A Compact, Syringe-Assisted, Vacuum-Driven Micropumping Device
Abstract
1. Introduction
2. Materials and Methods
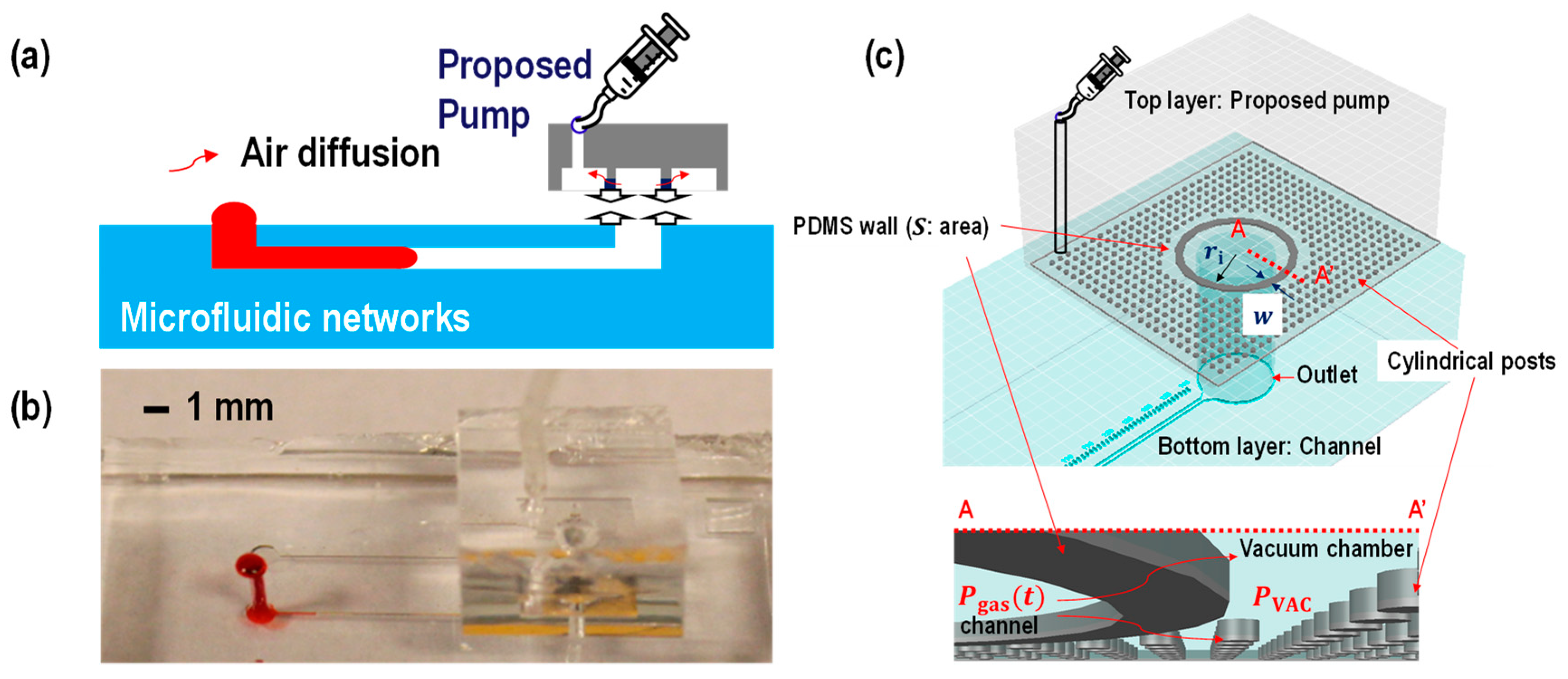
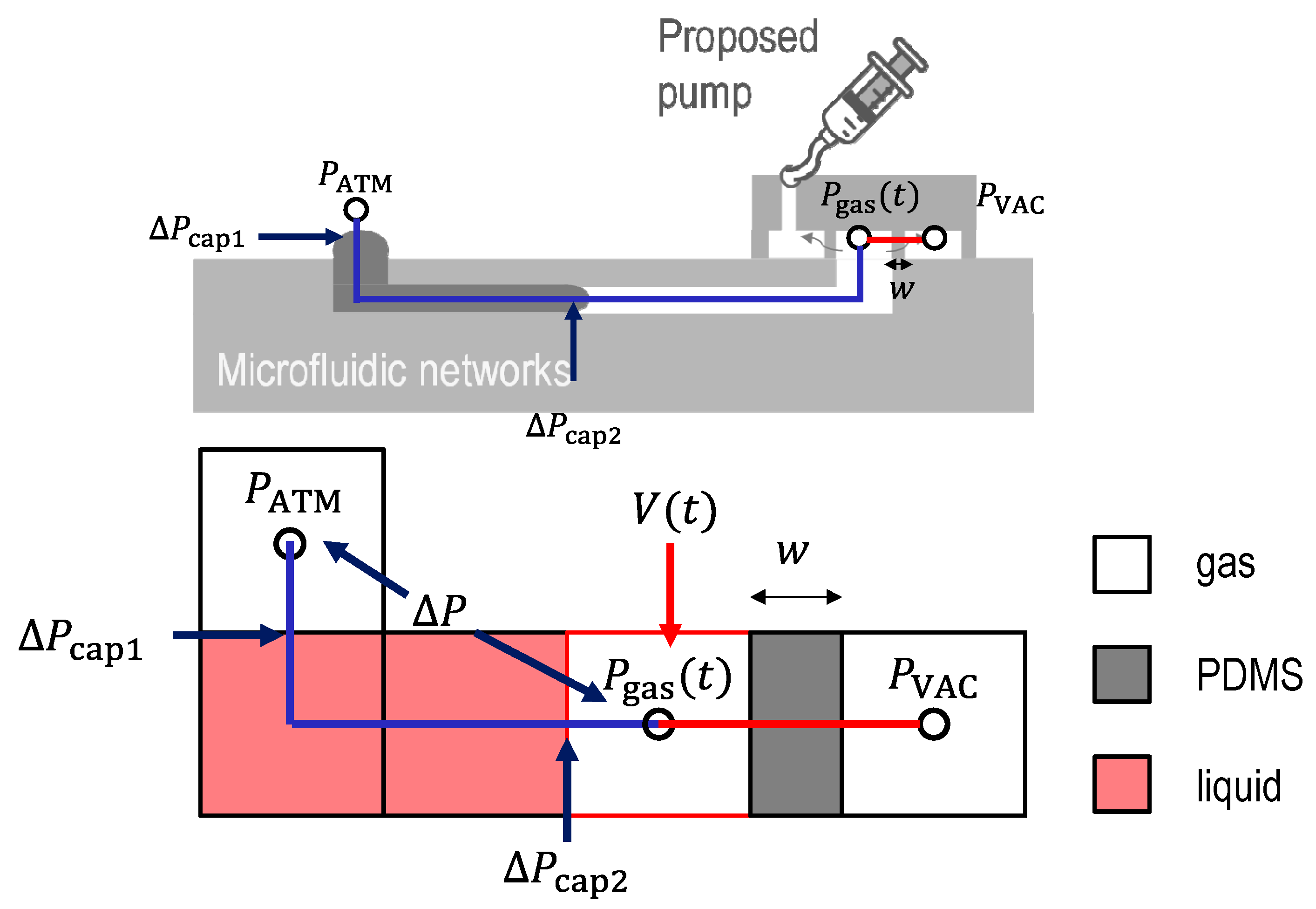
2.1. Theory
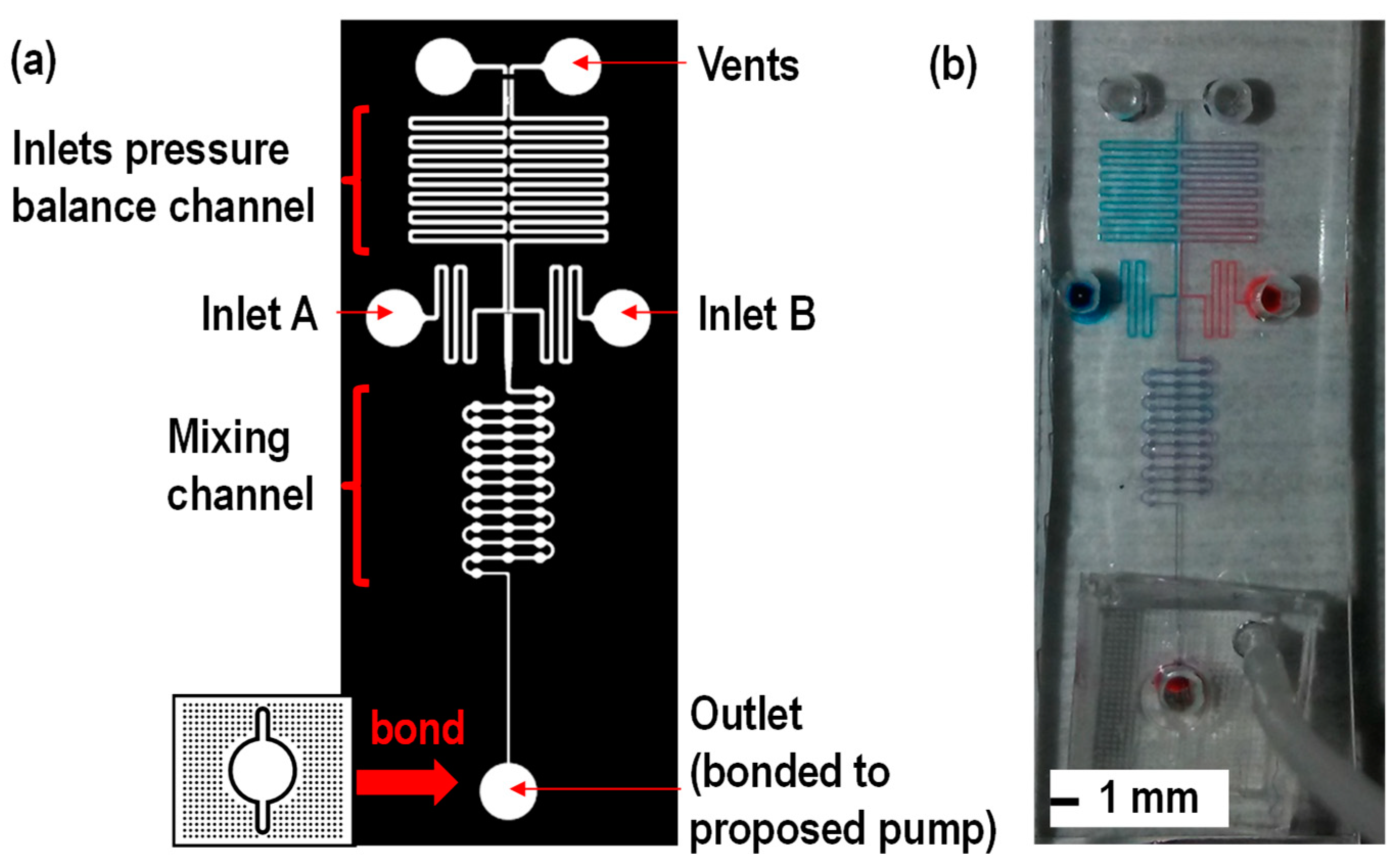
2.2. Device Fabrication
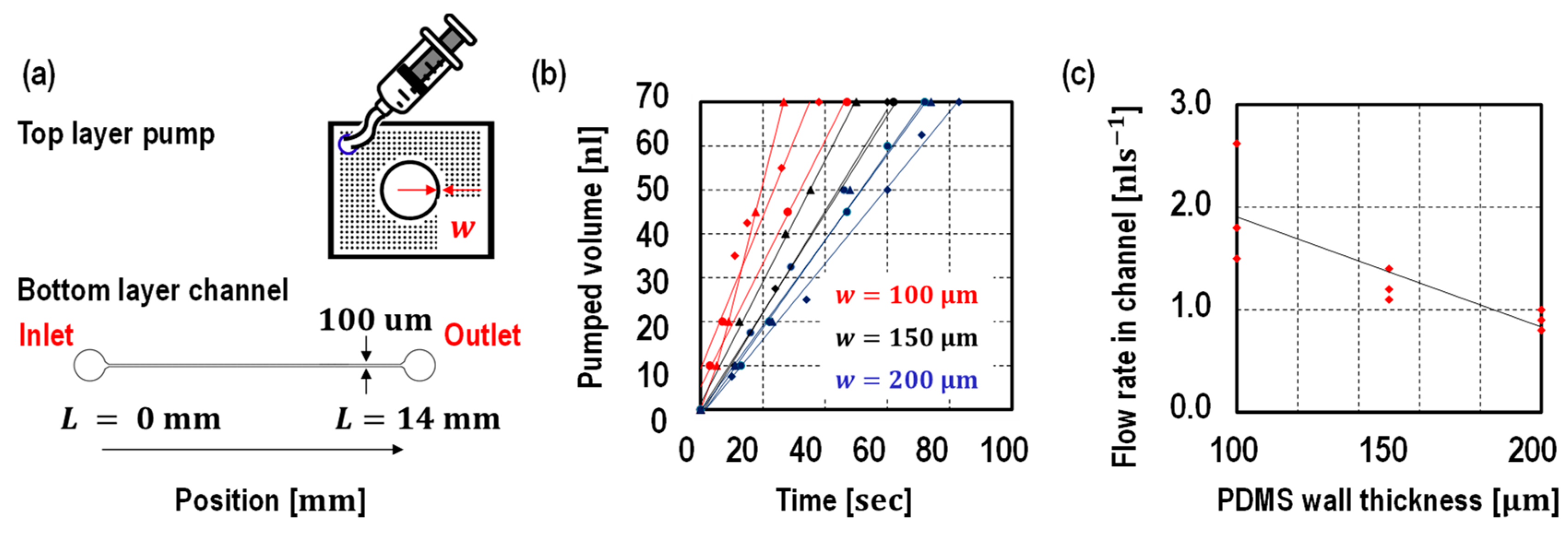
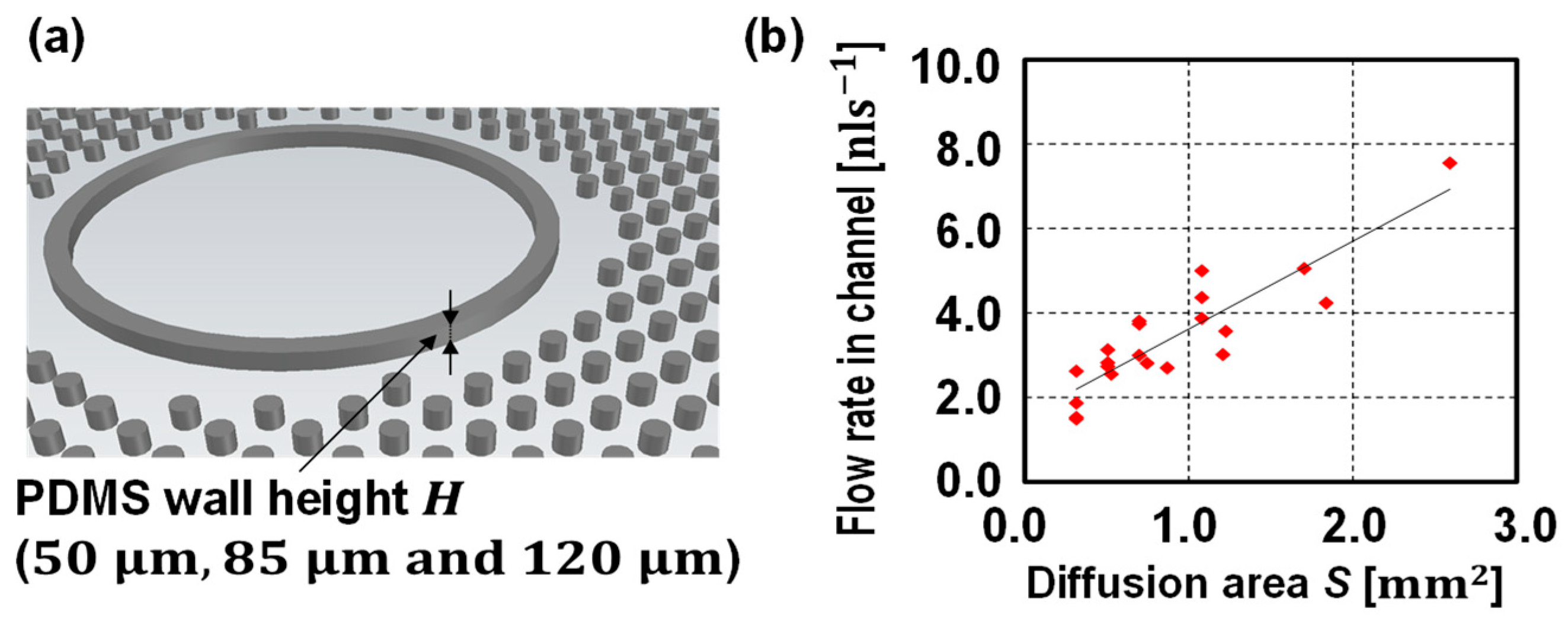
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, G.; Luo, Y.; Chen, Q.; Liao, L.; Zhao, J. A “Place n play” modular pump for portable microfluidic applications. Biomicrofluidics 2012, 6, 014118. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, K.; Sato, K.; Ichikaw, N.; Maeda, M. Power-free poly (dimethylsiloxane) microfluidic devices for gold nanoparticle-based DNA analysis. Lab Chip 2004, 4, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, K.; Omata, M.; Sato, K.; Maeda, M. Power-free sequential injection for microchip immunoassay toward point-of-care testing. Lab Chip 2006, 6, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, K.; Omata, M.; Maeda, M. Immunoassay on a power-free microchip with laminar flow-assisted dendritic amplification. Anal. Chem. 2007, 79, 6000–6004. [Google Scholar] [CrossRef] [PubMed]
- Juncker, D.; Schmid, H.; Drechsler, U.; Wolf, H.; Wolf, M.; Michel, B.; de Rooij, N.; Delamarche, E. Autonomous microfluidic capillary system. Anal. Chem. 2002, 74, 6139–6144. [Google Scholar] [CrossRef] [PubMed]
- Olanrewaju, A.O.; Robillard, A.; Dagher, M.; Juncker, D. Autonomous microfluidic capillaric circuits replicated from 3D-printed molds. Lab Chip 2016, 16, 3804–3814. [Google Scholar] [CrossRef] [PubMed]
- Olanrewaju, A.O.; Ng, A.; Decorwin-Martin, P.; Robillard, A.; Juncker, D. Microfluidic Capillaric Circuit for Rapid and Facile Bacteria Detection. Anal. Chem. 2017, 89, 6846–6853. [Google Scholar] [CrossRef] [PubMed]
- Olanrewaju, A.; Beaugrand, M.; Yafia, M.; Juncker, D. Capillary microfluidics in microchannels: from microfluidic networks to capillaric circuits. Lab Chip 2018, 18, 2323–2347. [Google Scholar] [CrossRef]
- Xu, L.; Lee, H.; Jetta, D.; Oh, K.W. Vacuum-driven powerfree microfluidics utilizing the gas solubility or permeability of polydimethylsiloxane (PDMS). Lab Chip 2015, 15, 3962–3979. [Google Scholar] [CrossRef]
- Xu, L.; Lee, H.; Oh, K.W. Syringe-assisted point-of-care micropumping utilizing the gas permeability of polydimethylsiloxane. Microfluid. Nanofluid. 2014, 17, 745–750. [Google Scholar] [CrossRef]
- Xu, L.; Lee, H.; Pinheiro, M.V.B.; Schneider, P.; Jetta, D.; Oh, K.W. Phaseguide-assisted blood separation microfluidic device for point-of-care applications. Biomicrofluidics 2015, 9, 014106. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Wang, A.; Koh, D.; Schneider, P.; Oh, K.W. A robust, portable and backflow-free micromixing device based on both capillary- and vacuum-driven flows. Lab Chip 2018, 18, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Koh, D.; Wang, A.; Schneider, P.; Oh, K.W. Hermetic encapsulation of negative-pressure-driven PDMS microfluidic devices using paraffin wax and glass. Microsyst. Technol. 2017, 24, 2035–2043. [Google Scholar] [CrossRef]
- Liu, B.; Li, M.; Tian, B.; Yang, X.; Yang, J. A positive pressure-driven PDMS pump for fluid handling in microfluidic chips. Microfluid. Nanofluid. 2018, 22, 94. [Google Scholar] [CrossRef]
- Tang, X.J.; Bo, Z. A PDMS viscometer for assaying endoglucanase activity. Analyst 2011, 136, 1222–1226. [Google Scholar] [CrossRef] [PubMed]
- Yeh, E.C.; Fu, C.C.; Hu, L.; Thakur, R.; Feng, J.; Lee, L.P. Self-powered integrated microfluidic point-of-care low-cost enabling (SIMPLE) chip. Sci. Adv. 2017, 3, e1501645. [Google Scholar] [CrossRef]
- Merkel, T.C.; Bondar, V.I.; Nagai, K.; Freeman, B.D.; Pinnau, I. Gas sorption, diffusion, and permeation in poly (dimethylsiloxane). J. Polym. Sci. Part B Polym. Phys. 2000, 38, 415–434. [Google Scholar] [CrossRef]
- Firpo, G.; Angeli, E.; Repetto, L.; Valbusa, U. Permeability thickness dependence of polydimethylsiloxane (PDMS) membranes. J. Membr. Sci. 2015, 481, 1–8. [Google Scholar] [CrossRef]
- Lamberti, A.; Marasso, S.L.; Cocuzza, M. PDMS membranes with tunable gas permeability for microfluidic applications. Rsc Adv. 2014, 4, 61415–61419. [Google Scholar] [CrossRef]
- Prabhakar, R.S.; Merkel, T.C.; Freeman, B.D.; Imizu, T.; Higuchi, A. Sorption and transport properties of propane and perfluoropropane in poly (dimethylsiloxane) and poly (1-trimethylsilyl-1-propyne). Macromolecules 2005, 38, 1899–1910. [Google Scholar] [CrossRef]
- Skelley, A.M.; Voldman, J. An active bubble trap and debubbler for microfluidic systems. Lab Chip 2008, 8, 1733–1737. [Google Scholar] [CrossRef]
- McDonald, J.C.; Duffy, D.C.; Anderson, J.R.; Chiu, D.T.; Wu, H.; Schueller, O.J.A.; Whitesides, G.M. Fabrication of microfluidic systems in poly(dimethylsiloxane). Electrophoresis 2000, 21, 27–40. [Google Scholar] [CrossRef]
- Lee, H.; Lee, K.; Ahn, B.; Xu, J.; Xu, L.; Oh, K.W. A new fabrication process for uniform SU-8 thick photoresist structures by simultaneously removing edge bead and air bubbles. J. Micromech. Microeng. 2011, 21, 125006. [Google Scholar] [CrossRef]
- Koh, D.; Wang, A.; Schneider, P.; Bosinski, B.; Oh, K.W. Introduction of a chemical-free metal PDMS thermal bonding for fabrication of flexible electrode by metal transfer onto PDMS. Micromachines 2017, 8, 280. [Google Scholar]
- Feng, Y.; Zhou, Z.; Ye, X.; Xiong, J. Passive valves based on hydrophobic microfluidics. Sens. Actuators A 2003, 108, 138–143. [Google Scholar] [CrossRef]
- Hansang, C.; Kim, H.Y.; Kang, J.Y.; Kim, T.S. How the capillary burst microvalve works. J. Colloid Interface Sci. 2007, 306, 379–385. [Google Scholar]
- Alvaro, M.; Fleischman, A.J.; Roy, S. Fabrication of multi-layer SU-8 microstructures. J. Micromech. Microeng. 2006, 16, 276. [Google Scholar]
- Aránzazu, d.C.; Greiner, C. SU-8: a photoresist for high-aspect-ratio and 3D submicron lithography. J. Micromech. Microeng. 2007, 17, R81. [Google Scholar]
- Addison, P.S. Fractals and Chaos: An Illustrated Course; CRC Press: Boca Raton, FL, USA, 1997; pp. 16–21. [Google Scholar]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, A.; Koh, D.; Schneider, P.; Breloff, E.; Oh, K.W. A Compact, Syringe-Assisted, Vacuum-Driven Micropumping Device. Micromachines 2019, 10, 543. https://doi.org/10.3390/mi10080543
Wang A, Koh D, Schneider P, Breloff E, Oh KW. A Compact, Syringe-Assisted, Vacuum-Driven Micropumping Device. Micromachines. 2019; 10(8):543. https://doi.org/10.3390/mi10080543
Chicago/Turabian StyleWang, Anyang, Domin Koh, Philip Schneider, Evan Breloff, and Kwang W. Oh. 2019. "A Compact, Syringe-Assisted, Vacuum-Driven Micropumping Device" Micromachines 10, no. 8: 543. https://doi.org/10.3390/mi10080543
APA StyleWang, A., Koh, D., Schneider, P., Breloff, E., & Oh, K. W. (2019). A Compact, Syringe-Assisted, Vacuum-Driven Micropumping Device. Micromachines, 10(8), 543. https://doi.org/10.3390/mi10080543





