2D Materials in Development of Electrochemical Point-of-Care Cancer Screening Devices
Abstract
:1. Introduction
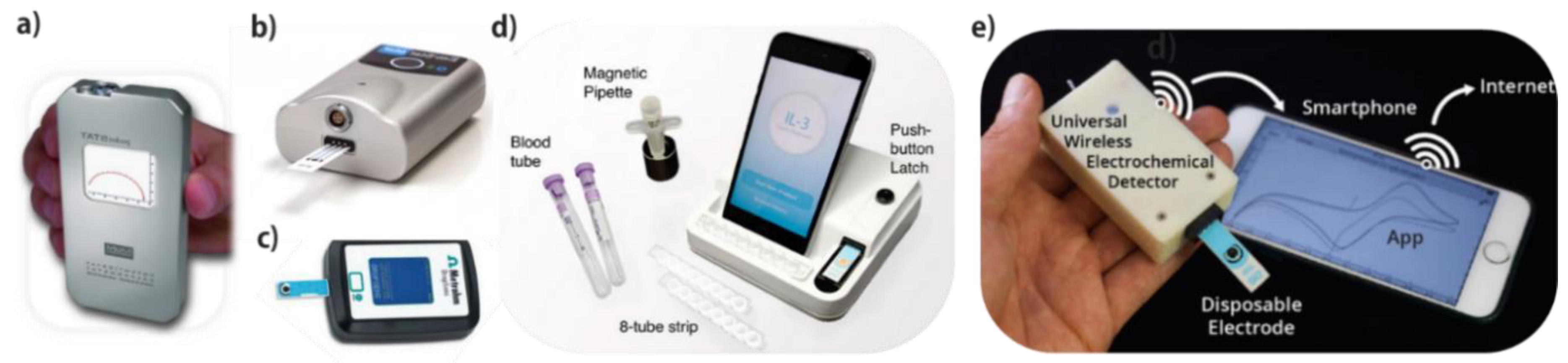
2. POC Screening
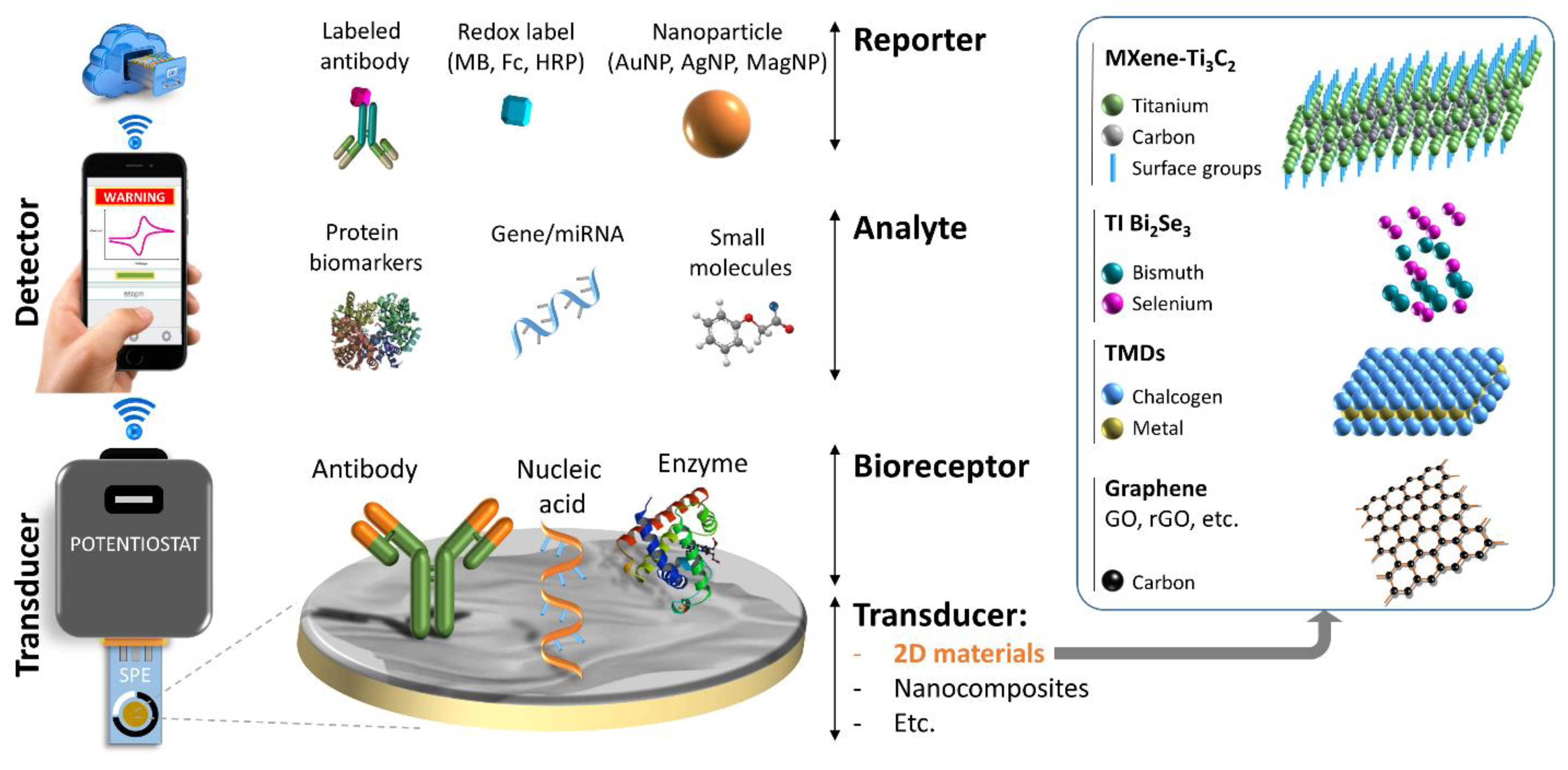
3. Electrochemical Biosensors
Electrochemical Biosensors/Sensors for Cancer Biomarker Detection
4. 2D Materials and Their Biomedical Applications
4.1. Carbon-Based 2D Materials
4.2. Graphene-Like 2D Materials
4.3. Transition Metal Dichalcogenides (TMDs)
4.4. Topological Insulator Bi2Se3
4.5. MXene
5. Graphene-Based Electrochemical Biosensors/Sensors for Cancer Diagnostics
5.1. Graphene-Based Nucleic Acid Detection
5.2. Graphene-Based Protein Detection
5.3. Graphene-Based Small Molecule Detection
6. MoS2-Based Electrochemical Biosensors/Sensors for Cancer Diagnostics
6.1. MoS2-Based Nucleic Acid Detection
6.2. MoS2-Based Protein Detection
6.3. MoS2-Based Small Molecule Detection
6.3.1. Non-Enzymatic Detection
6.3.2. Enzymatic Detection
7. Bi2Se3-Based Electrochemical Biosensors
8. MXene-Based Electrochemical Biosensors
9. Electrochemical Biosensors/Sensors Based on the Other 2D Materials
10. Pros and Cons of Different Detection Systems
10.1. Type of 2D Material
10.2. Reproducibility and Stability
10.3. Type of Biomarker
10.4. Multiplexing
10.5. Sample Preparation and Clinical Validation
10.6. POC Devices
11. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes, B.; Murphy, C.; Crawley, A.; O’Kennedy, R. Developments in point-of-care diagnostic technology for cancer detection. Diagnostics 2018, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Sandbhor Gaikwad, P.; Banerjee, R. Advances in point-of-care diagnostic devices in cancers. Analyst 2018, 143, 1326–1348. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, J.A.; Weinstein, J.N. Biomarkers in cancer staging, prognosis and treatment selection. Nat. Rev. Cancer 2005, 5, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Henry, N.L.; Hayes, D.F. Cancer biomarkers. Mol. Oncol. 2012, 6, 140–146. [Google Scholar] [CrossRef] [Green Version]
- Early detection: A long road ahead. Nat. Rev. Cancer 2018, 18, 401. [CrossRef] [PubMed]
- Wu, L.; Qu, X. Cancer biomarker detection: Recent achievements and challenges. Chem. Soc. Rev. 2015, 44, 2963–2997. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.D. Translational genomics: The challenge of developing cancer biomarkers. Genome Res. 2012, 22, 183–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sher, M.; Zhuang, R.; Demirci, U.; Asghar, W. Paper-based analytical devices for clinical diagnosis: Recent advances in the fabrication techniques and sensing mechanisms. Expert. Rev. Mol. Diagn. 2017, 17, 351–366. [Google Scholar] [CrossRef]
- Sajid, M.; Kawde, A.-N.; Daud, M. Designs, formats and applications of lateral flow assay: A literature review. J. Saudi Chem. Soc. 2015, 19, 689–705. [Google Scholar] [CrossRef] [Green Version]
- Mohammadniaei, M.; Park, C.; Min, J.; Sohn, H.; Lee, T. Fabrication of Electrochemical-Based Bioelectronic Device and Biosensor Composed of Biomaterial-Nanomaterial Hybrid. In Biomimetic Medical Materials: From Nanotechnology to 3D Bioprinting; Noh, I., Ed.; Springer: Singapore, 2018; pp. 263–296. [Google Scholar]
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef]
- Min, J.; Nothing, M.; Coble, B.; Zheng, H.; Park, J.; Im, H.; Weber, G.F.; Castro, C.M.; Swirski, F.K.; Weissleder, R.; et al. Integrated biosensor for rapid and point-of-care sepsis diagnosis. ACS Nano 2018, 12, 3378–3384. [Google Scholar] [CrossRef] [PubMed]
- Ainla, A.; Mousavi, M.P.S.; Tsaloglou, M.-N.; Redston, J.; Bell, J.G.; Fernández-Abedul, M.T.; Whitesides, G.M. Open-source potentiostat for wireless electrochemical detection with smartphones. Anal. Chem. 2018, 90, 6240–6246. [Google Scholar] [CrossRef] [PubMed]
- Maclin, E.; Mahoney, W.C. Point-of-care testing technology. J. Clin. Ligand Assay 1995, 18, 21–33. [Google Scholar]
- Quesada-González, D.; Merkoçi, A. Nanomaterial-based devices for point-of-care diagnostic applications. Chem. Soc. Rev. 2018, 47, 4697–4709. [Google Scholar] [CrossRef]
- Kaur, N.; Toley, B.J. Paper-based nucleic acid amplification tests for point-of-care diagnostics. Analyst 2018, 143, 2213–2234. [Google Scholar] [CrossRef] [PubMed]
- Pashchenko, O.; Shelby, T.; Banerjee, T.; Santra, S. A comparison of optical, electrochemical, magnetic, and colorimetric point-of-care biosensors for infectious disease diagnosis. ACS Infect. Dis. 2018, 4, 1162–1178. [Google Scholar] [CrossRef]
- Laocharoensuk, R. Development of electrochemical immunosensors towards point-of-care cancer diagnostics: Clinically relevant studies. Electroanalysis 2016, 28, 1716–1729. [Google Scholar] [CrossRef]
- Hughes, M.D. The business of self-monitoring of blood glucose: A market profile. J. Diabetes Sci. Technol. 2009, 3, 1219–1223. [Google Scholar] [CrossRef]
- Matthews, D.R.; Bown, E.; Watson, A.; Holman, R.R.; Steemson, J.; Hughes, S.; Scott, D. Pen-sized digital 30-second blood glucose meter. Lancet 1987, 329, 778–779. [Google Scholar] [CrossRef]
- Fineberg, S.E.; Bergenstal, R.M.; Bernstein, R.M.; Laffel, L.M.; Schwartz, S.L. Use of an automated device for alternative site blood glucose monitoring. Diabetes Care 2001, 24, 1217–1220. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.D.; Turner, A.P.F. Home blood glucose biosensors: A commercial perspective. Biosens. Bioelectron. 2005, 20, 2435–2453. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Cuellar, H.E.; Kuang, R.; Caurin, H.F.N.; Goswami, D.; Martinez, R.V. Self-powered, paper-based electrochemical devices for sensitive point-of-care testing. Adv. Mater. Technol. 2017, 2, 1700130. [Google Scholar] [CrossRef]
- Davis, J.W. Biomarker classification, validation, and what to look for in 2017 and beyond. BJU Int. 2017, 119, 812–814. [Google Scholar] [CrossRef] [PubMed]
- Halford, W.P. The essential prerequisites for quantitative RT-PCR. Nat. Biotech. 1999, 17, 835. [Google Scholar] [CrossRef] [PubMed]
- Shendure, J.; Ji, H. Next-generation DNA sequencing. Nat. Biotechnol. 2008, 26, 1135. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ruan, K. MicroRNA detection by microarray. Anal. Bioanal. Chem. 2009, 394, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Goda, S.K.; Minton, N.P. A simple procedure for gel electrophoresis and northern blotting of RNA. Nucleic Acids Res. 1995, 23, 3357–3358. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, E.T.S.G.; Souto, D.E.P.; Barragan, J.T.C.; de Giarola, J.F.; de Moraes, A.C.M.; Kubota, L.T. Electrochemical biosensors in point-of-care devices: Recent advances and future trends. ChemElectroChem 2017, 4, 778–794. [Google Scholar] [CrossRef]
- Mas-Balleste, R.; Gomez-Navarro, C.; Gomez-Herrero, J.; Zamora, F.J.N. 2D materials: To graphene and beyond. Nanoscale 2011, 3, 20–30. [Google Scholar] [CrossRef]
- Yu, J.; Ma, D.; Mei, L.; Gao, Q.; Yin, W.; Zhang, X.; Yan, L.; Gu, Z.; Ma, X.; Zhao, Y. Peroxidase-like activity of MoS2 nanoflakes with different modifications and their application for H2O2 and glucose detection. J. Mater. Chem. B 2018, 6, 487–498. [Google Scholar] [CrossRef]
- Maduraiveeran, G.; Sasidharan, M.; Ganesan, V. Electrochemical sensor and biosensor platforms based on advanced nanomaterials for biological and biomedical applications. Biosens. Bioelectron. 2018, 103, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Antiochia, R.; Tortolini, C.; Tasca, F.; Gorton, L.; Bollella, P. Chapter 1 - Graphene and 2D-like nanomaterials: Different biofunctionalization pathways for electrochemical biosensor development. In Graphene Bioelectronics; Tiwari, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–35. [Google Scholar]
- Wang, L.; Xiong, Q.; Xiao, F.; Duan, H. 2D nanomaterials based electrochemical biosensors for cancer diagnosis. Biosens. Bioelectron. 2017, 89, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Mohammadniaei, M.; Yoon, J.; Choi, H.K.; Placide, V.; Bharate, B.G.; Lee, T.; Choi, J.-W. Multifunctional nanobiohybrid material composed of Ag@Bi2Se3/RNA three-way junction/mirna/retinoic acid for neuroblastoma differentiation. ACS Appl. Mater. Interfaces 2019, 11, 8779–8788. [Google Scholar] [CrossRef]
- Jayakumar, A.; Surendranath, A.; Pv, M. 2D materials for next generation healthcare applications. Int. J. Pharm. 2018, 551, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Zhang, L.; Liu, M.; Zhang, Z. Biomedical applications of graphene. Theranostics 2012, 2, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Merlo, A.; Mokkapati, V.R.S.S.; Pandit, S.; Mijakovic, I. Boron nitride nanomaterials: Biocompatibility and bio-applications. Biomater. Sci. 2018, 6, 2298–2311. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.R.; Yong, K.W.; Choi, J.Y.; Nilghaz, A.; Lin, Y.; Xu, J.; Lu, X. Black phosphorus and its biomedical applications. Theranostics 2018, 8, 1005–1026. [Google Scholar] [CrossRef]
- Agarwal, V.; Chatterjee, K. Recent advances in the field of transition metal dichalcogenides for biomedical applications. Nanoscale 2018, 10, 16365–16397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-D.; Chen, J.; Min, Y.; Park, G.B.; Shen, X.; Song, S.-S.; Sun, Y.-M.; Wang, H.; Long, W.; Xie, J.; et al. Metabolizable Bi2Se3 nanoplates: Biodistribution, toxicity, and uses for cancer radiation therapy and imaging. Adv. Funct. Mater. 2014, 24, 1718–1729. [Google Scholar] [CrossRef]
- Soleymaniha, M.; Shahbazi, M.-A.; Rafieerad, A.R.; Maleki, A.; Amiri, A. Promoting role of MXene nanosheets in biomedical sciences: Therapeutic and biosensing innovations. Adv. Healthc. Mater. 2019, 8, 1801137. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Choi, T.K.; Lee, Y.B.; Cho, H.R.; Ghaffari, R.; Wang, L.; Choi, H.J.; Chung, T.D.; Lu, N.; Hyeon, T.; et al. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat. Nanotechnol. 2016, 11, 566. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Fang, Y. 9 - Biomedical Applications of Graphene. In Graphene; Zhu, H., Xu, Z., Xie, D., Fang, Y., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 215–232. [Google Scholar]
- Bollella, P.; Fusco, G.; Tortolini, C.; Sanzò, G.; Favero, G.; Gorton, L.; Antiochia, R. Beyond graphene: Electrochemical sensors and biosensors for biomarkers detection. Biosens. Bioelectron. 2017, 89, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, M.; Kang, F. Graphene derivatives: Graphane, fluorographene, graphene oxide, graphyne and graphdiyne. J. Mater. Chem. A 2014, 2, 13193–13206. [Google Scholar] [CrossRef]
- Shi, S.; Chen, F.; Ehlerding, E.B.; Cai, W. Surface engineering of graphene-based nanomaterials for biomedical applications. Bioconj. Chem. 2014, 25, 1609–1619. [Google Scholar] [CrossRef]
- Hu, Z.; Niu, T.; Guo, R.; Zhang, J.; Lai, M.; He, J.; Wang, L.; Chen, W. Two-dimensional black phosphorus: Its fabrication, functionalization and applications. Nanoscale 2018, 10, 21575–21603. [Google Scholar] [CrossRef]
- Yang, G.; Zhu, C.; Du, D.; Zhu, J.; Lin, Y. Graphene-like two-dimensional layered nanomaterials: Applications in biosensors and nanomedicine. Nanoscale 2015, 7, 14217–14231. [Google Scholar] [CrossRef]
- Chhowalla, M.; Liu, Z.; Zhang, H. Two-dimensional transition metal dichalcogenide (TMD) nanosheets. Chem. Soc. Rev. 2015, 44, 2584–2586. [Google Scholar] [CrossRef]
- Lv, R.; Robinson, J.A.; Schaak, R.E.; Sun, D.; Sun, Y.; Mallouk, T.E.; Terrones, M. Transition metal dichalcogenides and beyond: Synthesis, properties, and applications of single- and few-layer nanosheets. Acc. Chem. Res. 2015, 48, 56–64. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Huang, K.-J.; Wu, X. Recent advances in transition-metal dichalcogenides based electrochemical biosensors: A review. Biosens. Bioelectron. 2017, 97, 305–316. [Google Scholar] [CrossRef]
- Yoshimi, R.; Tsukazaki, A.; Kikutake, K.; Checkelsky, J.G.; Takahashi, K.S.; Kawasaki, M.; Tokura, Y. Dirac electron states formed at the heterointerface between a topological insulator and a conventional semiconductor. Nat. Mater. 2014, 13, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Kane, C.L. Topological insulators with inversion symmetry. Phys. Rev. B 2007, 76, 045302. [Google Scholar] [CrossRef] [Green Version]
- König, M.; Wiedmann, S.; Brüne, C.; Roth, A.; Buhmann, H.; Molenkamp, L.W.; Qi, X.-L.; Zhang, S.-C. Quantum spin hall insulator state in HgTe quantum wells. Science 2007, 318, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, D.; Xia, Y.; Qian, D.; Wray, L.; Meier, F.; Dil, J.H.; Osterwalder, J.; Patthey, L.; Fedorov, A.V.; Lin, H.; et al. Observation of Time-Reversal-Protected Single-Dirac-Cone Topological-Insulator States in Bi2Te3 and Sb2Te3. Phys. Rev. Lett. 2009, 103, 146401. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Zhu, A.; Xu, Q.; Chen, Y.; Xu, J.; Weng, J. Colorimetric biosensor for detection of cancer biomarker by Au nanoparticle-decorated Bi2Se3 nanosheets. ACS Appl. Mater. Interfaces 2017, 9, 6931–6940. [Google Scholar] [CrossRef]
- Mohammadniaei, M.; Lee, T.; Bharate, B.G.; Yoon, J.; Choi, H.K.; Park, S.-j.; Kim, J.; Kim, J.; Choi, J.-W. Bifunctional Au@Bi2Se3 core–shell nanoparticle for synergetic therapy by SERS-traceable AntagomiR delivery and photothermal treatment. Small 2018, 14, 1802934. [Google Scholar] [CrossRef]
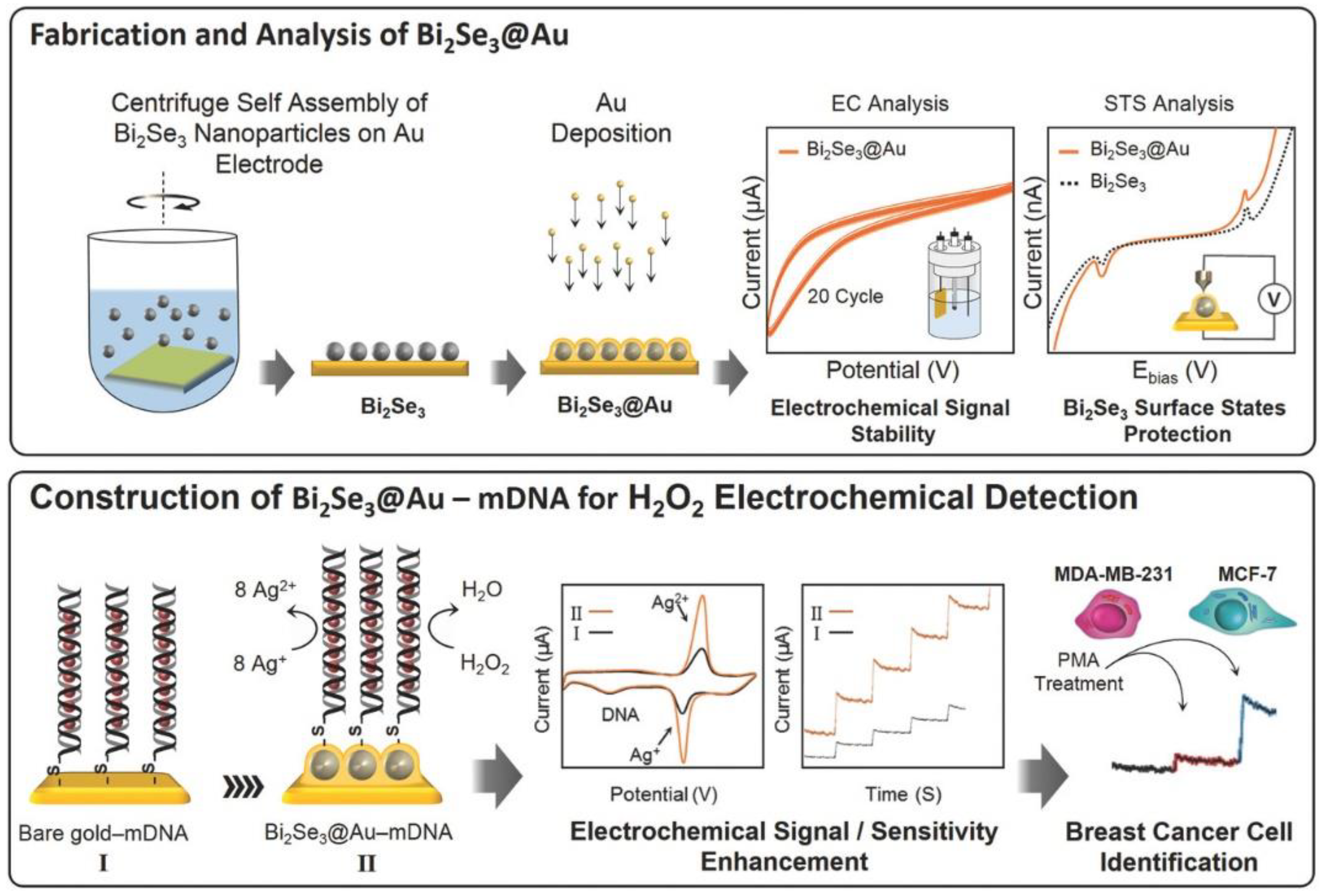
- Mohammadniaei, M.; Yoon, J.; Lee, T.; Bharate, B.G.; Jo, J.; Lee, D.; Choi, J.-W. Electrochemical biosensor composed of silver ion-mediated dsDNA on Au-encapsulated Bi2Se3 nanoparticles for the detection of H2O2 released from breast cancer cells. Small 2018, 14, 1703970. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Ha, E.; Zhao, G.; Zhou, Y.; Huang, D.; Yue, G.; Hu, L.; Sun, N.; Wang, Y.; Lee, L.Y.S.; et al. Recent advance in MXenes: A promising 2D material for catalysis, sensor and chemical adsorption. Coord. Chem. Rev. 2017, 352, 306–327. [Google Scholar] [CrossRef]
- Fan, X.; Liu, L.; Jin, X.; Wang, W.; Zhang, S.; Tang, B. MXene Ti3C2Tx for phase change composite with superior photothermal storage capability. J. Mater. Chem. A 2019, 7, 14319–14327. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Jiang, X.; Xu, J.; Huang, W.; Zhang, F.; Liu, J.; Yang, F.; Song, Y.; Ge, Y.; et al. MXene Ti3C2Tx: A promising photothermal conversion material and application in all-optical modulation and all-optical information loading. Adv. Opt. Mater. 2019, 7, 1900060. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, X.; Zhang, R.; Zhang, Y.; Wu, L.; Lu, W.; Li, J.; Li, Y.; Zhang, H. MXene-enabled electrochemical microfluidic biosensor: Applications toward multicomponent continuous monitoring in whole blood. Adv. Funct. Mater. 2019, 29, 1807326. [Google Scholar] [CrossRef]
- Lorencova, L.; Bertok, T.; Dosekova, E.; Holazova, A.; Paprckova, D.; Vikartovska, A.; Sasinkova, V.; Filip, J.; Kasak, P.; Jerigova, M.; et al. Electrochemical performance of Ti3C2Tx MXene in aqueous media: Towards ultrasensitive H2O2 sensing. Electrochim. Acta 2017, 235, 471–479. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, B.; Benedetti, E.; Cimini, A.; Giordano, A. MicroRNAs: A puzzling tool in cancer diagnostics and therapy. Anticancer Res. 2016, 36, 5571–5575. [Google Scholar] [CrossRef] [PubMed]
- Suraj, S.; Dhar, C.; Srivastava, S. Circulating nucleic acids: An analysis of their occurrence in malignancies. Biomed. Rep. 2017, 6, 8–14. [Google Scholar] [CrossRef] [PubMed]
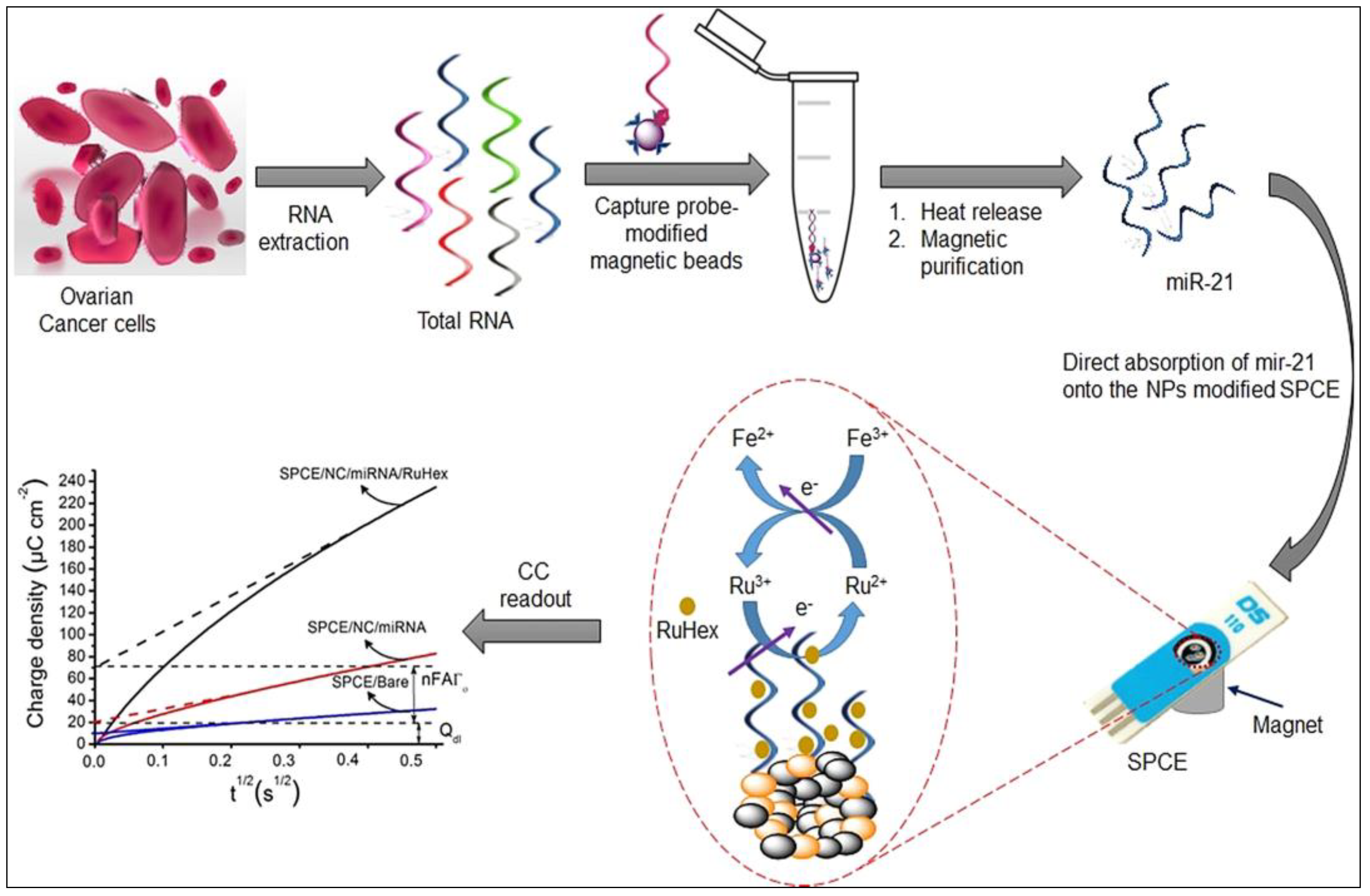
- Mohammadniaei, M.; Go, A.; Chavan, S.G.; Koyappayil, A.; Kim, S.-E.; Yoo, H.J.; Min, J.; Lee, M.-H. Relay-race RNA/barcode gold nanoflower hybrid for wide and sensitive detection of microRNA in total patient serum. Biosens. Bioelectron. 2019, 141, 111468. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, P.A.; Sandhyarani, N. Graphene-DNA electrochemical sensor for the sensitive detection of BRCA1 gene. Sensors Actuators B Chem. 2014, 204, 777–782. [Google Scholar] [CrossRef]
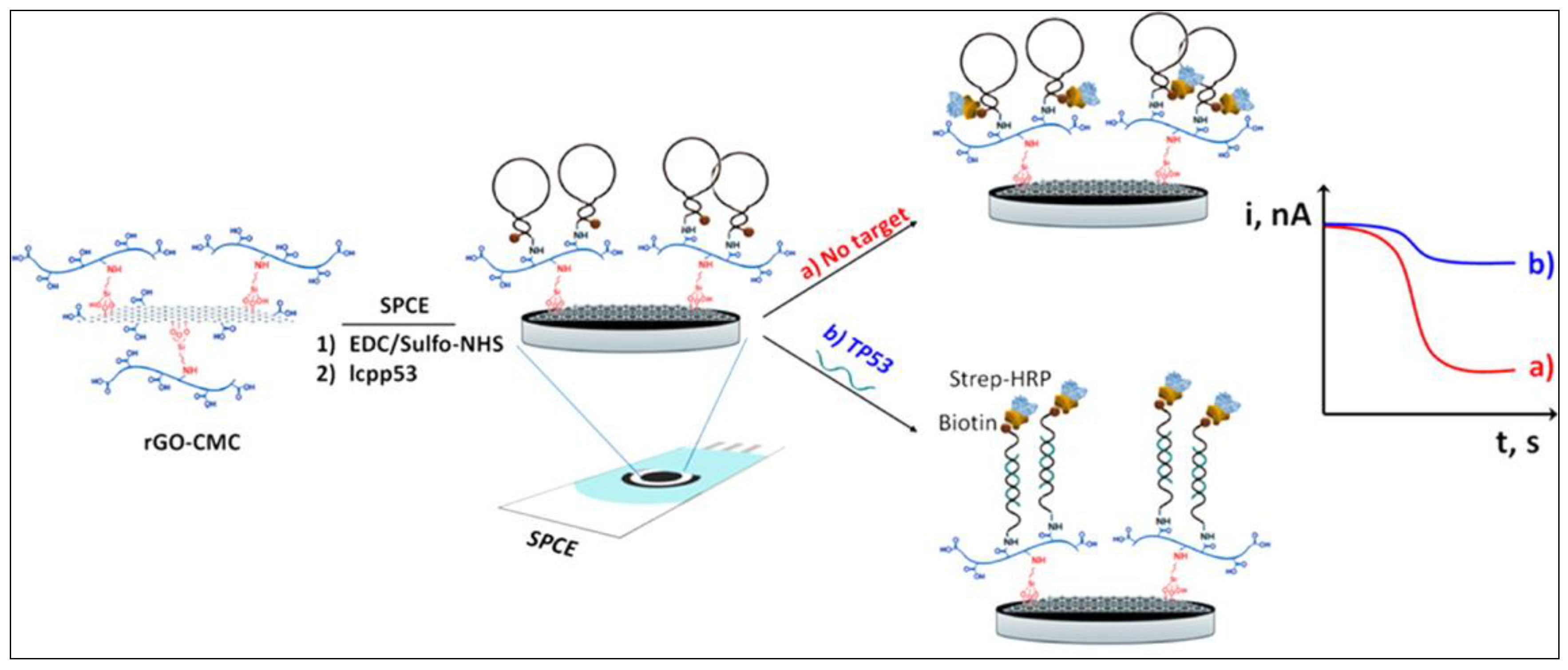
- Esteban-Fernández de Ávila, B.; Araque, E.; Campuzano, S.; Pedrero, M.; Dalkiran, B.; Barderas, R.; Villalonga, R.; Kiliç, E.; Pingarrón, J.M. Dual functional graphene derivative-based electrochemical platforms for detection of the TP53 gene with single nucleotide polymorphism selectivity in biological samples. Anal. Chem. 2015, 87, 2290–2298. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, F.; Lu, Y.; Jin, L.; Tan, S.; Zhang, Y.; Li, C.-P. Ultrasensitive electrochemical detection of alternative cleavage and polyadenylation of CCND2 gene at the single-cell level. Sens. Actuators B Chem. 2019, 285, 553–561. [Google Scholar] [CrossRef]
- Yin, H.; Zhou, Y.; Zhang, H.; Meng, X.; Ai, S. Electrochemical determination of microRNA-21 based on graphene, LNA integrated molecular beacon, AuNPs and biotin multifunctional bio bar codes and enzymatic assay system. Biosens. Bioelectron. 2012, 33, 247–253. [Google Scholar] [CrossRef]
- Tran, H.V.; Piro, B.; Reisberg, S.; Huy Nguyen, L.; Dung Nguyen, T.; Duc, H.T.; Pham, M.C. An electrochemical ELISA-like immunosensor for miRNAs detection based on screen-printed gold electrodes modified with reduced graphene oxide and carbon nanotubes. Biosens. Bioelectron. 2014, 62, 25–30. [Google Scholar] [CrossRef]
- Azimzadeh, M.; Rahaie, M.; Nasirizadeh, N.; Ashtari, K.; Naderi-Manesh, H. An electrochemical nanobiosensor for plasma miRNA-155, based on graphene oxide and gold nanorod, for early detection of breast cancer. Biosens. Bioelectron. 2016, 77, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Shuai, H.-L.; Huang, K.-J.; Zhang, W.-J.; Cao, X.; Jia, M.-P. Sandwich-type microRNA biosensor based on magnesium oxide nanoflower and graphene oxide–gold nanoparticles hybrids coupling with enzyme signal amplification. Sens. Actuators B Chem. 2017, 243, 403–411. [Google Scholar] [CrossRef]
- Salahandish, R.; Ghaffarinejad, A.; Omidinia, E.; Zargartalebi, H.; Majidzadeh-A, K.; Naghib, S.M.; Sanati-Nezhad, A. Label-free ultrasensitive detection of breast cancer miRNA-21 biomarker employing electrochemical nano-genosensor based on sandwiched AgNPs in PANI and N-doped graphene. Biosens. Bioelectron. 2018, 120, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Gorgannezhad, L.; Masud, M.K.; Tanaka, S.; Hossain, M.S.A.; Yamauchi, Y.; Nguyen, N.-T.; Shiddiky, M.J.A. Graphene-oxide-loaded superparamagnetic iron oxide nanoparticles for ultrasensitive electrocatalytic detection of microRNA. ChemElectroChem 2018, 5, 2488–2495. [Google Scholar] [CrossRef]
- Bharti, A.; Agnihotri, N.; Prabhakar, N. A voltammetric hybridization assay for microRNA-21 using carboxylated graphene oxide decorated with gold-platinum bimetallic nanoparticles. Mikrochim. Acta 2019, 186, 185. [Google Scholar] [CrossRef] [PubMed]
- Ruiyi, L.; Ling, L.; Hongxia, B.; Zaijun, L. Nitrogen-doped multiple graphene aerogel/gold nanostar as the electrochemical sensing platform for ultrasensitive detection of circulating free DNA in human serum. Biosens. Bioelectron. 2016, 79, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Gorgannezhad, L.; Umer, M.; Kamal Masud, M.; Hossain, M.S.A.; Tanaka, S.; Yamauchi, Y.; Salomon, C.; Kline, R.; Nguyen, N.-T.; Shiddiky, M.J.A. Detection of FGFR2 : FAM76A fusion gene in circulating tumor RNA based on catalytic signal amplification of graphene oxide-loaded magnetic nanoparticles. Electroanalysis 2018, 30, 2293–2301. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Y.; Su, H.; Mao, L.; Jiang, X.; Zhang, T.; Dai, X. Three-dimensional electrochemical DNA biosensor based on 3D graphene-Ag nanoparticles for sensitive detection of CYFRA21-1 in non-small cell lung cancer. Sens. Actuators B Chem. 2018, 255, 2910–2918. [Google Scholar] [CrossRef]
- Shoja, Y.; Kermanpur, A.; Karimzadeh, F. Diagnosis of EGFR exon21 L858R point mutation as lung cancer biomarker by electrochemical DNA biosensor based on reduced graphene oxide /functionalized ordered mesoporous carbon/Ni-oxytetracycline metallopolymer nanoparticles modified pencil graphite electrode. Biosens. Bioelectron. 2018, 113, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Zou, Z.; Shin, Y.; Wang, J.; Wu, H.; Engelhard, M.H.; Liu, J.; Aksay, I.A.; Lin, Y. Sensitive immunosensor for cancer biomarker based on dual signal amplification strategy of graphene sheets and multienzyme functionalized carbon nanospheres. Anal. Chem. 2010, 82, 2989–2995. [Google Scholar] [CrossRef]
- Wu, Y.; Xue, P.; Kang, Y.; Hui, K.M. Paper-based microfluidic electrochemical immunodevice integrated with nanobioprobes onto graphene film for ultrasensitive multiplexed detection of cancer biomarkers. Anal. Chem. 2013, 85, 8661–8668. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Wang, Q.; Mandler, D.; Li, M.; Boukherroub, R.; Szunerits, S. Detection of folic acid protein in human serum using reduced graphene oxide electrodes modified by folic-acid. Biosens. Bioelectron. 2016, 75, 389–395. [Google Scholar] [CrossRef]
- Khan, M.S.; Dighe, K.; Wang, Z.; Srivastava, I.; Daza, E.; Schwartz-Dual, A.S.; Ghannam, J.; Misra, S.K.; Pan, D. Detection of prostate specific antigen (PSA) in human saliva using an ultra-sensitive nanocomposite of graphene nanoplatelets with diblock-co-polymers and Au electrodes. Analyst 2018, 143, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Tabasi, A.; Noorbakhsh, A.; Sharifi, E. Reduced graphene oxide-chitosan-aptamer interface as new platform for ultrasensitive detection of human epidermal growth factor receptor 2. Biosens. Bioelectron. 2017, 95, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kumar, S.; Srivastava, S.; Yadav, B.K.; Lee, S.H.; Sharma, J.G.; Doval, D.C.; Malhotra, B.D. Reduced graphene oxide modified smart conducting paper for cancer biosensor. Biosens. Bioelectron. 2015, 73, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Sharafeldin, M.; Bishop, G.W.; Bhakta, S.; El-Sawy, A.; Suib, S.L.; Rusling, J.F. Fe3O4 nanoparticles on graphene oxide sheets for isolation and ultrasensitive amperometric detection of cancer biomarker proteins. Biosens. Bioelectron. 2017, 91, 359–366. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, J.; Liu, J.; Sun, S.; Xiong, Y.; Ma, Y.; Yan, S.; Yang, Y.; Yin, H.; Cai, X. Label-free microfluidic paper-based electrochemical aptasensor for ultrasensitive and simultaneous multiplexed detection of cancer biomarkers. Biosens. Bioelectron. 2019, 136, 84–90. [Google Scholar] [CrossRef]
- Fan, Y.; Shi, S.; Ma, J.; Guo, Y. A paper-based electrochemical immunosensor with reduced graphene oxide/thionine/gold nanoparticles nanocomposites modification for the detection of cancer antigen 125. Biosens. Bioelectron. 2019, 135, 1–7. [Google Scholar] [CrossRef]
- Wei, B.; Mao, K.; Liu, N.; Zhang, M.; Yang, Z. Graphene nanocomposites modified electrochemical aptamer sensor for rapid and highly sensitive detection of prostate specific antigen. Biosens. Bioelectron. 2018, 121, 41–46. [Google Scholar] [CrossRef]
- Martins, G.V.; Tavares, A.P.M.; Fortunato, E.; Sales, M.G.F. Paper-based sensing device for electrochemical detection of oxidative stress biomarker 8-Hydroxy-2’-deoxyguanosine (8-OHdG) in point-of-care. Sci. Rep. 2017, 7, 14558. [Google Scholar] [CrossRef]
- Li, N.; Wang, Y.; Cao, W.; Zhang, Y.; Yan, T.; Du, B.; Wei, Q. An ultrasensitive electrochemical immunosensor for CEA using MWCNT-NH2 supported PdPt nanocages as labels for signal amplification. J. Mater. Chem. B 2015, 3, 2006–2011. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Wang, T.; Li, D.; Xi, F.; Wang, J.; Wang, E. Three-dimensional electrochemical immunosensor for sensitive detection of carcinoembryonic antigen based on monolithic and macroporous graphene foam. Biosens. Bioelectron. 2015, 65, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Chai, Y.; Zhuo, Y.; Yuan, R. Ultrasensitive simultaneous detection of four biomarkers based on hybridization chain reaction and biotin-streptavidin signal amplification strategy. Biosens. Bioelectron. 2015, 68, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sharma, J.G.; Maji, S.; Malhotra, B.D. Nanostructured zirconia decorated reduced graphene oxide based efficient biosensing platform for non-invasive oral cancer detection. Biosens. Bioelectron. 2016, 78, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Wu, D.; Ma, H.; Pang, X.; Fan, D.; Wei, Q.; Du, B. Ultrasensitive label-free electrochemical immunosensor based on multifunctionalized graphene nanocomposites for the detection of alpha fetoprotein. Sci. Rep. 2017, 7, 42361. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour, S.; Hasanzadeh, M.; Saadati, A.; Shadjou, N.; Soleymani, J.; Jouyban, A. A novel paper based immunoassay of breast cancer specific carbohydrate (CA 15.3) using silver nanoparticles-reduced graphene oxide nano-ink technology: A new platform to construction of microfluidic paper-based analytical devices (μPADs) towards biomedical analysis. Microchem. J. 2019, 146, 345–358. [Google Scholar] [CrossRef]
- Pothipor, C.; Wiriyakun, N.; Putnin, T.; Ngamaroonchote, A.; Jakmunee, J.; Ounnunkad, K.; Laocharoensuk, R.; Aroonyadet, N. Highly sensitive biosensor based on graphene–poly (3-aminobenzoic acid) modified electrodes and porous-hollowed-silver-gold nanoparticle labelling for prostate cancer detection. Sens. Actuators B Chem. 2019, 296, 126657. [Google Scholar] [CrossRef]
- Proenca, C.A.; Baldo, T.A.; Freitas, T.A.; Materon, E.M.; Wong, A.; Duran, A.A.; Melendez, M.E.; Zambrano, G.; Faria, R.C. Novel enzyme-free immunomagnetic microfluidic device based on Co0.25Zn0.75Fe2O4 for cancer biomarker detection. Anal. Chim. Acta 2019, 1071, 59–69. [Google Scholar] [CrossRef]
- Ma, L.; Hu, L.; Feng, X.; Wang, S. Nitrate and nitrite in health and disease. Aging Dis. 2018, 9, 938–945. [Google Scholar] [CrossRef]
- Wu, P.; Cai, Z.; Gao, Y.; Zhang, H.; Cai, C. Enhancing the electrochemical reduction of hydrogen peroxide based on nitrogen-doped graphene for measurement of its releasing process from living cells. Chem. Commun. 2011, 47, 11327–11329. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, H.; Rui, Q.; Tian, Y. Detection of extracellular H2O2 released from human liver cancer cells based on TiO2 nanoneedles with enhanced electron transfer of cytochrome c. Anal. Chem. 2009, 81, 3035–3041. [Google Scholar] [CrossRef] [PubMed]
- Korde Choudhari, S.; Chaudhary, M.; Bagde, S.; Gadbail, A.R.; Joshi, V. Nitric oxide and cancer: A review. World J. Surg. Oncol. 2013, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Peña-Bahamonde, J.; Nguyen, H.N.; Fanourakis, S.K.; Rodrigues, D.F. Recent advances in graphene-based biosensor technology with applications in life sciences. J. Nanobiotechnol. 2018, 16, 75. [Google Scholar] [CrossRef] [PubMed]
- Si, P.; Huang, Y.; Wang, T.; Ma, J. Nanomaterials for electrochemical non-enzymatic glucose biosensors. RSC Adv. 2013, 3, 3487–3502. [Google Scholar] [CrossRef]
- Guo, C.X.; Zheng, X.T.; Lu, Z.S.; Lou, X.W.; Li, C.M. Biointerface by cell growth on layered graphene–artificial peroxidase–protein nanostructure for in situ quantitative molecular detection. Adv. Mater. 2010, 22, 5164–5167. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-R.; Kong, F.-Y.; Liu, J.; Liang, T.-M.; Xu, J.-J.; Chen, H.-Y. Synthesis of potassium-modified graphene and its application in nitrite-selective sensing. Adv. Funct. Mater. 2012, 22, 1981–1988. [Google Scholar] [CrossRef]
- Xi, J.; Zhang, Y.; Wang, Q.; Xiao, J.; Chi, K.; Duan, X.; Chen, J.; Tang, C.; Sun, Y.; Xiao, F.; et al. Multi-element doping design of high-efficient carbocatalyst for electrochemical sensing of cancer cells. Sens. Actuators B Chem. 2018, 273, 108–117. [Google Scholar] [CrossRef]
- Maji, S.K.; Sreejith, S.; Mandal, A.K.; Ma, X.; Zhao, Y. Immobilizing gold nanoparticles in mesoporous silica covered reduced graphene oxide: A hybrid material for cancer cell detection through hydrogen peroxide sensing. ACS Appl. Mater. Interfaces 2014, 6, 13648–13656. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, X.; Wang, X.; Shiu, K.-K.; Zhu, Y.; Jiang, H. Highly sensitive graphene–Pt nanocomposites amperometric biosensor and its application in living cell H2O2 detection. Anal. Chem. 2014, 86, 9459–9465. [Google Scholar] [CrossRef]
- Bai, J.; Jiang, X. A Facile one-pot synthesis of copper sulfide-decorated reduced graphene oxide composites for enhanced detecting of H2O2 in biological environments. Anal. Chem. 2013, 85, 8095–8101. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Y.; Cheng, C.; Liu, X.; Jiang, H.; Wang, X. Highly sensitive electrochemical biosensor for evaluation of oxidative stress based on the nanointerface of graphene nanocomposites blended with gold, Fe3O4, and platinum nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 18441–18449. [Google Scholar] [CrossRef]
- Kumar, V.; Gupta, R.K.; Gundampati, R.K.; Singh, D.K.; Mohan, S.; Hasan, S.H.; Malviya, M. Enhanced electron transfer mediated detection of hydrogen peroxide using a silver nanoparticle–reduced graphene oxide–polyaniline fabricated electrochemical sensor. RSC Adv. 2018, 8, 619–631. [Google Scholar] [CrossRef]
- Zhu, L.; Guo, X.; Liu, Y.; Chen, Z.; Zhang, W.; Yin, K.; Li, L.; Zhang, Y.; Wang, Z.; Sun, L.; et al. High-performance Cu nanoparticles/three-dimensional graphene/Ni foam hybrid for catalytic and sensing applications. Nanotechnology 2018, 29, 145703. [Google Scholar] [CrossRef] [PubMed]
- Zan, X.; Fang, Z.; Wu, J.; Xiao, F.; Huo, F.; Duan, H. Freestanding graphene paper decorated with 2D-assembly of Au@Pt nanoparticles as flexible biosensors to monitor live cell secretion of nitric oxide. Biosens. Bioelectron. 2013, 49, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; He, K.; Zhang, Z.; Zhou, A.; Duan, H. Real-time electrochemical detection of hydrogen peroxide secretion in live cells by Pt nanoparticles decorated graphene–carbon nanotube hybrid paper electrode. Biosens. Bioelectron. 2015, 68, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.; Tran, V.-K.; Son, S.E.; Hur, W.; Choi, H.; Seong, G.H. Characterization of Au@PtNP/GO nanozyme and its application to electrochemical microfluidic devices for quantification of hydrogen peroxide. Sens. Actuators B Chem. 2019, 294, 166–176. [Google Scholar] [CrossRef]
- Li, C.; Wu, R.; Zou, J.; Zhang, T.; Zhang, S.; Zhang, Z.; Hu, X.; Yan, Y.; Ling, X. MNPs@anionic MOFs/ERGO with the size selectivity for the electrochemical determination of H2O2 released from living cells. Biosens. Bioelectron. 2018, 116, 81–88. [Google Scholar] [CrossRef]
- Dou, B.; Li, J.; Jiang, B.; Yuan, R.; Xiang, Y. DNA-templated in situ synthesis of highly dispersed AuNPs on nitrogen-doped graphene for real-time electrochemical monitoring of nitric oxide released from live cancer cells. Anal. Chem. 2019, 91, 2273–2278. [Google Scholar] [CrossRef]
- Dong, W.; Ren, Y.; Bai, Z.; Yang, Y.; Chen, Q. Fabrication of hexahedral Au-Pd/graphene nanocomposites biosensor and its application in cancer cell H2O2 detection. Bioelectrochemistry 2019, 128, 274–282. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, Z.; Shen, T.; Qin, Y. Graphene/Gold nanoparticle composite-based paper sensor for electrochemical detection of hydrogen peroxide. Fuller. Nanotub. Carb. Nanostruct. 2018, 27, 23–27. [Google Scholar] [CrossRef]
- Amala, G.; Saravanan, J.; Jin Yoo, D.; Kim, A.R.; Gnana kumar, G. An environmentally benign one pot green synthesis of reduced graphene oxide based composites for the enzyme free electrochemical detection of hydrogen peroxide. New J. Chem. 2017, 41, 4022–4030. [Google Scholar] [CrossRef]
- Ju, J.; Chen, W. In situ growth of surfactant-free gold nanoparticles on nitrogen-doped graphene quantum dots for electrochemical detection of hydrogen peroxide in biological environments. Anal. Chem. 2015, 87, 1903–1910. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Chen, W. In situ synthesis of MoS2/graphene nanosheet composites with extraordinarily high electrochemical performance for lithium ion batteries. Chem. Commun. 2011, 47, 4252–4254. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wong, S.L. Functionalization of 2D transition metal dichalcogenides for biomedical applications. Mater. Sci. Eng. C 2017, 70, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Liu, W.; Xie, X.; Anselmo, A.C.; Mitragotri, S.; Banerjee, K. MoS2 field-effect transistor for next-generation label-free biosensors. ACS Nano 2014, 8, 3992–4003. [Google Scholar] [CrossRef]
- Liu, T.; Liu, Z. 2D MoS2 nanostructures for biomedical applications. Adv. Healthc. Mater. 2018, 7, 1701158. [Google Scholar] [CrossRef]
- Wang, T.; Zhu, R.; Zhuo, J.; Zhu, Z.; Shao, Y.; Li, M. Direct detection of DNA below ppb level based on thionin-functionalized layered MoS2 electrochemical sensors. Anal. Chem. 2014, 86, 12064–12069. [Google Scholar] [CrossRef]
- Chu, Y.; Cai, B.; Ma, Y.; Zhao, M.; Ye, Z.; Huang, J. Highly sensitive electrochemical detection of circulating tumor DNA based on thin-layer MoS2/graphene composites. RSC Adv. 2016, 6, 22673–22678. [Google Scholar] [CrossRef]
- Su, S.; Cao, W.; Liu, W.; Lu, Z.; Zhu, D.; Chao, J.; Weng, L.; Wang, L.; Fan, C.; Wang, L. Dual-mode electrochemical analysis of microRNA-21 using gold nanoparticle-decorated MoS2 nanosheet. Biosens. Bioelectron. 2017, 94, 552–559. [Google Scholar] [CrossRef]
- Zhu, D.; Liu, W.; Zhao, D.; Hao, Q.; Li, J.; Huang, J.; Shi, J.; Chao, J.; Su, S.; Wang, L. Label-free electrochemical sensing platform for MicroRNA-21 detection using thionine and gold nanoparticles co-functionalized MoS2 nanosheet. ACS Appl. Mater. Interfaces 2017, 9, 35597–35603. [Google Scholar] [CrossRef]
- Shuai, H.-L.; Huang, K.-J.; Chen, Y.-X.; Fang, L.-X.; Jia, M.-P. Au nanoparticles/hollow molybdenum disulfide microcubes based biosensor for microRNA-21 detection coupled with duplex-specific nuclease and enzyme signal amplification. Biosens. Bioelectron. 2017, 89, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-X.; Wu, X.; Huang, K.-J. A sandwich-type electrochemical biosensing platform for microRNA-21 detection using carbon sphere-MoS2 and catalyzed hairpin assembly for signal amplification. Sens. Actuators B Chem. 2018, 270, 179–186. [Google Scholar] [CrossRef]
- Wang, X.; Nan, F.; Zhao, J.; Yang, T.; Ge, T.; Jiao, K. A label-free ultrasensitive electrochemical DNA sensor based on thin-layer MoS2 nanosheets with high electrochemical activity. Biosens. Bioelectron. 2015, 64, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Dutta Chowdhury, A.; Biswas, S.; Park, E.Y.; Agnihotri, N.; De, A.; De, S. Development of an effective electrochemical platform for highly sensitive DNA detection using MoS2-polyaniline nanocomposites. Biochem. Eng. J. 2018, 140, 130–139. [Google Scholar] [CrossRef]
- Zhang, W.; Dai, Z.; Liu, X.; Yang, J. High-performance electrochemical sensing of circulating tumor DNA in peripheral blood based on poly-xanthurenic acid functionalized MoS2 nanosheets. Biosens. Bioelectron. 2018, 105, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Soni, A.; Pandey, C.M.; Pandey, M.K.; Sumana, G. Highly efficient Polyaniline-MoS2 hybrid nanostructures based biosensor for cancer biomarker detection. Anal. Chim. Acta 2019, 1055, 26–35. [Google Scholar] [CrossRef]
- Yang, J.; Yin, X.; Zhang, W. Electrochemical determination of PIK3CA gene associated with breast cancer based on molybdenum disulfide nanosheet-supported poly(indole-6-carboxylic acid). Anal. Methods 2019, 11, 157–162. [Google Scholar] [CrossRef]
- Chand, R.; Ramalingam, S.; Neethirajan, S. A 2D transition-metal dichalcogenide MoS2 based novel nanocomposite and nanocarrier for multiplex miRNA detection. Nanoscale 2018, 10, 8217–8225. [Google Scholar] [CrossRef]
- Liu, L.; Wei, Y.; Jiao, S.; Zhu, S.; Liu, X. A novel label-free strategy for the ultrasensitive miRNA-182 detection based on MoS2/Ti3C2 nanohybrids. Biosens. Bioelectron. 2019, 137, 45–51. [Google Scholar] [CrossRef]
- Tian, R.; Li, Y.; Bai, J. Hierarchical assembled nanomaterial paper based analytical devices for simultaneously electrochemical detection of microRNAs. Anal. Chim. Acta 2019, 1058, 89–96. [Google Scholar] [CrossRef]
- Fang, L.-X.; Huang, K.-J.; Liu, Y. Novel electrochemical dual-aptamer-based sandwich biosensor using molybdenum disulfide/carbon aerogel composites and Au nanoparticles for signal amplification. Biosens. Bioelectron. 2015, 71, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Jing, P.; Yi, H.; Xue, S.; Chai, Y.; Yuan, R.; Xu, W. A sensitive electrochemical aptasensor based on palladium nanoparticles decorated graphene–molybdenum disulfide flower-like nanocomposites and enzymatic signal amplification. Anal. Chim. Acta 2015, 853, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chu, C.; Shen, L.; Deng, W.; Yan, M.; Ge, S.; Yu, J.; Song, X. An ultrasensitive electrochemical immunosensor based on the catalytical activity of MoS2-Au composite using Ag nanospheres as labels. Sens. Actuators B Chem. 2015, 206, 30–36. [Google Scholar] [CrossRef]
- Su, S.; Zou, M.; Zhao, H.; Yuan, C.; Xu, Y.; Zhang, C.; Wang, L.; Fan, C.; Wang, L. Shape-controlled gold nanoparticles supported on MoS2 nanosheets: Synergistic effect of thionine and MoS2 and their application for electrochemical label-free immunosensing. Nanoscale 2015, 7, 19129–19135. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Han, X.; Lu, Z.; Liu, W.; Zhu, D.; Chao, J.; Fan, C.; Wang, L.; Song, S.; Weng, L.; et al. Facile synthesis of a MoS2–prussian blue nanocube nanohybrid-based electrochemical sensing platform for hydrogen peroxide and carcinoembryonic antigen detection. ACS Appl. Mater. Interfaces 2017, 9, 12773–12781. [Google Scholar] [CrossRef]
- Su, S.; Sun, Q.; Wan, L.; Gu, X.; Zhu, D.; Zhou, Y.; Chao, J.; Wang, L. Ultrasensitive analysis of carcinoembryonic antigen based on MoS2-based electrochemical immunosensor with triple signal amplification. Biosens. Bioelectron. 2019, 140, 111353. [Google Scholar] [CrossRef]
- Wang, X.; Deng, W.; Shen, L.; Yan, M.; Yu, J. A 3D electrochemical immunodevice based on an Au paper electrode and using Au nanoflowers for amplification. New J. Chem. 2016, 40, 2835–2842. [Google Scholar] [CrossRef]
- He, B. A sandwich-type electrochemical biosensor for alpha-fetoprotein based on Au nanoparticles decorating a hollow molybdenum disulfide microbox coupled with a hybridization chain reaction. New J. Chem. 2017, 41, 11353–11360. [Google Scholar] [CrossRef]
- Duan, F.; Zhang, S.; Yang, L.; Zhang, Z.; He, L.; Wang, M. Bifunctional aptasensor based on novel two-dimensional nanocomposite of MoS2 quantum dots and g-C3N4 nanosheets decorated with chitosan-stabilized Au nanoparticles for selectively detecting prostate specific antigen. Anal. Chim. Acta 2018, 1036, 121–132. [Google Scholar] [CrossRef]
- Kukkar, M.; Tuteja, S.K.; Kumar, P.; Kim, K.H.; Bhadwal, A.S.; Deep, A. A novel approach for amine derivatization of MoS2 nanosheets and their application toward label-free immunosensor. Anal. Biochem. 2018, 555, 1–8. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, G.; Zhang, Y.; Pang, X.; Cao, W.; Du, B.; Wei, Q. Sandwich-type electrochemical immunosensor for CEA detection based on Ag/MoS2@Fe3O4 and an analogous ELISA method with total internal reflection microscopy. Sens. Actuators B Chem. 2018, 266, 561–569. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Wu, D.; Ma, H.; Zhang, Y.; Fan, D.; Pang, X.; Du, B.; Wei, Q. Label-free electrochemical immunosensor based on flower-like Ag/MoS2/rGO nanocomposites for ultrasensitive detection of carcinoembryonic antigen. Sens. Actuators B Chem. 2018, 255, 125–132. [Google Scholar] [CrossRef]
- Liu, H.; Chen, X.; Su, X.; Duan, C.; Guo, K.; Zhu, Z. Flower-like MoS2 modified reduced graphene oxide nanocomposite: Synthesis and application for lithium-ion batteries and mediator-free biosensor. J. Electrochem. Soc. 2015, 162, B312–B318. [Google Scholar] [CrossRef]
- Lin, X.; Ni, Y.; Kokot, S. Electrochemical and bio-sensing platform based on a novel 3D Cu nano-flowers/layered MoS2 composite. Biosens. Bioelectron. 2016, 79, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Du, X. Molybdenum disulfide nanosheets supported Au-Pd bimetallic nanoparticles for non-enzymatic electrochemical sensing of hydrogen peroxide and glucose. Sens. Actuators B Chem. 2017, 239, 536–543. [Google Scholar] [CrossRef]
- Xue, Y.; Maduraiveeran, G.; Wang, M.; Zheng, S.; Zhang, Y.; Jin, W. Hierarchical oxygen-implanted MoS2 nanoparticle decorated graphene for the non-enzymatic electrochemical sensing of hydrogen peroxide in alkaline media. Talanta 2018, 176, 397–405. [Google Scholar] [CrossRef]
- Cheng, Z.; Shen, Q.; Yu, H.; Han, D.; Zhong, F.; Yang, Y.J.M.A. Non-enzymatic sensing of hydrogen peroxide using a glassy carbon electrode modified with the layered MoS2-reduced graphene oxide and Prussian Blue. Microchim. Acta 2017, 184, 4587–4595. [Google Scholar] [CrossRef]
- Lin, D.; Su, Z.; Wei, G. Three-dimensional porous reduced graphene oxide decorated with MoS2 quantum dots for electrochemical determination of hydrogen peroxide. Mater. Today Chem. 2018, 7, 76–83. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, Y.; Xu, P.; Wen, W.; Li, X.; Xu, J. PtW/MoS2 hybrid nanocomposite for electrochemical sensing of H2O2 released from living cells. Biosens. Bioelectron. 2016, 80, 601–606. [Google Scholar] [CrossRef]
- Dai, H.; Chen, D.; Cao, P.; Li, Y.; Wang, N.; Sun, S.; Chen, T.; Ma, H.; Lin, M. Molybdenum sulfide/nitrogen-doped carbon nanowire-based electrochemical sensor for hydrogen peroxide in living cells. Sens. Actuators B Chem. 2018, 276, 65–71. [Google Scholar] [CrossRef]
- Shu, Y.; Chen, J.; Xu, Q.; Wei, Z.; Liu, F.; Lu, R.; Xu, S.; Hu, X. MoS2 nanosheet–Au nanorod hybrids for highly sensitive amperometric detection of H2O2 in living cells. J. Mater. Chem. B 2017, 5, 1446–1453. [Google Scholar] [CrossRef]
- Dou, B.; Yang, J.; Yuan, R.; Xiang, Y. Trimetallic hybrid nanoflower-decorated MoS2 nanosheet sensor for direct in situ monitoring of H2O2 secreted from live cancer cells. Anal. Chem. 2018, 90, 5945–5950. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, P.; Wen, F.; Zhu, Y.; Zhang, Y. Flower-like Fe2O3@MoS2 nanocomposite decorated glassy carbon electrode for the determination of nitrite. Sens. Actuators B Chem. 2015, 220, 749–754. [Google Scholar] [CrossRef]
- Wang, H.; Wen, F.; Chen, Y.; Sun, T.; Meng, Y.; Zhang, Y. Electrocatalytic determination of nitrite based on straw cellulose/molybdenum sulfide nanocomposite. Biosens. Bioelectron. 2016, 85, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.L.; Li, B.L.; Luo, H.Q.; Li, N.B. 0D-2D heterostructures of Au nanoparticles and layered MoS2 for simultaneous detections of dopamine, ascorbic acid, uric acid, and nitrite. Sens. Actuators B Chem. 2017, 253, 352–360. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, Y.; Chen, Y.; Zheng, J. Synthesis of gold nanoparticles coated on flower-like MoS2 microsphere and their application for electrochemical nitrite sensing. J. Electroanal. Chem. 2019, 839, 195–201. [Google Scholar] [CrossRef]
- Kim, H.-U.; Kim, H.; Ahn, C.; Kulkarni, A.; Jeon, M.; Yeom, G.Y.; Lee, M.-H.; Kim, T. In situ synthesis of MoS2 on a polymer based gold electrode platform and its application in electrochemical biosensing. RSC Adv. 2015, 5, 10134–10138. [Google Scholar] [CrossRef]
- Chao, J.; Zou, M.; Zhang, C.; Sun, H.; Pan, D.; Pei, H.; Su, S.; Yuwen, L.; Fan, C.; Wang, L. A MoS2–based system for efficient immobilization of hemoglobin and biosensing applications. Nanotechnology 2015, 26, 274005. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, H.; Hou, S. Layered MoS2–graphene composites for biosensor applications with sensitive electrochemical performance. Anal. Methods 2016, 8, 3780–3787. [Google Scholar] [CrossRef]
- Yoon, J.; Shin, J.-W.; Lim, J.; Mohammadniaei, M.; Bharate Bapurao, G.; Lee, T.; Choi, J.-W. Electrochemical nitric oxide biosensor based on amine-modified MoS2/graphene oxide/myoglobin hybrid. Colloids Surf. B. Biointerfaces 2017, 159, 729–736. [Google Scholar] [CrossRef]
- Lin, D.; Li, Y.; Zhang, P.; Zhang, W.; Ding, J.; Li, J.; Wei, G.; Su, Z. Fast preparation of MoS2 nanoflowers decorated with platinum nanoparticles for electrochemical detection of hydrogen peroxide. RSC Adv. 2016, 6, 52739–52745. [Google Scholar] [CrossRef]
- Yoon, J.; Lee, T.; Bapurao, G.B.; Jo, J.; Oh, B.K.; Choi, J.W. Electrochemical H2O2 biosensor composed of myoglobin on MoS2 nanoparticle-graphene oxide hybrid structure. Biosens. Bioelectron. 2017, 93, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.X.; Tang, L.N.; Yang, F.; Liang, F.X.; Wang, H.; Li, Y.T.; Zhang, G.J. MoS2/Pt nanocomposite-functionalized microneedle for real-time monitoring of hydrogen peroxide release from living cells. Analyst 2017, 142, 4322–4329. [Google Scholar] [CrossRef] [PubMed]
- Sha, R.; Vishnu, N.; Badhulika, S. Bimetallic Pt-Pd nanostructures supported on MoS2 as an ultra-high performance electrocatalyst for methanol oxidation and nonenzymatic determination of hydrogen peroxide. Mikrochim. Acta 2018, 185, 399. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, X.; Yi, R.; Zhang, J.; Su, Z.; Wei, G. Electrochemical sensor based on novel two-dimensional nanohybrids: MoS2 nanosheets conjugated with organic copper nanowires for simultaneous detection of hydrogen peroxide and ascorbic acid. Inorg. Chem. Fronti. 2018, 5, 112–119. [Google Scholar] [CrossRef]
- Mutyala, S.; Kinsly, J.; Sharma, G.V.R.; Chinnathambi, S.; Jayaraman, M. Non-enzymatic electrochemical hydrogen peroxide detection using MoS2-Interconnected porous carbon heterostructure. J. Electroanal. Chem. 2018, 823, 429–436. [Google Scholar] [CrossRef]
- Žalnėravičius, R.; Gedminas, A.; Ruzgas, T.; Jagminas, A. Nanoplatelet MoS2 arrays decorated with Pt nanoparticles for non-enzymatic detection of hydrogen peroxide. J. Electroanal. Chem. 2019, 839, 274–282. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, P.; Wen, F.; Yuan, B.; Wang, H. Fe3O4 nanospheres on MoS2 nanoflake: Electrocatalysis and detection of Cr(VI) and nitrite. J. Electroanal. Chem. 2016, 761, 14–20. [Google Scholar] [CrossRef]
- Ghanei-Motlagh, M.; Taher, M.A. A novel electrochemical sensor based on silver/halloysite nanotube/molybdenum disulfide nanocomposite for efficient nitrite sensing. Biosens. Bioelectron. 2018, 109, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, J.; Zhao, Z.; Liu, J.; Shi, J.; Li, G.; Li, P.; Zhang, W.; Lian, K.; Zhuiykov, S. Synthesis and electrochemical properties of rGO-MoS2 heterostructures for highly sensitive nitrite detection. Ionics 2017, 24, 577–587. [Google Scholar] [CrossRef]
- Sha, R.; Gopalakrishnan, A.; Sreenivasulu, K.V.; Srikanth, V.V.S.S.; Badhulika, S. Template-cum-catalysis free synthesis of α-MnO2 nanorods-hierarchical MoS2 microspheres composite for ultra-sensitive and selective determination of nitrite. J. Alloys Compd. 2019, 794, 26–34. [Google Scholar] [CrossRef]
- Huang, H.; Lv, L.; Xu, F.; Liao, J.; Liu, S.; Wen, H.-R. PrFeO3-MoS2 nanosheets for use in enhanced electro-oxidative sensing of nitrite. Microchim. Acta 2017, 184, 4141–4149. [Google Scholar] [CrossRef]
- Fan, H.; Zhang, S.; Ju, P.; Su, H.; Ai, S. Flower-like Bi2Se3 nanostructures: Synthesis and their application for the direct electrochemistry of hemoglobin and H2O2 detection. Electrochim. Acta 2012, 64, 171–176. [Google Scholar] [CrossRef]
- Dong, S.; Li, M.; Wei, W.; Liu, D.; Huang, T. An convenient strategy for IgG electrochemical immunosensor: The platform of topological insulator materials Bi2Se3 and ionic liquid. J. Electrochem. Soc. 2017, 21, 793–801. [Google Scholar] [CrossRef]
- Liu, H.; Duan, C.; Yang, C.; Shen, W.; Wang, F.; Zhu, Z. A novel nitrite biosensor based on the direct electrochemistry of hemoglobin immobilized on MXene-Ti3C2. Sens. Actuators B Chem. 2015, 218, 60–66. [Google Scholar] [CrossRef]
- Wang, F.; Yang, C.; Duan, C.; Xiao, D.; Tang, Y.; Zhu, J. An organ-like titanium carbide material (MXene) with multilayer structure encapsulating hemoglobin for a mediator-free biosensor. J. Electrochem. Soc. 2015, 162, B16–B21. [Google Scholar] [CrossRef]
- Wang, F.; Yang, C.; Duan, M.; Tang, Y.; Zhu, J. TiO2 nanoparticle modified organ-like Ti3C2 MXene nanocomposite encapsulating hemoglobin for a mediator-free biosensor with excellent performances. Biosens. Bioelectron. 2015, 74, 1022–1028. [Google Scholar] [CrossRef]
- Zheng, J.; Diao, J.; Jin, Y.; Ding, A.; Wang, B.; Wu, L.; Weng, B.; Chen, J. An inkjet printed Ti3C2-GO electrode for the electrochemical sensing of hydrogen peroxide. J. Electrochem. Soc. 2018, 165, B227–B231. [Google Scholar] [CrossRef]
- Kumar, S.; Lei, Y.; Alshareef, N.H.; Quevedo-Lopez, M.A.; Salama, K.N. Biofunctionalized two-dimensional Ti3C2 MXenes for ultrasensitive detection of cancer biomarker. Biosens. Bioelectron. 2018, 121, 243–249. [Google Scholar] [CrossRef]
- Toh, R.J.; Mayorga-Martinez, C.C.; Sofer, Z.; Pumera, M. 1T-Phase WS2 Protein-Based Biosensor. Adv. Funct. Mater. 2017, 27, 1604923. [Google Scholar] [CrossRef]
- Tang, J.; Quan, Y.; Zhang, Y.; Jiang, M.; Al-Enizi, A.M.; Kong, B.; An, T.; Wang, W.; Xia, L.; Gong, X.; et al. Three-dimensional WS2 nanosheet networks for H2O2 produced for cell signaling. Nanoscale 2016, 8, 5786–5792. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yin, X.; Xia, M.; Zhang, W. Tungsten disulfide nanosheets supported poly(xanthurenic acid) as a signal transduction interface for electrochemical genosensing applications. RSC Adv. 2018, 8, 39703–39709. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Yin, H.; Zhou, Y.; Sui, C.; Wang, Y.; Meng, X.; Waterhouse, G.I.N.; Ai, S. Photoelectrochemical biosensor for microRNA detection based on a MoS2/g-C3N4/black TiO2 heterojunction with Histostar@AuNPs for signal amplification. Biosensors Bioelectron. 2019, 128, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Xu, J.; Chen, J.; Xu, Q.; Xiao, X.; Jin, D.; Pang, H.; Hu, X. Ultrasensitive electrochemical detection of H2O2 in living cells based on ultrathin MnO2 nanosheets. Sens. Actuators B Chem. 2017, 252, 72–78. [Google Scholar] [CrossRef]
- Lilja, H.; Ulmert, D.; Vickers, A.J. Prostate-specific antigen and prostate cancer: Prediction, detection and monitoring. Nat. Rev. Cancer 2008, 8, 268. [Google Scholar] [CrossRef] [PubMed]

| Electrode Architect | Target | Detection Method | Label | LOD | Linear Range | Real Sample | Device | Ref. |
|---|---|---|---|---|---|---|---|---|
| Ab2-HRP/Ab1/ssDNA/o-MWCNT/RGO/GSP | miR-141 and miR-29b-1 | DPV | anti-HRP | 10 fM | 10 fM to 1 nM | No | No | [73] |
| OB/ssDNA/GO/GNR/GCE | miR-155 | DPV | Oracet Blue (OB)) | 0.6 fM | 2.0 fM to 8.0 pM | Human plasma samples | No | [74] |
| ssDNA/SA/Au-PtBNPs/CGO/FTO | miRNA-21 | DPV | No | 1 fM | 1 fM to 1 μM | Spiked human serum | No | [78] |
| SA-ALP/ssDNA-GO-AuNPs/ssDNA/AuNPs/MgO/GCE | miRNA-21 | DPV | SA-ALP | 0.05 pM | 0.0001 to 100 pM | Human serum samples | No | [75] |
| ssDNA/NFG/AgNPs//FTO | miRNA-21 | DPV | No | 0.2 fM | 10 fM to 10 μM | Human blood samples | No | [76] |
| SA-dynabeads /ssRNA/GO-IO/SPCE | miRNA-21 | CA | SA-dynabeads | 1.0 fM | 1.0 fM to 1.0 nM | No | SPCE | [77] |
| N-doped-Multiple Graphene Agarose /AuNPs/GCE | ctDNA | DPV | No | 3.9 ng mL−22 | 1.0 × 10−21 to 1.0 × 10−16 ng mL−1 | Human serum samples | No | [79] |
| SA-dynabeads /ssRNA/GO-NPFe2O3/SPCE | ctNAs- FGFR2:FAM76A | CA | SA dynabeads | 1 fM | 1.0 fM to 1.0 nM | Human plasma samples | No | [80] |
| Au@ SCX8- Au@SCX8-rGO-TB-LP/AP/ssRNA/Au@Fe3O4/SPCE | mRNA (CCND2-S and CCND2-L) | DPV | Au@SCX8-RGO-TB-LP | CCND2-S: 0.176 aM; CCND2-L: 9.5 aM | CCND2-S: 10−18 to 10−11 and CCND2-L: 10−17 to 10−11 M | Lung cancer cells | SPCE | [71] |
| MB/ssDNA/3D GF/AgNPs | CYFRA21-1 DNA | DPV | MB (MethylBlue) | 1.0 × 10−14 M | 1.0 × 10−14 to 1.0 × 10−7 M | Lung cancer cells | No | [81] |
| Ni-OTC NPs/ssDNA-OMC/rGO/PGE | EGFR exon 21point mutation | DPV | Ni-OTC NPs | 120 nM | 0.1 μM to 3 μM | No | No | [82] |
| SA-HRP /ssDNA/rGO-CMC/SPCE | TP53 Gene | A | SA-HRP | sccp53: 3.4 nM, lcpp53: 2.9 nM | 0.01 to 0.1 μM | Total human serum and saliva | SPCE | [70] |
| ssDNA reporter-AuNP/ssDNA /Gr/ITO | BRCA1 gene | CA | DNA-AuNP | 1 fM | 1 fM to 1 nM | No | No | [69] |
| Electrode Architect | Target | Detection Method | Label | LOD | Linear Range | Real Sample | Device | Ref. |
|---|---|---|---|---|---|---|---|---|
| rGO-FA/Au | Folic acid protein (FP) | CV/DPV | No | 1 pM | 1–200 pM | No | No | [85] |
| Ab/PEDOT:PSS/rGO/Whatman filter paper electrode | CEA | EIS/ CA | No | 25.8 µA ng−1 mL cm−2 | 1–8 ng·mL−1 | Human serum samples | No | [88] |
| Ab/AuNPs/Thi/rGO/Pure cellulose paper electrode | CA125 | DPV | No | 0.01 U mL−1 | 0.1 U·mL−1–200 U·mL−1 | Human serum samples | No | [91] |
| Apt/AuNPs/rGO/THI/Cellulose paper electrode | PSA | CV/DPV | No | 10 pg mL−1 | 0.05 to 200 ng·mL−1 | Human serum samples | Paper microfluidic device | [92] |
| MB/Apt/rGO-Chit/GCE | HER2 | DPV | MB | 0.22 ng mL−1 | 2–75 ng·mL−1 | Human serum samples | No | [87] |
| MWCNT-COOH /PEDOT/Graphene/Cellulose paper electrode | OS- 8-OHdG | CV/DPV | No | 14.4 ng mL−1 | 50–1000 ng·mL−1 | Human serum samples | Paper-Based Sensing Device | [93] |
| Ab2/PdPt nanocages/MWCNT/Ab1/NH2-GS/GCE | CEA | A | PdPt nanocages | 0.2 pg mL−1 | 0.001–20 ng·mL−1 | Human serum samples | No | [94] |
| Ab-HRP/pDA/3D-Graphene | CEA | DPV | HRP | 90 pg mL−1 | 0.1–750.0 ng·mL−1 | Human serum samples | No | [95] |
| SA-HCR/Ab2/Ab1/ Graphene–Au/GCE | AFP, CEA, CA125, PSA | DPV | SA-HCR | 62, 48, 77 and 60 fg mL−1 | 0.2 to 800 pg·mL−1 for AFP, 0.2 to 600 pg·mL−1 for CEA, 0.2 to 1000 pg·mL−1 for CA125, 0.2 to 800 pg·mL−1 for PSA | Human serum samples | No | [96] |
| Ab/ZrO2–rGO/ITO | CYFRA-21-1 | DPV | No | 0.122 ng mL−1 | 2–22 ng mL−1 | Human saliva samples | No | [97] |
| HRP/Ab2/Ab1-Fe3O4@GO/Carbon electrode | PSA and PSMA | A | HRP | 15 fg·mL−1 for PSA and 4.8 fg·mL−1 for PSMA | 61 fg·mL−1 - 3.9 pg·mL−1 for PSA and9.8–624 pg·mL−1 for PSMA | Human serum samples | Microfluidic immunoarray device | [89] |
| Ab/TB/AuNPs/Fe3O4-rGO/GCE | AFP | A | No | 2.7 fg·mL−1 | 1.0 × 10−5–10 ng·mL−1 | No | No | [98] |
| Ab/CysA-Au NPs/Ag-rGO/Photo paper | CA153 | CA | No | 15 U·mL−1 | 15–125 U·mL−1 | Human plasma samples | μPAD | [99] |
| Ab2-porous-hollowed-Ag-Au/Ab1/Graphene/SPCE | PSA | DPV | Metal-ion-loaded AuNP | 0.13 pg·mL−1 | 0 - 80 ng·mL−1 | Human serum samples | SPCE | [100] |
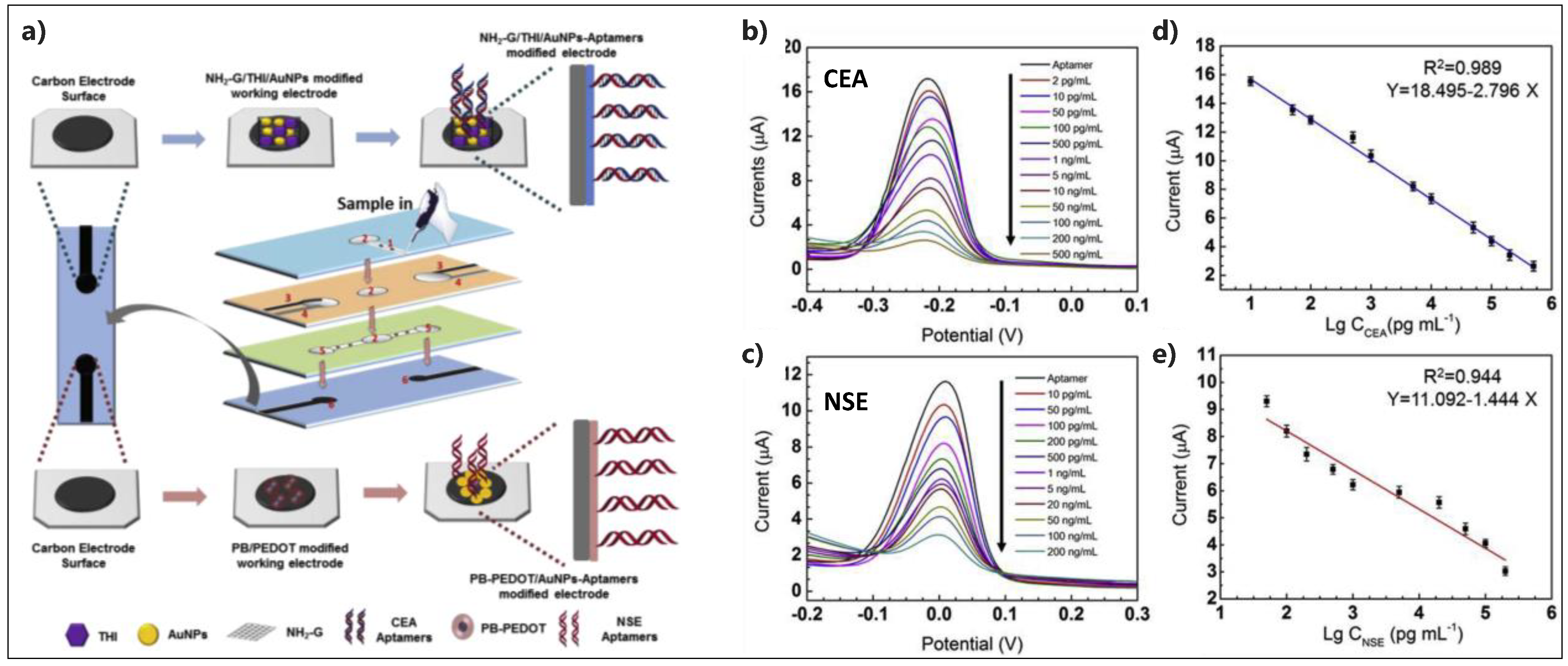
| Apt/NH2- Graphene-THI-AuNPs and PB-PEDOT- AuNPs/SPE | CEA, NSE | DPV | No | 2 pg·mL−1 for CEA and 10 pg·mL−1 for NSE | 0.01–500 ng·mL−1 | Human serum samples | Microfluidic paper-based device | [90] |
| HRP-Ab2-SiO2/Ab1/GO/chitosan/Pure cellulose paper | AFP, CEA, CA125, CA153 | DPV | HRP | 0.001 ng·mL−1 for AFP, 0.005 ng·mL−1 for CEA, 0.001 ng·mL−1 for CA125, and 0.005 ng·mL−1 for CA153 | 0.001−100 ng·mL−1 for AFP,0.005−100 ng·mL−1 for CEA, 0.001−100 ng·mL−1 for CA125, and 0.005−100 ng·mL−1 for CA153 | Human serum samples | μPAD | [84] |
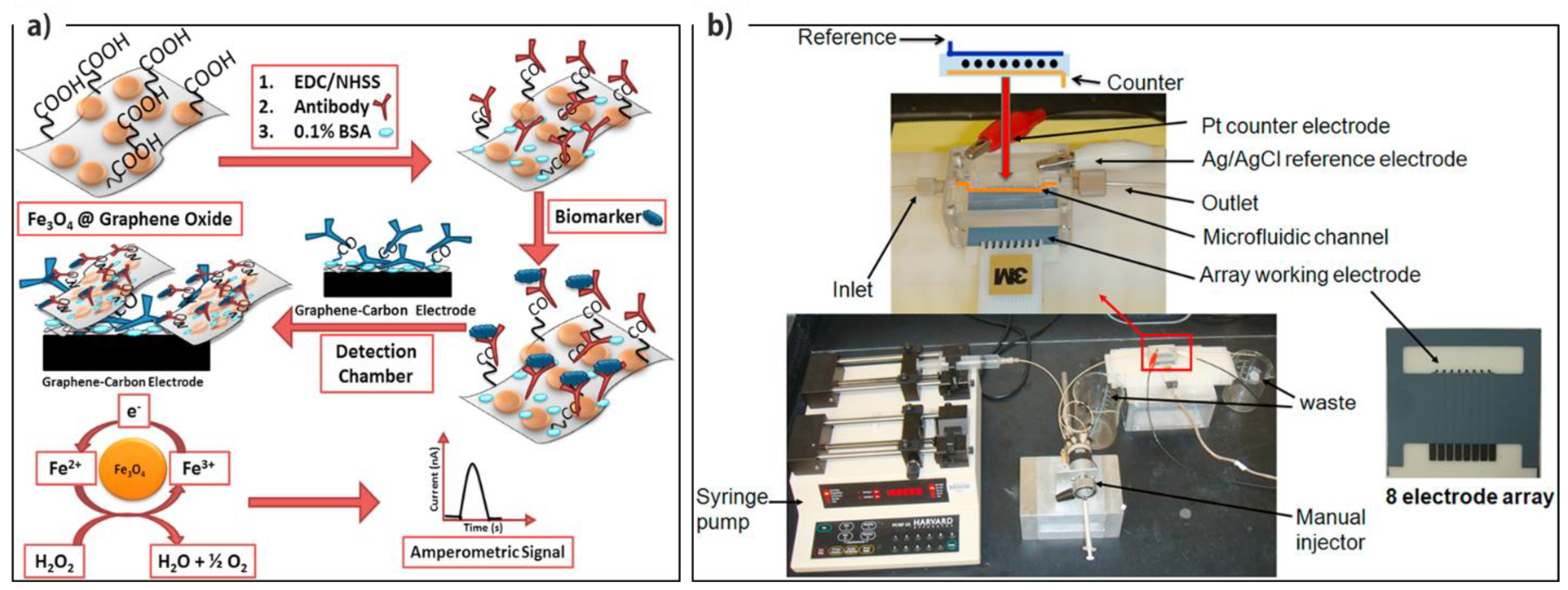
| FeONPs and CoZnFeONPs-/Ab2/Ab1/GO/Carbon-based electrode | CYFRA 21-1 | A | No | 0.3 fg·mL−1 for FeONPs and 0.19 fg·mL−1 for CoZnFeONs | 3.9 to 500 fg·mL−1 for FeONPs and 3.9 to 1000 fg·mL−1 for CoZnFeONs | Human serum samples | μIDs | [101] |
| Electrode Architect | Target | Detection Method | LOD | Linear Range | Real Sample | Device | Ref. |
|---|---|---|---|---|---|---|---|
| MNPs@Y-1,4-NDC-MOF/ERGO/GCE (M = Ag, Cu) | H2O2 | A | 0.18 μM | 4 to 11,000 μM | Living cells | No | [120] |
| NSG/GCE | H2O2 | A | 1 μM | 4 to 50 μM | Living cells | No | [110] |
| AuNPs-ctDNA-NGS/SPCE | NO | A | 0.8 nM | 2 to 500 nM | Living cells | SPCE | [121] |
| Au-Pd/rGO/GCE | H2O2 | A | 0.004 μM | 0.005 to 3500 μM | Human breast cancer cells | No | [122] |
| rGo/AuNP/Paper electrode | H2O2 | CV | 15 μM | 8.53 to 17.35 mM | No | No | [123] |
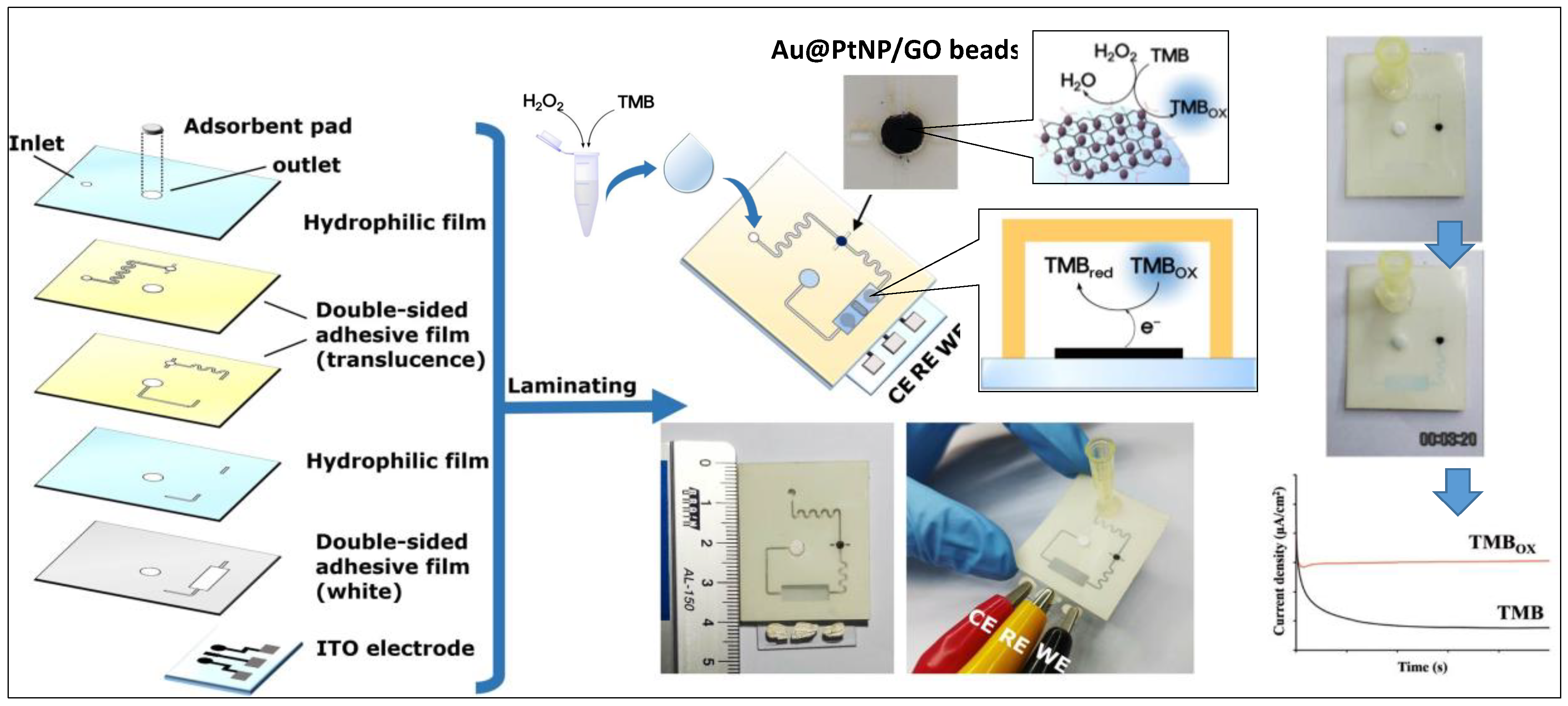
| Au@PtNP/GO nanozymes/ITO | H2O2 | CA | 1.62 μM | 0.001 to 3 mM | Human urine samples | Electrochemical microfluidic devices | [119] |
| Ag-PdNPs/rGO/GCE | H2O2 | CV/A | 1.1 μM | 0.005 to 14.65 mM | Human urine samples | No | [124] |
| Pt/Graphene-CNT/Paper electrode | H2O2 | A | 10 nM | 0.1 To 25 μM | Living cells | No | [118] |
| AuNPs/N-GQDs/GCE | H2O2 | CV/A | 0.12 μM | 0.25 to 13327 μM | Human serum samples and living cells | No | [125] |
| N-Graphene/GCE | H2O2 | A | 0.05 μM | 0.5 mM to 1.2 mM | Living cells | No | [103] |
| (Graphene-AP-laminin)10/ITO | H2O2 | A | 0.1 μM | Up to 100 μM | Living cells | No | [108] |
| Electrode Architect | Target | Detection Method | Label | LOD | Linear Range | Real Sample | Fabricated Device | Ref. |
|---|---|---|---|---|---|---|---|---|
| ssDNA/nanoMoS2/CPE | ssDNA | DPV | No | 1.9 × 10−17 M | 10−16–10−10 M | No | No | [136] |
| ssDNA/MoS2-graphene/CGE | ctDNA | DPV | No | 10−17 M | 10−16–10−13 M | No | No | [131] |
| ssDNA/MoS2-polyaniline/Pt | ssDNA | DPV | No | 10−15 M | 10−15–10−6 M | Human serum | No | [137] |
| ssDNA/PXA-MoS2/CPE | ctDNA | EIS | No | 1.8 × 10−17 M | 10−16–10−10 M | No | No | [138] |
| ssDNA/PANI-MoS2/ITO | ssDNA | EIS | No | 3 × 10−18 M | 10−6–10−17 M | CML positive patient samples | No | [139] |
| ssDNA/PIn6COOH-MoS2/CPE | ctDNA (PIK3CA gene) | EIS | No | 1.5 × 10−17 M | 10−16 M–10−1 M | No | No | [140] |
| DNA2/DNA1/AuNPs@MoS2/GCE | miRNA-21 | DPV; EIS | No | 0.78 fM (DPV); 0.45 fM (EIS) | 10 fM–1 nM | Human serum | No | [132] |
| ssDNA/AuNPs@MoS2-Thi/GCE | miRNA-21 | DPV | No | 0.26 pM | 1.0 pM–10 nM | Human serum | No | [133] |
| ssDNA/AuNPS@hollow MoS2 microcubes/GCE | miRNA-21 | DPV | No | 0.086 fM | 0.0001–100 pM | Human breast cancer serum | No | [134] |
| H2/H1/cDNA/AuNPs@Carbon sphere-MoS2/GCE | miRNA-21 | DPV | HRP | 1.6 × 10−17 M | 0.0001–100 pM | Human serum | No | [135] |
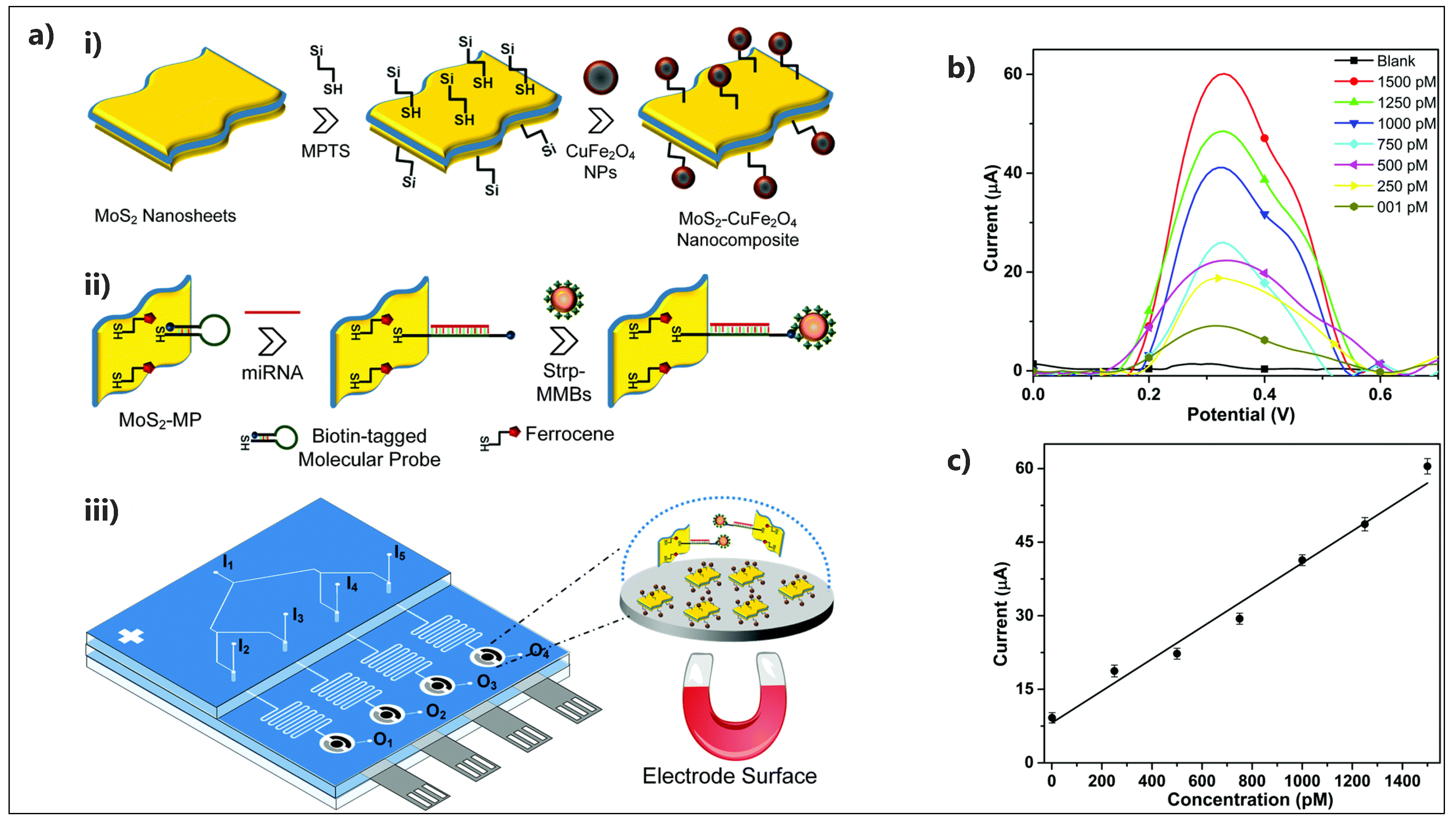
| Strp-MMBs/ssDNA/MoS2-CuFe2O4 | multiplexed miRNA | SWV | FcSH | 0.48 pM | 1 pM–1.5 nM | Human serum; positive clinical samples - blood and fecal samples | Microfluidic biosensor | [141] |
| ssRNA/AuNPs@MoS2-Ti3C2/GCE | miRNA-182 | DPV | No | 0.43 fM | 1 fM–0.1 nM | Human serum | No | [142] |
| PtCuMOF-DNA3/4/DNA1/2/MoS2/AuNPs/AgNW | miRNA-141; miRNA-21 | SWV | G4/Hemin | 0.1 fM | 1 fM–1 nM | Human serum | Paper-based biosensor | [143] |
| Electrode Architect | Target | Detection Method | Label | LOD | Linear Range | Real Sample | Device | Ref. |
|---|---|---|---|---|---|---|---|---|
| Fc-AuNPs-Apt2/Apt1/AuNPs/MoS2/carbon aerogel/GCE | PDGF-BB | DPV | FcSH | 0.3pM | 0.001–10nM | Human serum | No | [144] |
| Anti-CEA/AuNPs-Thi-MoS2/GCE | CEA | SWV | No | 0.52 pg/mL | 1 pg/mL–10 ng/mL | Human serum | No | [147] |
| Ab2/Ab1/MoS2-Au/GCE | CEA | DPV | Ag nanosphere | 0.27 pg/mL | 1 pg/mL–50 ng/mL | Human serum | No | [148] |
| Hemin/PdNPs/PDDA-G-MoS2/TBA-NH2/GCE | Thrombin | DPV | G4/Hemin | 0.062 pM | 0.0001–40 nM | Human blood serum | No | [145] |
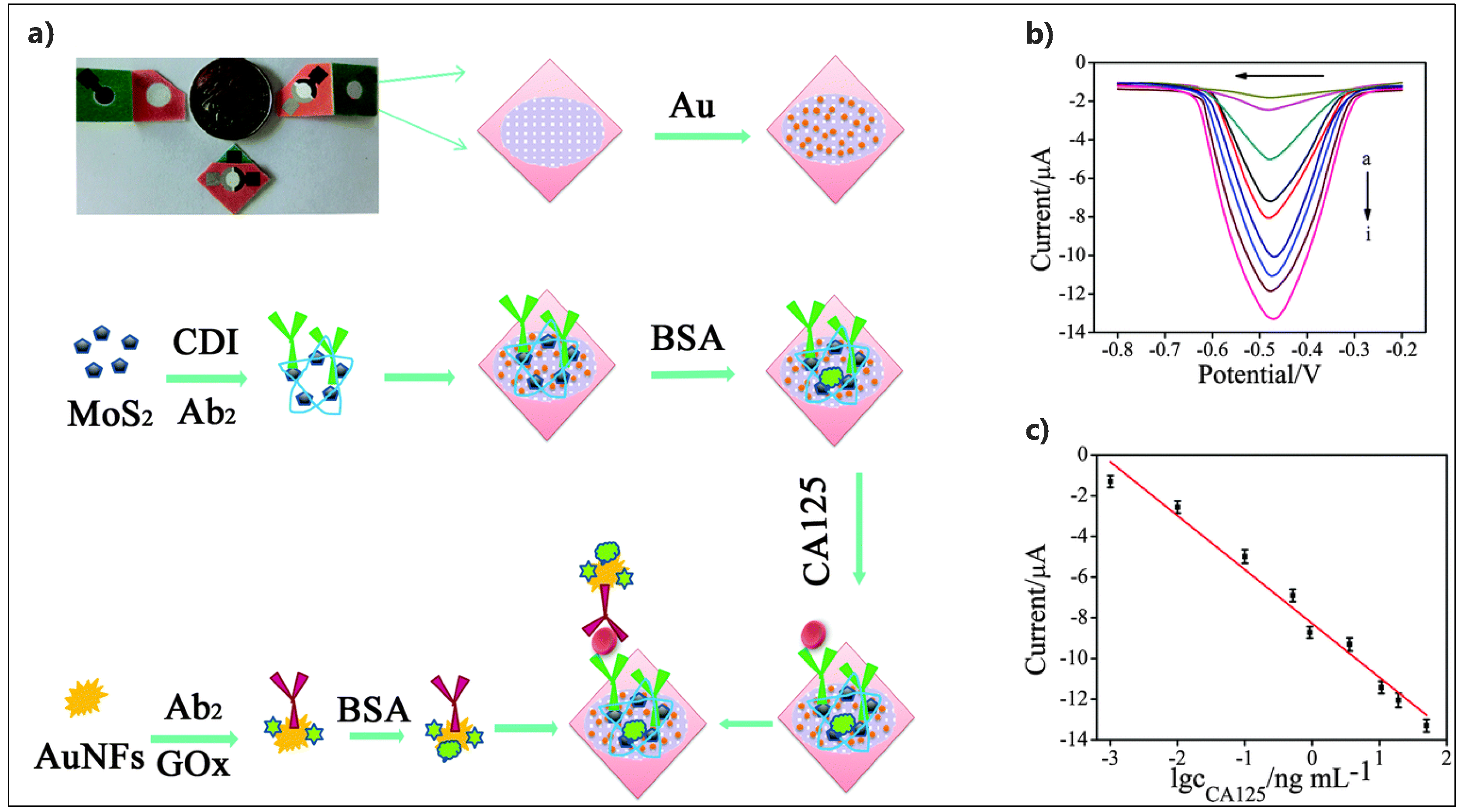
| GOx-AuNF@Ab2/Ab1/MoS2-Au/Paper | CA125 | DPV | GOx | 0.38 pg/mL | 0.001–50 ng/mL | Human serum | Microfluidic paper-based | [150] |
| HRP-DNA/Ab2/Ab1/AuNPs@hollow MoS2 microbox/GCE | AFP | DPV | HRP | 2.0 fg/mL | 0.00005–75 ng/mL | Human serum | No | [151] |
| Apt/MoS2QDs@g-C3N4@CSAuNPs | PSA | EIS | No | 0.72 ng/mL | 1.0–250 ng/mL | Human serum | No | [152] |
| Anti-PSA/MoS2/Au | PSA | EIS | No | 0.001 ng/mL | 0.001–200 ng/mL | Human serum | No | [153] |
| Anti-PSA-Ag/MoS2@Fe3O4/AgNPs/GCE | CEA | DPV | No | 0.03 pg/mL | 0.0001–20 ng/mL | Human serum | No | [154] |
| Anti-CEA/MoS2-PBNCs/GCE | CEA | DPV | No | 0.54 pg/mL | 0.005–10 ng/mL | Human serum | No | [148] |
| Anti-CEA/ Ag/MoS2/rGO/GCE | CEA | A | No | 1.6 fg/mL | 0.01 pg/mL–100 ng/mL | Human serum | No | [155] |
| HRP-Ab2/Ab1/MoS2-AuNPs/GCE | CEA | DPV | HRP | 1.2 fg/mL | 10 fg/mL–1 ng/mL | Human serum | No | [149] |
| Electrode Architect | Target | Detection Method | LOD | Linear Range | Real Sample | Device | Ref. |
|---|---|---|---|---|---|---|---|
| Hb/AuNPs@MoS2/GCE | H2O2; NO | CV | H2O2: 4 µM; NO: 5 µM | H2O2: 10–300 µM; NO: 10–1100 µM | No | No | [171] |
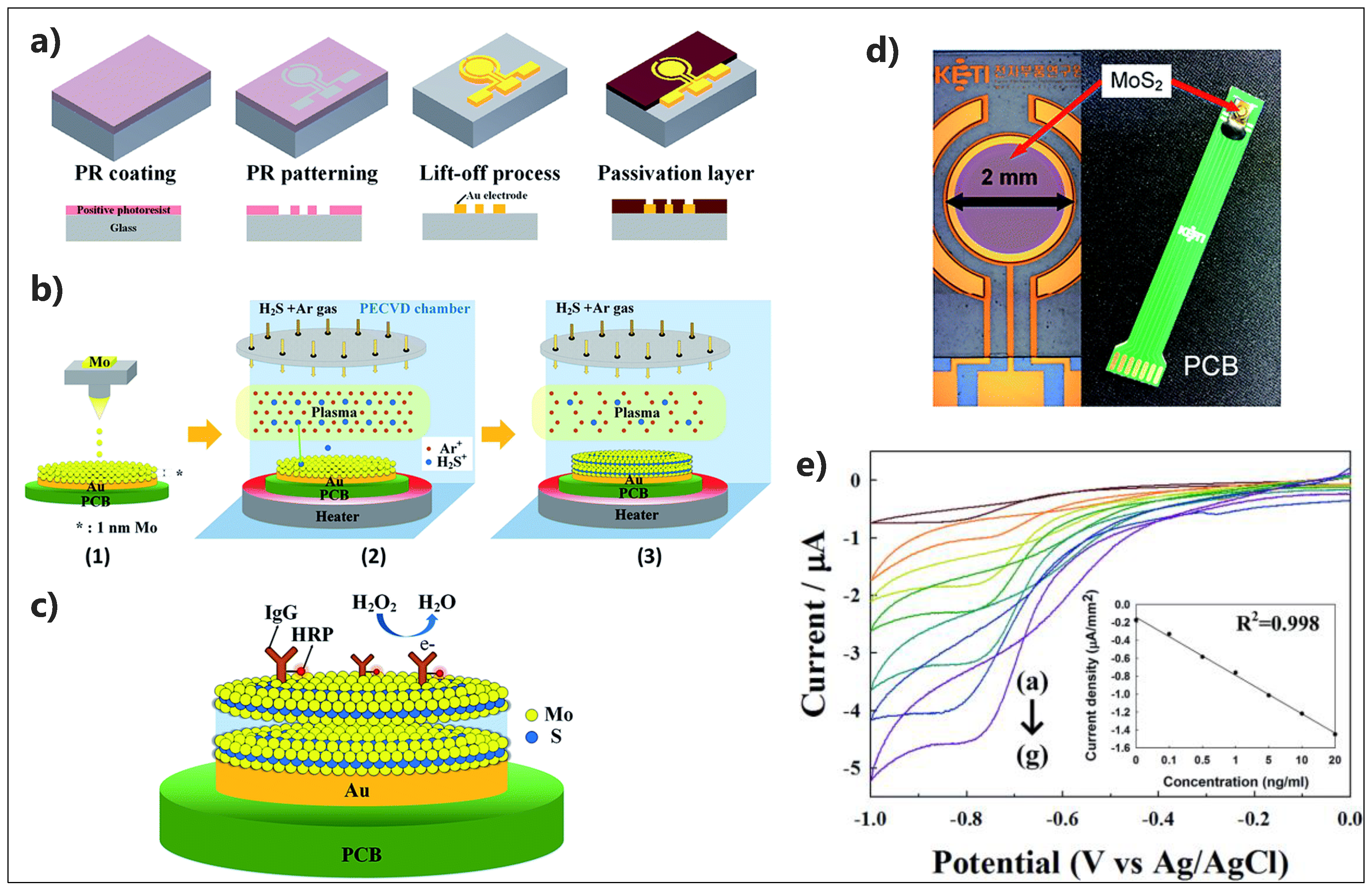
| IgG-HRP/MoS2-Au/PCB | H2O2 | CV | No | 0–20 ng/mL | No | No | [172] |
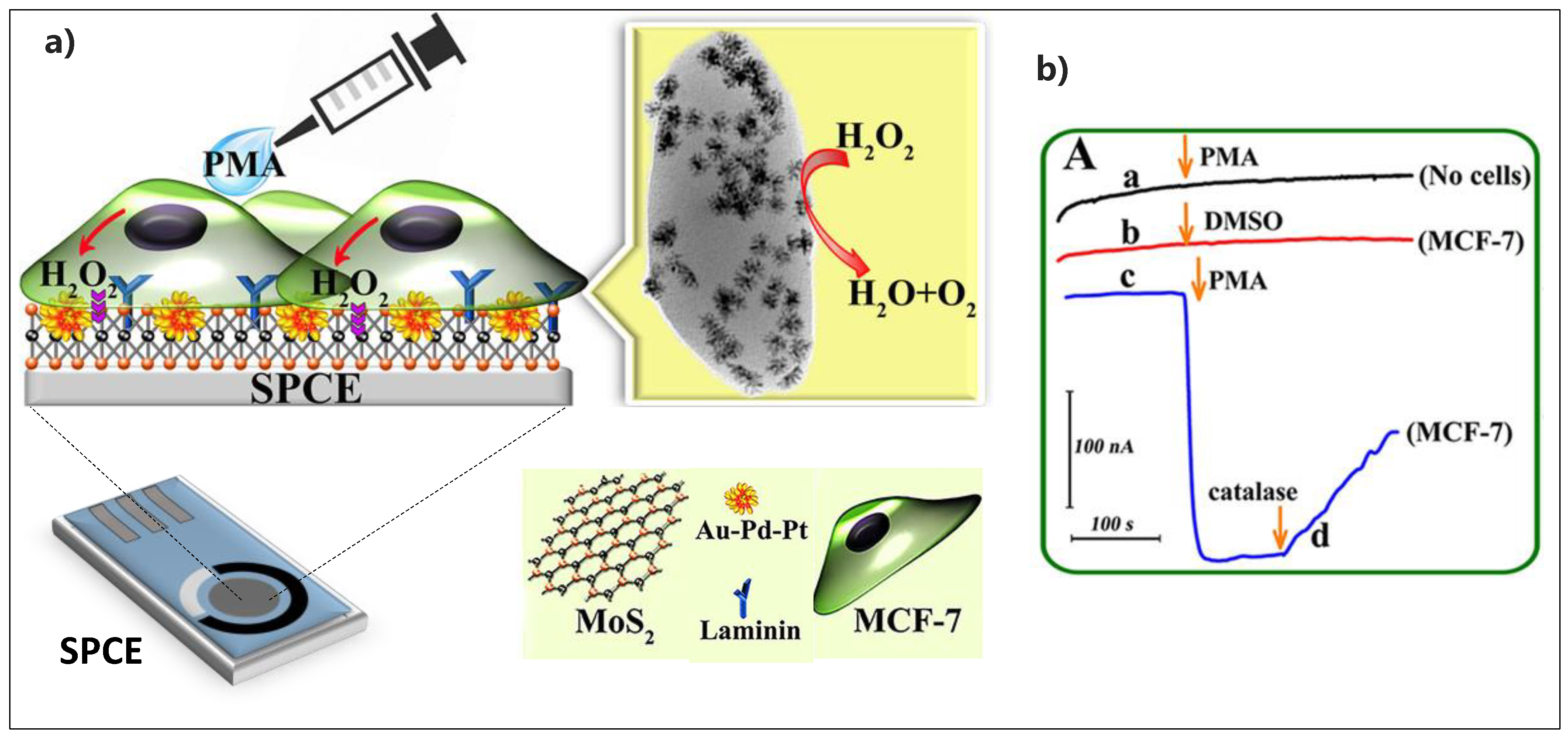
| Laminin/Au−Pd−Pt/MoS2/SPCE | H2O2 | CV | 0.3 nM | 1–100 nM | MCF-7 cancer cells | SPCE | [165] |
| Nafion/Hb/MoS2-rGO/GCE | H2O2 | CV | 25 nM | 0.1–250 µM | No | No | [156] |
| PtW/MoS2/GCE | H2O2 | A | No | Up to 5 nM | Breast cancer 4T1 cells | No | [162] |
| Nafion/Mb/MoS2 -graphene/ Nafion/GCE | H2O2; NaNO2 | CV | H2O2: 1.25 µM; NaNO2: 0.125 mM | H2O2: 6.25–225 µM; NaNO2: 1.25–12.5 mM | H2O2: Milk sample | No | [172] |
| CuNFs/MoS2/GCE | H2O2 | A | 0.021 µM | 0.04–1.88 µM | Tap water | No | [157] |
| MoS2-PtNP/GCE | H2O2 | A | 0.345 µM | 0.02–4.72 mM | No | No | [174] |
| MoS2-PBNCs/GCE | H2O2 | A | 4.1 nM | 0.01–300 µM | No | No | [148] |
| Mb/GO@MoS2/Au | H2O2 | A | 20 nM | - | No | No | [175] |
| Catalase/MoS2-Au/chitosan/GCE | H2O2 | A | 10−7 M | 5 × 10−7–2 × 10−4 M | SP2/0 cells | No | [164] |
| Au-Pd/MoS2/GCE | H2O2 | DPV | 0.16 µM | 0.8 µM–10 mM | No | No | [158] |
| MoS2/PtNPs/Au/AN | H2O2 | DPV; A | 0.686 µM | 1–100 µM | HeLa cells | No | [176] |
| PB/MoS2-rGO/GCE | H2O2 | A | 0.14 µM | 0.0003–1.15 mM | Tap & river water | No | [160] |
| O-MoS2/graphene/GCE | H2O2 | A | 0.12 µM | 0.25–16 mM | Water | No | [159] |
| Pt-Pd/MoS2/GCE | H2O2 | A | 3.4 µM | 10 to 80 µM | No | No | [177] |
| MoS2-OCu/GCE | H2O2 | A | 0.0767 µM | 0.085–38.0 mM | No | No | [178] |
| MoS2-ICPC/GCE | H2O2 | A | 11.8 µM | 20–300 µM | No | No | [179] |
| 3D RGO-MoS2QDs/GCE | H2O2 | A | 1.90 µM | 0.01–5.57 mM | No | No | [161] |
| MoS2/CN NWs/GCE | H2O2 | A | 0.73 µM | 2–500 µM | Epithelial cells (A549 cells) | No | [163] |
| Pt/MoS2/Ti | H2O2 | A | 0.87 µM | 10–160 µM | No | No | [180] |
| Fe2O3@MoS2/Nafion/GCE | NO2− | A | 1.0 µM | 2.0–6730 µM | Drinking water; River water | No | [166] |
| TOSC-MoS2/GCE | NO2− | A | 2 µM | 6–3140, 3140–4200 µM | Drinking water; River water | No | [167] |
| Fe3O4/MoS2/GCE | NO2− | A | 0.5 µM | 1–2630 µM | No | No | [181] |
| Mb/GO/Amine-modified MoS2/Au | NO | A | 3.6 nM | - | No | No | [173] |
| AuNPs@MoS2-NSs/GCE | NO2− | DPV | 0.5 µmol/L | 5.0–260.0 µmol/L | Human serum | No | [168] |
| Ag/HNT/MoS2/CPE | NO2− | A | 0.7 µM | 2–425 µM | Tap water and aqueduct water | No | [182] |
| rGO-MoS2/GCE | NO2− | A | 0.17 µM | 0.2–4800 µM | Tap water | No | [183] |
| AuNPs/MoS2/GCE | NO2− | A | 1.67 µM | 5.0 µM–27.8 mM | Tap water | No | [169] |
| α-MnO2-MoS2/GCE | NO2− | A | 16 µM | 100–800 µM | Drinking water | No | [184] |
| PrFeO3-MoS2/GCE | NO2− | CV | 1.67 µM | 0.005–3 mM | Tap water; Local river water and waste water | No | [185] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammadniaei, M.; Nguyen, H.V.; Tieu, M.V.; Lee, M.-H. 2D Materials in Development of Electrochemical Point-of-Care Cancer Screening Devices. Micromachines 2019, 10, 662. https://doi.org/10.3390/mi10100662
Mohammadniaei M, Nguyen HV, Tieu MV, Lee M-H. 2D Materials in Development of Electrochemical Point-of-Care Cancer Screening Devices. Micromachines. 2019; 10(10):662. https://doi.org/10.3390/mi10100662
Chicago/Turabian StyleMohammadniaei, Mohsen, Huynh Vu Nguyen, My Van Tieu, and Min-Ho Lee. 2019. "2D Materials in Development of Electrochemical Point-of-Care Cancer Screening Devices" Micromachines 10, no. 10: 662. https://doi.org/10.3390/mi10100662
APA StyleMohammadniaei, M., Nguyen, H. V., Tieu, M. V., & Lee, M.-H. (2019). 2D Materials in Development of Electrochemical Point-of-Care Cancer Screening Devices. Micromachines, 10(10), 662. https://doi.org/10.3390/mi10100662





