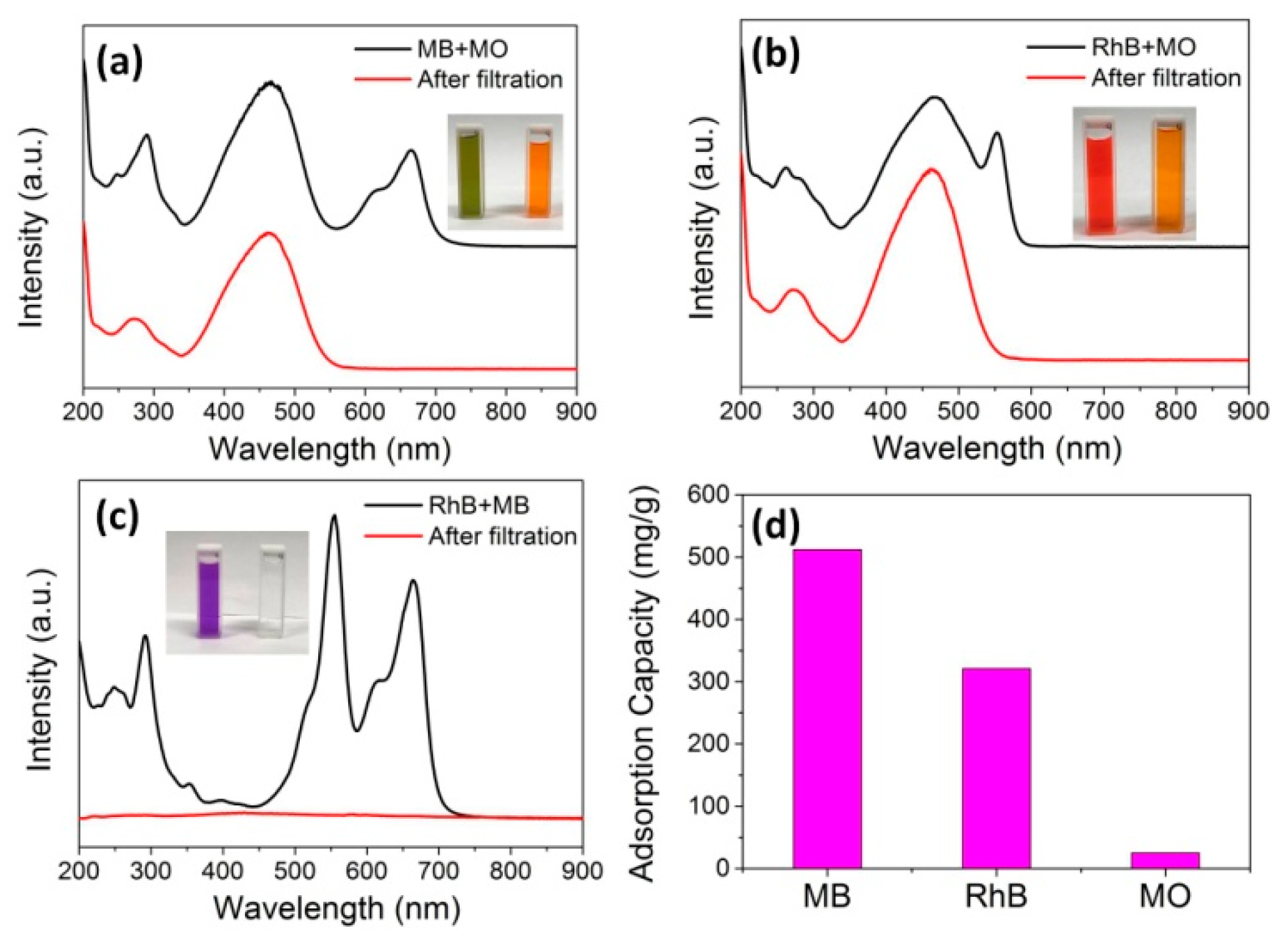
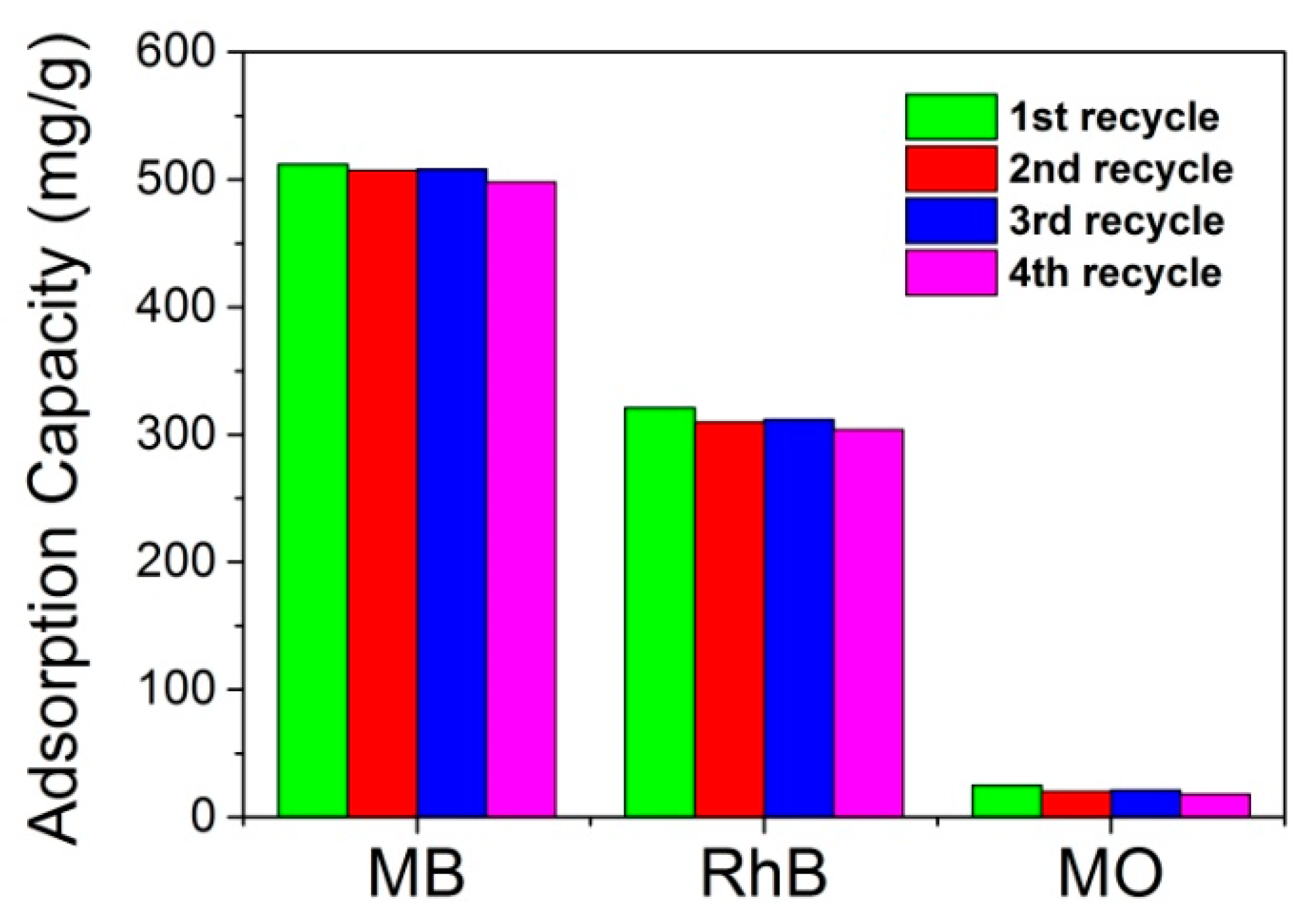
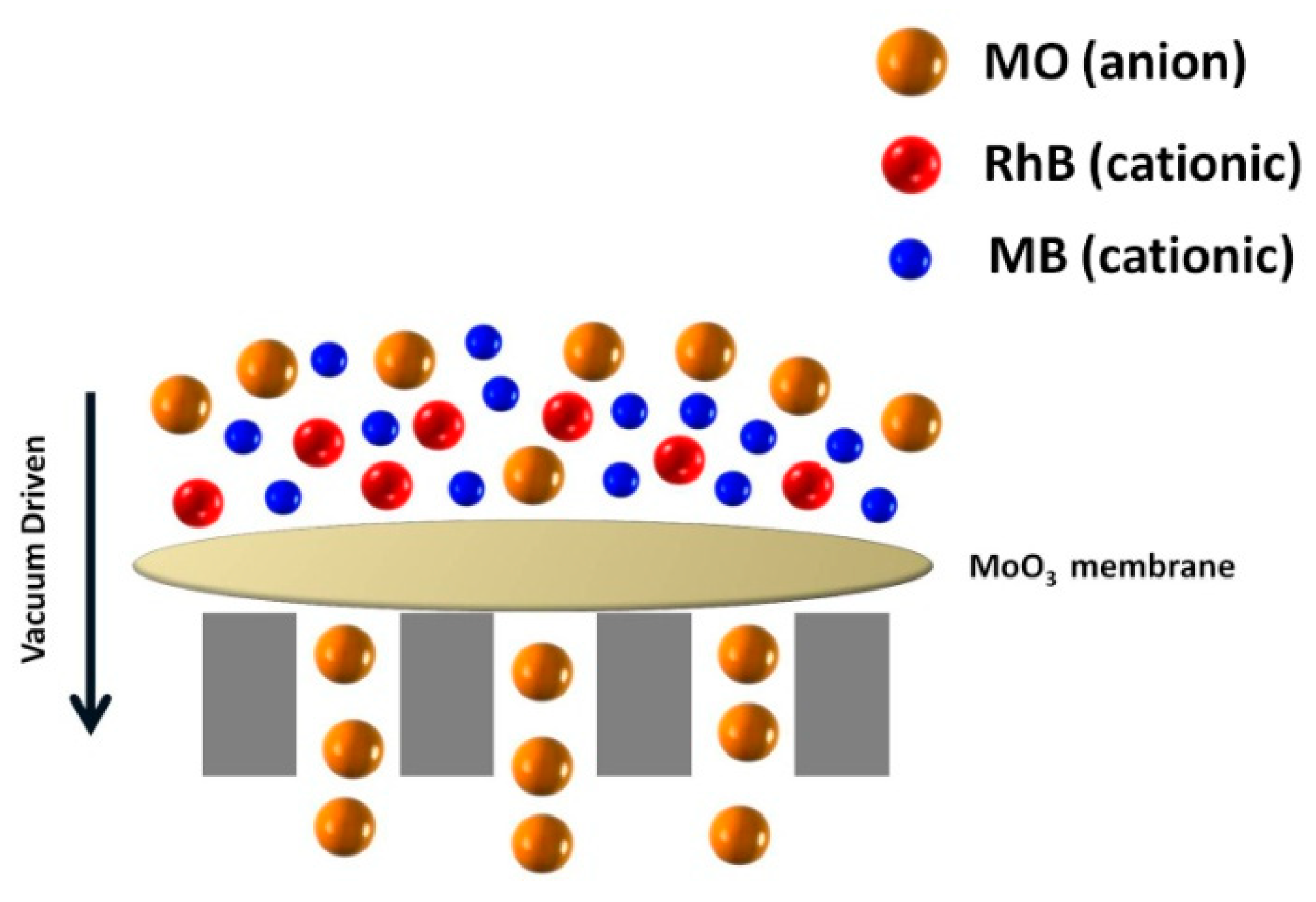
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Borthakur, P.; Das, M.R. Hydrothermal assisted decoration of NiS2 and CoS nanoparticles on the reduced graphene oxide nanosheets for sunlight driven photocatalytic degradation of azo dye: Effect of background electrolyte and surface charge. J. Colloid Interface Sci. 2018, 516, 342–354. [Google Scholar] [CrossRef]
- Ezugwu, C.I.; Asraf, M.A.; Li, X.; Liu, S.; Kao, C.M.; Zhuiykov, S.; Verpoort, F. Selective and adsorptive removal of anionic dyes and CO2 with azolium-based metal-organic frameworks. J. Colloid Interface Sci. 2018, 519, 214–223. [Google Scholar] [CrossRef]
- Yang, S.; Xu, C.Y.; Zhang, B.Y.; Yang, L.; Hu, S.P.; Zhen, L. Ca(II) doped beta-In2S3 hierarchical structures for photocatalytic hydrogen generation and organic dye degradation under visible light irradiation. J. Colloid Interface Sci. 2017, 491, 230–237. [Google Scholar] [CrossRef]
- He, Y.-C.; Yang, J.; Kan, W.-Q.; Zhang, H.-M.; Liu, Y.-Y.; Ma, J.-F. A new microporous anionic metal–organic framework as a platform for highly selective adsorption and separation of organic dyes. J. Mater. Chem. A 2015, 3, 1675–1681. [Google Scholar] [CrossRef]
- Zhang, Y.; Park, M.; Kim, H.-Y.; El-Newehy, M.; Rhee, K.Y.; Park, S.-J. Effect of TiO2 on photocatalytic activity of polyvinylpyrrolidone fabricated via electrospinning. Composites Part B 2015, 80, 355–360. [Google Scholar] [CrossRef]
- Wu, G.; Zhao, Y.; Li, Y.; Ma, H.; Zhao, J. pH-dependent synthesis of iodine-deficient bismuth oxyiodide microstructures: Visible-light photocatalytic activity. J. Colloid Interface Sci. 2018, 510, 228–236. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, B.J. Ammonia removal of activated carbon fibers produced by oxyfluorination. J. Colloid Interface Sci. 2005, 291, 597–599. [Google Scholar] [CrossRef]
- Liu, T.; Li, B.; Hao, Y.; Yao, Z. MoO3-nanowire membrane and Bi2Mo3O12/MoO3 nano-heterostructural photocatalyst for wastewater treatment. Chem. Eng. J. 2014, 244, 382–390. [Google Scholar] [CrossRef]
- Yu, L.; Ruan, S.; Xu, X.; Zou, R.; Hu, J. One-dimensional nanomaterial-assembled macroscopic membranes for water treatment. Nano Today 2017, 17, 79–95. [Google Scholar] [CrossRef]
- Xu, Y.; Yuan, D.; Bao, J.; Xie, Y.; He, M.; Shi, Z.; Chen, S.; He, C.; Zhao, W.; Zhao, C. Nanofibrous membranes with surface migration of functional groups for ultrafast wastewater remediation. J. Mater. Chem. A 2018, 6, 13359–13372. [Google Scholar] [CrossRef]
- Zhang, Y.; Park, S.-J. Incorporation of RuO2 into charcoal-derived carbon with controllable microporosity by CO2 activation for high-performance supercapacitor. Carbon 2017, 122, 287–297. [Google Scholar] [CrossRef]
- Li, B.; Jiang, X.; Yin, J. Multi-responsive microgel of hyperbranched poly(ether amine) (hPEA-mGel) for the selective adsorption and separation of hydrophilic fluorescein dyes. J. Mater. Chem. 2012, 22, 17976. [Google Scholar] [CrossRef]
- Park, S.-J.; Seo, M.-K.; Lee, Y.-S. Surface characteristics of fluorine-modified PAN-based carbon fibers. Carbon 2003, 41, 723–730. [Google Scholar] [CrossRef]
- Xie, S.; Zheng, B.; Kuang, Q.; Wang, X.; Xie, Z.; Zheng, L. Synthesis of layered protonated titanate hierarchical microspheres with extremely large surface area for selective adsorption of organic dyes. CrystEngComm 2012, 14, 7715. [Google Scholar] [CrossRef]
- Zhang, Y.; Park, S.-J. Au–pd bimetallic alloy nanoparticle-decorated BiPO4 nanorods for enhanced photocatalytic oxidation of trichloroethylene. J. Catal. 2017, 355, 1–10. [Google Scholar] [CrossRef]
- Zhu, Z.; Bai, Y.L.; Zhang, L.; Sun, D.; Fang, J.; Zhu, S. Two nanocage anionic metal-organic frameworks with rht topology and {[M(H2O)6]6}12+ charge aggregation for rapid and selective adsorption of cationic dyes. Chem. Commu. 2014, 50, 14674–14677. [Google Scholar] [CrossRef]
- Deng, S.; Wang, R.; Xu, H.; Jiang, X.; Yin, J. Hybrid hydrogels of hyperbranched poly(ether amine)s (hPEAs) for selective adsorption of guest molecules and separation of dyes. J. Mater. Chem. 2012, 22, 10055. [Google Scholar] [CrossRef]
- Deng, S.; Xu, H.; Jiang, X.; Yin, J. Poly(vinyl alcohol) (PVA)-Enhanced Hybrid Hydrogels of Hyperbranched Poly(ether amine) (hPEA) for Selective Adsorption and Separation of Dyes. Macromolecules 2013, 46, 2399–2406. [Google Scholar] [CrossRef]
- Li, H.-H.; Li, Z.-Y.; Wu, X.-L.; Zhang, L.-L.; Fan, C.-Y.; Wang, H.-F.; Li, X.-Y.; Wang, K.; Sun, H.-Z.; Zhang, J.-P. Shale-like Co3O4 for high performance lithium/sodium ion batteries. J. Mater. Chem. A 2016, 4, 8242–8248. [Google Scholar] [CrossRef]
- Hassan, M.F.; Guo, Z.P.; Chen, Z.; Liu, H.K. Carbon-coated MoO3 nanobelts as anode materials for lithium-ion batteries. J. Power Sources 2010, 195, 2372–2376. [Google Scholar] [CrossRef]
- Wang, C.; Wu, L.; Wang, H.; Zuo, W.; Li, Y.; Liu, J. Fabrication and Shell Optimization of Synergistic TiO2-MoO3 Core-Shell Nanowire Array Anode for High Energy and Power Density Lithium-Ion Batteries. Adv. Funct. Mater. 2015, 25, 3524–3533. [Google Scholar] [CrossRef]
- Wang, G.; Ni, J.; Wang, H.; Gao, L. High-performance CNT-wired MoO3 nanobelts for Li-storage application. J. Mater. Chem. A 2013, 1, 4112. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, A. Nanostructured transition metal oxides as advanced anodes for lithium-ion batteries. Sci. Bull. 2015, 60, 823–838. [Google Scholar] [CrossRef]
- Mai, L.; Yang, F.; Zhao, Y.; Xu, X.; Xu, L.; Hu, B.; Luo, Y.; Liu, H. Molybdenum oxide nanowires: Synthesis & properties. Mater. Today 2011, 14, 346–353. [Google Scholar]
- Chernova, N.A.; Roppolo, M.; Dillon, A.C.; Whittingham, M.S. Layered vanadium and molybdenum oxides: Batteries and electrochromics. J. Mater. Chem. 2009, 19, 2526. [Google Scholar] [CrossRef]
- Lou, S.N.; Ng, Y.H.; Ng, C.; Scott, J.; Amal, R. Harvesting, storing and utilising solar energy using MoO3: Modulating structural distortion through pH adjustment. ChemSusChem 2014, 7, 1934–1941. [Google Scholar] [CrossRef]
- Lou, S.N.; Yap, N.; Scott, J.; Amal, R.; Ng, Y.H. Influence of MoO3 (110) crystalline plane on its self-charging photoelectrochemical properties. Sci. Rep. 2014, 4, 7428. [Google Scholar] [CrossRef]
- Riley, L.A.; Lee, S.-H.; Gedvilias, L.; Dillon, A.C. Optimization of MoO3 nanoparticles as negative-electrode material in high-energy lithium ion batteries. J. Power Sources 2010, 195, 588–592. [Google Scholar] [CrossRef]
- Tsumura, T.; Inagaki, M. Lithium insertion/extraction reaction on crystalline MoO3. Solid State Ionics 1997, 104, 183–189. [Google Scholar] [CrossRef]
- Madhu Mohan, V.; Bin, H.; Chen, W. Enhancement of electrochemical properties of MoO3 nanobelts electrode using PEG as surfactant for lithium battery. J. Solid State Electrochem. 2010, 14, 1769–1775. [Google Scholar] [CrossRef]
- Chen, T.; Duan, M.; Shi, P.; Fang, S. Ultrathin nanoporous membranes derived from protein-based nanospheres for high-performance smart molecular filtration. J. Mater. Chem. A 2017, 5, 20208–20216. [Google Scholar] [CrossRef]
- Ghosal, A.; Shah, J.; Kotnala, R.K.; Ahmad, S. Facile green synthesis of nickel nanostructures using natural polyol and morphology dependent dye adsorption properties. J. Mater. Chem. A 2013, 1, 12868. [Google Scholar] [CrossRef]
- Li, J.; Huang, Y.; Liu, Z.; Zhang, J.; Liu, X.; Luo, H.; Ma, Y.; Xu, X.; Lu, Y.; Lin, J.; et al. Chemical activation of boron nitride fibers for improved cationic dye removal performance. J. Mater. Chem. A 2015, 3, 8185–8193. [Google Scholar] [CrossRef]
- Shen, Y. Carbothermal synthesis of metal-functionalized nanostructures for energy and environmental applications. J. Mater. Chem. A 2015, 3, 13114–13188. [Google Scholar] [CrossRef]
- Yener, J.; Kopac, T.; Dogu, G.; Dogu, T. Dynamic analysis of sorption of Methylene Blue dye on granular and powdered activated carbon. Chem. Eng. J. 2008, 144, 400–406. [Google Scholar] [CrossRef]
- Liu, T.; Li, Y.; Du, Q.; Sun, J.; Jiao, Y.; Yang, G.; Wang, Z.; Xia, Y.; Zhang, W.; Wang, K.; et al. Adsorption of methylene blue from aqueous solution by graphene. Colloids Surf. B 2012, 90, 197–203. [Google Scholar] [CrossRef]
- Li, Y.; Du, Q.; Liu, T.; Peng, X.; Wang, J.; Sun, J.; Wang, Y.; Wu, S.; Wang, Z.; Xia, Y.; et al. Comparative study of methylene blue dye adsorption onto activated carbon, graphene oxide, and carbon nanotubes. Chem. Eng. Res. Des. 2013, 91, 361–368. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, F.; Chen, M.; Xu, Z.; Zhu, Z. Adsorption behavior of methylene blue on carbon nanotubes. Bioresour. Technol. 2010, 101, 3040–3046. [Google Scholar] [CrossRef]
- Deng, H.; Lu, J.; Li, G.; Zhang, G.; Wang, X. Adsorption of methylene blue on adsorbent materials produced from cotton stalk. Chem. Eng. J. 2011, 172, 326–334. [Google Scholar] [CrossRef]
- Fan, L.; Luo, C.; Li, X.; Lu, F.; Qiu, H.; Sun, M. Fabrication of novel magnetic chitosan grafted with graphene oxide to enhance adsorption properties for methyl blue. J. Hazard. Mater. 2012, 215–216, 272–279. [Google Scholar] [CrossRef]
| Adsorbent. | pH | Temp/K | qmax (mg/g) | References |
|---|---|---|---|---|
| Activated carbon | 7.0 | 293 | 91.0 | [35] |
| Graphene | 3.0 | 293 | 153.85 | [36] |
| Graphene Oxide | 6.0 | 298 | 243.9 | [37] |
| Carbon nanotubes | 7.0 | 298 | 46.2 | [38] |
| Cotton stalk | 7.0 | 308 | 147.06 | [39] |
| Chitosan/graphene oxide | 5.3 | 303 | 95.16 | [40] |
| MoO3 nanowires | 7.0 | 298 | 521.0 | This study |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Park, S.-J. Fabrication of MoO3 Nanowire-based Membrane Devices for the Selective Adsorption of Cationic Dyes from Aqueous Solutions with High Performance and Reusability. Micromachines 2019, 10, 586. https://doi.org/10.3390/mi10090586
Zhang Y, Park S-J. Fabrication of MoO3 Nanowire-based Membrane Devices for the Selective Adsorption of Cationic Dyes from Aqueous Solutions with High Performance and Reusability. Micromachines. 2019; 10(9):586. https://doi.org/10.3390/mi10090586
Chicago/Turabian StyleZhang, Yifan, and Soo-Jin Park. 2019. "Fabrication of MoO3 Nanowire-based Membrane Devices for the Selective Adsorption of Cationic Dyes from Aqueous Solutions with High Performance and Reusability" Micromachines 10, no. 9: 586. https://doi.org/10.3390/mi10090586
APA StyleZhang, Y., & Park, S.-J. (2019). Fabrication of MoO3 Nanowire-based Membrane Devices for the Selective Adsorption of Cationic Dyes from Aqueous Solutions with High Performance and Reusability. Micromachines, 10(9), 586. https://doi.org/10.3390/mi10090586




