Paper-Based Resazurin Assay of Inhibitor-Treated Porcine Sperm
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Porcine Sperm Preparation and Inhibitor Treatment Experiments
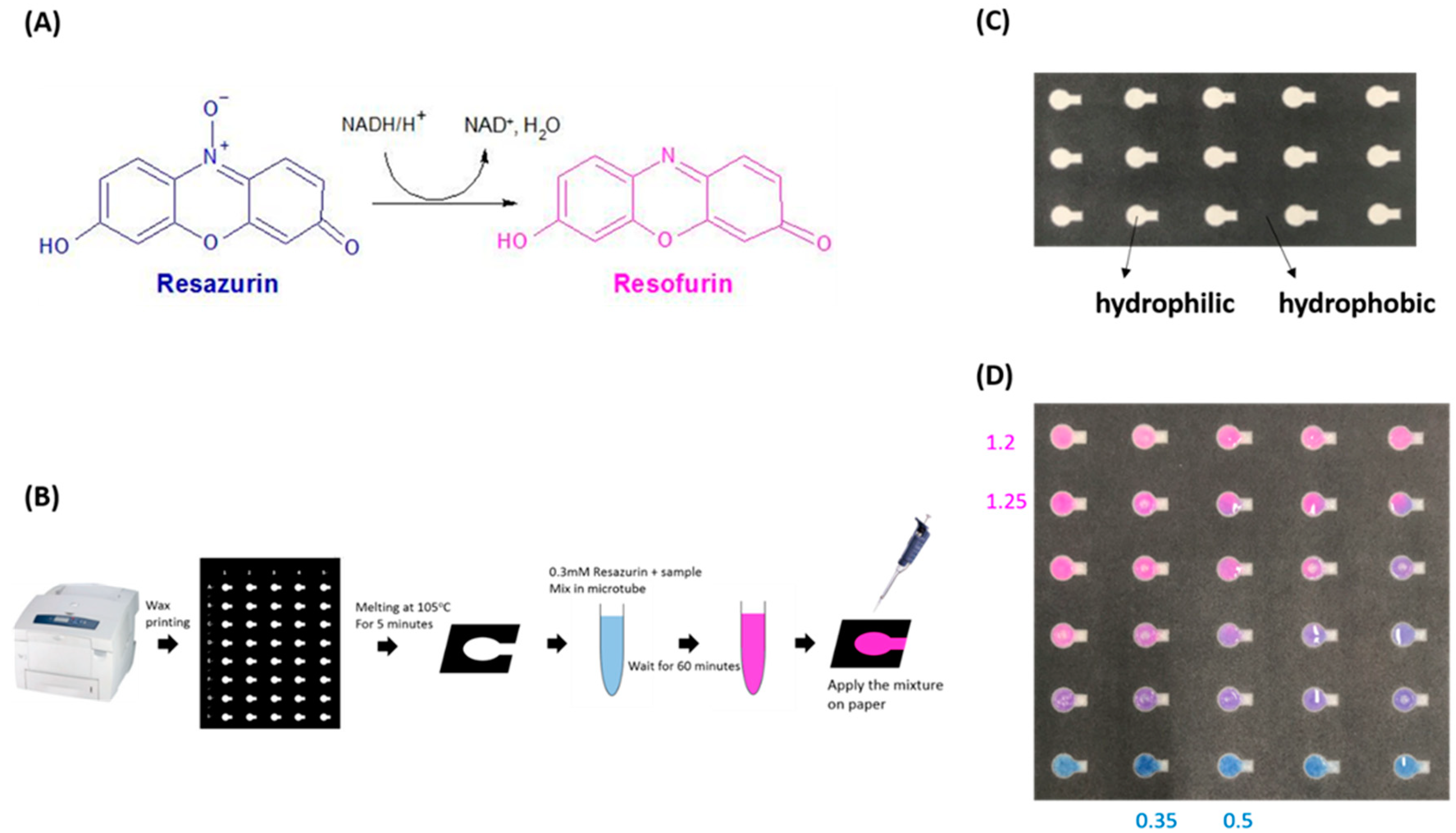
2.3. Sperm Motility and Resazurin Assays
2.4. Statistical Analysis
3. Result
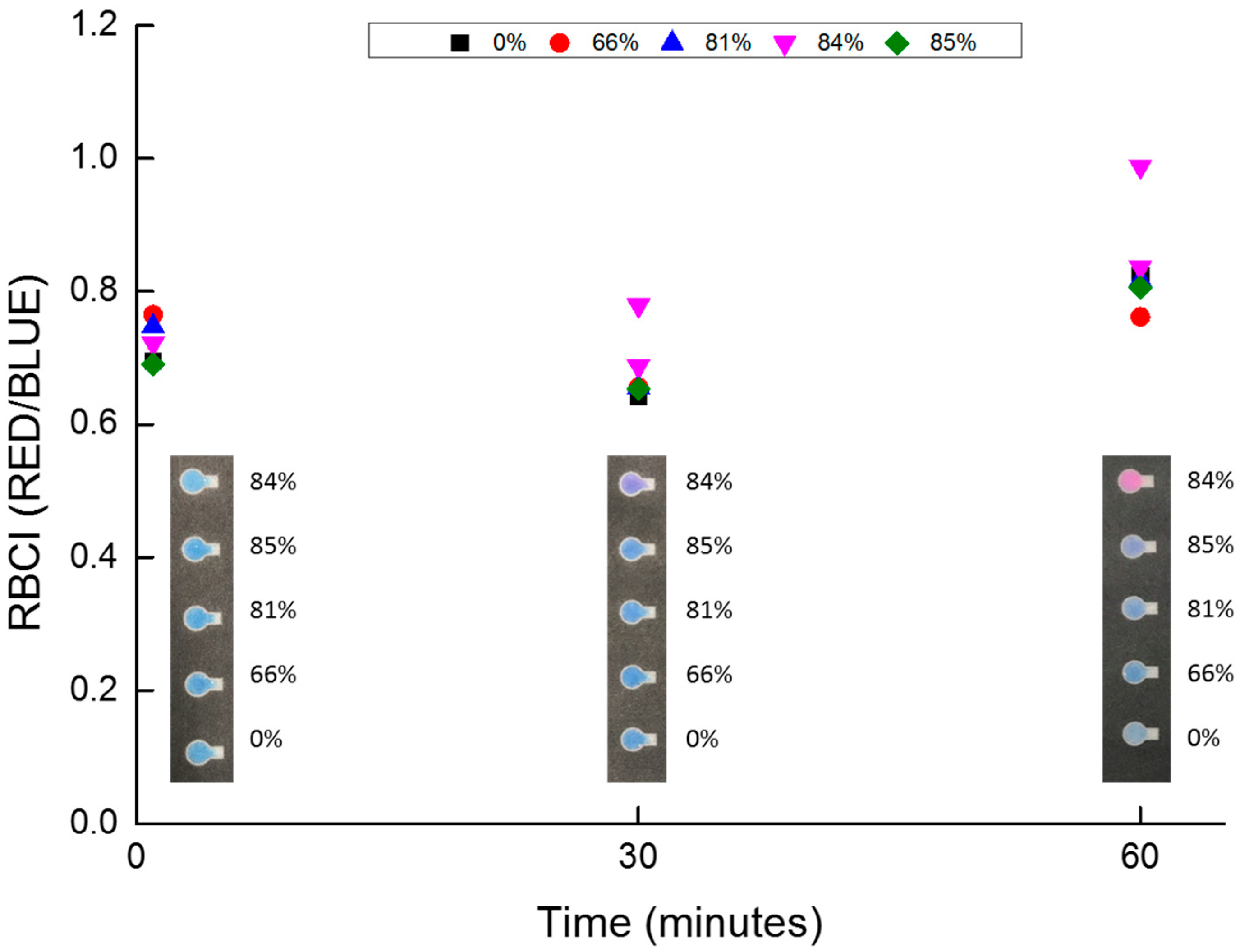
3.1. Resazurin Assay and Semen Parameters
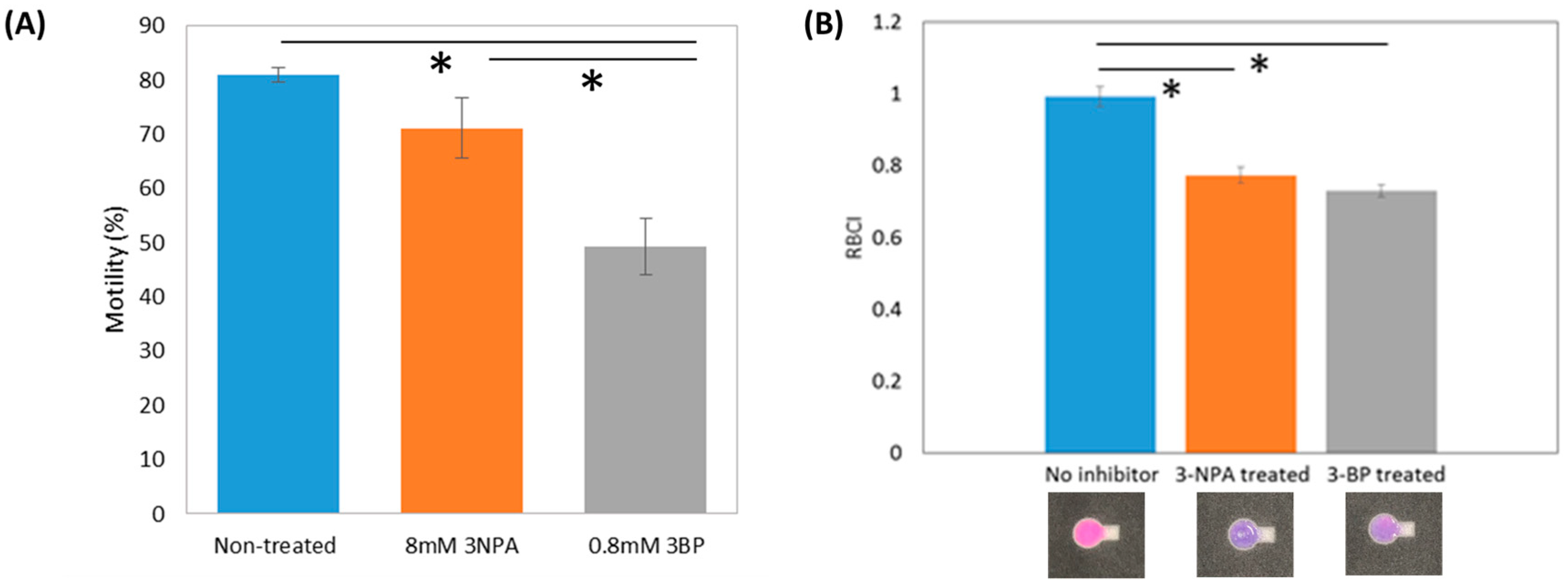
3.2. Inhibitor Treatment Effects and Inhibition Mechanism
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tratnyek, P.G.; Reilkoff, T.E.; Lemon, A.W.; Scherer, M.M.; Balko, B.A.; Feik, L.M.; Henegar, B.D. Visualizing Redox Chemistry: Probing Environmental Oxidation? Reduction Reactions with Indicator Dyes. Chem. Educ. 2001, 6, 172–179. [Google Scholar] [CrossRef]
- Bitenc, M.; Horvat, B.; Likozar, B.; Dražič, G.; Orel, Z.C. The impact of ZnO load, stability and morphology on the kinetics of the photocatalytic degradation of caffeine and resazurin. Appl. Catal. B Environ. 2013, 136, 202–209. [Google Scholar] [CrossRef]
- Safavi, A.; Rahmani, A.; Hosseini, V.N. Kinetic-spectrophotometric determination of sulfide by its reaction with resazurin. Anal. Bioanal. Chem. 1996, 354, 502–504. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, Y.; Li, W.; Zhang, Q.; Chen, J.; Zhou, H.; Yu, C. A facile method for detection of alkaline phosphatase activity based on the turn-on fluorescence of resorufin. Anal. Methods 2014, 6, 6105–6109. [Google Scholar] [CrossRef]
- Chou, C.K.; Penniston, J.T.; Brubaker, R.F. Ascorbic acid in the anterior chamber: Can it be measured noninvasively? Trans. Am. Ophthalmol. Soc. 1986, 84, 269–279. [Google Scholar]
- Guerin, T.F.; Mondido, M.; McClenn, B.; Peasley, B. Application of resazurin for estimating abundance of contaminant-degrading micro-organisms. Lett. Appl. Microbiol. 2001, 32, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Geusens, N.; Luyten, C.; Pijnenborg, R.; Al-Nasiry, S.; Hanssens, M. The use of Alamar Blue assay for quantitative analysis of viability, migration and invasion of choriocarcinoma cells. Hum. Reprod. 2007, 22, 1304–1309. [Google Scholar]
- Koyanagi, M.; Kawakabe, S.; Arimura, Y. A comparative study of colorimetric cell proliferation assays in immune cells. Cytotechnology 2016, 68, 1489–1498. [Google Scholar] [CrossRef]
- Helm, K.; Beyreis, M.; Mayr, C.; Ritter, M.; Jakab, M.; Kiesslich, T.; Plaetzer, K. In Vitro Cell Death Discrimination and Screening Method by Simple and Cost-Effective Viability Analysis. Cell. Physiol. Biochem. 2017, 41, 1011–1019. [Google Scholar] [CrossRef]
- Borra, R.C.; Lotufo, M.A.; Gagioti, S.M.; Barros, F.D.M.; Andrade, P.M. A simple method to measure cell viability in proliferation and cytotoxicity assays. Braz. Oral Res. 2009, 23, 255–262. [Google Scholar] [CrossRef]
- Aleshin, V.A.; Artiukhov, A.V.; Oppermann, H.; Kazantsev, A.V.; Lukashev, N.V.; Bunik, V.I. Mitochondrial Impairment May Increase Cellular NAD(P)H: Resazurin Oxidoreductase Activity, Perturbing the NAD(P)H-Based Viability Assays. Cells 2015, 4, 427–451. [Google Scholar] [CrossRef] [PubMed]
- Zrimek, P.; Zrimšek, P. Evaluation of a New Method and Diagnostic Test in Semen Analysis. In Artificial Insemination in Farm Animals; IntechOpen: London, UK, 2011; p. 10. [Google Scholar]
- Dart, M.G.; Mesta, J.; Crenshaw, C.; Ericsson, S.A. Modified Resazurin Reduction Test for Determining the Fertility Potential of Bovine Spermatozoa. Arch. Androl. 1994, 33, 71–75. [Google Scholar] [CrossRef] [PubMed]
- A Zalata, A.; Lammertijn, N.; Christophe, A.; Comhaire, F.H. The correlates and alleged biochemical background of the resazurin reduction test in semen. Int. J. Androl. 1998, 21, 289–294. [Google Scholar] [CrossRef]
- Mrkun, J.; Zrimšek, P.; Kunc, J.; Kosec, M. Spectrophotometric application of resazurin reduction assay to evaluate boar semen quality. Int. J. Androl. 2004, 27, 57–62. [Google Scholar]
- Zrimšek, P.; Kosec, M.; Kunc, J.; Mrkun, J. Determination of the diagnostic value of the resazurin reduction assay for evaluating boar semen by receiver operating characteristic analysis. Asian J. Androl. 2006, 8, 343–348. [Google Scholar] [CrossRef]
- El-Battawy, K.A.; El-Nattat, W. Evaluation of rabbit semen quality using Resazurin reduction test. Glob. Vet. 2013, 11, 767–770. [Google Scholar]
- Sabés-Alsina, M.; Planell, N.; Gil, S.; Tallo-Parra, O.; Maya-Soriano, M.J.; Taberner, E.; Piles, M.; Sabés, M.; Lopez-Bejar, M. Metabolic activity of sperm cells: Correlation with sperm cell concentration, viability and motility in the rabbit. Zygote 2016, 24, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Rafał, S.; Krystyna, F.; Marta, S.; Władysław, K. Spectrophotometric Analysis of the Resazurin Reduction Test as a Tool for Assessing Canine Semen Quality. Bull. Vet. Inst. Pulawy 2013, 57, 281–285. [Google Scholar] [CrossRef]
- Aziz, D.; Ahlswede, L.; Enbergs, H.; Aziz, D. Application of MTT reduction assay to evaluate equine sperm viability. Theriogenology 2005, 64, 1350–1356. [Google Scholar] [CrossRef]
- Aziz, D.; Aziz, D. Assessment of bovine sperm viability by MTT reduction assay. Anim. Reprod. Sci. 2006, 92, 1–8. [Google Scholar] [CrossRef]
- Byun, J.W.; Choo, S.H.; Kim, H.H.; Kim, Y.J.; Hwang, Y.J.; Kim, D.Y. Evaluation of Boar Sperm Viability by MTT Reduction Assay in Beltsville Thawing Solution Extender. Asian Australas. J. Anim. Sci. 2008, 21, 494–498. [Google Scholar] [CrossRef]
- Guo, H.; Gong, Y.; He, B.; Zhao, R. Relationships between mitochondrial DNA content, mitochondrial activity, and boar sperm motility. Theriogenology 2017, 87, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Samplaski, M.K.; Dimitromanolakis, A.; Lo, K.C.; Grober, E.D.; Mullen, B.; Garbens, A.; Jarvi, K.A. The relationship between sperm viability and DNA fragmentation rates. Reprod. Biol. Endocrinol. 2015, 13, 2324. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Pesini, E.; Diez, C.; Lapeña, A.C.; Pérez-Martos, A.; Montoya, J.; Alvarez, E.; Arenas, J.; López-Pérez, M.J. Correlation of sperm motility with mitochondrial enzymatic activities. Clin. Chem. 1998, 44, 1616–1620. [Google Scholar] [PubMed]
- Piomboni, P.; Focarelli, R.; Stendardi, A.; Ferramosca, A.; Zara, V. The role of mitochondria in energy production for human sperm motility. Int. J. Androl. 2012, 35, 109–124. [Google Scholar] [CrossRef]
- Troiano, L.; Granata, A.R.; Kalashnikova, G.; Bianchi, R.; Pini, G.; Carani, C.; Cossarizza, A.; Tropea, F.; Franceschi, C. Mitochondrial Membrane Potential and DNA Stainability in Human Sperm Cells: A Flow Cytometry Analysis with Implications for Male Infertility. Exp. Cell Res. 1998, 241, 384–393. [Google Scholar] [CrossRef]
- Donnelly, E.T. Differences in nuclear DNA fragmentation and mitochondrial integrity of semen and prepared human spermatozoa. Hum. Reprod. 2000, 15, 1552–1561. [Google Scholar] [CrossRef]
- Marchetti, C.; Obert, G.; Deffosez, A.; Formstecher, P. Study of mitochondrial membrane potential, reactive oxygen species, DNA fragmentation and cell viability by flow cytometry in human sperm. Hum. Reprod. 2002, 17, 1257–1265. [Google Scholar] [CrossRef]
- Espinoza, J.A.; Schulz, M.A.; Sanchez, R.; Villegas, J.V. Integrity of mitochondrial membrane potential reflects human sperm quality. Andrologia 2009, 41, 51–54. [Google Scholar] [CrossRef]
- Matsuura, K.; Chen, K.-H.; Tsai, C.-H.; Li, W.; Asano, Y.; Naruse, K.; Cheng, C.-M. Paper-based diagnostic devices for evaluating the quality of human sperm. Microfluid. Nanofluid. 2014, 16, 857–867. [Google Scholar] [CrossRef]
- Matsuura, K.; Komiyama, J.; Okitsu, O. Relationship between human spermatozoa motility and emzymatic reactivity. Fertil. Steril. 2015, 104, e239. [Google Scholar] [CrossRef][Green Version]
- Matsuura, K.; Huang, H.-W.; Chen, M.-C.; Chen, Y.; Cheng, C.-M. Relationship between Porcine Sperm Motility and Sperm Enzymatic Activity using Paper-based Devices. Sci. Rep. 2017, 7, 46213. [Google Scholar] [CrossRef] [PubMed]
- Nosrati, R.; Gong, M.M.; Gabriel, M.C.S.; Pedraza, C.E.; Zini, A.; Sinton, D. Paper-Based Quantification of Male Fertility Potential. Clin. Chem. 2016, 62, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Gabrielson, K.L.; Hogue, B.A.; Bohr, V.A.; Cardounel, A.J.; Nakajima, W.; Kofler, J.; Zweier, J.L.; Rodriguez, E.R.; Martin, L.J.; De Souza-Pinto, N.C.; et al. Mitochondrial Toxin 3-Nitropropionic Acid Induces Cardiac and Neurotoxicity Differentially in Mice. Am. J. Pathol. 2001, 159, 1507–1520. [Google Scholar] [CrossRef]
- Ayala, A. Mitochondrial toxins and neurodegenerative diseases. Front. Biosci. 2007, 12, 986. [Google Scholar] [CrossRef] [PubMed]
- Coles, C.J.; Edmondson, D.E.; Singer, T.P. Inactivation of succinate dehydrogenase by 3-nitropropionate. J. Biol. Chem. 1979, 254, 5161–5167. [Google Scholar] [PubMed]
- Francis, K.; Smitherman, C.; Nishino, S.F.; Spain, J.C.; Gadda, G. The biochemistry of the metabolic poison propionate 3-nitronate and its conjugate acid, 3-nitropropionate. IUBMB Life 2013, 65, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Shoshan, M.C. 3-bromopyruvate: Targets and outcomes. J. Bioenerg. Biomembr. 2012, 44, 7–15. [Google Scholar] [CrossRef]
- Davis, M.I.; Shen, M.; Simeonov, A.; Hall, M.D. Diaphorase Coupling Protocols for Red-Shifting Dehydrogenase Assays. ASSAY Drug Dev. Technol. 2016, 14, 207–212. [Google Scholar] [CrossRef]
- Atanassov, B.; Kyurkchiev, S.; Georgiev, G.; Kehayov, I. Characterization of NAD(P)H diaphorase from boar spermatozoa using specific monoclonal antibodies. Int. J. Biochem. 1990, 22, 1471–1478. [Google Scholar] [CrossRef]
- Tourmente, M.; Villar-Moya, P.; Rial, E.; Roldan, E.R.S. Differences in ATP Generation via Glycolysis and Oxidative Phosphorylation and Relationships with Sperm Motility in Mouse Species. J. Biol. Chem. 2015, 290, 20613–20626. [Google Scholar] [CrossRef] [PubMed]
- Takei, G.L.; Miyashiro, D.; Mukai, C.; Okuno, M. Glycolysis plays an important role in energy transfer from the base to the distal end of the flagellum in mouse sperm. J. Exp. Biol. 2014, 217, 1876–1886. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.; Lourenço, B.; Marques, M.; Ramalho-Santos, J. Mitochondria functionality and sperm quality. Reproduction 2013, 146, R163–R174. [Google Scholar] [CrossRef] [PubMed]
- Ferramosca, A.; Zara, V. Bioenergetics of Mammalian Sperm Capacitation. BioMed Res. Int. 2014, 2014, 902953. [Google Scholar] [CrossRef] [PubMed]
- Ferramosca, A.; Focarelli, R.; Piomboni, P.; Coppola, L.; Zara, V. Oxygen uptake by mitochondria in demembranated human spermatozoa: A reliable tool for the evaluation of sperm respiratory efficiency. Int. J. Androl. 2008, 31, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Moscatelli, N.; Spagnolo, B.; Pisanello, M.; Lemma, E.D.; De Vittorio, M.; Zara, V.; Pisanello, F.; Ferramosca, A. Single-cell-based evaluation of sperm progressive motility via fluorescent assessment of mitochondria membrane potential. Sci. Rep. 2017, 7, 17931. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuura, K.; Wang, W.-H.; Ching, A.; Chen, Y.; Cheng, C.-M. Paper-Based Resazurin Assay of Inhibitor-Treated Porcine Sperm. Micromachines 2019, 10, 495. https://doi.org/10.3390/mi10080495
Matsuura K, Wang W-H, Ching A, Chen Y, Cheng C-M. Paper-Based Resazurin Assay of Inhibitor-Treated Porcine Sperm. Micromachines. 2019; 10(8):495. https://doi.org/10.3390/mi10080495
Chicago/Turabian StyleMatsuura, Koji, Wen-Hsin Wang, Alex Ching, Yu Chen, and Chao-Min Cheng. 2019. "Paper-Based Resazurin Assay of Inhibitor-Treated Porcine Sperm" Micromachines 10, no. 8: 495. https://doi.org/10.3390/mi10080495
APA StyleMatsuura, K., Wang, W.-H., Ching, A., Chen, Y., & Cheng, C.-M. (2019). Paper-Based Resazurin Assay of Inhibitor-Treated Porcine Sperm. Micromachines, 10(8), 495. https://doi.org/10.3390/mi10080495





