Construction of a CQDs/Ag3PO4/BiPO4 Heterostructure Photocatalyst with Enhanced Photocatalytic Degradation of Rhodamine B under Simulated Solar Irradiation
Abstract
:1. Introduction
2. Materials and Methods
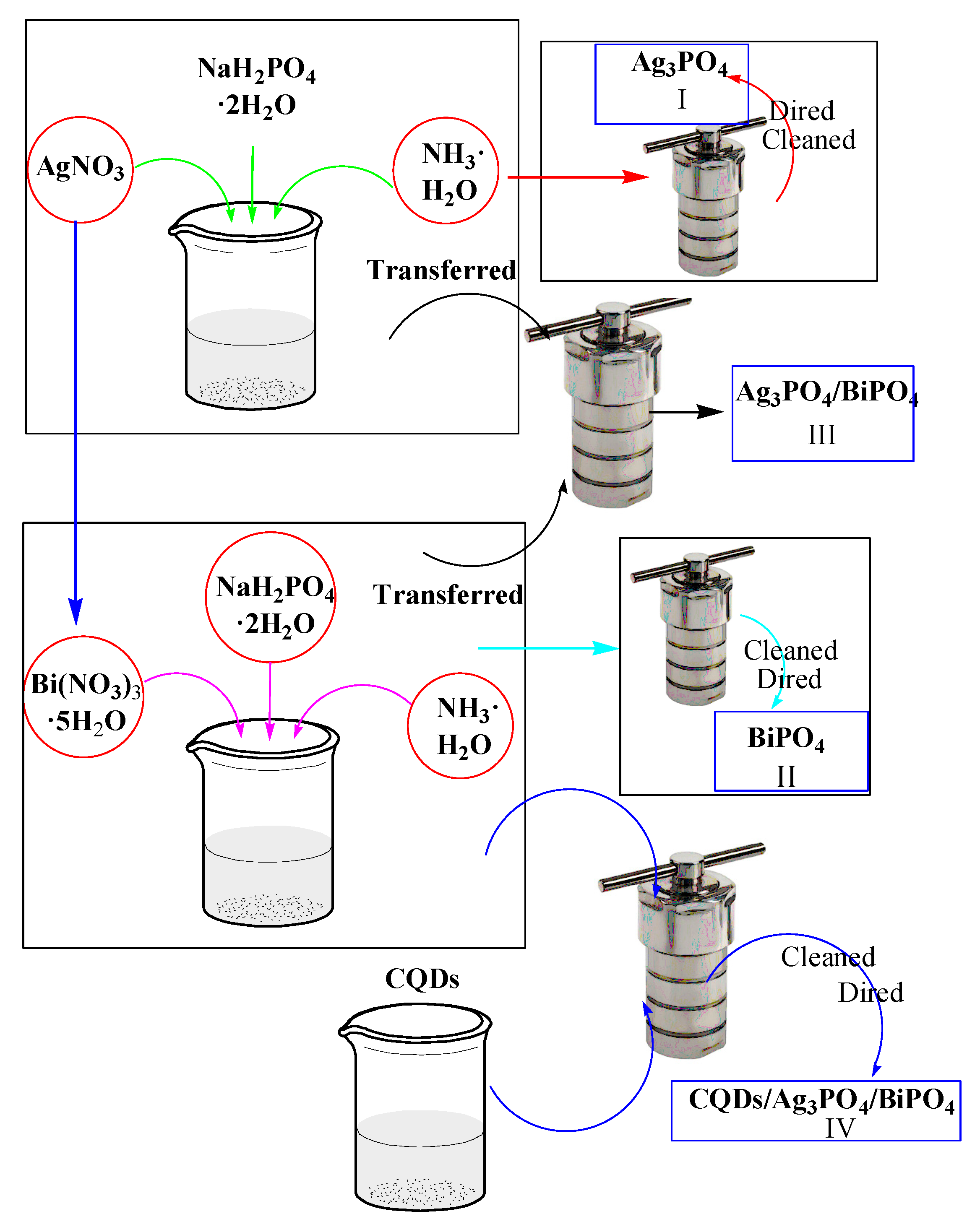
2.1. Synthesis of the Ag3PO4 Photocatalyst
2.2. Synthesis of the BiPO4 Photocatalyst
2.3. Synthesis of Ag3PO4/BiPO4 Photocatalyst
2.4. Synthesis of CQDs/Ag3PO4/BiPO4 Photocatalyst
2.5. Sample Characterization
2.6. Photocatalytic Testing
3. Results and Discussion
3.1. Phase Structural Analysis
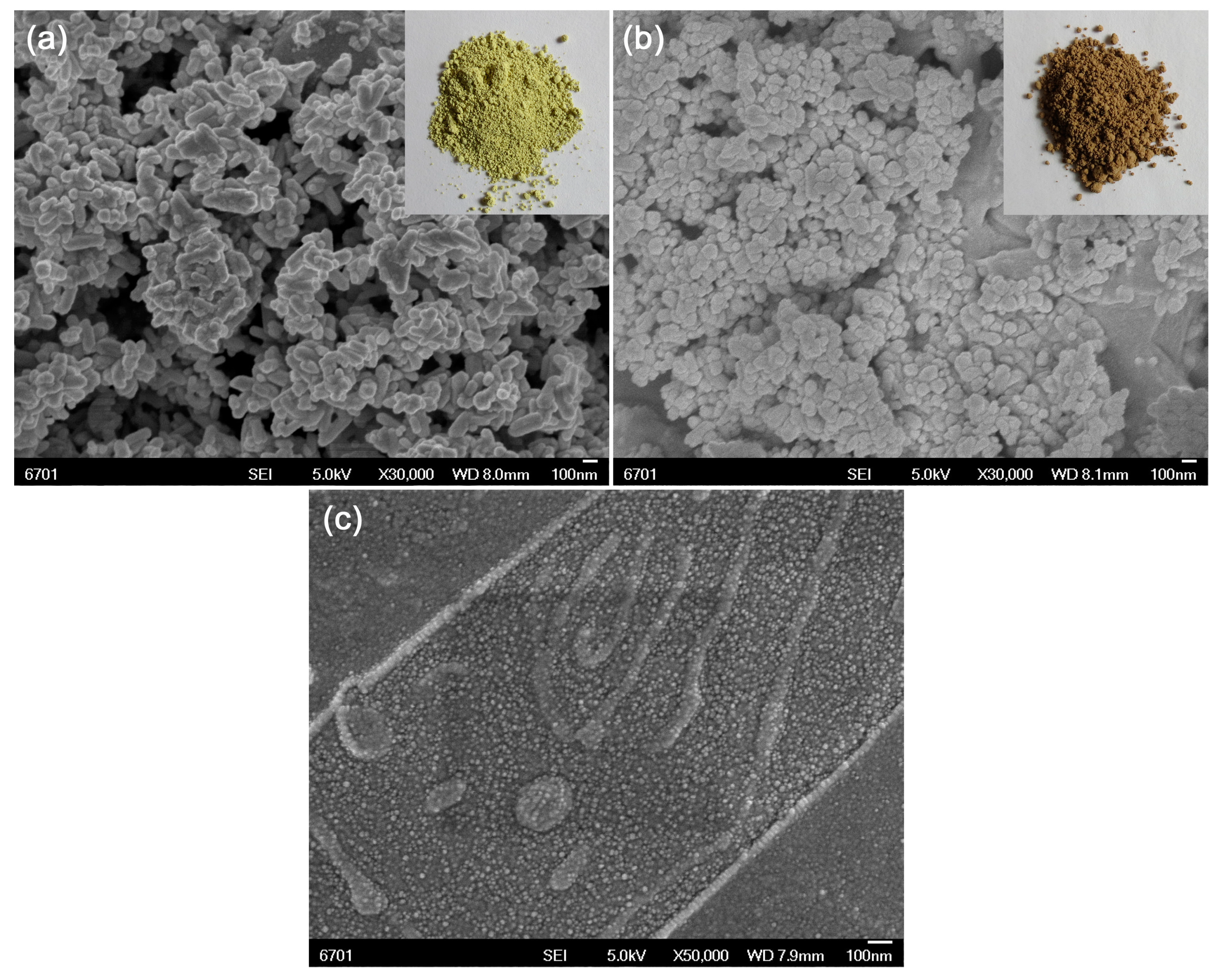
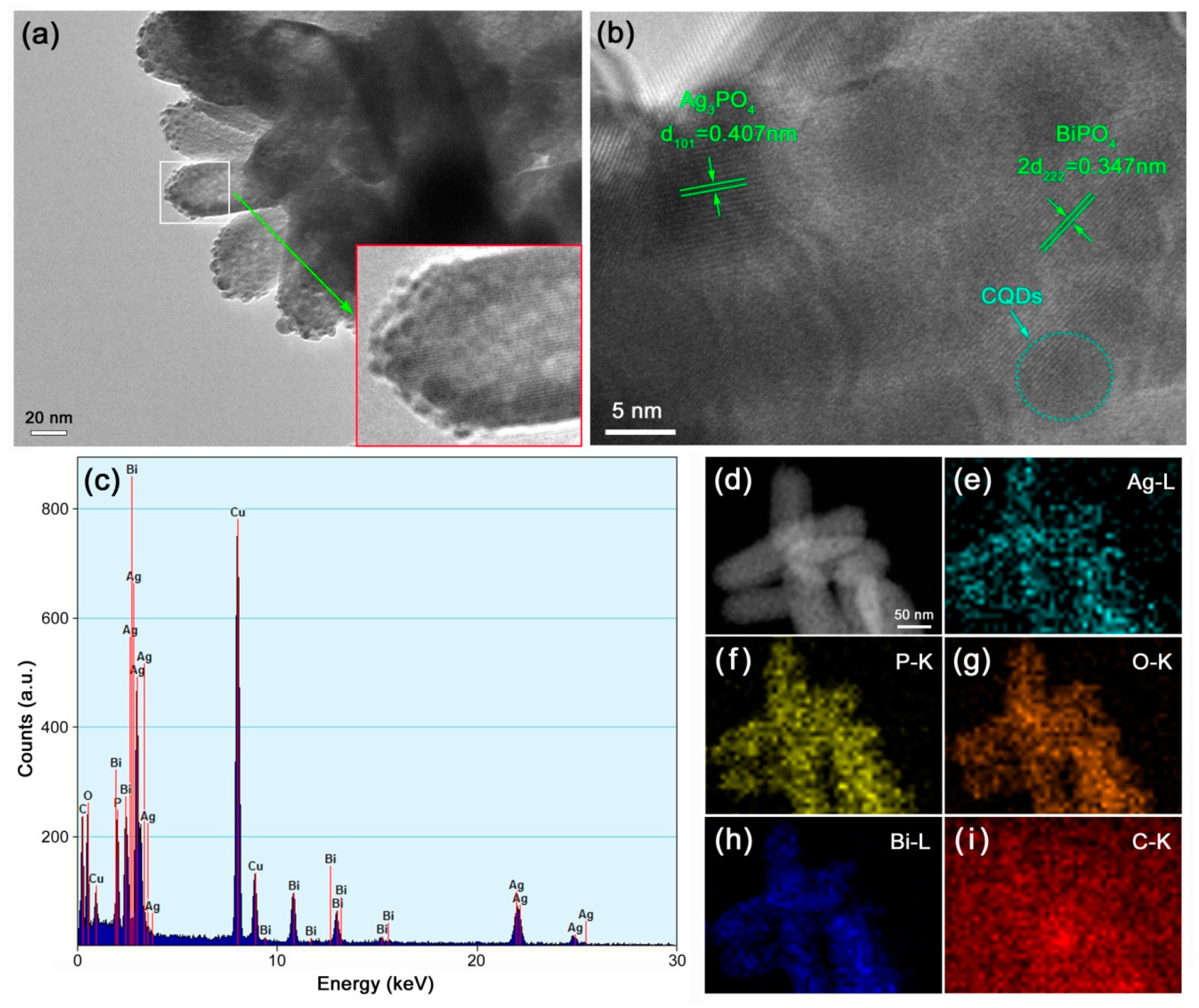
3.2. Surface Morphology and Elemental Component Analysis
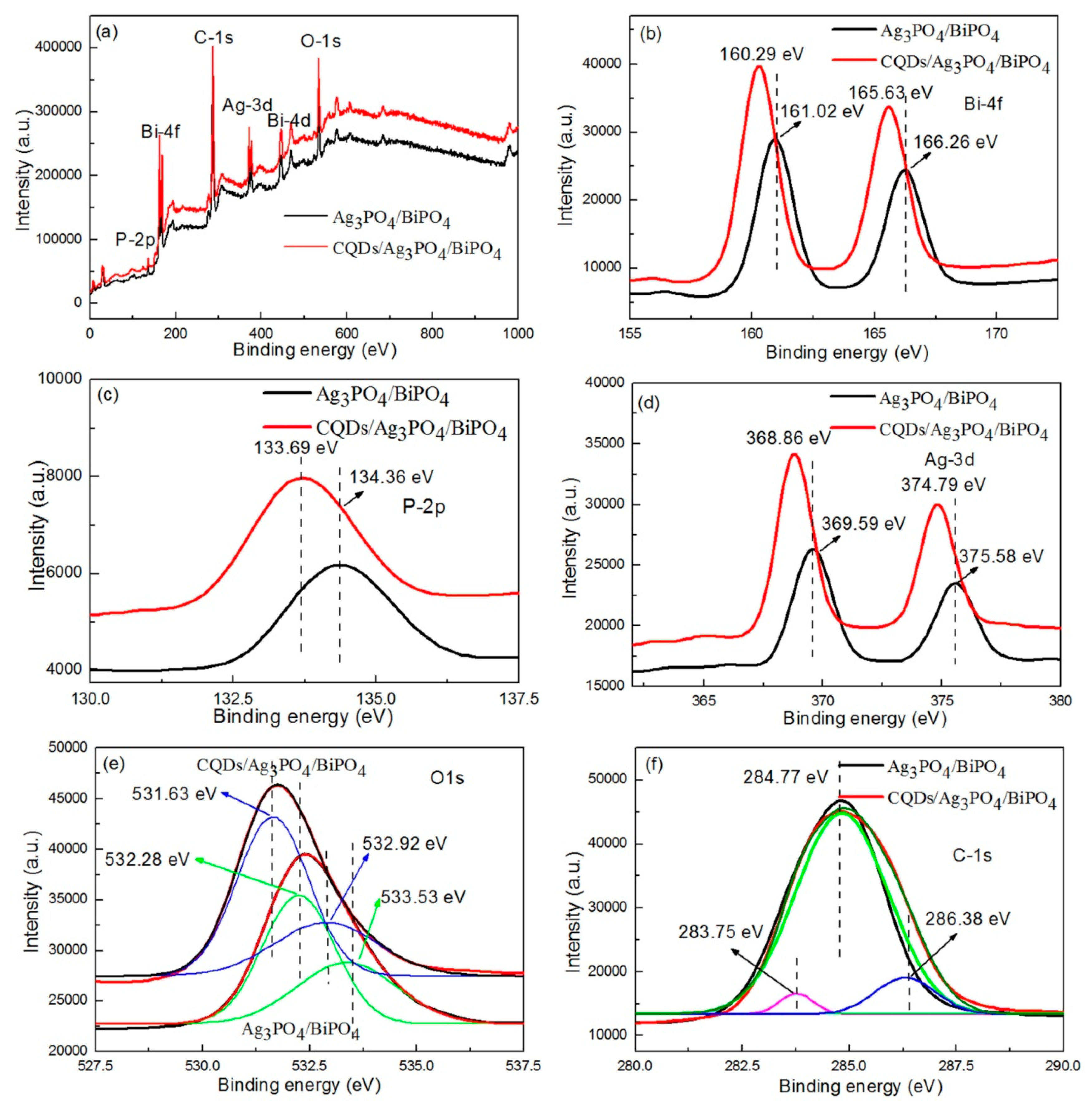
3.3. XPS Analysis
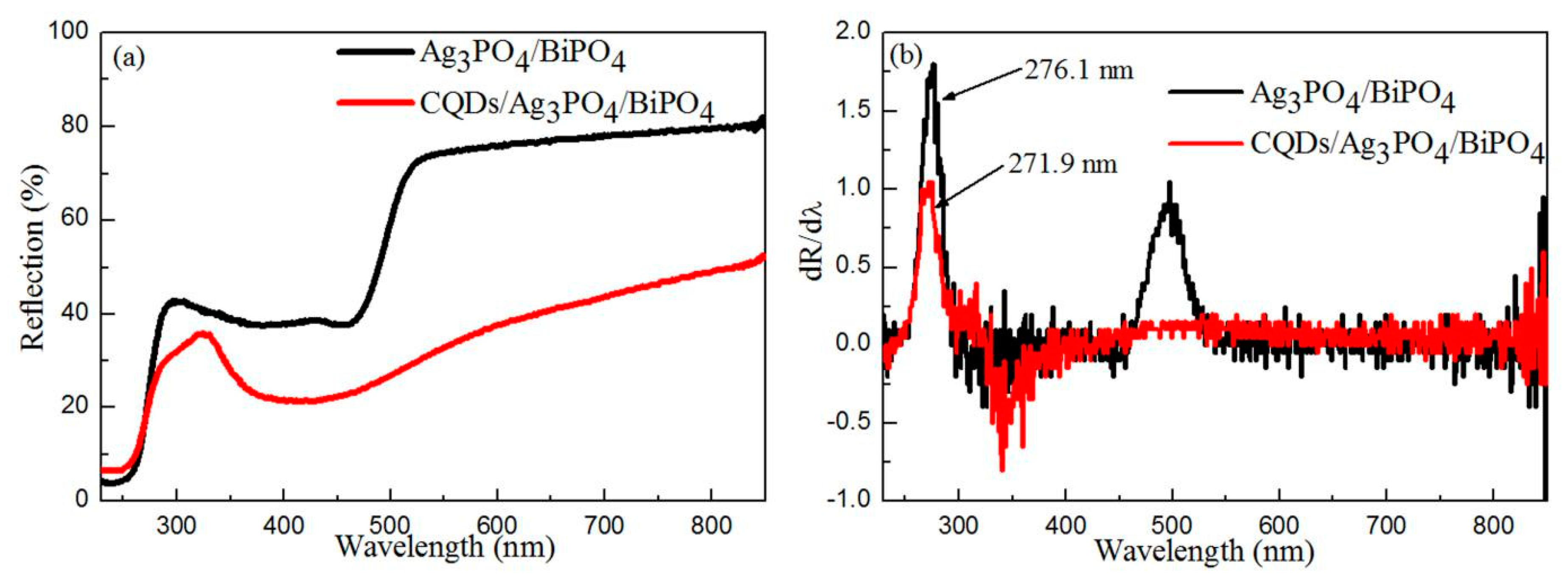
3.4. Optical Properties
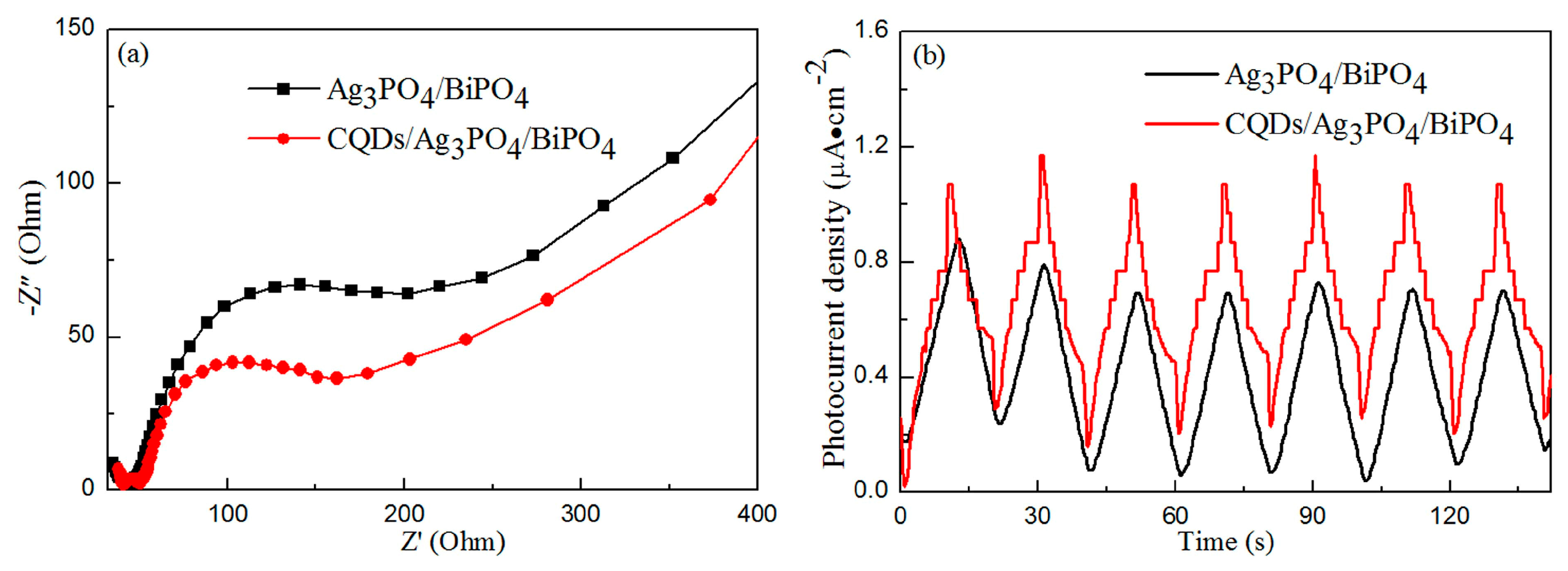
3.5. Photoelectrochemical Properties
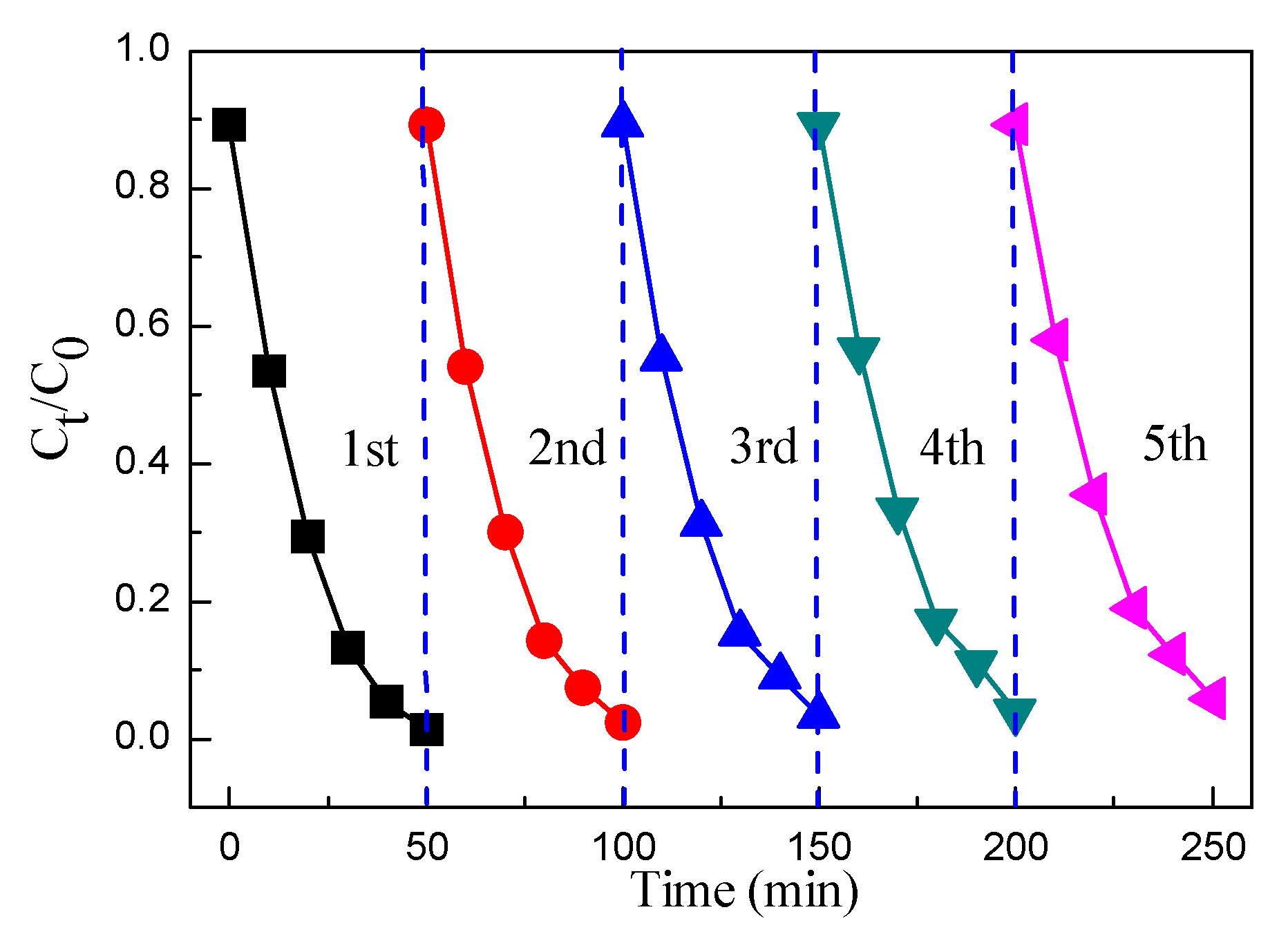
3.6. Photocatalytic Activity
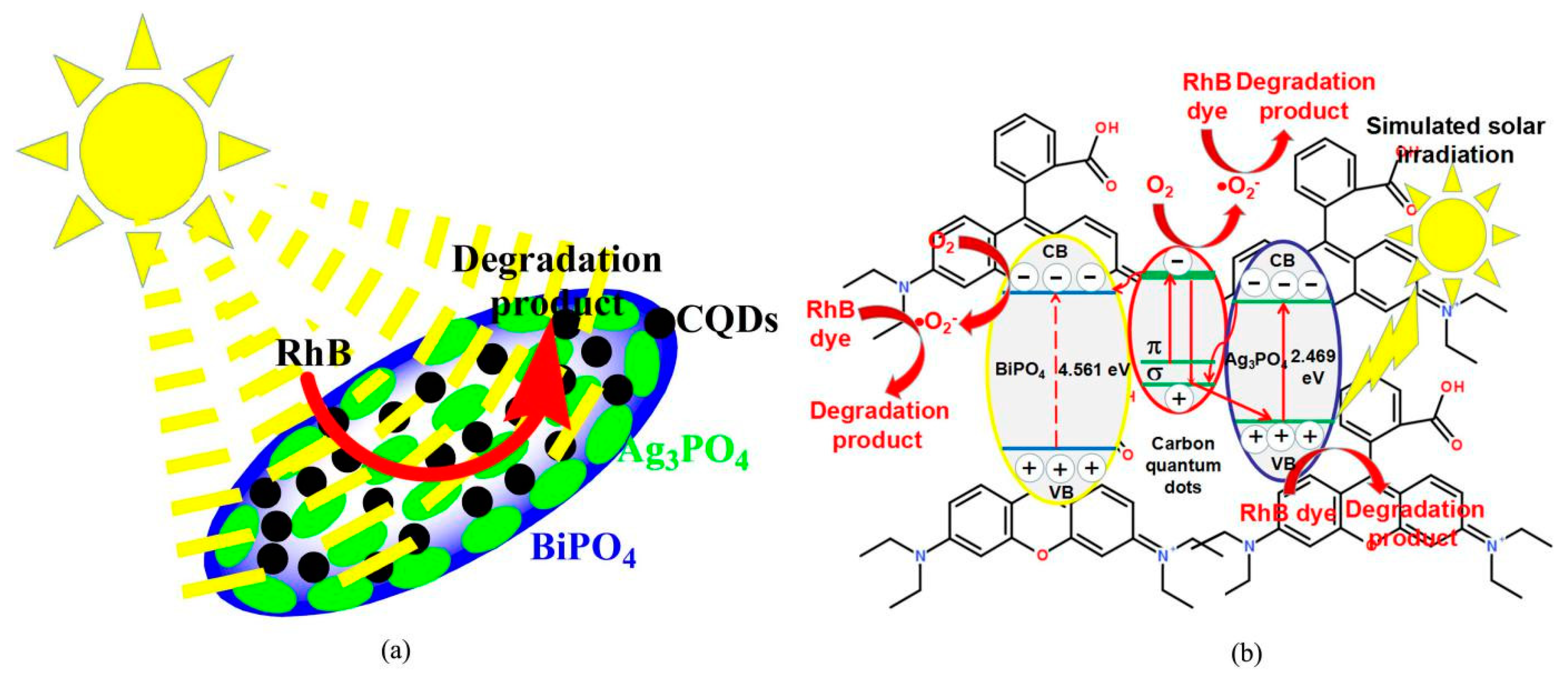
3.7. Photocatalytic Mechanism
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Darkwah, W.K.; Adormaa, B.B.; Sandrine, M.K.C.; Ao, Y. Modification strategies for enhancing the visible light responsive photocatalytic activity of the BiPO4 nano-based composite photocatalysts. Catal. Sci. Technol. 2019, 9, 546–566. [Google Scholar] [CrossRef]
- Zhao, X.X.; Yang, H.; Li, S.H.; Cui, Z.M.; Zhang, C.R. Synthesis and theoretical study of large-sized Bi4Ti3O12 square nanosheets with high photocatalytic activity. Mater. Res. Bull. 2018, 107, 180–188. [Google Scholar] [CrossRef]
- Police, A.K.R.; Vattikuti, S.V.P.; Mandari, K.K.; Chennaiahgari, M.; Sharma, P.; Valluri, D.K.; Byon, C. Bismuth oxide cocatalyst and copper oxide sensitizer in Cu2O/TiO2/Bi2O3 ternary photocatalyst for efficient hydrogen production under solar light irradiation. Ceram. Int. 2018, 44, 11783–11791. [Google Scholar] [CrossRef]
- Vattikuti, S.V.P.; Police, A.K.R.; Shim, J.; Byon, C. In situ fabrication of the Bi2O3-V2O5 hybrid embedded with graphitic carbon nitride nanosheets: Oxygen vacancies mediated enhanced visible-light-driven photocatalytic degradation of organic pollutants and hydrogen evolution. Appl. Surf. Sci. 2018, 447, 740–756. [Google Scholar] [CrossRef]
- Vattikuti, S.V.P.; Police, A.K.R.; Shim, J.; Byon, C. Visible-Light-Driven photocatalytic activity of SnO2-ZnO quantum dots anchored on g-C3N4 nanosheets for photocatalytic pollutant degradation and H2 production. ACS Omega 2018, 3, 7587–7602. [Google Scholar] [CrossRef]
- Vattikuti, S.V.P.; Police, A.K.R.; Shim, J.; Byon, C. Sacrifcial-template-free synthesis of core-shell C@Bi2S3 heterostructures for efcient supercapacitor and H2 production applications. Sci. Rep. 2018, 8, 4194. [Google Scholar] [CrossRef] [PubMed]
- He, Z.M.; Tang, B.; Su, J.B.; Xia, Y.M. Fabrication of novel Cu2O/Bi24O31Br10 composites and excellent photocatalytic performance. J. Mater. Sci. Mater. Electron. 2018, 29, 19544–19553. [Google Scholar] [CrossRef]
- Zangeneh, H.; Zinatizadeh, A.A.L.; Habibi, M.; Akia, M.; Isa, M.H. Photocatalytic oxidation of organic dyes and pollutants in wastewater using different modified titanium dioxides: A comparative review. J. Ind. Eng. Chem. 2015, 26, 1–36. [Google Scholar] [CrossRef]
- Zheng, C.X.; Yang, H.; Cui, Z.M.; Zhang, H.M.; Wang, X.X. A novel Bi4Ti3O12/Ag3PO4 heterojunction photocatalyst with enhanced photocatalytic performance. Nanoscale Res. Lett. 2017, 12, 608. [Google Scholar] [CrossRef]
- Wang, X.; Ma, J.; Kong, Y.; Fan, C.; Peng, M.; Komarneni, S. Synthesis of p-n heterojunction Ag3PO4/NaTaO3 composite photocatalyst for enhanced visible-light-driven photocatalytic performance. Mater. Lett. 2019, 251, 192–195. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Si, M.; Zhang, H. Carbon cloth-supported MoS2/Ag2S/Ag3PO4 composite with high photocatalytic activity and recyclability. ChemCatChem 2019, 11, 1017–1025. [Google Scholar]
- Zheng, C.X.; Yang, H. Assembly of Ag3PO4 nanoparticles on rose flower-like Bi2WO6 hierarchical architectures for achieving high photocatalytic performance. J. Mater. Sci. Mater. Electron. 2018, 29, 9291–9300. [Google Scholar] [CrossRef]
- Naresh, G.; Lee, A.T.; Meena, V.; Satyanarayana, M.; Huang, M.H. Photocatalytic activity suppression of Ag3PO4-deposited Cu2O octahedra and rhombic dodecahedra. J. Phys. Chem. C 2019, 123, 2314–2320. [Google Scholar] [CrossRef]
- Tran, T.T.T.; Doan, V.D.; Ho, T.T.T.; Le, V.T.; Nguyen, H.T. Novel of TiO2/Ag3PO4/bentonite composite photocatalyst: Preparation, characterization, and application for degradation of methylene blue in aqueous solution. Environ. Eng. Sci. 2019, 36, 71–80. [Google Scholar] [CrossRef]
- Geng, Z.; Yang, M.; Qi, X.; Li, Z.; Yang, X.; Huo, M.; Crittenden, J.C. Co3(PO4)2/Ag3PO4 with enhanced simulated sunlight photocatalytic activity toward ofloxacin degradation and mechanism insight. J. Chem. Technol. Biotechnol. 2019, 94, 1660–1669. [Google Scholar] [CrossRef]
- Di, L.J.; Yang, H.; Xian, T.; Chen, X.J. Facile synthesis and enhanced visible-light photocatalytic activity of novel p-Ag3PO4/n-BiFeO3 heterojunction composites for dye degradation. Nanoscale Res. Lett. 2018, 13, 257. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Zhu, Y.; Zhu, Y. Enhanced photocatalytic performance for the BiPO4−x nanorod induced by surface oxygen vacancy. J. Phys. Chem. C 2013, 117, 18520. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, B.; Huang, H.; He, Y.; Fei, B.; Lv, F. BiPO4/reduced graphene oxidecomposites photocatalyst with high photocatalytic activity. Appl. Surf. Sci. 2014, 319, 272–277. [Google Scholar] [CrossRef]
- Lin, H.; Ye, H.; Xu, B.; Cao, J.; Chen, S. Ag3PO4 quantum dot sensitized BiPO4: A novel p–n junction Ag3PO4/BiPO4 with enhanced visible-light photocatalytic activity. Catal. Commun. 2013, 37, 55–59. [Google Scholar] [CrossRef]
- Wu, S.; Zheng, H.; Wu, Y.; Lin, W.; Xu, T.; Guan, M. Hydrothermal synthesis and visible light photocatalytic activity enhancement of BiPO4/Ag3PO4 composites for degradation of typical dyes. Ceram. Int. 2014, 40, 14613–14620. [Google Scholar] [CrossRef]
- Mohaghegh, N.; Rahimi, E.; Gholami, M.R. Ag3PO4/BiPO4 p–n heterojunction nanocomposite prepared in room-temperature ionic liquid medium with improved photocatalytic activity. Mater. Sci. Semicond. Process. 2015, 39, 506–514. [Google Scholar] [CrossRef]
- Mohaghegh, N.; Tasviri, M.; Rahimi, E.; Gholami, M.R. A novel p–n junction Ag3PO4/BiPO4-based stabilized Pickering emulsion for highly efficient photocatalysis. RSC Adv. 2015, 5, 12944–12955. [Google Scholar] [CrossRef]
- Li, J.; Yuan, H.; Zhu, Z. In situ growth of Ag3PO4 on N-BiPO4 nanorod: A core–shell heterostructure for high performance photocatalyst. J. Colloid Interface Sci. 2016, 462, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Jiang, H.; Wang, L. Enhanced photo-stability and photocatalytic activity of Ag3PO4 via modification with BiPO4 and polypyrrole. Appl. Surf. Sci. 2017, 420, 43–52. [Google Scholar] [CrossRef]
- Mohaghegh, N.; Tasviri, M.; Rahimi, E.; Gholami, M.R. Comparative studies on Ag3PO4/BiPO4–metal-organic framework–graphene-based nanocomposites for photocatalysis application. Appl. Surf. Sci. 2015, 351, 216–224. [Google Scholar] [CrossRef]
- Wang, S.Y.; Yang, H.; Wang, X.X.; Feng, W.J. Surface disorder engineering of flake-like Bi2WO6 crystals for enhanced photocatalytic activity. J. Electron. Mater. 2019, 48, 2067–2076. [Google Scholar] [CrossRef]
- Wang, S.F.; Gao, H.J.; Wei, Y.; Li, Y.W.; Yang, X.H.; Fang, L.M.; Lei, L. Insight into the optical, color, photoluminescence properties, and photocatalytic activity of the N-O and C-O functional groups decorating spinel type magnesium aluminate. CrystEngComm 2019, 21, 263–277. [Google Scholar] [CrossRef]
- He, Z.M.; Xia, Y.M.; Tang, B.; Jiang, X.F.; Su, J.B. Fabrication and photocatalytic property of ZnO/Cu2O core-shell nanocomposites. Mater. Lett. 2016, 184, 148–151. [Google Scholar] [CrossRef]
- Zhao, X.X.; Yang, H.; Li, R.S.; Cui, Z.M.; Liu, X.Q. Synthesis of heterojunction photocatalysts composed of Ag2S quantum dots combined with Bi4Ti3O12 nanosheets for the degradation of dyes. Environ. Sci. Pollut. Res. 2019, 26, 5524–5538. [Google Scholar] [CrossRef]
- Fernando, K.A.S.; Sahu, S.P.; Liu, Y.; Lewis, W.K.; Guliants, E.; Jafariyan, A.; Wang, P.; Bunker, C.E.; Sun, Y.P. Carbon quantum dots and applications in photocatalytic energy conversion. ACS Appl. Mater. Interfaces 2015, 7, 8363–8376. [Google Scholar] [CrossRef]
- Yi, Z.; Huang, J.; Cen, C.L.; Chen, X.F.; Zhou, Z.G.; Tang, Y.J.; Wang, B.Y.; Yi, Y.G.; Wang, J.; Wu, P.H. Nanoribbon-ring cross perfect metamaterial graphene multi-band absorber in THz range and the sensing application. Results Phys. 2019, 14, 102367. [Google Scholar] [CrossRef]
- Cen, C.L.; Zhang, Y.B.; Liang, C.P.; Chen, X.F.; Yi, Z.; Duan, T.; Tang, Y.J.; Ye, X.; Yi, Y.G.; Xiao, S.Y. Numerical investigation of a tunable dual-band metamaterial perfect absorber consisting of two-intersecting graphene nanorings arrays. Phys. Lett. A 2019, 383, 3030–3035. [Google Scholar] [CrossRef]
- Yi, Z.; Liang, C.P.; Chen, X.F.; Zhou, Z.G.; Tang, Y.J.; Ye, X.; Yi, Y.G.; Wang, J.Q.; Wu, P.H. Dual-band plasmonic perfect absorber based on graphene metamaterials for refractive index sensing application. Micromachines 2019, 10, 443. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Zhu, J.K.; Tong, H.; Yang, X.D.; Wu, X.X.; Pang, Z.Y.; Yang, H.; Qi, Y.P. A theoretical study of a plasmonic sensor comprising a gold nano-disk array on gold film with an SiO2 spacer. Chin. Phys. B 2019, 28, 044201. [Google Scholar] [CrossRef]
- Wang, X.X.; Zhu, J.K.; Wen, X.L.; Wu, X.X.; Wu, Y.; Su, Y.W.; Tong, H.; Qi, Y.P.; Yang, H. Wide range refractive index sensor based on a coupled structure of Au nanocubes and Au film. Opt. Mater. Express 2019, 9, 3079–3088. [Google Scholar] [CrossRef]
- Cen, C.L.; Yi, Z.; Zhang, G.F.; Zhang, Y.B.; Liang, C.P.; Chen, X.F.; Tang, Y.J.; Ye, X.; Yi, Y.G.; Wang, J.Q.; et al. Theoretical design of a triple-band perfect metamaterial absorber in the THz frequency range. Results Phys. 2019, 14, 102463. [Google Scholar] [CrossRef]
- Li, M.W.; Liang, C.P.; Zhang, Y.B.; Yi, Z.; Chen, X.F.; Zhou, Z.G.; Yang, H.; Tang, Y.J.; Yi, Y.G. Terahertz wideband perfect absorber based on open loop with cross nested structure. Results Phys. 2019, in press. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Cen, C.L.; Liang, C.P.; Yi, Z.; Chen, X.F.; Li, M.W.; Zhou, Z.G.; Tang, Y.J.; Yi, Y.G.; Zhang, G.F. Dual-band switchable terahertz metamaterial absorber based on metal nanostructure. Results Phys. 2019, 14, 102422. [Google Scholar] [CrossRef]
- Wang, X.X.; Bai, X.L.; Pang, Z.Y.; Zhu, J.K.; Wu, Y.; Yang, H.; Qi, Y.P.; Wen, X.L. Surface-enhanced Raman scattering by composite structure of gold nanocube-PMMA-gold film. Opt. Mater. Express 2019, 9, 1872–1881. [Google Scholar] [CrossRef]
- Tong, H.; Xu, Y.Q.; Su, Y.W.; Wang, X.X. Theoretical study for fabricating elliptical subwavelength nanohole arrays by higher-order waveguide-mode interference. Results Phys. 2019, 14, 102460. [Google Scholar] [CrossRef]
- Yan, Y.X.; Yang, H.; Yi, Z.; Li, R.S.; Wang, X.X. Enhanced photocatalytic performance and mechanism of Au@CaTiO3 composites with Au nanoparticles assembled on CaTiO3 nanocuboids. Micromachines 2019, 10, 254. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Lu, G.; Hu, X.; Xie, W.; Xia, Q.; Luo, S. Ag/Ag3PO4/BiPO4 nanocomposites with high photocatalytic degradation activity for 2,4-dichlorophenol. Mater. Lett. 2017, 188, 392–395. [Google Scholar] [CrossRef]
- Di, L.J.; Yang, H.; Xian, T.; Chen, X.J. Construction of Z-scheme g-C3N4/CNT/Bi2Fe4O9 composites with improved simulated-sunlight photocatalytic activity for the dye degradation. Micromachines 2018, 9, 613. [Google Scholar] [CrossRef] [PubMed]
- Alansi, A.M.; Al-Qunaibit, M.; Alade, I.O.; Qahtan, T.F.; Saleh, T.A. Visible-light responsive BiOBr nanoparticles loaded on reduced graphene oxide for photocatalytic degradation of dye. J. Mol. Liq. 2018, 253, 297–304. [Google Scholar] [CrossRef]
- Gao, H.J.; Wang, F.; Wang, S.F.; Wang, X.X.; Yi, Z.; Yang, H. Photocatalytic activity tuning in a novel Ag2S/CQDs/CuBi2O4 composite: Synthesis and photocatalytic mechanism. Mater. Res. Bull. 2019, 115, 140–149. [Google Scholar] [CrossRef]
- Di, L.J.; Xian, T.; Sun, X.F.; Li, H.Q.; Zhou, Y.J.; Ma, J.; Yang, H. Facile preparation of CNT/Ag2S nanocomposites with improved visible and NIR light photocatalytic degradation activity and their catalytic mechanism. Micromachines 2019, 10, 503. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.F.; Zu, X.T.; Sun, G.Z.; Li, D.M.; He, C.D.; Xiang, X.; Liu, W.; Han, S.B.; Li, S. Highly dispersed spinel (Mg, Ca, Ba)-ferrite nanoparticles: Tuning the particle size and magnetic properties through a modified polyacrylamide gel route. Ceram. Int. 2016, 42, 19133–19140. [Google Scholar] [CrossRef]
- Wang, S.F.; Zhang, C.F.; Sun, G.G.; Yuan, Y.G.; Chen, L.; Xiang, X.; Ding, Q.P.; Chen, B.; Li, Z.J.; Zu, X.T. Self-assembling synthesis of α-Al2O3–carbon composites and a method to increase their photoluminescence. J. Lumin. 2014, 153, 393–400. [Google Scholar] [CrossRef]
- Pan, C.; Li, D.; Ma, X.; Chen, Y.; Zhu, Y. Effects of distortion of PO4 tetrahedron on the photocatalytic performances of BiPO4. Catal. Sci. Technol. 2011, 1, 1399–1405. [Google Scholar] [CrossRef]
- Chen, X.; Dai, Y.; Wang, X. Methods and mechanism for improvement of photocatalytic activity and stability of Ag3PO4: A review. J. Alloys Compd. 2015, 649, 910–932. [Google Scholar] [CrossRef]
- Ye, Y.C.; Yang, H.; Zhang, H.M.; Jiang, J.L. A promising Ag2CrO4/LaFeO3 heterojunction photocatalyst applied to photo-Fenton degradation of RhB. Environ. Technol. 2018, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, S.; Zhou, J.; Li, D.; Zhou, X.; Ge, C.; Fang, Y. Novel mesoporous g-C3N4 and BiPO4 nanorods hybrid architectures and their enhanced visible-light-driven photocatalytic performances. Chem. Eng. J. 2014, 241, 344–351. [Google Scholar] [CrossRef]
- Cruz-Filho, J.F.; Costa, T.M.S.; Lima, M.S.; Silva, L.J.; Santos, R.S.; Cavalcante, L.S.; Longo, E.; Luz, G.E., Jr. Effect of different synthesis methods on the morphology, optical behavior, and superior photocatalytic performances of Ag3PO4 sub-microcrystals using white-light-emitting diodes. J. Photochem. Photobiol. A Chem. 2019, 377, 14–25. [Google Scholar] [CrossRef]
- Pooladi, M.; Shokrollahi, H.; Lavasani, S.A.N.H.; Yang, H. Investigation of the structural, magnetic and dielectric properties of Mn-doped Bi2Fe4O9 produced by reverse chemical co-precipitation. Mater. Chem. Phys. 2019, 229, 39–48. [Google Scholar] [CrossRef]
- Yan, Y.X.; Yang, H.; Zhao, X.X.; Zhang, H.M.; Jiang, J.L. A hydrothermal route to the synthesis of CaTiO3 nanocuboids using P25 as the titanium source. J. Electron. Mater. 2018, 47, 3045–3050. [Google Scholar] [CrossRef]
- Wang, S.F.; Zhang, C.F.; Sun, G.G.; Chen, B.; Xiang, X.; Wang, H.; Fang, L.M.; Tian, Q.; Ding, Q.P.; Zu, X.T. Fabrication of a novel light emission material AlFeO3 by a modified polyacrylamide gel route and characterization of the material. Opt. Mater. 2013, 36, 482–488. [Google Scholar] [CrossRef]
- Gupta, A.; Verma, N.C.; Khan, S.; Nandi, C.K. Carbon dots for naked eye colorimetric ultrasensitive arsenic and glutathione detection. Biosens. Bioelectron. 2016, 81, 465–472. [Google Scholar] [CrossRef]
- Munoz-Sandoval, E.; Cortes-Lopez, A.J.; Flores-Gomez, B.; Fajardo-Diaz, J.L.; Sanchez-Salas, R.; Lopez-Urias, F. Carbon sponge-type nanostructures based on coaxial nitrogen-doped multiwalled carbon nanotubes grown by CVD using benzylamine as precursor. Carbon 2017, 115, 409–421. [Google Scholar] [CrossRef]
- Wang, X.X.; Pang, Z.Y.; Yang, H.; Qi, Y.P. Theoretical study of subwavelength circular grating fabrication based on continuously exposed surface plasmon interference lithography. Results Phys. 2019, 14, 102446. [Google Scholar] [CrossRef]
- Gao, H.; Yang, H.; Wang, S.; Zhao, X. Optical and electrochemical properties of perovskite type MAlO3 (M=Y, La, Ce) pigments synthesized by a gamma-ray irradiation assisted polyacrylamide gel route. Ceram. Int. 2018, 44, 14754–14766. [Google Scholar] [CrossRef]
- Zhao, X.X.; Yang, H.; Zhang, H.M.; Cui, Z.M.; Feng, W.J. Surface-disorder-engineering-induced enhancement in the photocatalytic activity of Bi4Ti3O12 nanosheets. Desalin. Water Treat. 2019, 145, 326–336. [Google Scholar] [CrossRef]
- Yan, Y.X.; Yang, H.; Yi, Z.; Xian, T.; Wang, X.X. Direct Z-scheme CaTiO3@BiOBr composite photocatalysts with enhanced photodegradation of dyes. Environ. Sci. Pollut. Res. 2019, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.M.; He, Z.M.; Su, J.B.; Hu, K.J. Construction of novel Cu2O/PbBiO2Br composites with enhanced photocatalytic activity. J. Mater. Sci. Mater. Electron. 2019, 30, 9843–9854. [Google Scholar] [CrossRef]
- Zhao, X.X.; Yang, H.; Cui, Z.M.; Yi, Z.; Yu, H. Synergistically enhanced photocatalytic performance of Bi4Ti3O12 nanosheets by Au and Ag nanoparticles. J. Mater. Sci. Mater. Electron. 2019, 30, 13785–13796. [Google Scholar] [CrossRef]
- Raizadaa, P.; Sudhaik, A.; Singh, P.; Shandilya, P.; Gupta, V.K.; Hosseini-Bandegharaei, A.; Agrawal, S. Ag3PO4 modified phosphorus and sulphur co-doped graphitic carbon nitride as a direct Z-scheme photocatalyst for 2, 4-dimethyl phenol degradation. J. Photochem. Photobiol. A Chem. 2019, 374, 22–35. [Google Scholar] [CrossRef]
- Xia, Y.M.; He, Z.M.; Hu, K.J.; Tang, B.; Su, J.B.; Liu, Y.; Li, X.P. Fabrication of n-SrTiO3/p-Cu2O heterojunction composites with enhanced photocatalytic performance. J. Alloys Compd. 2018, 753, 356–363. [Google Scholar] [CrossRef]
- Lei, Y.; Wang, G.; Guo, P.; Song, H.C. Silver phosphate based plasmonic photocatalyst: Highly active visible-light-enhanced photocatalytic property and photosensitized degradation of pollutants. Funct. Mater. Lett. 2012, 5, 1250047. [Google Scholar] [CrossRef]
- Shekofteh-Gohari, M.; Habibi-Yangjeh, A. Photosensitization of Fe3O4/ZnO by AgBr and Ag3PO4 to fabricate novel magnetically recoverable nanocomposites with significantly enhanced photocatalytic activity under visible-light irradiation. Ceram. Int. 2016, 42, 15224–15234. [Google Scholar] [CrossRef]
- Du, X.; Wan, J.; Jia, J.; Pan, C.; Hu, X.; Fan, J.; Liu, E. Photocatalystic degradation of RhB over highly visible-light-active Ag3PO4-Bi2MoO6 heterojunction using H2O2 electron capturer. Mater. Des. 2017, 119, 113–123. [Google Scholar] [CrossRef]
- Dong, C.; Wu, K.L.; Li, M.R.; Liu, L.; Wei, X.W. Synthesis of Ag3PO4–ZnO nanorod composites with high visible-light photocatalytic activity. Catal. Commun. 2014, 46, 32–35. [Google Scholar] [CrossRef]
- Vu, T.A.; Dao, C.D.; Hoang, T.T.T.; Nguyen, K.T.; Le, G.H.; Dang, P.T.; Tran, H.T.K.; Nguyen, T.V. Highly photocatalytic activity of novel nano-sized Ag3PO4 for Rhodamine B degradation under visible light irradiation. Mater. Lett. 2013, 92, 57–60. [Google Scholar] [CrossRef]
- Ye, H.; Lin, H.; Cao, J.; Chen, S.; Chen, Y. Enhanced visible light photocatalytic activity and mechanism of BiPO4 nanorods modified with AgI nanoparticles. J. Mol. Catal. A Chem. 2015, 397, 85–92. [Google Scholar] [CrossRef]
- Mohaghegh, N.; Rahimi, E. BiPO4 photocatalyst employing synergistic action of Ag/Ag3PO4 nanostructure and graphene nanosheets. Solid State Sci. 2016, 56, 10–15. [Google Scholar] [CrossRef]
- Wang, S.F.; Gao, H.J.; Fang, L.M.; Wei, Y.; Li, Y.W.; Lei, L. Synthesis and characterization of BaAl2O4 catalyst and its photocatalytic activity towards degradation of methylene blue dye. Z. Phys. Chem. 2018, 233, 1161–1181. [Google Scholar] [CrossRef]
- Di, L.J.; Yang, H.; Xian, T.; Liu, X.Q.; Chen, X.J. Photocatalytic and photo-Fenton catalytic degradation activities of Z-scheme Ag2S/BiFeO3 heterojunction composites under visible-light irradiation. Nanomaterials 2019, 9, 399. [Google Scholar] [CrossRef] [PubMed]
- Li, H.T.; Kang, Z.H.; Liu, Y.; Lee, S.T. Carbon nanodots: Synthesis, properties and applications. J. Mater. Chem. 2012, 22, 24230–24253. [Google Scholar] [CrossRef]
- Wang, S.Y.; Yang, H.; Yi, Z.; Wang, X.X. Enhanced photocatalytic performance by hybridization of Bi2WO6 nanoparticles with honeycomb-like porous carbon skeleton. J. Environ. Manag. 2019, 248, 109341. [Google Scholar] [CrossRef]


| Sample | Color Coordinates | Eg of Ag3PO4 (eV) | Eg of BiPO4 (eV) | |||||
|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | c* | Ho | ECIE* | |||
| Ag3PO4/BiPO4 | 87.110 | −3.666 | 30.235 | 30.456 | −83.087 | 92.281 | 2.469 | 4.491 |
| CQDs/Ag3PO4/BiPO4 | 63.817 | 4.286 | 15.421 | 16.006 | 74.468 | 65.794 | - | 4.561 |
| Samples | Light Source | Cphotocatalyst (g L−1) | CRhB (mg L−1) | Irradiation Time (min) | D% | Reference |
|---|---|---|---|---|---|---|
| CQDs/Ag3PO4/BiPO4 | 200 W Xe lamp | 1 | 5 | 50 | 98.7 | This work |
| 20wt%Ag3PO4/Bi2WO6 | 200 W Xe lamp | 0.5 | 5 | 120 | 94 | [9] |
| 10% Bi4Ti3O12/Ag3PO4 | 200 W Xe lamp | 0.2 | 5 | 30 | 99.5 | [6] |
| Ag-Ag3PO4 | 30 W fluorescent light lamp (λ ≥ 420 nm) | 0.75 | 10 | 60 | 70 | [67] |
| Fe3O4/ZnO/Ag3PO4 | 50 W LED lamp | 0.4 | 12 (10−5 mol/L) | 100 | 75 | [68] |
| 15 wt% Ag3PO4-Bi2MoO6 | 300 W Xe lamp with a 400-nm cutoff filter | 1 | 10 | 100 | 39 | [69] |
| Ag3PO4-ZnO (1:40) | 300 W Xe lamp with a 400-nm cutoff filter | 0.67 | 12 (10−5 mol/L) | 30 | 93 | [70] |
| Ag3PO4 | 15 W four fluorescent lamp | 0.3 | 15 | 60 | 75 | [71] |
| AgI/BiPO4 | 500 W Xe lamp with a 420-nm cutoff filter | 1.67 | 10 | 60 | 92.2 | [72] |
| Ag2S/CQDs/CuBi2O4 | 200 W Xe lamp | 1 | 5 | 60 | 99.3 | [45] |
| BiPO4/Ag/Ag3PO4 | 150 W Xe lamp with a 420-nm cutoff filter | 0.1 | 20 | 120 | 65 | [73] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, H.; Zheng, C.; Yang, H.; Niu, X.; Wang, S. Construction of a CQDs/Ag3PO4/BiPO4 Heterostructure Photocatalyst with Enhanced Photocatalytic Degradation of Rhodamine B under Simulated Solar Irradiation. Micromachines 2019, 10, 557. https://doi.org/10.3390/mi10090557
Gao H, Zheng C, Yang H, Niu X, Wang S. Construction of a CQDs/Ag3PO4/BiPO4 Heterostructure Photocatalyst with Enhanced Photocatalytic Degradation of Rhodamine B under Simulated Solar Irradiation. Micromachines. 2019; 10(9):557. https://doi.org/10.3390/mi10090557
Chicago/Turabian StyleGao, Huajing, Chengxiang Zheng, Hua Yang, Xiaowei Niu, and Shifa Wang. 2019. "Construction of a CQDs/Ag3PO4/BiPO4 Heterostructure Photocatalyst with Enhanced Photocatalytic Degradation of Rhodamine B under Simulated Solar Irradiation" Micromachines 10, no. 9: 557. https://doi.org/10.3390/mi10090557
APA StyleGao, H., Zheng, C., Yang, H., Niu, X., & Wang, S. (2019). Construction of a CQDs/Ag3PO4/BiPO4 Heterostructure Photocatalyst with Enhanced Photocatalytic Degradation of Rhodamine B under Simulated Solar Irradiation. Micromachines, 10(9), 557. https://doi.org/10.3390/mi10090557





